Abstract
While most animals live in a three-dimensional world, they move through it to different extents depending on their mode of locomotion: terrestrial animals move vertically less than do swimming and flying animals. As nearly everything we know about how animals learn and remember locations in space comes from two-dimensional experiments in the horizontal plane, here we determined whether the use of three-dimensional space by a terrestrial and a flying animal was correlated with memory for a rewarded location. In the cubic mazes in which we trained and tested rats and hummingbirds, rats moved more vertically than horizontally, whereas hummingbirds moved equally in the three dimensions. Consistent with their movement preferences, rats were more accurate in relocating the horizontal component of a rewarded location than they were in the vertical component. Hummingbirds, however, were more accurate in the vertical dimension than they were in the horizontal, a result that cannot be explained by their use of space. Either as a result of evolution or ontogeny, it appears that birds and rats prioritize horizontal versus vertical components differently when they remember three-dimensional space.
Keywords: spatial cognition, three-dimensional navigation, hummingbirds, rats, locomotory style
1. Introduction
Although all animals inhabit a three-dimensional world, they move through it to different extents. For example, terrestrial animals tend to move more horizontally than they do vertically, whereas arboreal, swimming and flying animals may move relatively equally in the horizontal and vertical planes. Regardless of how animals move in space, they all need to orient themselves and navigate to relevant three-dimensional locations (e.g. to find food, mates and nests) within their environment. Until recently, however, the vast majority of research on navigation and spatial cognition has addressed the question of how animals return to rewarded locations in the horizontal plane, largely ignoring the vertical component ([1–6] but see [7]).
Where animals have been tested in a spatial task that requires them to move up or down through a test apparatus, animals attend to the vertical component and its presence can facilitate the learning of the horizontal component of a rewarded location. For example, in the first study to include the vertical component explicitly in a spatial experiment, rats trained to return to a rewarded location situated either on a vertical wall or on a surface tilted at an angle of 45° required fewer trials to learn that location than when they were trained on a floor [8]. Similar effects have been seen in birds and humans: pigeons learning a location on a tilted arena made fewer errors in returning to that location than when the arena was flat and humans pedalling on a bicycle simulator coupled to a tilted virtual environment made fewer navigation errors than when the virtual landscape was placed on a flat surface [9–11].
It is possible that this facilitation of learning by including a vertical component to spatial tasks occurs because there is a greater energetic cost to moving vertically, leading all animals to pay more attention to the vertical component of space than they pay to its horizontal component, so that they can minimize movement in that dimension [8,12]. The finding that rats learned first the vertical component of a three-dimensional location then its horizontal component when trained on a cubic maze would be consistent with this interpretation [8]. However, while energy expenditure might explain why hummingbirds relocate a single rewarded location more accurately in the vertical dimension than in the horizontal [13], and why rats move less vertically than they do horizontally when free-foraging [12], energetic cost does not explain why the sighted morph of the fish Astyanax fasciatus, trained on a Y-maze, when forced to choose one or the other dimension, consistently chose the vertical over the horizontal [14,15]. Rather than minimizing movement in the vertical the fish appear to prefer to move in that dimension [16]. Furthermore, hummingbirds trained and tested on a one-dimensional (linear) array learned a rewarded location only when the array was oriented horizontally rather than vertically. Additionally, when trained to a rewarded location in a two-dimensional array (presented on the diagonal) birds appeared to favour the horizontal over the vertical component when they had to choose between the components [17]. Neither of these results is consistent with accuracy of memory being associated with energetic cost.
