Abstract
Anti-predator behaviour affects prey population dynamics, mediates cascading effects in food webs and influences the likelihood of rapid extinctions. Predator manipulations in natural settings provide a rare opportunity to understand how prey anti-predator behaviour is affected by large-scale changes in predators. Here, we couple a long-term, island-wide manipulation of an important rodent predator, the island fox (Urocyon littoralis), with nearly 6 years of measurements on foraging by deer mice (Peromyscus maniculatus) to provide unequivocal evidence that prey closely match their foraging behaviour to the number of fox predators present on the island. Peromyscus maniculatus foraging among exposed and sheltered microhabitats (a measure of aversion to predation risk) closely tracked fox density, but the nature of this effect depended upon nightly environmental conditions known to affect rodent susceptibility to predators. These effects could not be explained by changes in density of deer mice over time. Our work reveals that prey in natural settings are cognizant of the dynamic nature of their predators over timescales that span many years, and that predator removals spanning many generations of prey do not result in a loss of anti-predator behaviour.
Keywords: anti-predator behaviour, predation risk, predator–prey interactions, relaxed selection
1. Introduction
Anti-predator behaviour affects prey distribution [1–4], prey population dynamics [5,6], mediates cascading effects in food webs [5,7], may alter the course of biological invasions [8] and influences the likelihood of rapid extinctions [9]. The degree to which prey exhibit anti-predator behaviour is a function of many factors, such as prey hunger, the distance to refuge habitat, predator hunting mode and the density of predators [5,9–14]. Understanding how anti-predator behaviour of prey is affected by the density of predators is especially important because the large-scale and widespread loss of top predators has led to shifts in ecosystem function, health and stability [15], which has catalysed proposals to reintroduce predators into regions from which they have been extirpated [16]. Although anti-predator behaviour can influence the persistence of prey and predators, and although changes in the abundance of top predators are increasingly widespread, it remains unclear whether prey behaviour closely tracks predator abundance in natural systems over time [17,18]. This gap is likely due, in part, to the difficulty of manipulating predator abundance and observing changes in prey behaviour over timescales that are ecologically relevant.
Predator manipulations in natural settings, although rare, provide a unique and powerful opportunity to understand how prey anti-predator behaviour is affected by large-scale changes in predators that are typical of contemporary ecological communities [15,16]. Islands are excellent systems for understanding evolution [19,20] and predator–prey dynamics [21,22]. Island systems may be particularly useful in the context of the ecology and evolution of anti-predator behaviour [23,24], because dynamics of predators and prey can often be known with greater precision than in mainland systems, because islands have relatively discrete system boundaries compared with more open mainland systems. Here, we leverage the strength of both approaches by using the island-wide manipulation of a top predator to examine how changes in prey anti-predator behaviour respond to changes in the density of a top predator.
We examined the anti-predator behaviour of deer mice (Peromyscus maniculatus) on San Miguel Island for nearly 6 years following a period of long-term, island-wide reduction of an important rodent predator, the island fox (Urocyon littoralis). Following a precipitous population decline (figure 1) [25,26], remaining foxes were removed from the wild and held as part of a captive breeding programme [27]. By examining the anti-predator behaviour of island mice over a 6-year period following the reintroduction of foxes from the wild, we use a system where the abundance of a primary rodent predator is known to provide a relatively long-term perspective on the degree to which prey behaviour responds to changes in the density of top predators.
Figure 1.
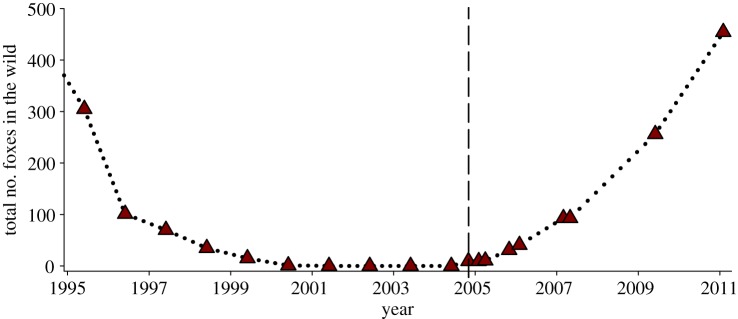
Long-term changes in the island-wide density of a top predator, the island fox (U. littoralis). Fox abundance was dramatically reduced in 1998 just prior to the initiation of a captive breeding programme that removed all foxes from the wild in 2000. The dashed line just prior to 2005 indicates when foxes were released back into the wild. (Online version in colour.)
