Abstract
The istiophorid family of billfishes is characterized by an extended rostrum or ‘bill’. While various functions (e.g. foraging and hydrodynamic benefits) have been proposed for this structure, until now no study has directly investigated the mechanisms by which billfishes use their rostrum to feed on prey. Here, we present the first unequivocal evidence of how the bill is used by Atlantic sailfish (Istiophorus albicans) to attack schooling sardines in the open ocean. Using high-speed video-analysis, we show that (i) sailfish manage to insert their bill into sardine schools without eliciting an evasive response and (ii) subsequently use their bill to either tap on individual prey targets or to slash through the school with powerful lateral motions characterized by one of the highest accelerations ever recorded in an aquatic vertebrate. Our results demonstrate that the combination of stealth and rapid motion make the sailfish bill an extremely effective feeding adaptation for capturing schooling prey.
Keywords: predator–prey interactions, fish schools, animal weapons, billfishes
1. Introduction
The billfishes (i.e. swordfish, spearfish, sailfish and marlins) are some of the most enigmatic marine species and are among the fastest swimmers in the ocean [1]. They have a global distribution and some species can reach body lengths of up to 5 m (see the electronic supplementary material, table S1). Billfishes are top predators, highly specialized for life in the pelagic environment [2] and have unique body adaptations in the form of an extended rostrum or bill. The primary functions of this structure have been the subject of much speculation. Information on billfish predation comes mainly from studies of stomach content, which reported gashes on the bodies of prey fish found in the stomachs of billfishes [3,4] and from these injuries studies have inferred that the bill might be used as a weapon for prey capture. However, more recent work has shown that the prey's bodies can be injury-free [5] which is consistent with the observation of billfishes that are in good body condition but whose bills have broken off or are severely bent [6]. This has called into question whether the bills are in fact required for prey capture [6]. Alternatively, it has also been suggested that the main function of the bill is a reduction in drag while swimming [1,7].
To date no study has directly examined the role of the bill in billfish feeding behaviour nor quantified the response of the prey to billfish attacks. In contrast to most billfish, the Atlantic sailfish's (Istiophorus albicans) diurnal and social feeding ecology offers a rare and accessible opportunity to study bill use in these pelagic predators. Sailfish typically hunt in groups to drive large schools of their prey to the surface for presumably easier capture. This feature of their foraging behaviour allows a unique opportunity for relatively easy tracking from boats (see Material and methods) as well as close underwater observations of predator–prey interactions. Here, we present the first direct evidence of how the bill is used to capture evasive prey through a combination of stealth and extremely rapid motion.
2. Material and methods
To investigate the role of the bill in predation and the anti-predator response of the prey, we collected high-speed and high-definition video footage of group-hunting Atlantic sailfish (I. albicans) that attacked schools of adult sardine (Sardinella aurita), 30–70 km offshore from Cancun, Mexico (21 28.3–41.15 N, 86 38.41–41.30 W). Observation was carried out near the surface (0–5 m depth; water depth 30–40 m) between 10.00 and 16.00 h over a 6-day period in February 2012. Sailfish–sardine interactions were located by tracking groups of avian predators (e.g. frigate birds, Fregata magnificens; pelicans, Pelecanus occidentalis) flying above the sardine schools. High-speed and high-definition video recordings of predation events were made using Casio EX-FH100 high-speed cameras and a HD GOPRO HERO video camera, respectively. In total, 180 and 84 min of recording were obtained for high-definition and high-speed videos, respectively. Based on video footage, we estimated the number of sailfish involved in attacks on sardine prey schools (n = 6–40) and the size of the school itself upon encounter (10–1000+). Fin extension (dorsal and pelvic fins) in sailfish was recorded during predatory events. Gut content analysis of sardines from a sailfish specimen (180 cm from the tip of the bill to the end of caudal fin) caught by professional fishermen, showed that prey size was relatively uniform (n = 14; LB, mean total body length ± s.d. = 19.0 ± 0.2 cm, range 18.5–19.3 cm) and typical of adult sardines (www.fishbase.org).
From the recordings, we gathered (i) quantitative analyses of the behavioural sequence of predation events (Markov chain), (ii) kinematic analyses of bill motion and (iii) behavioural responses of the prey.
(a). Markov chain
Markov chain analysis was based on high-definition videos. All modelled sequences start in state ‘approach’ and end in state ‘departure’. The probabilities of each state of predation behaviour were estimated from the observed relative frequencies of the state changes. The durations of the states are not part of the model (i.e. the model's probability for a state being followed by the same state is always 0) and the probabilities for leaving the end state ‘departure’ are left unspecified because our observations ended in this state. A sailfish might start another attack sequence, swim away, or perform another action, which we did not observe.