An alternative explanation for why an animal may prefer to move in one spatial dimension over another is because the animal is more accurate in its memory for that component of a spatial location [18]. This differential accuracy might be a result of greater neural precision in the encoding of horizontal over vertical information (or vice versa), which may result from different selection pressures imposed on animals that vary in their locomotory style. For example, rats might remember the horizontal component of a three-dimensional location more precisely than its vertical component owing to the different firing patterns of grid cells, the neurons responsible for the metric encoding of space [19], while animals that move freely through three-dimensions such as fish and hummingbirds might not be impaired in the vertical dimension. Support for such neural variation correlated with locomotory style comes from the similar degree of encoding accuracy by fishes in both the horizontal and vertical dimensions of a Y-maze [16,20]. It may also be that such equivalence is enabled owing to their ability to estimate absolute depth through their swim bladder using hydrostatic pressure cues [21]. That hummingbirds, which move through three-dimensional space but do not have a swim bladder, are more accurate in the vertical than in the horizontal when relocating a three-dimensional location on open space [13] may suggest that their neural encoding is more similar to that of fishes than it is to that of rats.
A third possibility is that animals are more accurate in the dimension through which they move most frequently. To determine whether animals encode three-dimensional space in relation to their experience of three-dimensional during the task, we compared the memory for a rewarded location within a three-dimensional array by a terrestrial animal (rats) and a flying animal (hummingbirds). If differential spatial learning in three-dimensional tasks is a consequence of the animal's spatial experience during the task, then we would expect the use of space to predict accuracy, such that the more the animal moves in one spatial dimension, the greater the accuracy in that dimension at test. Given that we expected the hummingbirds to move through the vertical dimension of our three-dimensional apparatus more often than did rats, we expected the birds to be more accurate than the rats in their memory of the vertical component of a three-dimensional location. Accuracy could, however, result from a better encoding on one dimension than the other, regardless of experience owing to differences in the ways that place and grid cells are thought to encode three-dimensional information [18]. This being the case we expected the rats to be more accurate on the horizontal dimension.
2. Material and methods
(a). Study animals
We used experimentally naive male Lister hooded rats Rattus norvegicus (n = 6, aged two months on arrival, with a mean ± standard error weight of 276 ± 3 g; further details on housing, see the electronic supplementary material). Two weeks prior to experimentation, we began habituating the animals to handling to decrease the stress of being moved from cage to maze. During the experiment, which was carried out 7 days a week between 09.00 and 18.00 (the light phase) and in compliance with national (Animals (Scientific Procedures) Act, 1986) and international (European Communities Council Directive of 24 November 1986 (86/609/EEC)) legislation governing the maintenance of laboratory animals and their use in scientific experiments, rats were maintained at no less than 90% of their free-feeding body weight.
We also tested eight free-living male rufous hummingbirds Selasphorus rufus. This part of the experiment was run in a valley in the eastern Rocky Mountains, 20 km southwest of Beaver Mines, Alberta, Canada (49°20′56.61″ N, 114°24′38.49″ W). On return from overwintering in Mexico, males set up territories (figure 1a) along this valley centred on artificial feeders containing 14% sucrose solution [28,29]. Territorial males were caught and colour marked with non-toxic waterproof ink on the chest for individual identification.
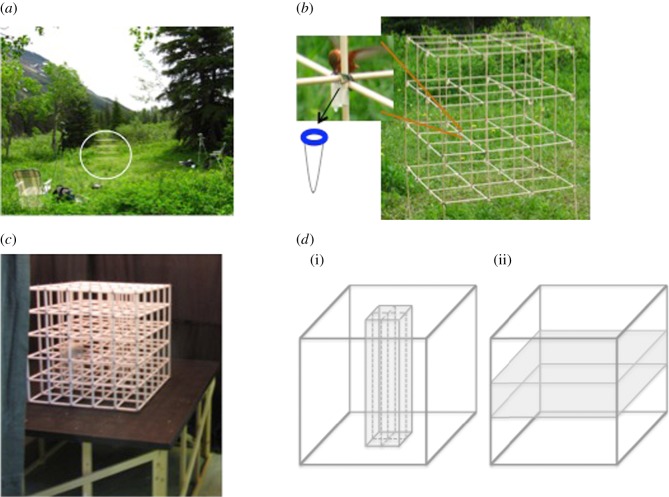
Figure 1.