2. Material and methods
(a). Study system
Deer mice are the only abundant terrestrial rodent on San Miguel Island [28]. The only terrestrial predator of deer mice is the island fox, U. littoralis. Evidence suggests that island foxes are strong agents of selection on mice: mice can comprise a substantial fraction of fox diet [27], mice on fox-free islands are often much more abundant than mice on the mainland [28] and when foxes were removed from the wild, mouse populations reached densities that were greater than those observed in mainland systems [27,28]. Moreover, relaxed selection over evolutionary timescales has presumably led to the loss of responses to fox cues on an island where rodents have existed without foxes for over 6000 years [29]. Avian predators (owls, hawks and harriers) also consume P. maniculatus and were present for the duration of this study, yet no comprehensive data were available on changes in avian predator densities over time (see Discussion).
Fox abundance data and rodent abundance data were obtained from published reports from the National Park Service [30]; see the electronic supplementary material for additional information. These data were used to evaluate whether temporal variation in prey behaviour was driven by changes in fox abundance (figure 1), as well as density-dependent mechanisms (see below).
(b). Quantifying rodent foraging
We used foraging trays to quantify giving-up densities to assess risk perception by rodents [31]; see the electronic supplementary material for additional information regarding this approach. Rodent foraging was assessed over eight periods spanning 6 years: 6–8 February 2005; 24–29 March 2005; 17–21 November 2005; 8–10 February 2006; 28 February–4 March 2007; 2–6 May 2007; 16–23 June 2009; 16–22 February 2011. Because rodent foraging can be sensitive to nightly climatic conditions that affect predation risk, such as changes in moon illumination, precipitation and overcast conditions [32–34], we classified each night of foraging observations as safe (i.e. fraction of the moon illuminated less than 0.5 or rainfall more than 0.001 mm) or risky (i.e. nights with no precipitation and high moon illumination). This dichotomous classification is strongly supported by the bimodal nature of our data (see the electronic supplementary material and figure S1 for additional information).
Foraging trays were translucent round plastic containers (21 cm diameter, 16 cm height) with translucent plastic lids [33]. Two holes were cut in the side of each foraging tray to allow rodent entry; lids and the small size of entry holes likely precluded foraging by avian granivores (no signs of bird foraging in the trays were ever observed over the course of this work). Foraging trays were provisioned with 1 dram of heat-sterilized millet seeds (4.73 ± 0.03 g of seeds, N = 39) mixed with 0.47 l of sand. Pairs of foraging trays were established at eight sites within an area of approximately 50 × 50 m; all pairs of trays were separated by at least 10 m to reduce the likelihood that the same individuals were foraging among multiple pairs of trays (see the electronic supplementary material). For each pair, one foraging tray was located in a sheltered microhabitat (below a shrub, Lupinus chamissonis) and the other foraging tray was located less than 1 m away, in an exposed microhabitat outside of the cover of the shrub. We selected this study area because it is representative of one of the dominant habitat types on the island, and it is also a habitat type where there is a clear distinction between risky and safe microhabitats. Trays were visited daily, remaining seeds were collected and trays were recharged with fresh seeds. This design is commonly used to examine anti-predator behaviour because it manipulates predation risk while holding other costs of foraging constant [31,32], such that the difference between foraging trays in sheltered and exposed habitats are robust to changes in background food availability and small mammal density [4,31,32,35]. However, absolute values of foraging in both exposed and sheltered habitats may be affected by rodent density [36]; all fox and rodent foraging data are presented in the electronic supplementary material.
While these foraging data are designed to interpret predation risk in rodents, understanding the extent to which rodents changed in abundance over time with fox abundance is also of interest for understanding whether density-dependent changes in foraging were occurring. We use rodent abundance data collected by the National Park Service as an independent measure to determine whether foxes affected rodent abundance and whether rodent abundance was linked to patterns in anti-predator behaviour we observed from foraging trays.