We identified 10 main states of sailfish predation behaviour: (i) prey herding, (ii) chasing, (iii) approach, (iv) imminent attack (bill inside or in close proximity to school), (v) attack (including both slash and tap), (vi) prey contact (when the bill makes physical contact with one or more sardines), (vii) prey handling (redirection of prey towards mouth using bill), (viii) capture/ingestion, (ix) reapproach (in an instance when capture/ingestion was unsuccessful) or alternatively, (x) departure. This process can be repeated until every individual in the prey school is caught and consumed.
(b). Kinematic analyses of bill motion
We performed kinematic analyses of bill motion during slashing focusing on speed and acceleration since both can play an important role in predator–prey interactions of fish [8]. High-speed video footage of slashing events was recorded at 240 fps and analysed using WinAnalyze (www.winanalyze.com). From high-speed video footage, the X and Y coordinates of the sailfish's centre of the head (Hsf; the point centred between the eyes), the front of the head (Fsf, the point where the front of the head meets the base of the bill) and tip of the bill (Tsf) for each focal sailfish were digitized for every frame (with 4.167 ms between frames) beginning at five frames before a slashing event and ending 25 frames after the slashing event. Observations of slashing manoeuvres (n = 15) were based on top-view recordings of slashing events allowing two-dimensional analyses of the motion. In some instance, a few frames of footage for Tsf were obscured by sardines, although Hsf and Fsf were always visible. In these cases, Tsf was estimated based on the fixed length of the bill (i.e. the fixed distance between Fsf and Tsf, as a prolongation of the segment Hsf–Fsf). Distances were estimated on the basis of the sardines (i.e. length = 19 cm based on sailfish stomach content data) affected by the slash and therefore in the same plane as the sailfish bill, and judged to be swimming in the plane perpendicular to the camera lens. Sailfish and bill length in specimens that were fully visible were also estimated (n = 5).
The following variables were analysed: (i) maximum slashing speed and acceleration at three points Hsf, Fsf and Tsf; (ii) mean slashing speed at Tsf; (iii) mean turning rate (TRmean), which is a measure of the angular velocity of the bill during the slashing manoeuvre. TRmean was calculated as the angle (αtot) between the segment joining the points Fsf and Tsf, at the beginning and end of the slashing motion, divided by the slashing duration (Ds). Hence TRmean = αtot/Ds. A five-point differentiation-based smoothing method was then applied for each derivative procedure (i.e. speed and acceleration [9]).
(c). Behavioural responses of the prey
To analyse the sardines’ behavioural response to the presence of the sailfish bill, 28 slashing events from our high-speed recordings were analysed. Two variables were measured: (i) the tail beat frequency (number of tail beats (a complete oscillation cycle of the tail) per second) and (ii) overtaking behaviour (i.e. the number of body lengths that a focal fish gained on an individual swimming directly in front of it, expressed in number of body lengths overtaken per second). We focused on three different phases (pre-bill contact, bill contact and post-bill contact). Since not all phases were visible in all recorded events, we present final sample sizes per phase. In the pre-bill-contact phase, the bill approaches the prey but does not enter the school. The end of this phase is marked by bill entrance in the school (n = 23; mean duration ± s.d. = 0.4 ± 0.05 s, range = 0.21–0.42 s). The bill-contact phase begins when the bill of the sailfish enters the sardine school and ends when the actual slashing event starts (n = 21, mean duration ± s.d. = 0.28 ± 0.07 s, range = 0.19–0.42 s). The post-bill-contact phase starts after the actual slash and lasted for a maximum of 0.42 s (100 frames) or shorter when the fish could not be observed on the recordings for that period (n = 28, mean duration ± s.d. = 0.39 ± 0.06 s, range = 0.22–0.42 s). A target fish was defined as the closest observable fish to the bill of the sailfish. A control fish was defined as the first observable fish that was swimming in front of the target fish and was not within the bill strike zone (i.e. the area that was impacted by the slash).