Pictures and representations of both cubic mazes: a photograph of (a) a territory of one bird with a circle showing the maze secured to the ground and two video cameras to film the experiment; (b) the hummingbird 3 × 3 × 3 unit cubic maze (in which each unit was 25 × 25 × 25 cm) with a vial attached to every junction (64 in total). The inset shows a male feeding from the rewarded vial while perching and the arrow points to a schematic of the vial that contained the sucrose; (c) the rat 5 × 5 × 5 unit cubic maze (in which each unit was 10 × 10 × 10 cm) on the 120 × 90 cm platform; and (d) a schematic diagram of a generalized cubic maze showing the location of the reward, marked by an asterisk. (i) The light grey column represents the vertical region through which the animal could move while remaining in the correct x–y component of the reward. (ii) The dark grey plane represents the horizontal region through which the animal could move while remaining in the correct vertical component of the reward. (Online version in colour.)
(b). Apparatus
Training and testing were conducted in a cubic maze constituted of 27 (in a 3 × 3 × 3) and 125 (in a 5 × 5 × 5) units for hummingbirds and rats, respectively (figure 1b,c). The mazes were built using wooden rods (hummingbirds) and PVC (rats). The length of each side of each unit in the hummingbird maze was 25 and 10 cm in the rat maze. The hummingbird maze also contained a 200 µl vial on each junction (n = 64). Each vial was surrounded by a coloured disc (diameter, 1 cm = a ‘flower’; all flowers were either yellow, orange, pink, red, green or blue; figure 1b). All flowers were filled with water except one, which was filled with 25% sucrose solution.
The hummingbird maze was raised off the ground by approximately 30 cm (figure 1b). The rat maze was placed on a rectangular wooden platform, which was itself sitting on a wooden frame 40 cm from the ground. Black curtains surrounded the platform. We attached to the curtains one two-dimensional cue (a piece of blue and green plastic 30 × 4 cm) at 35 cm from the edge of the maze and 45 cm higher than the top of the maze and a second two-dimensional cue (a piece of coloured cardboard 45 × 30 cm) to a tripod, also placed 35 cm from the edge of the platform at the same height as the central levels of the maze (figure 1c).
(c). Experimental training and testing
(i). Rats
All rats received 6 days of training with four trials per day followed by two test days (for habituation and pre-training, see the electronic supplementary material). A reward (a 0.7 cm diameter piece of the same food reward used during maze habituation) was placed on the third and fourth levels of the maze at one of the 32 junctions where there were pegs radiating in all six directions. The reward was always located in the same place for each rat for the duration of the experiment but randomized across rats (electronic supplementary material, figure S1). Once the reward was on the maze, we placed the rat at one of the four corners of the platform randomly chosen for each trial. We considered a trial finished when the rat found the reward or after 10 min. The maze was cleaned and rotated in three-dimensions between trials and tests to prevent rats from using odour and visual cues intrinsic to the maze to relocate the reward.
Training was considered complete by the end of day 6 as all rats had considerably decreased their time to reach the reward (electronic supplementary material, figure S2). On days 7 and 10, we conducted probe tests, which consisted of placing the rat in the arena with the maze unbaited for 10 min. There was no training between days 7 and 10.
(ii). Hummingbirds
Hummingbirds were allowed to visit any of the 64 flowers in the array. Typically, a bird would leave the maze once he had drunk from the rewarded flower (200 µl is considerably more than a territorial male hummingbird consumes in a single foraging trip). The rewarded flower was refilled whenever he left the maze. Each bird was trained with a flower at a different rewarded location, which remained constant throughout the experiment for each bird. As for the rats, the reward was located on either level 3 or 4 at junctions from which pegs radiated in all six directions (electronic supplementary material). Experimental training continued until the bird made three consecutive visits to the array during which he visited the rewarded flower first. He then received a single test trial, for which we removed the rewarded flower.
All trials and tests were video recorded for both rats and hummingbirds, and all of the data for analysis were taken from the videos except for one bird (electronic supplementary material).
(d). Assessment of performance: learning
To evaluate the rats’ and birds’ ability to learn a three-dimensional location, we counted the number of crossings within the maze and the time taken to reach the rewarded location across trials and days. We then compared the average of the first three trials with the average of the last three trials.