(c). Statistical methods
We used general linear mixed models to evaluate changes in rodent foraging for nights with risky foraging conditions and nights with safe foraging conditions. We fit two types of models. First, we considered the log-transformed difference in rodent foraging between sheltered and exposed trays as a response variable, including sampling session and the interaction of session with day of sampling within a session and sampling site as random effects and fox abundance (log-transformed), risky versus safe night (night) and the interaction of fox abundance and night as fixed effects. The log-transformed difference between sheltered and exposed micohabitats reflects the change in foraging attributable to predation risk [32] and is thought to be less sensitive to changes in rodent density [35], as confirmed by our analyses (see Results; see also the electronic supplementary material). Second, we also considered the absolute value of seeds remaining in each tray as a response variable, with fox abundance, microhabitat (sheltered versus exposed) and the interaction of fox abundance and microhabitat as fixed effects, and the same random effects as above. To simplify model structure for this second model type, we fit separate models for nights with risky conditions (i.e. nights without precipitation or high moon illumination) and for nights with safe conditions (i.e. nights with precipitation or low moon illumination). Because data taken at the same site on consecutive days within a session may not be independent, we evaluated the fit of modelling the residual covariance among days within a session by comparing a first-order autoregressive structure, a compound symmetric structure and a variance components structure. Overall, a compound symmetric structure (assuming a constant temporal dependence among days) fit the data best based on model selection criteria (Akaike's information criterion, adjusted for sample size, all best models more than 2.2 AICc units better than first-order autoregressive and variance components models); results shown come from this model structure. We use the Kenward–Rogers approximation to determine appropriate degrees of freedom for hypothesis tests [37].
We used correlation analysis to evaluate the potential relationships between the abundance of foxes, the abundance of rodents while we were collecting foraging data and giving-up density data collected from foraging trays. To further interpret whether fox-mediated changes in rodent density were affecting our inferences, we also evaluated the mixed models described above using rodent abundance as a covariate and allowing all possible interactions. Because the inclusion of rodent abundance was not significant in any of these models (all p > 0.37), we did not include it in our final analyses.
3. Results
The release of island foxes back into the wild starting in November 2004 led to a steady increase in the total number of fox predators on the island over the course of the study (figure 1). Island mice rapidly altered their anti-predator behaviour upon the reintroduction of foxes to the island (figure 2a): the difference in foraging among exposed and sheltered microhabitats, a measure of aversion to predation risk [32], was significantly affected by fox density, but the nature of this effect depended upon nightly environmental