3. Results
Individual sailfish either made predation attempts or redirected the sardines by swimming through or around the prey school. All observed attacks were carried out by single sailfish that approached the sardine school. Multiple sailfish were never observed attacking the school at the same time. An attack was initiated when no other sailfish was within approximately one sailfish body length of the school. After approaching the school of sardines from a posterior position, sailfish inserted their bill into the school and then used it in one of two distinct ways: ‘slashing’ (figure 1; see the electronic supplementary material, movie S1) and ‘tapping’ (see electronic supplementary material, movie S2). Slashing consisted of a forceful, rapid lateral movement of the bill through a large section of the school wherein the bill typically made physical contact with multiple fish causing bodily damage to the prey (figure 1; see the electronic supplementary material, movie S1). Given its relatively low success rate in terms of direct prey capture (10%), the primary function of slashing was presumably to inflict injury to facilitate later capture. Tapping, by contrast, consisted of a targeted short-range movement of the bill which destabilizes a single sardine (see the electronic supplementary material, movie S2) and more often resulted in successful prey capture (33%). The effectiveness of capturing single prey using tapping may be related to the surface properties (i.e. presence of lateral denticles) of the bill (see the electronic supplementary material, figure S1). All of our videos of the attacks show that sailfish always swam with the dorsal fin (i.e. the sail) and their pelvic fins extended, both during and prior to slashing and tapping events. Furthermore, lateral sides of the sailfish's body, which are normally bluish-silver, darkened to almost black just before an attack. In addition, in some cases, sailfish also displayed vertical stripes and lateral blue and orange spots during attack and posturing around the sardine schools.
Figure 1.
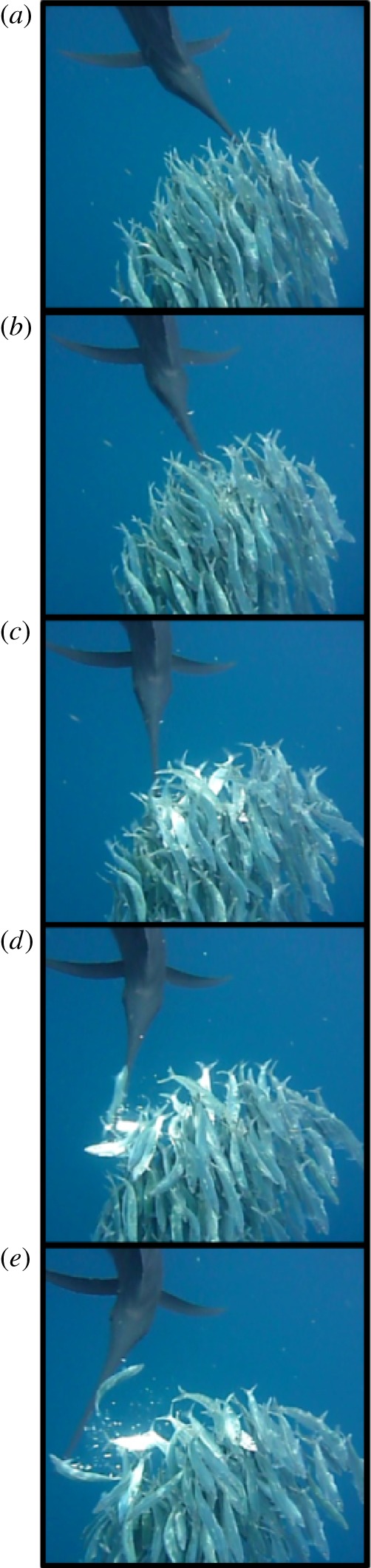
(a–e) A slashing sequence (interval between panels: 10 frames, i.e. 41.67 ms) of a sailfish ‘slash’ attack. In (c–e), the bill makes contact with at least five sardines and the detached scales from injured fish can be seen. (Online version in colour.)