(e). Use of the three spatial dimensions
To determine movement in each dimension of three-dimensional space, we compared the number of times each animal crossed from one unit (the smallest cubic subcomponent of the maze) to another in each of the axes x, y and z in the maze and the time they spent doing so, where x corresponded to right/left, y to forward/backward with respect to the video camera and z corresponded to up/down.
We considered a ‘crossing’ to have occurred when the head of the rat or bird crossed from one unit to another.
(f). Search strategy
To determine whether the animals moved went to the correct horizontal location of the reward and searched widely vertically (figure 1d(i)) or went to the correct vertical location of the reward and searched widely horizontally (figure 1d(ii)), we recorded the number of times the animals crossed units in these directions. Note that we combine data for x and y here for comparison with the data for the z component for two reasons: (i) this is the typical way to examine these data; (ii) although the dimension we code as x or y is arbitrary, the z-dimension is not, as z has polarity owing to gravity. In addition, the x–y plane could be rotated about the z-axis with no effect on behaviour.
Because each component contained a different number of units, we transformed these data into proportions. In the rats’ cubic maze, the animals could have made 25 crossings in the correct vertical component (i.e. all units with the same z as the goal), but only 20 in the horizontal component (all units sharing the x, y coordinates with the goal; figure 1d(i)(ii)) because owing to the reward being located at an intersection of units, there were four units representing the reward at each of the five levels.
In the birds’ cubic maze, the vertical component of the reward's location consisted of nine units while any of the four units around the reward at each level was considered the horizontal component. Therefore, the reward's horizontal component consisted of 16 units (four per level; figure 1b,d).
(g). Accuracy
For the test trial, the rewarded location in the rat maze was left empty while for the birds we removed the rewarded vial. To determine the accuracy with which rats and birds reached the rewarded location in the test trial and to assess whether they were more accurate in the vertical than in the horizontal plane, we looked at which unit they crossed first, once they had reached the rewarded location and found it to be empty (electronic supplementary material). For the animal to be more accurate in the vertical component than in the horizontal, we mean that the animal remained at the correct height and moved horizontally in search of the missing reward.
3. Results
(a). Task acquisition: learning
The performance of both species improved with experience decreasing time taken and number of crossings to reach the rewarded location (see the electronic supplementary material, figures S2 and S3).
(b). Use of the three spatial dimensions
During training, the rats crossed more units and spent more time moving in the z-axis than either in x or y (Friedman; crossings: 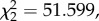 p < 0.001, figure 2a; time:
p < 0.001, figure 2a; time: 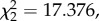 p < 0.001; mean ± s.e., z crossings: 8.46 ± 0.67; time: 9.7 ± 0.93, or x crossings: 6.84 ± 0.56, y crossings: 6.35 ± 0.58; time, 7.5 s ± 0.69, 7.9 s ± 0.88, respectively).
p < 0.001; mean ± s.e., z crossings: 8.46 ± 0.67; time: 9.7 ± 0.93, or x crossings: 6.84 ± 0.56, y crossings: 6.35 ± 0.58; time, 7.5 s ± 0.69, 7.9 s ± 0.88, respectively).
Figure 2.
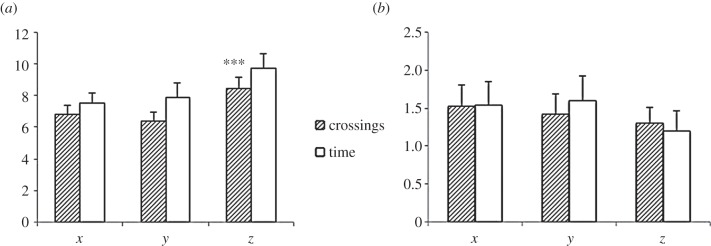
Use of space. The data are the mean number of crossings (hatched bars) on each axis (x, y and z) and time (white bars) in seconds by (a) rats (mean ± s.e. for six rats) and (b) hummingbirds (mean ± s.e. for seven hummingbirds). Asterisks represent a significance level of p < 0.001.