conditions that are thought to affect rodent susceptibility to predators (interaction between nightly environment and fox abundance F1,36.7 = 12.91, p = 0.001; environment main effect F1,26.8 = 9.36, p = 0.005, fox main effect F1,37.7 = 0.77, p = 0.386). On clear, moonlit nights that are typically considered risky nights for foraging rodents because predators may more readily detect and capture mice [38,39], rodent foraging exhibited less aversion to predation risk as foxes became more abundant (figure 2a). On nights with precipitation or low moonlight, which are typically considered to be relatively safe for rodent foraging [34], mouse foraging was increasingly averse to predation risk as the number of foxes increased (figure 2a).
Figure 2.
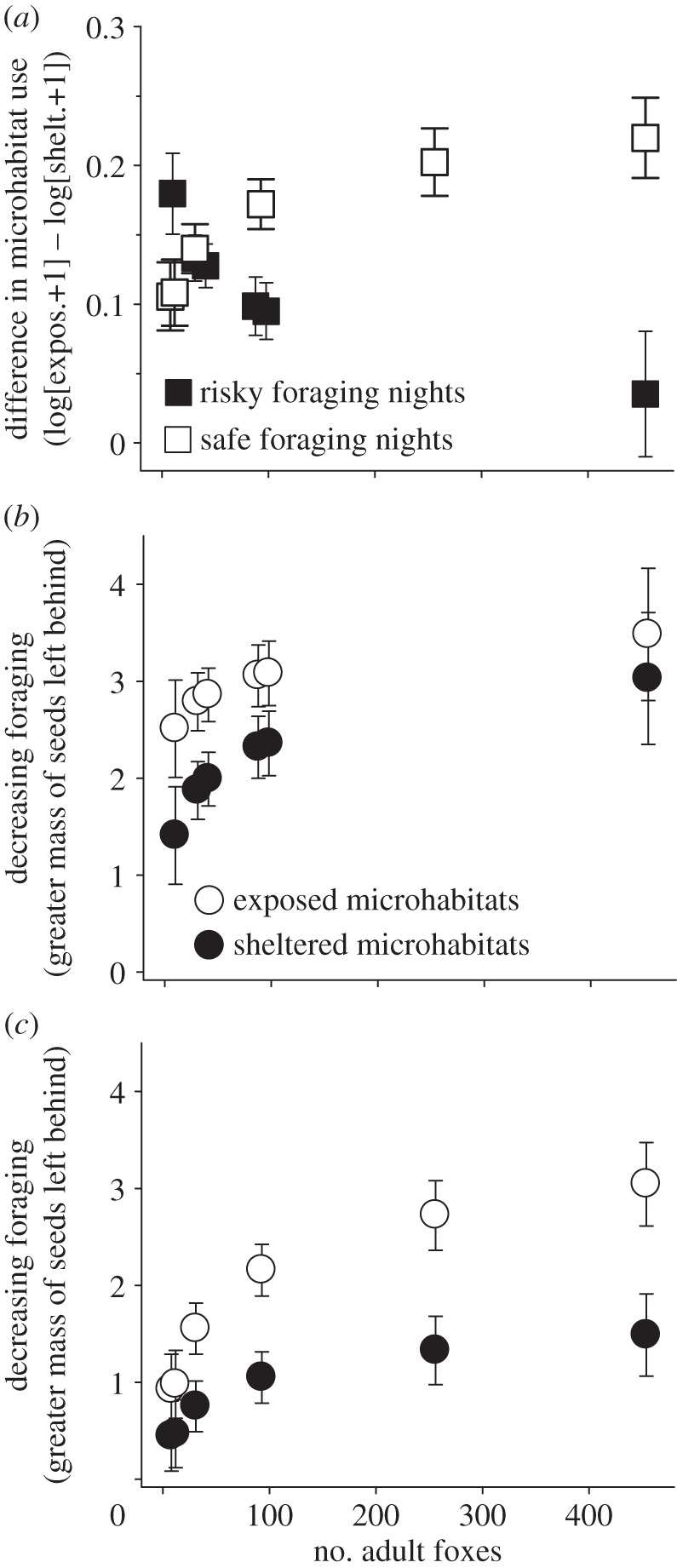
(a) After fox reintroduction in late 2004, behaviour indicating aversion to predation risk, measured as the difference in rodent foraging between exposed and sheltered microhabitats, became more evident as the abundance of foxes increased on nights when foraging conditions were safe (higher values on the y-axis indicate that rodents foraged less in the risky exposed microhabitat relative to the sheltered microhabitat, i.e. these data are the differences in the log-transformed values of overall foraging presented in (b,c)). On nights when climatic conditions are typically safer for foraging rodents, rodents exhibited a foraging preference for sheltered microhabitats over exposed microhabitats. Patterns of mouse foraging in exposed and sheltered microhabitats show that overall foraging decreased on both risky (b) and safe nights (c) as fox abundance increased. However, the nature of foraging among microhabitats was different depending upon whether the night was safe or risky.
The differences in rodent foraging on safe and risky nights arose because of differences in patterns of usage among sheltered and exposed foraging microhabitats. On nights with risky foraging conditions, rodents reduced foraging in both exposed and sheltered microhabitats (figure 2b), but the reduction in foraging occurred more rapidly in sheltered microhabitats as fox abundance increased (interaction between foraging microhabitat and fox abundance F1,80 = 4.66, p = 0.034; foraging microhabitat main effect F1,80 = 15.44, p < 0.001, fox main effect F1,22.4 = 2.06, p = 0.165). On nights when precipitation or low levels of moon illumination likely reduced risk to rodents, there was a clear effect of fox abundance affecting rodent foraging in both microhabitats (figure 2c), but the reduction in foraging occurred more rapidly in exposed microhabitats as fox abundance increased (interaction between foraging microhabitat and fox abundance F1,93.1 = 7.27, p = 0.008; foraging microhabitat main effect F1,92.5 = 0.52, p = 0.472, fox main effect F1,18.4 = 7.57, p = 0.013).
We found a significant negative correlation between our estimate of fox density during foraging tray trials and mean estimates of mouse abundance from National Park Service data (r = −0.78, p = 0.021, d.f. = 6; see the electronic supplementary material). There was no significant relationship between mean estimates of mouse abundance and rodent anti-predator behaviour in foraging trays measured as the mean log-transformed difference between sheltered and exposed trays (r = 0.46, p = 0.26, d.f. = 6).