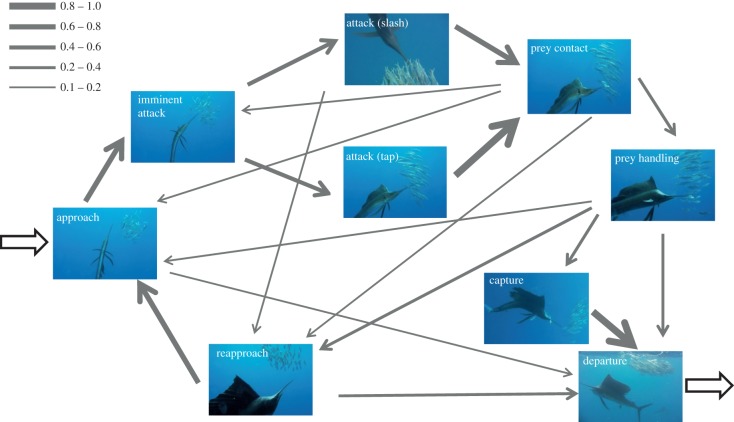
The feeding behaviour of the sailfish was highly ordered and the behavioural states of attack sequences for the sailfish were modelled using transition probabilities of a first-order Markov chain (figure 2). Total observed frequencies of the main states were: approach 245, imminent attack 235, attack (tap) 103, attack (slash) 111, prey contact 172, prey handling 75, capture/ingestion 43, reapproach 74. Behavioural states 1 and 2 (herding and chasing) are not included in the Markov chain because they are not part of the actual attack sequence. The most probable transitions are the following: approach almost always leads to the bill going into a school (i.e. imminent attack state, 79%) and this is followed by attack (i.e. slash, 44%; tap, 42%). Both slash and tap lead to contact, in most cases (i.e. 71% and 89%, respectively). After contact, however, the following behavioural states are less predictable. Nevertheless, the path with the highest probability gives a clear account of a typical capture sequence: approach, imminent attack, tap/slash, prey contact, prey handling, capture/ingestion and departure. Spearing was never observed in sailfish hunting on sardines.
Figure 2.
State transitions of a first-order Markov chain modelling changes of behavioural states in attack sequences of sailfish. Screen captures from videos are shown for each behavioural state. The line widths of the edges are proportional to the transition probabilities (see inset). For the sake of clarity, only transitions with a probability of at least 0.1 were included. (Online version in colour.)
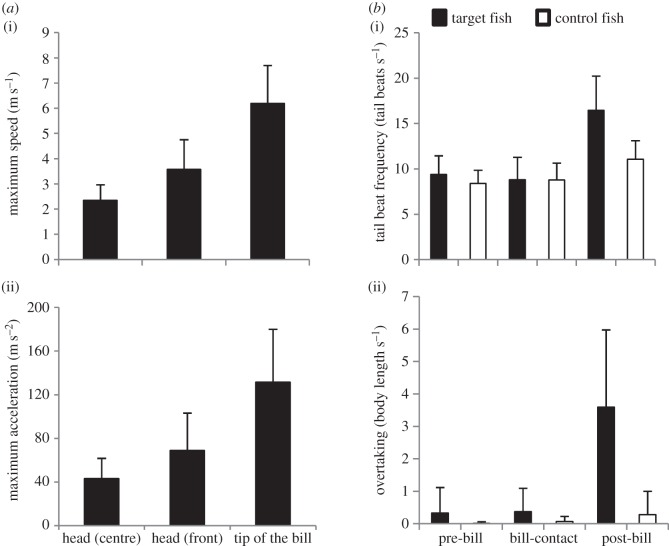
The kinematic analysis of slashing motion shows that the bill reaches a TRmean of 575.1 ± 205.2° s−1. These values are in line with expectations for a 1.5 m long fish (i.e. a sailfish without its 30 cm bill ‘extension’ (see the electronic supplementary material, text)). Much of the effectiveness of slashing can be attributed to the rapid lateral rotation of the bill and consequent swift motion of the bill tip. Because of the rotation, the maximum speed and acceleration measured at Tsf were higher (6.2±1.5 m s−1 and 131.6±48.5 m s−2, respectively) than those measured at Fsf (3.6±1.2 m s−1 and 69.0±34.2 m s−2, respectively) and Hsf (2.3±0.6 m s−1 and 43.2±18.5 m s−2, respectively; figure 3a). Speed and acceleration differed among Hsf, Fsf and Tsf (figure 3a; one-way repeated measures ANOVA p < 0.001 in both cases; Tukey post-hoc test, all p < 0.01). Based on the speed and acceleration of the bill tip, the estimated maximum swimming performance and the reaction time of a fish the size of a sardine (see the electronic supplementary material, text), sardines are not expected to be able to avoid being hit by a slashing bill.
Figure 3.
(a) Kinematic variables of an attack sequence; maximum speed (i) and acceleration (ii) measured at the front and centre of the head, and the tip of the bill during a slashing manoeuvre. (b) Behavioural response of the prey; tail beat frequency (i) and overtaking behaviour (ii) of target (filled bars) and control (open bars) fish during different phases of a sailfish attack sequence. The target fish was the sardine closest to the bill of the sailfish and impacted by a slash. A control fish was the first measurable sardine that was swimming directly in front of the target fish but was not directly impacted by the slashing event. Data are presented as mean ± s.d.
