Abstract
Culturally transmitted tool use has important ecological and evolutionary consequences and has been proposed as a significant driver of human evolution. Such evidence is still scarce in other animals. In cetaceans, tool use has been inferred using indirect evidence in one population of Indo-Pacific bottlenose dolphins (Tursiops sp.), where particular dolphins (‘spongers’) use marine sponges during foraging. To date, evidence of whether this foraging tactic actually provides access to novel food items is lacking. We used fatty acid (FA) signature analysis to identify dietary differences between spongers and non-spongers, analysing data from 11 spongers and 27 non-spongers from two different study sites. Both univariate and multivariate analyses revealed significant differences in FA profiles between spongers and non-spongers between and within study sites. Moreover, FA profiles differed significantly between spongers and non-spongers foraging within the same deep channel habitat, whereas the profiles of non-spongers from deep channel and shallow habitats at this site could not be distinguished. Our results indicate that sponge use by bottlenose dolphins is linked to significant differences in diet. It appears that cultural transmission of tool use in dolphins, as in humans, allows the exploitation of an otherwise unused niche.
Keywords: culture, niche exploitation, tool use, foraging specialization, intra-specific competition
1. Introduction
Animal tool use may have important ecological and evolutionary consequences, particularly due to its potential association with the exploration of novel niches and its contribution to niche variation within populations [1,2]. The ecological niche concept is one of the pillars of ecological theory [3]. It posits that the ecology of individuals (or species) in sympatry with similar constraints (i.e. niches) is adjusted by differentiation into new niches. Thus, the exploitation of novel niches will reduce competition for food, space or other important resources. There is mounting evidence that many animal populations are composed of specialized individuals, whose niches are narrower than that of the overall population [1] for reasons other than sex, age or morphology [4]. Such substantial individual variation in, for example, diet, microhabitat preferences and foraging behaviour, appears to be ubiquitous in the animal kingdom and has been described in highly divergent taxonomic groups, including gastropods, insects, fishes, reptiles, birds and mammals (reviewed in [1]).
In most organisms, niche exploitation appears to be under genetic control [2]. ‘Cultural’ niche exploitation, which is induced by stable trans-generational transfer of innovations through social learning not under genetic or environmental control [2], has therefore received special attention [5,6]. It has been proposed that cultural niche exploitation was a significant driver of human evolution [7]. For instance, social transmission of dairy farming practices appears to have changed selection pressures, a process referred to as ‘niche construction’ [8]. It is thought that the genes responsible for lactose absorption rose in frequency in pastoralist communities because of this, as the ability to digest lactose in adults is closely correlated with the occurrence of genes responsible for lactose digestion in human populations [7]. Gene-culture coevolutionary models provide support for this hypothesis when cultural transmission is very reliable [9].
There is increasing evidence that variation in behavioural patterns and tool use in primates [10–12], cetaceans [13] and other animals [14] appear to reflect cultural processes. Thus, investigating to what extent tool use affects niche exploitation in other cultural species may help us to identify those factors enabling niche construction in humans and other animals.
In almost all species, niche exploitation is achieved by direct interactions with the environment [1]. To our knowledge, there are only a few documented cases in which tool use (sensu [15]) was found to allow the exploitation of otherwise unused niches and, subsequently, resulted in significant differences in diets between tool users and non-tool users [16]. Although tool use appears to be widespread in the animal kingdom in terms of taxonomic breadth [14], it is rare in mammals, having been described in only a few primate [17] and non-primate mammal species [14]. In cetaceans, evidence of tool use has thus far only been inferred in a subset of Indo-Pacific bottlenose dolphins (Tursiops sp., ‘bottlenose dolphins’ hereafter) within the broader population of Shark Bay, Western Australia [18], although whether these dolphins use the sponges as tools is still regarded as contentious by some [19]. Particular matrilines of dolphins are thought to use marine sponges as foraging tools and previous studies invoked cultural transmission as a significant determinant of ‘sponging’ behaviour [13,20,21]. Cultural variation in primates is often observed between geographically distinct populations [10], but see [22]. However, behavioural variants in cetaceans often occur in sympatry [23]. Thus, cetacean populations provide an excellent opportunity to study the potential of culturally mediated niche variation involving tools within populations.
Bottlenose dolphins in Shark Bay exhibit a remarkable diversity of foraging specializations, some of which appear to be facilitated via social transmission [20] and involve apparent tool use, such as sponging [18] and, perhaps, shelling [24]. The sponges are thought to be used as a protective tool against abrasion from sharp objects, stingray barbs or noxious organisms [18,25]. Previous evidence suggests that sponging may enable dolphins to target fishes without swim bladders, as these prey items are not easily detectable with echolocation against a complex substrate [25]. Sponging is well documented in the two gulfs of Shark Bay, where its occurrence seems largely confined to deep-water channels [26,27]. With more than 60% of all females engaging in this behaviour, sponging is the most common foraging tactic in deep-water channels [28]. Although there is a female bias to sponging, recent findings suggest that the proportion of sponging males may be substantially higher than previously reported (at least 25% of all spongers in the western gulf are male (M. Krützen & S. J. Allen 2014, unpublished data) and up to 50% of males born to spongers in the eastern gulf become spongers [29]). Importantly, dolphins that do not use tools (‘non-spongers’) also forage in these deep-water channels [13,20], providing an ideal opportunity for a direct comparison of the diets of both foraging types without habitat being a confounding factor. Relatedness patterns differ between both gulfs. In the eastern gulf, spongers are more closely related than expected by chance [13], while this is not the case in the western gulf [30].
As direct observations of prey capture and feeding events in dolphins are rare, it remains unclear as to what extent the use of sponges permits access to otherwise unavailable prey items for Shark Bay dolphins. Thus, applications of techniques enabling the inference of dietary differences between groups of animals are required. Traditional methods, such as analyses of stomach contents or faecal samples have major drawbacks in that they only provide information about very recent diet and will be unlikely to reflect long-term dietary differences. Further, such samples are infrequently obtained or unfeasible due to their reliance on subject capture or mortality. In recent years, the analysis of fatty acids (FAs) has been shown to provide a powerful tool in diet studies [31–33]. The underlying premise is that FAs of consumed prey are deposited in a predictable manner into the adipose tissue of predators, thereby creating a unique profile of a number of FAs in various proportions, reflecting long-term diet [34]. As FA biosynthesis in animals is limited to saturated and mono-unsaturated FAs, those of dietary and non-dietary origin can be delineated and so serve as powerful biochemical tracers [34].