4. Discussion
In finding that rodent anti-predator behaviour tracked the island-wide abundance of a top predator over 6 years, our results suggest that manipulations of predators at the large scales that are typical of anthropogenic activities have direct impacts on prey anti-predator behaviour over timescales that span many years and several prey generations. Our results have several implications. First, they illustrate that prey are highly cognizant of risk in their environment and they use this information to reduce their exposure to predators when predators are more abundant. Second, contrasting patterns of foraging between sheltered and exposed microhabitats on safe and risky nights suggest that changes in rodent foraging may reflect the joint action of both fox predators and avian predators. Third, our results illustrate that relaxed selection on rodents during the period of fox removal did not lead to a change in rodent anti-predator behaviour, suggesting that predator manipulations in natural systems may not lead to loss of anti-predator behaviour in prey over contemporary timescales.
Although theory suggests that prey should alter their behaviour to match the level of risk present in the environment ([32] and references therein), this prediction is rarely evaluated in natural settings where the system-wide abundance of a key predator is subject to experimental manipulation over long timescales. Our findings suggest that mice are indeed cognizant of the abundance of their fox predators, and that the way mice respond to this increase in fox foraging depends upon environmental context. Moreover, our long-term time series provides support for changes in behaviour predicted by theory and smaller scale studies [32]: within the range of resources provided in our foraging trays (i.e. 4.73 g of seeds), anti-predator behaviour of mice is asymptotic (figure 2). This is especially apparent on safe foraging nights, where rodents continue to forage heavily in sheltered microhabitats as they reduce allocation to risky exposed microhabitats as fox abundance increases (figure 2c). On risky foraging nights, the asymptotic nature of rodent foraging may also reflect the absolute value of resources in a tray (i.e. rodents are foraging very little in a tray, leaving nearly 4 g of seeds behind). However, even on these nights, rodents left 3.48 (±0.68) g of seeds, suggesting that they consumed over 25% of available seeds on risky foraging nights even when foxes were abundant. Coupled with the even greater removal of seeds on safe foraging nights when foxes were abundant (e.g. consumption of over 35% of available seeds in exposed microhabitats and over 68% from sheltered microhabitats; figure 2c), our findings suggest that rodent prey trade-off predation risk against other activities that increase their fitness and that the benefits of predation risk are eventually offset by the costs (e.g. avoiding predation may also reduce access to food or mates) or that interference among predators leads to diminishing risk as the number of predators increases [40]. These results suggest that mice must have some means of assessing fox abundance; such information could come in the form of increasing exposure to fox cues (e.g. faeces, urine) as foxes become more abundant. The finding that rodents recognize and avoid fox faeces on the study island [29] suggests that this mechanism may be plausible.
The long-term nature of our study provides insight into how prey shift allocation of foraging behaviour as the density of fox predators is increasing and also yields an interesting and initially counterintuitive result. On nights when clear weather likely made foraging more risky for mice, the difference in rodent foraging between sheltered and exposed microhabitats is expected to increase as fox abundance (and thus predation risk) increases because rodents should spend more time foraging in safe microhabitats relative to risky ones [31,32]. On nights when overcast conditions, low moon illumination, or precipitation made foraging less risky for mice, we would expect reduced differences between sheltered and exposed trays, because overall foraging conditions are expected to be equalized between these habitats on safe nights. However, we find that the difference in foraging effort between sheltered and exposed microhabitats decreased on risky nights (rather than increased) as foxes became more abundant (figure 2b), with the reduction in the difference being caused by a greater decrease in foraging in sheltered microhabitats as fox abundance increased (figure 2b). At least three alternative hypotheses could explain this pattern. First, habitat quality might, by chance, vary systematically during the course of our study, leading to a reduction in foraging that is biased towards sheltered microhabitats (because exposed microhabitats are presumably still more risky). However, this hypothesis seems unlikely, as total precipitation input, a measure of grassland productivity [41], was not correlated with foraging in sheltered microhabitats (r = −0.33, p = 0.41, d.f. = 6) or correlated with fox abundance (r = 0.37, p = 0.37, d.f. = 6). Second, as there is evidence that foxes affect rodent abundance [27,28], and mouse density was likely changing during the course of our study (see Results), patterns in rodent foraging we observed may be due to density-dependent effects. Yet, the pattern we observed is not likely to be due to differences in mouse density: theory and empirical data both suggest that changes in rodent density should not alter the difference in foraging between adjacent (1-m separation) sheltered and exposed microhabitats [31,32,35], which was also confirmed by our data (see Material and methods, Results and the electronic supplementary material, figure S2). Moreover, the difference between sheltered and exposed microhabitats on safe nights was increasing with fox abundance, which is also inconsistent with density-mediated effects.