The birds made a similar number of crossings and spent a similar amount of time flying in each of the three axes (x, y, z; Friedman; crossings: 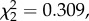 p = 0.857; figure 2b; time:
p = 0.857; figure 2b; time: 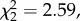 p = 0.274). Birds performed a similar number of crossings and spent a similar amount of time in each of the three axes (mean ± s.e., crossings: 1.52 ± 0.28, 1.42 ± 0.26, 1.3 ± 0.21 and time: 1.53 s ± 0.32, 1.6 s ± 0.32, 1.2 s ± 0.26).
p = 0.274). Birds performed a similar number of crossings and spent a similar amount of time in each of the three axes (mean ± s.e., crossings: 1.52 ± 0.28, 1.42 ± 0.26, 1.3 ± 0.21 and time: 1.53 s ± 0.32, 1.6 s ± 0.32, 1.2 s ± 0.26).
(c). Search strategy during training
While the use of the three spatial dimensions refers to the animal's movement in the whole maze, the search strategy concerns their crossings from one unit to another in the subset of the maze that constituted only the sections that contained the reward's vertical and horizontal components (figure 1d(i)(ii)). During training, the rats moved vertically more than they did horizontally (proportion of crossings: mean ± s.e., 0.33 ± 0.02 versus 0.19 ± 0.02; Wilcoxon, Z = 7.314, p < 0.001, figure 3a; proportion of time: mean ± s.e., 0.42 ± 0.02 versus 0.24 ± 0.02; Wilcoxon, Z = 6.416, p < 0.001). The rats’ search strategy (electronic supplementary material) remained constant across trials and days (linear mixed model; trials, proportion of time: F5,113 = 1.435, p = 0.236; proportion of crossings: F5,113 = 0.663, p = 0.179; days, proportion of time: F5,113 = 1.565, p = 0.176; proportion of crossings: F5,113 = 1.255, p = 0.288).
Figure 3.
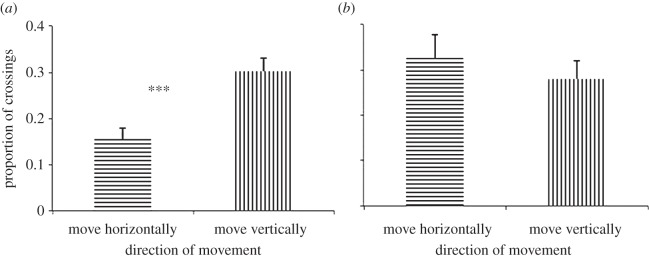
Search strategy in rats and hummingbirds. The data are the mean (±s.e.) proportion of the number of units crossed in the reward's z and x–y components across the training sessions by (a) rats and (b) hummingbirds. The direction of the lines within the bars represents the direction of crossings. The asterisks represent a significance level of p < 0.001.
Unlike the rats, during training, the birds crossed a similar proportion of units within the reward's vertical component as they did within its horizontal component (proportion of crossings: mean ± s.e., 0.33 ± 0.05 and 0.28 ± 0.04; Wilcoxon; Z = 1.062, p = 0.288; figure 3b). Furthermore, while rats moved more vertically than they do horizontally (they cross a higher proportion of units in the reward's horizontal component than in its vertical component), birds do not and the difference in the amount of movement through space by the two species was significantly different (two-factor ANOVA, species × dimension interaction: F1,22 = 2 0.849, p ≤ 0.0001; figure 3).
(d). Accuracy
In test 1, after reaching the rewarded location and finding it empty, rats tended to move to a location that was vertically adjacent (day 7 test: binomial with an expected proportion of 0.33 (V), 0.67 (H) (four of six rats), Z = 1.32, p = 0.097). In the second test, this tendency was significant (day 10 test: binomial (five of six rats), Z = 2.188, p = 0.014; figure 4a).
Figure 4.