The comparison of the behaviour of target fish in each phase to that of control fish allowed us to test whether sardines in the strike zone reacted to the presence of the bill by anticipating a slashing event. Surprisingly, we found no significant behavioural differences between target and control fish when the bill was inserted in the prey school during the bill-contact phase (Wilcoxon matched pairs test, p > 0.05; figure 3b). After a slash (i.e. post-bill-contact phase), however, both target and control fish had increased values for all behaviours (p < 0.01) and the behavioural change was significantly higher for target than for control fish (p < 0.01; see the electronic supplementary material, text). This increase in speed after a slash shows that sardines are not swimming at maximum performance when the bill is inserted in the fish school. Nevertheless, they do not show any avoidance behaviour when the bill is inserted which strongly suggests that the presence of the bill goes undetected.
4. Discussion
Using a combination of behavioural and kinematic analyses, we identified the underlying mechanistic basis of an extreme morphological feeding adaptation that is specially suited to the capture of schooling prey.
Our analyses show that sailfish use their bill to isolate and capture prey through two main attack strategies (i.e. tapping and slashing) and that bill-tip acceleration during slashing is comparable to the highest values ever recorded in any aquatic vertebrate, including both swimming and body part movements [10–13]. The recorded speed of the bill tip was much higher than that potentially obtainable for the swimming motion of a fish the size of a sardine (see the electronic supplementary material, text). Expected reaction time and maximum speed of sardines (see the electronic supplementary material, text) further corroborate that they are unlikely to be able to avoid the strike. Therefore, by having a thin and rigid prolongation at the head, sailfish manage to move part of their body (i.e. the bill tip), at a translational speed that is too high for prey to react in time to avoid being struck, even though the sailfish rotational performance lies within expectation for a fish of the same body length but without bill extension (see the electronic supplementary material, text). Based on morphological data from other billfish species and the relationship between mean turning rate and body length, such high translational speeds at the bill tip are also expected for other billfish species (see the electronic supplementary material, text and table S1).
Despite the fact that billfishes are considered to be among the fastest fish species [1], sailfish did not rely on rapid swimming for prey capture. On the contrary, prior to the critical stages of an attack (i.e. slashing or tapping) sailfish swam directly behind and at similar speeds to the sardine schools (1.17 m s−1; see the electronic supplementary material, text), which are much slower than those theoretically attainable by such large predators [14]. Previous work on other billfishes (blue marlin Makaira nigricans) also suggests that they spend most of their time swimming slowly, i.e. at speeds less than 1.20 m s−1 for 97% of the time, reaching speeds of around 2 m s−1 only occasionally [15]. While early estimates (ca 1940s–1960s) of the swimming speeds of billfishes (reviewed in [15]) suggested that billfishes can reach swimming speeds as high as 36 m s−1, recent theoretical work suggests that the maximum swimming speed of fish and cetaceans can attain at shallow depth is in the order of 10–15 m s−1 [14]. Clearly, work using modern recording techniques (e.g. accelerometers) is needed to clarify this discrepancy and to obtain more accurate field measurements of maximum speeds in large aquatic vertebrates.
Based solely on speed, large fish are expected to eventually catch small fish, but when manoeuvrability and acceleration are taken into account, large fish are often at a disadvantage [11,16]. Therefore, the use of morphological adaptations that can be manoeuvred effectively, such as the sailfish bill, can be critical for overcoming these challenges thereby allowing large predators to catch their evasive smaller prey. Extended bills can also be found in non-billfish species (e.g. paddlefishes and sawfishes) where they are believed to be used primarily for sensory detection and prey manipulation [17,18] rather than direct capture. In some instances, posterior extensions of the body in a number of vertebrates (e.g. tails of killer whales Orcinus orca) can also be moved more rapidly than the whole body itself and are used by various large aquatic predators to facilitate prey capture [10,13,19,20]. Previous work suggests that the feeding behaviour of large aquatic vertebrates (both in fishes and cetaceans) involving whole-body attacks is largely determined by the predator–prey size ratio [11,16]. The smaller the prey is relative to its predator, the higher the prey's advantage in terms of manoeuvrability. Large aquatic predators such as billfishes, dolphins and humpback whales (Megaptera novaeangliae) can reduce the disadvantage between prey and predator manoeuvrability by concentrating, disturbing and disorienting prey [16]. This can result in alternatives to whole-body attacks on single prey, such as attacking as a group or the use of weapons (e.g. tails and bills) which can deal with a concentrated group of prey by slapping and slashing them and then consuming stunned and injured individuals [21]. When prey items are much smaller than their predators (i.e. less than 10−3 predator length) as in the case of baleen whales and whale sharks, filter feeding is used [16]. In these cases, while the small prey item may indeed have a higher manoeuvrability than their predator, the difference in size is so large that once the prey is targeted, its speed is too low to avoid the predator's huge gape [11].