A third explanation for the patterns we observed is that terrestrial fox predators are more effective than avian predators at attacking rodents in sheltered microhabitats below lupine shrubs, such that sheltered microhabitats become less safe as foxes become more abundant on the island, especially on risky nights. Rodents may have responded to this change in risk by reduced foraging in sheltered microhabitats (figure 2b). As sheltered microhabitats become riskier due to changes in fox abundance, foraging in sheltered microhabitats on risky nights eventually converges on the low foraging activity in exposed microhabitats, where we expect both foxes and avian predators to be generally more effective at attacking a mouse (relative to sheltered microhabitats). Predator hunting mode has been found to have strong effects on the foraging behaviour of shared prey in many taxa [13], including rodents [42,43]. We conclude that the convergence in rodent foraging activity between sheltered and exposed microhabitats arises because sheltered habitats where rodents are safe from avian attack are habitats where rodents might still be attacked by foxes: although exposed habitats are always risky because of foxes and avian predators, as fox abundance increases, formerly safe sheltered microhabitats also become more risky. While even sheltered microhabitats may not be safe for rodents on risky nights, the reduction in foraging on risky nights may have been offset to some degree by the continued use of sheltered microhabitats on safe nights (figure 2c), as predicted by models of optimal foraging under temporal variation in risk [44]. Foxes on a nearby island have been found to select lupine-dominated habitats [45], suggesting that foxes may be successful at capturing rodent prey in lupine-dominated habitats like the one used in this study. Fox preference for lupine habitats may be further increased if shifts in activity timing observed on some islands in response to large raptors [46] are also leading to increased time spent in sheltered lupine habitats.
The loss of anti-predator behaviour in evolutionary time can have important contemporary consequences [23], such as promoting the extinction of prey that have become naive due to the loss of anti-predator behaviour [47]. Because mice do not respond to fox cues on a nearby island that does not contain foxes, but does contain owl predators [29], relaxed selection resulting from loss of fox predators over evolutionary timescales can lead to the lack of a response to fox predators in island mice. However, our work shows that, despite the loss of 99.75% of the historical fox population for a period of time that spans approximately 24–28 prey generations (figure 1), rodent prey immediately and closely matched anti-predator behaviour to reflect the density of newly reintroduced foxes (figure 2). This immediate matching of risk by prey suggests that removals of top predators may have minimal impact on the evolutionary loss of anti-predator behaviour [48] over timescales spanning many prey generations, providing new empirical insight on the role of relaxed selection in affecting prey behaviour, an important problem in evolutionary biology, conservation and restoration [16,48,49].
Our work illustrates that prey are aware of the density of their predators, and that they alter their foraging behaviour accordingly. This work suggests that prey behaviour may be sensitive to changes in predator density caused by human activities and that shifts in prey behaviour may occur quickly, creating the potential for complex indirect effects to rapidly arise in systems once predators are manipulated [15]. An additional conclusion is that evolutionary loss of anti-predator behaviour did not occur over timescales of relaxed selection (i.e. 24–28 rodent generations) that may be relevant to contemporary problems in conservation biology [50]. Our work also highlights several important and interesting avenues for future work. For example, our work suggests that there may be interesting differences in the efficacy of fox and owl predators, at least from the perspective of a foraging rodent; future studies that evaluate the effectiveness of fox and owl predators, as well as the potential for interactions among fox and owl predators, would be very informative. Studies from other systems, where different types of predators are being closely tracked, will also be informative for comparing with our results. For example, owls are the only rodent predator on Santa Barbara Island [51], and results from foraging studies currently being conducted on that island (S. Thomsen 2014, personal communication) will be informative for evaluating how density of avian predators affect rodent foraging in the absence of fox predators.
Acknowledgements
We thank National Park Service staff from the Channel Islands National Park and T. Rusca for making this work possible. We thank T. Coonan, C. Drost, E. Preisser, O. J. Reichman, C. Schwemm and I. Williams for discussions, assistance and/or comments on the manuscript.
Funding statement
This work was supported by NSF (DEB-0502069); portions of this work were also done while J.L.O. was a Fellow at NCEAS, a Centre funded by NSF (grant no. DEB-0072909), the University of California and the Santa Barbara Campus.