In this study, we aimed to investigate whether sponge use during foraging by Shark Bay bottlenose dolphins may be associated with the acquisition of novel resources. We hypothesized that sponge use allows the exploitation of otherwise unused niche space, so sponging dolphins should have a significantly different FA profile from that of dolphins that forage in the same habitat, yet have not been observed using sponges. In order to test our hypothesis, we examined the diet of groups of sponging and non-sponging wild bottlenose dolphins based on the analysis of FA profiles of remotely obtained blubber samples.
2. Material and methods
(a). Study areas and sample collection
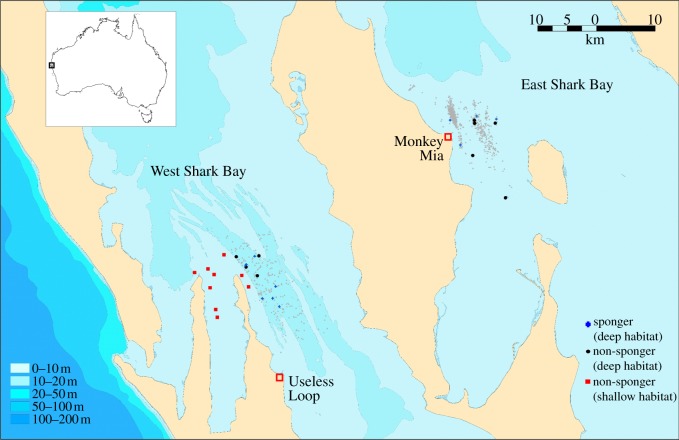
We studied Indo-Pacific bottlenose dolphins in the western (WSB) and eastern gulfs (ESB) of Shark Bay, Western Australia (figure 1). This semi-enclosed bay is largely comprised of shallow sea grass beds, broken up by deeper channels and embayment plains covered in sand or silt. In ESB, dolphins have been comprehensively studied since the mid-1980s [35]. We established a second study site in WSB, off the township of Useless Loop, in 2007. At both sites, we carried out standardized boat-based surveys to photographically identify dolphins [36], along with collection of data including group behaviour, group composition, sponger presence/absence, GPS location, water depth and substrate features. Similar to previous studies [26], we classified individual dolphins as spongers if they were sighted a minimum of three times on different days using a sponge. All other animals were categorized as non-spongers.
Figure 1.
Map of Shark Bay. Symbols identify sampling locations for spongers/deep habitat (blue crosses), non-spongers/deep habitat (black circles), and non-spongers/shallow habitat (red squares); grey triangles identify locations where sponging has been observed (ESB: 1990–2006; WSB: 2007–2010). Different shades of blue indicate approximate depth.
Biopsy samples were obtained from adult individuals using a remote biopsy system for small cetaceans [37]. We collected nine tissue samples in ESB during one month of sampling in 2002, where data were obtained ad libitum of known adult spongers and non-spongers [13] encountered within the same deep-water channels (figure 1). In ESB, we sampled in the deep habitat: four female spongers, two female and three male non-spongers. In WSB, we sampled in the deep habitat: seven female spongers, three female and one male non-spongers; in the moderate habitat: six female and two male non-spongers; in the shallow habitat: seven female and two male non-spongers (see data analysis section). In WSB, 29 samples from adult dolphins were collected in 2008 and 2009 during ad libitum surveys, resulting in a collection of tissue samples from sponging and non-sponging dolphins encountered in different habitats, namely shallow flats and deep-water channels.
After biopsy retrieval, the blubber portion of ESB samples was immediately cut off, placed in a chloroform–methanol solution (2 : 1 v/v) and stored at −20°C. WSB samples were wrapped in residue-free aluminium foil and placed in a cryo-vial, which was placed on ice and later transferred to a dry shipper filled with liquid nitrogen. The remaining epidermis, collected for genetic analyses, was kept in a saturated NaCl/20% dimethyl-sulfoxide solution [38] at −20°C. DNA samples were stored at −80°C in the laboratory. Genetic sexing was conducted on all samples as described previously [39].
(b). Laboratory procedures
Lipid extraction of the blubber biopsy samples was carried out following a modified Folch protocol [40]. All samples were transferred into a chloroform–methanol mixture (2 : 1 v/v) of 20-fold the sample/storage solution volume, with 0.01% butylated hydroxytoluene as an antioxidant for 24 h at 4°C. Potassium chloride in H2O (0.9%) was added to form a final emulsified mixture of chloroform, methanol and KCl/H2O (8 : 4 : 3 v/v/v). After centrifuging at 1800 r.p.m. at 0°C for 20 min, the lower layer of lipid and chloroform was removed. The solvent was evaporated in a rotary evaporator at 35°C and residual solvent was removed from the lipid using a vacuum pump. Lipid samples were then re-suspended in iso-hexane (2 ml) and stored under nitrogen at −20°C until trans-esterification. For the trans-esterification of triacylglycerides into FA methyl esters (FAME), first, iso-hexane was removed under a constant stream of nitrogen. Distilled toluene (1 ml) and sulfuric acid in methanol (1%, 2 ml) were added to each sample. The mixture was shaken and heated overnight at 50°C in a heating block. The extract was allowed to cool before H2O (5 ml) containing NaCl (5%), followed by iso-hexane (5 ml), was added. The samples were shaken and then left to settle until the layers separated. The top iso-hexane layer, containing the methyl esters, was transferred into a test tube. A further aliquot of iso-hexane was added to the original sample mixture and extraction of methyl esters was repeated. The combined organic layers were washed with 2% of potassium bicarbonate (4 ml) and, again, the upper layer was removed before being dried over anhydrous sodium sulfate (5 g). Samples were stored frozen prior to analysis. Prior to gas chromatography (GC), the extracts were diluted with iso-hexane to give a total FAME concentration of 0.01 mg ml−1
Trans-esterified lipids were analysed on a Hewlett Packard 5890 GC for ESB samples and a Hewlett Packard 6890 GC for WSB samples (Agilent Technologies, Berkshire, UK). Both were equipped with a flame ionization detector (GC-FID) and a cool on-column injector. An Agilent DB 23 fused silica capillary column (30 m × 0.2 mm i.d.) coated with 0.25 µm film of 50%-cyanopropyl-methylpolysiloxane (Crawford Scientific, Strathaven, UK) was used for the separation. Injections of 1 μl were carried out at 60°C, followed by an increase in temperature of 25°C min−1 to 150°C and 1°C min−1 up to 200°C. The temperature was then held constant for 10 min before final elevation at 5°C min−1 to 230°C, where it was held for 5 min. Nitrogen was used as the carrier gas.