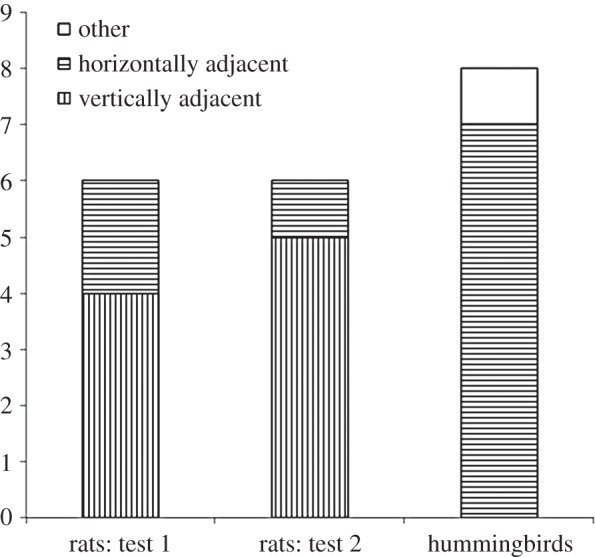
Test results for both rats and hummingbirds: the rats’ first choice in the two test trials (days 7 and 10) and the hummingbirds’ first choice during the test. For both species, the data are the number of animals that moved either to a vertical or horizontally adjacent location or to a non-adjacent location.
When the birds did not find a reward at the rewarded location, they tended to move to a location that was horizontally adjacent (binomial with an expectation of 0.33 (V), 0.67 (H) (seven of seven birds), Z = 1.455, p = 0.061; figure 4b). Furthermore, birds continued to visit flowers in this plane for their second choice (Wilcoxon; second choice: Z = 2.25, p = 0.024).
The direction in which each species moved when they found the rewarded location to be empty on the test differed with respect to the other (Fisher's exact test, p = 0.021), suggesting that the rats were more sure of the horizontal component while the birds were more sure of the vertical component.
4. Discussion
Rats and hummingbirds both learned a three-dimensional location within a cubic maze after only a few rewarded experiences. In learning the goal location, rats moved more in the z-dimension than they did in either the x or the y, whereas the hummingbirds moved equally in all three dimensions. However, once the animals had learned the goal location, rats and hummingbirds differed in their accuracy in the absence of the reward: rats searched up or down relative to the rewarded location, whereas hummingbirds searched horizontally around the rewarded location.
Although in previous experiments rats moved more in the horizontal plane than they did vertically [8,12], during the training prior to testing, our rats moved more in the vertical than they did in either of the two horizontal components and certainly more than did the hummingbirds. There are several possible explanations for the difference between our rats’ behaviour and that of the rats in the earlier work: (i) our rats did not minimize their vertical movements owing to their pre-experimental habituation; (ii) the maze was relatively easy to climb, because the rough texture of the rods reduced their slipperiness; (iii) in the Jovalekic task [12], the animals were provided with food throughout the maze, whereas our animals had to learn a single three-dimensional location. We would contend that the rats chose the more efficient strategy in each experiment. With regard to our experiment, however, the difference in the responses at test by rats and hummingbirds in the absence of the goal allows us to rule out experience during the task as an explanation for the difference in their three-dimensional accuracy.
Our alternative prediction was that a difference in accuracy between the species is owing to differences in their neural encoding of the spatial information. The rats’ better accuracy in the horizontal versus the vertical is consistent with three-dimensional behavioural and electrophysiological studies [8,19]: the firing fields of hippocampal place cells and entorhinal grid cells of rats climbing up either a pegboard or a helix (structures containing vertical and horizontal dimensions) appear vertically elongated relative to the hippocampal firing fields of rats moving around in a horizontal plane. This vertical elongation of the firing fields may mean that rats encode the vertical component with a lower resolution than they do the horizontal component and thus represent three-dimensional space anisotropically. Although little is yet known about the encoding of three-dimensional space by aerial or aquatic animals, it has been suggested that they may represent space as rats do [22]. The greater accuracy of our hummingbirds in the vertical than in the horizontal component would, however, suggest otherwise [13] as do data showing isotropic three-dimensional orientation in fishes [16]. In addition, the firing patterns of bat place cells also appear rather different from those of rats: place fields of free-flying bats did not appear to be compressed or elongated in any direction [23–25]. Whether or not bats’ grid cells also function differently from those of animals that do not move freely in three-dimensional is not yet clear [25].