References
- 1.Hua F, Fletcher RJ, Jr, Sieving KE, Dorazio RM. 2013. Too risky to settle: avian community structure changes in response to perceived predation risk on adults and offspring. Proc. R. Soc. B 280, 20130762 ( 10.1098/rspb.2013.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caro T. 2005. Antipredator defenses in birds and mammals, p. 591 Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Lima SL. 1998. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 27, 215–290. ( 10.1016/S0065-3454(08)60366-6) [DOI] [Google Scholar]
- 4.Morris DW, Davidson DL. 2000. Optimally foraging mice match patch use with habitat differences in fitness. Ecology 81, 2061–2066. ( 10.1890/0012-9658(2000)081[2061:OFMMPU]2.0.CO;2) [DOI] [Google Scholar]
- 5.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 6.Creel S, Christianson D, Liley S, Winnie JA., Jr 2007. Predation risk affects reproductive physiology and demography of elk. Science 315, 960 ( 10.1126/science.1135918) [DOI] [PubMed] [Google Scholar]
- 7.Schmitz O. 2008. Effect of predator hunting mode on grassland ecosystem function. Science 319, 952–954. ( 10.1126/science.1152355) [DOI] [PubMed] [Google Scholar]
- 8.Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor L, Preisser E, Rehage J, Vonesh JR. 2010. Predator–prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119, 610–621. ( 10.1111/j.1600-0706.2009.18039.x) [DOI] [Google Scholar]
- 9.Stankowich T, Blumstein DT. 2005. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634. ( 10.1098/rspb.2005.3251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144. ( 10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 11.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 12.Kats LB, Dill LM. 1998. The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5, 361–394. [Google Scholar]
- 13.Preisser EL, Orrock JL, Schmitz OJ. 2007. Predator hunting mode and habitat domain alter nonconsumptive effects in predator–prey interactions. Ecology 88, 2744–2751. ( 10.1890/07-0260.1) [DOI] [PubMed] [Google Scholar]
- 14.Orrock JL, Preisser EL, Grabowski JH, Trussell GC. 2013. The cost of safety: refuges increase the impact of predation risk in aquatic systems. Ecology 94, 573–579. ( 10.1890/12-0502.1) [DOI] [PubMed] [Google Scholar]
- 15.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Swenson JE, Persson IL. 2001. Recolonizing carnivores and naive prey: conservation lessons from Pleistocene extinctions. Science 291, 1036–1039. ( 10.1126/science.1056466) [DOI] [PubMed] [Google Scholar]
- 17.St Juliana JR, Kotler BP, Brown JS, Mukherjee S, Bouskila A. 2011. The foraging response of gerbils to a gradient of owl numbers. Evol. Ecol. Res. 13, 869–878. [Google Scholar]
- 18.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 19.Potsma E, Heinrich F, Koller U, Sardell RJ, Reid JM, Arcese P, Keller LF. 2011. Disentangling the effect of genes, the environment and chance on sex ratio variation in a wild bird population. Proc. R. Soc. B 278, 2996–3002. ( 10.1098/rspb.2010.2763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant BR, Grant PR. 1993. Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. Lond. B 251, 111–117. ( 10.1098/rspb.1993.0016) [DOI] [Google Scholar]
- 21.Post E, Peterson RO, Stenseth NC, McLaren BE. 1999. Ecosystem consequences of wolf behavioral response to climate. Nature 401, 905–907. ( 10.1038/44814) [DOI] [Google Scholar]
- 22.McLaren BE, Peterson RO. 1994. Wolves, moose, and tree rings on Isle Royale. Science 266, 1555–1558. ( 10.1126/science.266.5190.1555) [DOI] [PubMed] [Google Scholar]
- 23.Blumstein DT, Daniel JC. 2005. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668. ( 10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334, 1398–1401. ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 25.Coonan TJ, Schwemm CA, Roemer GW, Garcelon DK, Munson L. 2005. Decline of an island fox subspecies to near extinction. Southwest Nat. 50, 32–41. () [DOI] [Google Scholar]
- 26.Roemer GW, Donlan CJ, Courchamp F. 2002. Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc. Natl Acad. Sci. USA 99, 791–796. ( 10.1073/pnas.012422499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coonan TJ, Schwemm CA, Garcelon DK. 2010. Decline and recovery of the island fox: a case study for population recovery, p. 2012 New York, NY: Cambridge University Press. [Google Scholar]
- 28.Schwemm CA, Coonan TJ. 2001. Status and ecology of deer mice (Peromyscus maniculatus subspp.) on Anacapa, Santa Barbara, and San Miguel Islands, California: summary of monitoring 1992–2001. Ventura County, CA: National Park Service, Channel Islands National Park.