Twenty-nine FAME were determined: 14 : 0, 14 : 1 (n − 5), 15 : 0, 16 : 0, 16 : 1 (n − 7), 16 : 2 (n − 6), 16 : 3 (n − 6), 16 : 4 (n − 3), 18 : 0, 18 : 1 (n − 9), 18 : 1 (n − 7), 18 : 2 (n − 6), 18 : 3 (n − 6), 18 : 3 (n − 3),18 : 4 (n − 3), 20 : 0, 20 : 1 (n − 11), 20 : 1 (n − 9), 20 : 2 (n − 6), 20 : 3 (n − 3), 20 : 4 (n − 6), 20 : 4 (n − 3), 20 : 5 (n − 3), 21 : 5 (n − 3), 22 : 0, 22 : 1 (n − 11), 22 : 5 (n − 3), 22 : 6 (n − 3) and 24 : 1 (n − 9). Data were collected via a PE Nelson 610 link box and processed using TotalChromNavigator v. 6.3.1 software (Perkin-Elmer, Beaconsfield, UK). The normalized area percentages were calculated for each of the 29 individual FAME as percentage of the total area for all identified FAs. FAME were identified by comparison with retention time and profiles of various FAME in reference materials (Restek marine oil FAME standard, RESTEX, Bellafonte, PA, USA) and laboratory reference materials (LRM144 cod liver oil, LRM145 orange roughy oil and EO23 fish oil). These reference materials were also used to monitor quality control. Gas chromatography–mass spectrometry (GC–MS) using electron impact ionization had been used previously to confirm the identity of the FAME in the reference materials.
(c). Data analysis
Prior to statistical analysis, FA proportions were log-ratio transformed (division of the geometric mean of the sample followed by log transformation) to meet the criteria of parametric statistical tests. Values of 0 were set to 0.005%, as they could not be log transformed. This value was safely below the minimum detectable level [32].
All statistical analyses were carried out in JMP, v. 9.0 (SAS Institute). As sampling in both gulfs followed different regimes with regards to time and space, we combined samples from ESB and WSB to evaluate the influence of foraging type (spongers versus non-spongers), sampling location and sex. To do this, we conducted a principal component analyses (PCA) to produce a reduced set of orthogonal principal components (PCs) from a large number of original correlated variables that capture most of the variance of a sample. Only PCs with an eigenvalue over 1.0 were extracted. We then constructed a general linear model (GLM) to test for foraging type, sampling location and sex on the resultant PC scores. Effects that were significant on at least one PC were further examined by GLMs on individual FAs, where sequential Bonferroni corrections were used to reduce the risk of committing Type I errors [41].
We also carried out a discriminant analysis (DA) to predict group membership based on the maximization of mean differences between values of predefined grouping variables [42]. The null hypothesis that groups have identical means was tested with Wilk's lambda (Λ, significance level p < 0.05).
In addition, we conducted a further GLM analysis on WSB samples only to detect any effects of habitat and time of sampling on our results. In ESB, all samples were collected within 1 year (August 2002) and contained samples from only one habitat type (deep channel), therefore reducing the possible influence of confounding factors. WSB samples, however, were sampled randomly at different times (September 2008, April–July 2009) and in two different habitats, allowing us to investigate the effect of habitat on time of sampling FA profiles. For WSB, sampling depth was used as a proxy for habitat [27,43], categorizing samples into the depth classes shallow (less than 5 m), moderate (5–10 m) and deep (more than or equal to 10 m). This classification approximates the underwater topography of WSB, where sea grass coverage decreases to approximately 10% at a depth of 5 m due to limited light penetration, and sponge growth is only evident deeper than 10 m [27]. To minimize any potential edge effects, only samples obtained in shallow and deep areas were compared. In WSB, our previous research showed that individual dolphins exhibit almost exclusive preference to one particular habitat type [30]. Thus, we classified samples into foraging type and corresponding depth class, resulting in three foraging type/depth categories (non-spongers/shallow, non-spongers/deep and spongers/deep). To test for significance between the foraging type/depth categories, we used Tukey's honest significant difference test (Tukey HSD) for post hoc comparisons.
3. Result
FA profiles of spongers and non-spongers in both gulfs of Shark Bay differed significantly. Moreover, within WSB, FA profiles were significantly different between spongers and non-spongers from the same deep channel habitat, while FA profiles of non-spongers from deep and shallow habitats could not be distinguished.
We tested for 29 different FAs, 16 of which were used in statistical analyses as they occurred in quantities larger than 0.5% and not only trace amounts (figure 2). Seven of the 16 FAs were most likely primarily from direct dietary intake, while another eight originated from both diet and biosynthesis [44] (table 1). The six most abundant FAs were 14 : 0, 16 : 0, 16 : 1 (n − 7), 18 : 1 (n − 9), 18 : 1 (n − 7) and 22 : 6 (n − 3), which comprised about 70% of all FAs. Five of the six most abundant FAs in this study were products of both diet and biosynthesis, while 22 : 6 (n − 3) can only be acquired through dietary intake [44]. PCA (Varimax rotation) produced five PCs explaining 79.1% of the total variance in blubber FAs (table 1).