The data for hummingbirds, bats and fishes, then, suggest that volumetric animals differ from surface-bound ones (particularly from rats) in their three-dimensional spatial representation and use. If this were the case, then it would constitute an example of an adaptive specialization of learning and memory. We also think that these data highlight the importance of comparative studies for understanding three-dimensional navigation. Although such comparisons are often best done among taxonomically closely related species [26], when the type of locomotion and its possible three-dimensional representation varies, such comparisons are difficult, and we must be careful with the interpretation of the data. Our results suggest, however, that the animal's type of locomotion determines to a great extent the animal's memory accuracy in the three-dimensional, while further work is required to determine the nature of the neural encoding and whether that difference is largely ontogenetic or evolutionary.
Acknowledgements
We thank Andrew Burnley and Pete Wilcox in the workshop, Jerico Guzman Mejia for animal care, David Pritchard for being an outstanding field assistant, Jorge Fuentes Fernández for programming for our analyses and two anonymous referees for their helpful comments on an earlier version of the manuscript.
All of the work was carried out under permits from Environment Canada and Alberta Fish and Wildlife, with the ethical approval of the University of St Andrews and the University of Lethbridge, and was conducted in adherence with the ASAB Guidelines for the Treatment of Animals in Behavioural Research.
Funding statement
This research is supported by CONACYT (INFA: Consejo Nacional de Ciencia y Tecnología), the University of St Andrews (J.A.A.), The Association for the Study of Animal Behaviour (S.D.H.), the University of Lethbridge, and the Natural Sciences and Engineering Council of Canada (T.A.H.).
References
- 1.Poucet B, Chapuis N, Durup M, Thinus-Blanc C. 1986. A study of exploratory behavior as an index of spatial knowledge in hamsters. Learn. Behav. 14, 93–100. ( 10.3758/bf03200043) [DOI] [Google Scholar]
- 2.Poucet B, Durup M, Thinus-Blanc C. 1988. Short-term and long-term habituation of exploration in rats, hamsters and gerbils. Behav. Process. 16, 203–211. ( 10.1016/0376-6357(88)90040-x) [DOI] [PubMed] [Google Scholar]
- 3.Rifa H, Alonso Y, Ortega E, Naranjo JM. 1992. Use of space in open field by zebra finches. Percept. Motor Skills 75, 1127–1133. ( 10.2466/pms.1992.75.3f.1127) [DOI] [Google Scholar]
- 4.Tchernichovski O, Golani I. 1995. A phase plane representation of rat exploratory behavior. J. Neurosci. Methods 62, 21–27. ( 10.1016/0165-0270(95)00050-x) [DOI] [PubMed] [Google Scholar]
- 5.Healy SD, Hurly TA. 1995. Spatial memory in rufous hummingbirds (Selasphorus rufus): a field test. Anim. Learn. Behav. 23, 63–68. ( 10.3758/bf03198016) [DOI] [Google Scholar]
- 6.D'Hooge R, De Deyn PP. 2001. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 36, 60–90. ( 10.1016/s0165-0173(01)00067-4) [DOI] [PubMed] [Google Scholar]
- 7.Jeffery KJ, Jovalekic A, Verriotis M, Hayman R. 2013. Navigating in a three-dimensional world. Behav. Brain Sci. 36, 523–543. ( 10.1017/S0140525X12002476) [DOI] [PubMed] [Google Scholar]
- 8.Grobéty MC, Schenk F. 1992. Spatial learning in a three-dimensional maze. Anim. Behav. 43, 1011–1020. ( 10.1016/s0003-3472(06)80014-x) [DOI] [Google Scholar]
- 9.Henderson J, Hurly TA, Healy SD. 2001. Rufous hummingbirds’ memory for flower location. Anim. Behav. 61, 981–986. ( 10.1006/anbe.2000.1670) [DOI] [Google Scholar]