- 29.Orrock JL. 2010. When the ghost of predation has passed: do rodents from islands with and without fox predators exhibit aversion to fox cues? Ethology 116, 338–345. ( 10.1111/j.1439-0310.2010.01740.x) [DOI] [Google Scholar]
- 30.Coonan TJ. 2012. Island fox recovery program 2011 annual report. In National Park Service Natural Resource Report NPS/MEDN/NRR 2012/572. Ventura County, CA: National Park Service, Channel Islands National Park.
- 31.Brown JS. 1988. Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47. ( 10.1007/BF00395696) [DOI] [Google Scholar]
- 32.Brown JS, Kotler BP. 2004. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 7, 999–1014. ( 10.1111/j.1461-0248.2004.00661.x) [DOI] [Google Scholar]
- 33.Mattos KJ, Orrock JL. 2010. Behavioral consequences of plant invasion: an invasive plant alters rodent antipredator behavior. Behav. Ecol. 21, 556–561. ( 10.1093/beheco/arq020) [DOI] [Google Scholar]
- 34.Orrock JL, Danielson BJ, Brinkerhoff RJ. 2004. Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav. Ecol. 15, 433–437. ( 10.1093/beheco/arh031) [DOI] [Google Scholar]
- 35.Morris DW, Mukherjee S. 2007. Can we measure carrying capacity with foraging behavior? Ecology 88, 597–604. ( 10.1890/06-0389) [DOI] [PubMed] [Google Scholar]
- 36.Davidson DL, Morris DW. 2001. Density-dependent foraging effort of deer mice (Peromyscus maniculatus). Funct. Ecol. 15, 575–583. ( 10.1046/j.0269-8463.2001.00569.x) [DOI] [Google Scholar]
- 37.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. 2006. SAS for mixed models, p. 814 Cary, NC: SAS Institute. [Google Scholar]
- 38.Kotler BP, Brown JS, Hasson O. 1991. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72, 2249–2260. ( 10.2307/1941575) [DOI] [Google Scholar]
- 39.Clarke JA. 1983. Moonlight's influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behav. Ecol. Sociobiol. 13, 205–209. ( 10.1007/BF00299924) [DOI] [Google Scholar]
- 40.Skalski GT, Gilliam JF. 2001. Functional responses with predator interference: viable alternatives to the Holling type II model. Ecology 82, 3083–3092. ( 10.1890/0012-9658(2001)082[3083:FRWPIV]2.0.CO;2) [DOI] [Google Scholar]
- 41.Williams JW, Seabloom EW, Slayback D, Stoms DM, Viers JH. 2005. Anthropogenic impacts upon plant species richness and net primary productivity in California. Ecol. Lett. 8, 127–137. ( 10.1111/j.1461-0248.2004.00706.x) [DOI] [Google Scholar]
- 42.Bouskila A. 1995. Interactions between predation risk and competition: a field study of kangaroo rats and snakes. Ecology 76, 165–178. ( 10.2307/1940639) [DOI] [Google Scholar]
- 43.Kotler BP, Blaustein L, Brown JS. 1992. Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann. Zool. Fenn. 29, 199–206. [Google Scholar]
- 44.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 45.Drake EM. 2013. Home range and habitat use of Santa Rosa Island foxes (Urocyon littoralis santarosae). San Luis Obispo, CA: California Polytechnic State University. [Google Scholar]
- 46.Hudgens BR, Garcelon DK. 2011. Induced changes in island fox (Urocyon littoralis) activity do not mitigate the extinction threat posted by a novel predator. Oecologia 165, 699–705. ( 10.1007/s00442-010-1761-7) [DOI] [PubMed] [Google Scholar]
- 47.Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958. ( 10.1126/science.1101617) [DOI] [PubMed] [Google Scholar]
- 48.Blumstein DT. 2006. The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology 112, 209–217. ( 10.1111/j.1439-0310.2006.01209.x) [DOI] [Google Scholar]
- 49.Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster BL. 2009. Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496. ( 10.1016/j.tree.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 50.Stockwell CA, Hendry AP, Kinnison MT. 2003. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94–101. ( 10.1016/S0169-5347(02)00044-7) [DOI] [Google Scholar]
- 51.Drost CA, Fellers GM. 1991. Density cycles in an island population of deer mice, Peromyscus maniculatus. Oikos 60, 351–364. ( 10.2307/3545078) [DOI] [Google Scholar]