Figure 2.
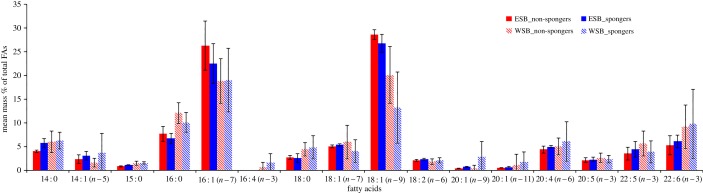
Overall FA profiles of spongers and non-spongers in WSB and ESB combined. Values are given in mass % ± s.e. Short-hand IUPAC nomenclature designates FAs based on carbon chain length: number of double bonds and the position (n − x) of the first double bond with respect to the methyl end. (Online version in colour.)
Table 1.
PC loadings of FAs sorted by magnitude.
| fatty acid | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| 16 : 0b | 0.868 | 0.125 | 0.110 | −0.094 | 0.176 |
| 18 : 0b | 0.824 | −0.154 | −0.066 | −0.052 | 0.250 |
| 15 : 0b | 0.764 | 0.442 | −0.077 | −0.113 | 0.304 |
| 20 : 1 (n − 9)a | −0.751 | −0.327 | −0.134 | 0.144 | 0.276 |
| 22 : 5 (n − 3)b | 0.737 | −0.103 | 0.011 | 0.523 | −0.030 |
| 14 : 1 (n − 5)c | 0.004 | 0.920 | 0.066 | −0.016 | −0.034 |
| 16 : 1 (n − 7)b | 0.195 | 0.718 | 0.328 | 0.439 | 0.026 |
| 14 : 0b | 0.540 | 0.636 | 0.002 | −0.002 | 0.211 |
| 18 : 2 (n − 6)a | 0.440 | 0.523 | 0.315 | 0.250 | 0.020 |
| 22 : 6 (n − 3)a | 0.255 | −0.500 | 0.446 | 0.007 | −0.193 |
| 18 : 1 (n − 9)b | 0.116 | 0.282 | 0.859 | −0.079 | −0.018 |
| 18 : 1 (n − 7)b | −0.053 | −0.093 | 0.816 | −0.211 | 0.378 |
| 16 : 4 (n − 3)a | 0.213 | −0.124 | −0.627 | −0.602 | 0.112 |
| 20 : 5 (n − 3)a | −0.290 | −0.043 | −0.148 | 0.749 | 0.064 |
| 20 : 4 (n − 6)a | 0.305 | 0.343 | −0.152 | 0.739 | 0.302 |
| 20 : 1 (n − 11)a | −0.284 | −0.115 | −0.151 | −0.191 | −0.839 |
| eigenvalue | 5.244 | 2.538 | 2.116 | 1.692 | 1.066 |
| percentage of variance | 32.8 | 15.9 | 13.2 | 10.6 | 6.7 |
| cumulative percentage | 32.8 | 48.6 | 61.9 | 72.4 | 79.1 |
aFAs that are primarily from direct dietary intake.
bFAs that can be from both dietary intake and biosynthesis.
cFAs that are primarily from biosynthesis (based on Iverson [44]).
We applied a GLM to infer whether spongers and non-spongers differed in their FA profiles, controlling for sampling location (WSB and ESB) and sex as additional effects. Interactions were not significant, so only main effects were further examined. The GLM revealed significant differences between spongers and non-spongers on PC1 (F = 5.023, p < 0.032) and a marginally non-significant trend was found on PC3 (F = 3.359, p < 0.076). WSB and ESB samples differed significantly on PC1 (F = 7.102, p < 0.012), PC3 (F = 11.304, p < 0.002) and PC4 (F = 4.175, p < 0.049). FA profiles did not differ with dolphin sex.
The GLM on individual FA percentages showed that FA 16 : 0 was significantly different between spongers and non-spongers after Bonferroni corrections (F = 10.997, p < 0.002), while we observed a trend on the FAs 18 : 1 (n − 9), 20 : 1 (n − 9) and 22 : 5 (n − 3). All of these FAs stem from diet and biosynthesis, except for 20 : 1(n − 9), which is predominantly of dietary origin [44]. An interaction between behaviour and sampling location was limited to 22 : 5 (n − 3), thus, the main effects were examined separately for all other FAs. The sampling location had a significant effect on FAs 16 : 4 (n − 3) and 18 : 1 (n − 9) (F = 33.316, p < 0.0001 and F = 12.701, p < 0.001, respectively), of which 16 : 4 (n − 3) is predominantly of dietary origin.
In order to further support differences between FA profiles of spongers and non-spongers across ESB and WSB, we applied a DA to determine how well FA profiles could be classified by foraging type. The DA clearly separated our dataset into the two foraging groups (Λ = 0.338, χ² = 30.891, d.f. = 15, p < 0.009, table 2). This test provided a correct assignment of 92.1% with a cross-validation error rate of 13.2%. The DA plot illustrates a clear separation of spongers and non-spongers, with the 95% confidence regions situated well apart (figure 3). FAs most closely related to the discriminant function were 16 : 0, 20 : 1 (n − 9), 22 : 5 (n − 3) and 18 : 1 (n − 9).
Table 2.
Classification results of DA for all individuals.
| predicted group membership |
||||
|---|---|---|---|---|
| behaviour | spongers | non-spongers | ||
| (a) original | count | spongers | 9 | 2 |
| non-spongers | 1 | 26 | ||
| % | sponger | 81.8 | 18.2 | |
| non-spongers | 3.7 | 96.3 | ||
| (b) cross-validated | count | spongers | 8 | 3 |
| non-spongers | 22 | |||
| % | sponger | 72.7 | 27.3 | |
| non-spongers | 12.9 | 87.1 | ||
Figure 3.