- 10.Restat JD, Steck SD, Mochnatzki HF, Mallot HA. 2004. Geographical slant facilitates navigation and orientation in virtual environments. Perception 33, 667–687. ( 10.1068/p5030) [DOI] [PubMed] [Google Scholar]
- 11.Nardi D, Bingman VP. 2009. Pigeon (Columba livia) encoding of a goal location: the relative importance of shape geometry and slope information. J. Comp. Psychol. 123, 204–216. ( 10.1037/a0015093) [DOI] [PubMed] [Google Scholar]
- 12.Jovalekic A, Hayman R, Becares N, Reid H, Thomas G, Wilson J, Jeffery K. 2011. Horizontal biases in rats’ use of three-dimensional space. Behav. Brain Res. 222, 279–288. ( 10.1016/j.bbr.2011.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurly TA, Franz S, Healy SD. 2010. Do rufous hummingbirds (Selasphorus rufus) use visual beacons? Anim. Cogn. 13, 377–383. ( 10.1007/s10071-009-0280-6) [DOI] [PubMed] [Google Scholar]
- 14.Holbrook RI, Burt de Perera T. 2009. Separate encoding of vertical and horizontal components of space during orientation in fish. Anim. Behav. 78, 241–245. ( 10.1016/j.anbehav.2009.03.021) [DOI] [Google Scholar]
- 15.Holbrook RI, Burt de Perera T. 2011. Three-dimensional spatial cognition: information in the vertical dimension overrides information from the horizontal. Anim. Cogn. 14, 613–619. ( 10.1007/s10071-011-0393-6) [DOI] [PubMed] [Google Scholar]
- 16.Holbrook RI, Burt de Perera T. 2013. Three-dimensional spatial cognition: freely swimming fish accurately learn and remember metric information in a volume. Anim. Behav. 86, 1077–1083. ( 10.1016/j.anbehav.2013.09.014) [DOI] [Google Scholar]
- 17.Flores-Abreu I, Hurly TA, Healy SD. 2013. Three-dimensional spatial learning in hummingbirds. Anim. Behav. 85, 579–584. ( 10.1016/j.anbehav.2012.12.019) [DOI] [Google Scholar]
- 18.Hampton RR. 2001. Rhesus monkeys know when they remember. Proc. Natl Acad. Sci. USA 98, 5359–5362. ( 10.1073/pnas.071600998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayman R, Verriotis MA, Jovalekic A, Fenton AA, Jeffery K. 2011. Anisotropic encoding of three-dimensional space by place cells and grid cells. Nat. Neurosci. 14, 1182–1188. ( 10.1038/nn.2892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burt de Perera T, Holbrook RI. 2012. Three-dimensional spatial representation in freely swimming fish. Cogn. Process. 13, 107–111. ( 10.1007/s10339-012-0473-9) [DOI] [PubMed] [Google Scholar]
- 21.Taylor GK, Holbrook RI, Burt de Perera T. 2010. Fractional rate of change of swim-bladder volume is reliably related to absolute depth during vertical displacements in teleost fish. J. R. Soc. Interface 7, 1379–1382. ( 10.1098/rsif.2009.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffery K. 2011. Navigating in a 3D world. In Animal thinking: contemporary issues in comparative cognition (eds Fischer J, Menzel) R, pp. 23–28. Cambridge, MA: The MIT Press. [Google Scholar]
- 23.Yartsev M, Witter MP, Ulanovsky N. 2011. Grid cells without theta oscillations in the enthorhinal cortex of bats. Nature 479, 103–107. ( 10.1038/nature10583) [DOI] [PubMed] [Google Scholar]
- 24.Yartsev MM, Ulanovsky N. 2013. Representation of three-dimensional space in the hippocampus of flying bats. Science 340, 367–372. ( 10.1126/science.1235338) [DOI] [PubMed] [Google Scholar]
- 25.Heys JG, MacLeod KM, Moss CF, Hasselmo ME. 2013. Bat and rat neurons differ in theta-frequency resonance despite similar coding of space. Science 340, 363–367. ( 10.1126/science.1233831) [DOI] [PubMed] [Google Scholar]
- 26.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]