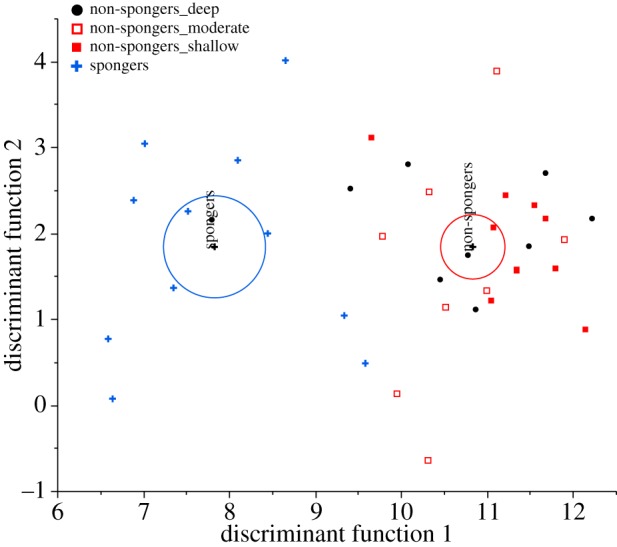
Plot of canonical function separating spongers and non-spongers. Circles indicate the 95% confidence region around group mean (black crosses).
The different sampling times in WSB (September 2008 and April–July 2009) did not have any significant effect on the first five PCs of FA profiles (data not shown). We found that non-spongers from different habitats in WSB (deep versus shallow water) had FA profiles that did not differ from each other, but that spongers were different from deep-water non-spongers. There was a significant effect of the foraging type/depth class (i.e. habitat) on PC1 (F = 6.378, p < 0.009). Post hoc comparisons on PC1 revealed a significant difference between spongers and deep-water non-spongers (p < 0.01), whereas FA profiles of non-spongers from the different habitats did not differ significantly (p = 0.07) (figure 4).
Figure 4.
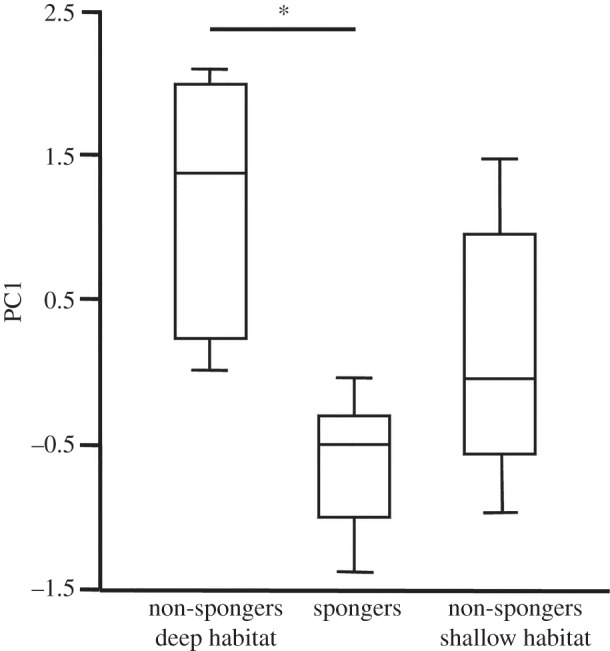
Box-plot of PC1 scores of deep habitat non-spongers and spongers, and shallow habitat non-spongers from Western Shark Bay. The asterisk indicates significance using Tukey HSD.
4. Discussion
Here, we provide evidence that the cultural transmission of sponge use during foraging by Shark Bay bottlenose dolphins is indeed associated with the continued exploitation of an otherwise unused niche. Niche theory suggests that foraging specializations are ultimately driven by intraspecific competition or by trade-offs related to either habitat heterogeneity or phenotypic differences [1,45–47]. The exploitation of otherwise unused resources can be beneficial as it offers a competitive refuge from conspecifics [4]. This will be particularly favourable in the deep-water channels of Shark Bay, which are known to be of lower productivity in terms of fish species richness and biomass [48].
Our results revealed significant FA profile differences between spongers and non-spongers. The DA grouped spongers and non-spongers with high accuracy (92.1% and 78.9% cross-validated, respectively, table 2). Our findings strongly suggests that sponge use in dolphins allows the exploitation of a novel niche and also leads to a significant long-term difference in diet between dolphins that use sponges and those that do not. Based on a widely recognized dietary analysis test [34], we were able to infer that a culturally transmitted foraging tactic can lead to exploitation of a niche that does not seem to be accessible to dolphins that do not use sponges.
The analysis of FA profiles obtained from superficial blubber biopsy samples provides a useful tool for making dietary inferences in bottlenose dolphins. The GLM on PC scores and individual FAs verified the results from the DA. We controlled for the effects of both sex and sampling locations/time (i.e. habitat) on FA profiles. Similar to a study using the same techniques on common bottlenose dolphins (Tursiops truncatus) around the Azores [49], we found no statistically significant differences in FA profiles by sex.
The different FA profiles of dolphins from WSB and ESB may be the result of there being dissimilar prey species abundance in the two gulfs. This is conceivable, given the distance (ca 120 km) between the sampling sites and the different sampling years (2002 for ESB and 2008/2009 for WSB). Furthermore, we used slightly different preservation methods for ESB and WSB samples, which may have influenced our results systematically. However, sampling regimes and preservation methods were consistent within each gulf. In ESB, all sampling occurred within just one month in the Austral winter, so it is unlikely that any temporal aspects affected our results. In WSB, sampling occurred randomly with respect to time and habitat over two consecutive Austral winters. Thus, the key finding of a difference in FA profiles between spongers and non-spongers using the same habitat in WSB is likely to be a genuine reflection of long-term dietary differences [34].
Differences in FA profiles between groups can reflect the consumption of different prey species, or can reflect a different mixture of prey species. Potential prey profiles need to be known in order to distinguish between these different sources of variation with certainty [44], which was not feasible in this study. A close examination of the WSB dataset renders it highly likely that spongers and non-spongers differ strongly in prey types. We found significantly different FA profiles between spongers and non-spongers, but not between non-spongers from the deep and shallow habitats (in which sponging does not occur), although sample size for non-spongers from the deep habitat was small. This was unexpected, as prey species abundance and biomass are known to differ considerably among the different habitat types in Shark Bay [43]. Thus, individuals foraging in the same habitat should have similar FA profiles, unless their dietary differences are profound. Our finding that spongers and non-spongers foraging in the same habitat differ significantly in their FA profiles suggests that spongers include exclusive prey in their diet and, therefore, have only marginal niche overlap with conspecifics.
Previous studies on Shark Bay dolphins have demonstrated that vertical social transmission in the appropriate environment is of critical importance for the acquisition and spread of sponging behaviour [13,20,21]. Social learning can be advantageous in a heterogeneous marine environment without boundaries, where resources are patchily distributed and mobile prey patches cannot be easily defended [50]. In particular, when foraging skills are transmitted vertically, competition on an intra-population level may be reduced, as vertical cultural transmission produces ecological similarity between parent and offspring. Thus, offspring benefit from their parents’ knowledge and the inclusive fitness landscape is modified as competition occurs mainly among kin. There is empirical evidence for these kinship effects in ESB, in which all adult spongers are more closely related than expected by chance [13], although this is not the case in WSB [30].
Furthermore, strong intra-population niche variation allows individuals in close spatial proximity to minimize competition through the exploitation of different resources [1]. This might have positive effects on population density, as niche segregation allows for increased carrying capacities compared with ecologically homogeneous populations [51–53]. In this context, it is important to note that Shark Bay appears to support one of the highest densities of bottlenose dolphins worldwide, estimated to be 0.53–0.92 dolphins km−2 in parts of the study area in WSB [54].
Foraging efficiency can be increased by specializing on a foraging behaviour associated with specific skills that, for example, improve searching or handling times [47]. The sponging specialization appears to involve some potentially costly features, such as spongers appearing to spend significantly more time foraging than other females and being more solitary [26,28]. However, these costs could be offset by increased foraging efficiency, resulting in similar fitness pay-offs in comparison to conspecifics within the population. In a previous study in ESB, spongers were found to have higher calving success than non-spongers [26], although these differences were not significant. Thus, it is questionable whether there are ultimate costs to this behaviour. It awaits further investigation to determine whether equal fitness pay-offs for spongers are reflected by a trade-off associated with some costly features, or whether sponging is frequency-dependent and, perhaps, depends on the foraging behaviours of other individuals in the population.
It would be interesting to speculate whether the creation of a novel foraging niche, as in the case of sponging, might actually cause an alteration of the selective environment through the process of cultural niche construction [8]. In this case, it would be conceivable that there may be selection for the mechanisms reinforcing behavioural innovations and traditions, i.e. vertical social learning, cognition and prolonged infancy period [55]. Although primarily documented in humans [56], some evidence of how vertical cultures may structure populations can be found in cetaceans [57]. Sperm whales (Physeter macrocephalus) live in stable, matrilineal, culturally determined clans that display significant variation in foraging tactics, habitat use patterns and vocal dialects. It has been proposed that the low effective mtDNA population sizes of sperm whales (and other matrilineal whales) are the result of selection on matrilineally transmitted cultural traits, upon which neutral mtDNA alleles ‘hitchhike’ [57], although this was hotly debated ([58] and discussion of thereafter, pp. 324–382). Culturally induced behavioural diversification may also have led to evolutionary diversification in killer whales (Orcinus orca). The ongoing sympatric speciation of ecotypes that differ in genetics, morphology, behaviour and acoustics has been suggested as the result of divergence through culturally acquired behaviours within stable social groups [59]. Furthermore, cultural niche constructing activities, such as habitat selection, can influence the genetic structure of populations [8]. Evidence can be found in the WSB bottlenose dolphin population, in which matrilineal haplotype distribution strongly correlates with habitat characteristics and is likely to be linked to vertical social transmission [30].
5. Conclusion
In this research, we inferred that culturally transmitted sponge use in dolphins allows the exploitation of a novel foraging niche, providing evidence for cultural niche exploitation in bottlenose dolphins. Our findings offer insights into the potential for cultural niche construction and selection pressures facilitating the evolution of cultural transmission of novel niches. They also suggest that the vertical cultural transmission of foraging tactics may provide an important mechanism for reducing intra-specific competition.
Acknowledgements
We thank Shark Bay Resources Pty Ltd, the Useless Loop community, Aspen Parks, Monkey Mia Resort and Monkey Mia Wildsights for logistical support. We also thank numerous field assistants, without whom this research would not have been possible. The Marine Environmental Assessment Group, Marine Scotland Science, Aberdeen, UK, provided resources and training to analyse the FA samples. We are grateful to Karin Isler for statistical advice and to Anna Lindholm and the journal club of the Anthropological Institute and Museum at the University of Zurich for constructive comments on the manuscript. This manuscript was markedly improved by incorporating the insightful comments provided by three anonymous reviewers.
This research was carried out under scientific investigation permits from the Department of Environment and Conservation and the Department of Local Government Research and Development, as well as animal ethics approval from the University of Zurich, Murdoch University and the University of New South Wales. This manuscript represents publication no. 9 of the Shark Bay Dolphin Innovation Project.
Data accessibility
Data for this manuscript will be made available on Dryad.
Funding statement
The National Geographic Society, Sea World Research and Rescue Foundation, W. V. Scott Foundation, Australian Research Council and the A. H. Schultz Stiftung provided financial support.
References
- 1.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 2.Laland KN, Sterelny K, Harrison R. 2006. Perspective: seven reasons (not) to neglect niche construction. Evolution 60, 1751–1762. [PubMed] [Google Scholar]
- 3.Hutchinson GE. 1959. Homage to Santa-Rosalia or why are there so many kinds of animals. Am. Nat. 93, 145–159. ( 10.1086/282070) [DOI] [Google Scholar]
- 4.Roughgarden J. 1972. Evolution of niche width. Am. Nat. 106, 683–718. ( 10.1086/282807) [DOI] [Google Scholar]
- 5.Boyd R, Richerson PJ, Henrich J. 2011. The cultural niche: why social learning is essential for human adaptation. Proc. Natl Acad. Sci. USA 108, 10 918–10 925. ( 10.1073/pnas.1100290108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd R, Richerson PJ, Henrich J. 2011. Rapid cultural adaptation can facilitate the evolution of large-scale cooperation. Behav. Ecol. Sociobiol. 65, 431–444. ( 10.1007/s00265-010-1100-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulijaszek SJ, Strickland SS. 1993. Nutritional anthropology: prospects and perspectives. London, UK: Smith-Gordon. [Google Scholar]
- 8.Odling-Smee FJ, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Feldman MW, Cavalli Sforza LL. 1989. On the theory of evolution under genetic and cultural transmission with application to the lactose absoprtion problem. In Mathematical evolutionary theory (ed. Feldman MW.). Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Krützen M, Willems EP, van Schaik CP. 2011. Culture and geographic variation in orang-utan behaviour. Curr. Biol. 21, 1808–1812. ( 10.1016/j.cub.2011.09.017) [DOI] [PubMed] [Google Scholar]
- 11.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham R, Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685. ( 10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 12.Visalberghi E. 1990. Tool use in Cebus. Folia Primatol. 54, 146–154. ( 10.1159/000156438) [DOI] [PubMed] [Google Scholar]
- 13.Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB. 2005. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA 102, 8939–8943. ( 10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentley-Condit VK, Smith E. 2010. Animal tool use: current definitions and an updated comprehensive catalog. Behaviour 147, 185 ( 10.1163/000579509X12512865686555) [DOI] [Google Scholar]
- 15.Beck B. 1980. Animal tool behavior. New York, NY: Garland STPM Press. [Google Scholar]
- 16.Rutz C, Bluff LA, Reed N, Troscianko J, Newton J, Inger R, Kacelnik A, Bearhop S. 2010. The ecological significance of tool use in New Caledonian crows. Science 329, 1523–1526. ( 10.1126/science.1192053) [DOI] [PubMed] [Google Scholar]
- 17.van Schaik CP, Deaner RO, Merrill MY. 1999. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 36, 719–741. ( 10.1006/jhev.1999.0304) [DOI] [PubMed] [Google Scholar]
- 18.Smolker R, Richards A, Connor R, Mann J, Berggren P. 1997. Sponge carrying by dolphins (Delphinidae, Tursiops sp.): a foraging specialization involving tool use? Ethology 103, 454–465. ( 10.1111/j.1439-0310.1997.tb00160.x) [DOI] [Google Scholar]
- 19.Manger PR. 2013. Questioning the interpretations of behavioral observations of cetaceans: is there really support for a special intellectual status for this Mammalian order? Neuroscience 250, 664–696. ( 10.1016/j.neuroscience.2013.07.041) [DOI] [PubMed] [Google Scholar]
- 20.Mann J, Sargeant B. 2003. Like mother, like calf: the ontogeny of foraging traditions in wild Indian ocean bottlenose dolphins (Tursiops sp.). In The biology of traditions (eds Fragaszy D, Perry S.), pp. 236–266. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 21.Bacher K, Allen S, Lindholm AK, Bejder L, Krützen M. 2010. Genes or culture: are mitochondrial genes associated with tool use in bottlenose dolphins (Tursiops sp.)? Behav. Genet. 40, 706–714. ( 10.1007/s10519-010-9375-8) [DOI] [PubMed] [Google Scholar]
- 22.McGrew WC, Marchant LF, Scott SE, Tutin CEG. 2001. Intergroup differences in a social custom of wild chimpanzees: the grooming hand-clasp of the Mahale Mountains. Curr. Anthropol. 42, 148–153. ( 10.1086/318441) [DOI] [Google Scholar]
- 23.Whitehead H, Rendell L, Osborne RW, Würsig B. 2004. Culture and conservation of non-humans with reference to whales and dolphins: review and new directions. Biol. Conserv. 120, 427–437. ( 10.1016/j.biocon.2004.03.017) [DOI] [Google Scholar]
- 24.Allen SJ, Bejder L, Krützen M. 2011. Why do Indo-Pacific bottlenose dolphins (Tursiops sp.) carry conch shells (Turbinella sp.) in Shark Bay, Western Australia? Mar. Mamm. Sci. 27, 449–454. ( 10.1111/j.1748-7692.2010.00409.x) [DOI] [Google Scholar]
- 25.Patterson EM, Mann J. 2011. The ecological conditions that favor tool use and innovation in wild bottlenose dolphins (Tursiops sp.). PLoS ONE 6, e22243 ( 10.1371/journal.pone.0022243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann J, Sargeant BL, Watson-Capps JJ, Gibson QA, Heithaus MR, Connor RC, Patterson E. 2008. Why do dolphins carry sponges? PLoS ONE 3, e3868 ( 10.1371/journal.pone.0003868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyne JA, Loneragan NR, Kopps AM, Allen SJ, Krützen M, Bejder L. 2012. Ecological characteristics contribute to sponge distribution and tool use in bottlenose dolphins Tursiops sp. Mar. Ecol. Prog. Ser. 444, 143–153. ( 10.3354/Meps09410) [DOI] [Google Scholar]
- 28.Kopps AM, Krützen M, Allen SJ, Bacher K, Sherwin WB. In press Characterizing the socially transmitted foraging tactic ‘sponging’ by bottlenose dolphins (Tursiops sp.) in the western gulf of Shark Bay, Western Australia. Mar. Mamm. Sci . ( 10.1111/mms.12089) [DOI] [Google Scholar]
- 29.Mann J, Patterson EM. 2013. Tool use by aquatic animals. Phil. Trans. R. Soc. B 368, 20120424 ( 10.1098/rstb.2012.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopps AM, Ackermann CY, Sherwin WB, Allen SJ, Bejder L, Krützen M. 2014. Cultural transmission of tool use combined with habitat specializations leads to fine-scale genetic structure in bottlenose dolphins. Proc. R. Soc. B 281, 20133245 ( 10.1098/rspb.2013.3245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman DP, Burrows DG, Wade PR, Durban JW, Matkin CO, LeDuc RG, Barrett-Lennard LG, Krahn MM. 2005. Feeding ecology of eastern North Pacific killer whales Orcinus orca from fatty acid, stable isotope, and organochlorine analyses of blubber biopsies. Mar. Ecol. Prog. Ser. 302, 275–291. ( 10.3354/meps302275) [DOI] [Google Scholar]
- 32.Iverson SJ, Frost KJ, Lang LC. 2002. Fat content and fatty acid composition of forage fish and invertebrates in Prince William Sound, Alaska: factors contributing to among and within species variability. Mar. Ecol. Prog. Ser. 241, 161–181. ( 10.3354/meps241161) [DOI] [Google Scholar]
- 33.Møller P. 2006. Lipids and stable isotopes in marine food webs in West Greenland: trophic relations and health implications, p. 212 PhD thesis, National Environmental Research Institute, Denmark. [Google Scholar]
- 34.Budge SM, Iverson SJ, Koopman HN. 2006. Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar. Mamm. Sci. 22, 759–801. ( 10.1111/j.1748-7692.2006.00079.x) [DOI] [Google Scholar]
- 35.Connor RC, Smolker RS. 1985. Habituated dolphins (Tursiops sp.) in western Australia. J. Mammal. 66, 398–400. ( 10.2307/1381260) [DOI] [Google Scholar]
- 36.Würsig B, Würsig M. 1977. The photographic determination of group size, composition, and stability of coastal porpoises (Tursiops truncatus). Science 198, 755–756. ( 10.1126/science.198.4318.755) [DOI] [Google Scholar]
- 37.Krützen M, Barre LM, Möller LM, Heithaus MR, Simms C, Sherwin WB. 2002. A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp. Mar. Mamm. Sci. 18, 863–878. ( 10.1111/j.1748-7692.2002.tb01078.x) [DOI] [Google Scholar]
- 38.Amos B, Hoelzel AR. 1992. Applications of molecular genetic techniques to the conservation of small populations. Biol Conserv. 61, 133–144. ( 10.1016/0006-3207(92)91103-Y) [DOI] [Google Scholar]
- 39.Krützen M, Barre LM, Connor RC, Mann J, Sherwin WB. 2004. ‘O father, where art thou?’: paternity analysis in an open fission–fusion society of wild bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia. Mol. Ecol. 13, 1975–1990. ( 10.1111/j.1365-294X.2004.02192.x) [DOI] [PubMed] [Google Scholar]
- 40.Folch J, Lees M, Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497. [PubMed] [Google Scholar]
- 41.Rice WR. 1989. Analyzing tables of statistical tests. Evolution 43, 223–225. ( 10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- 42.Sharma S. 1995. Applied multivariate techniques. New York, NY: John Wiley and Sons, Inc. [Google Scholar]
- 43.Heithaus MR, Dill LM. 2002. Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83, 480–491. ( 10.1890/0012-9658(2002)083[0480:FAATSP]2.0.CO;2) [DOI] [Google Scholar]
- 44.Iverson SJ, Field C, Don Bowen W, Blanchard W. 2004. Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol. Monogr. 74, 211–235. ( 10.1890/02-4105) [DOI] [Google Scholar]
- 45.Svanbäck R, Bolnick DI. 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B 274, 839 ( 10.1098/rspb.2006.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Partridge L, Green P. 1985. Intraspecific feeding specializations and population dynamics. In Behavioural ecology: ecological consequences of adaptive behavior (eds Sibly RM, Smith RH.), pp. 207–226. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 47.Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE. 2003. Individual variation in prey selection by sea otters: patterns, causes and implications. J. Anim. Ecol. 72, 144–155. ( 10.1046/j.1365-2656.2003.00690.x) [DOI] [Google Scholar]
- 48.Heithaus MR. 2004. Fish communities of subtropical seagrass meadows and associated habitats in Shark Bay, Western Australia. Bull. Mar. Sci. 75, 79–99. [Google Scholar]
- 49.Walton MJ, Silva MA, Magalhaes SM, Prieto R, Santos RS. 2007. Using blubber biopsies to provide ecological information about bottlenose dolphins (Tursiops truncatus) around the Azores. J. Mar. Biol. Assoc. UK 87, 223–230. ( 10.1017/S0025315407054537) [DOI] [Google Scholar]
- 50.Connor RC, Mann J, Tyack PL, Whitehead H. 1998. Social evolution in toothed whales. Trends Ecol. Evol. 13, 228–232. ( 10.1016/S0169-5347(98)01326-3) [DOI] [PubMed] [Google Scholar]
- 51.van Valen L. 1965. Morphological variation and width of ecological niche. Am. Nat. 99, 377–390. ( 10.1086/282379) [DOI] [Google Scholar]
- 52.Bürger R, Schneider KA. 2006. Intraspecific competitive divergence and convergence under assortative mating. Am. Nat. 167, 190–205. ( 10.1086/499375) [DOI] [PubMed] [Google Scholar]
- 53.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholson K, Bejder L, Allen SJ, Krützen M, Pollock KH. 2012. Abundance, survival and temporary emigration of bottlenose dolphins (Tursiops sp.) off Useless Loop in the western gulf of Shark Bay, Western Australia. Aust. J. Mar. Freshwater Res. 63, 1059–1068. ( 10.1071/MF12210) [DOI] [Google Scholar]
- 55.Whiten A, van Schaik CP. 2007. The evolution of animal ‘cultures’ and social intelligence. Phil. Trans. R. Soc. B 362, 603–620. ( 10.1098/rstb.2006.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laland KN, Odling-Smee J, Myles S. 2010. How culture shaped the human genome: bringing genetics and the human sciences together. Nat. Rev. Gen. 11, 137–148. ( 10.1038/nrg2734) [DOI] [PubMed] [Google Scholar]
- 57.Whitehead H. 1998. Cultural selection and genetic diversity in matrilineal whales. Science 282, 1708–1711. ( 10.1126/science.282.5394.1708) [DOI] [PubMed] [Google Scholar]
- 58.Rendell L, Whitehead H. 2001. Culture in whale and dolphins. Behav. Brain Sci. 24, 309–382. ( 10.1017/S0140525X0100396X) [DOI] [PubMed] [Google Scholar]
- 59.Baird RW, Whitehead H. 2000. Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105. ( 10.1139/z00-155) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this manuscript will be made available on Dryad.