While it is unlikely that the sardines could avoid a slash, the possibility that they actively avoided the bill prior to the swinging motion was also investigated. We observed no evasive behaviour by the sardines in response to the insertion of the sailfish bill into the school. The reaction of a fish to an approaching predator generally occurs at a distance that is related both to the speed of the predator and its body depth [22,23]. Predators with narrow profiles, such as sailfish, can get extremely close to their prey without eliciting an escape response [24]. The bill gives the sailfish an additional predatory advantage as the thin bill represents a stealthy object that creates minimal hydrodynamic or visual disturbance to the prey.
Slashing behaviour often resulted in the removal of scales but immediate prey death as a result of bill contact was never observed. Repeated slashing by different individual sailfish was observed, resulting in accumulated bodily damage to many sardines in each group. While the chasing of prey is widespread among predators in both aquatic and terrestrial ecosystems, inflicting bodily damage gradually and over prolonged periods is more typical of pack-hunting animals such as wild-dogs (Lycaon pictus), wolves (Canis lupus) and killer whales (Orcinus orca) with tightly regulated group membership based on individual recognition [25], whereas gregarious pelagic teleosts are generally believed to live in fission–fusion groups [26] (but see also [27]). By contrast, tapping behaviour is so subtle that it probably leaves little or no detectable traces on the body despite the fact that it is a highly efficient capture technique. The latter might explain why stomach content analyses have produced such inconsistent results (i.e. prey with and without gashes on their bodies [3,5]).
Sailfish attacks were accompanied by changes in body posture, colour and pattern which might have multiple functions. The erect dorsal fin (i.e. sail) and pelvic fins probably act as control surfaces to increase body stability [28,29]. We therefore postulate that these fin extensions enhance the accuracy of tapping and slashing. In addition, dorsal fin extension was also observed prior to attacks and therefore it may aid in ‘herding’ prey fish. The colour and pattern changes along the body might be related to intra-specific communication. Sailfish always attacked one at a time (even when up to 40 of them were present around a school of prey) presumably because of the risk of injury when slashing. Whether and how they signal to each other to establish feeding order is a topic in need of further investigation.
Morphological studies indicate large differences in bill morphology between different billfish species (e.g. long oval bills with lateral denticles in sailfish (see the electronic supplementary material, figure S1)) and shorter ones in marlins and very long smooth, flat, sword-like bills in swordfish [30,31] (see the electronic supplementary material, table S1) which strongly suggest that they serve different functions. Another striking morphological feature of sailfish is their large dorsal fin (i.e. the sail) which is considerably smaller in other billfishes. Its role in herding schooling fish and/or stabilizing the body of the sailfish during slashing requires further investigation. Clearly, comparative studies regarding the morphology and behaviour of different billfish species are needed to shed further light on the evolution of these remarkable morphological features.
Acknowledgements
We thank Rodrigo Friscione Wyssmann and the staff of Solo Buceo for their help in the field. We are also grateful to Christian Jørgensen, who provided comments on an earlier version of this paper and to Fabio Antognarelli for assistance with image analysis.
Funding statement
We acknowledge the Carlsberg Foundation for a grant to J.F.S.
References
- 1.Videler JJ. 1993. Fish swimming. London, UK: Chapman & Hall. [Google Scholar]
- 2.Brill RW. 1996. Selective advantages conferred by the high performance physiology of tunas, billfishes, and dolphin fish. Comp. Biochem. Physiol. A Comp. Physiol. 13A, 3–15. ( 10.1016/0300-9629(95)02064-0) [DOI] [Google Scholar]
- 3.Shimose T, Yokawa K, Saito H, Tachihara K. 2007. Evidence for use of the bill by blue marlin, Makaira nigricans, during feeding. Ichthyol. Res. 54, 420–422. ( 10.1007/s10228-007-0419-x) [DOI] [Google Scholar]
- 4.Scott WB, Tibbo SN. 1968. Food and feeding habits of swordfish, Xiphias gladius, in the Western North Atlantic. J. Fish. Res. Board Can. 25, 903–919. ( 10.1139/f68-084) [DOI] [Google Scholar]
- 5.Vaske T, Jr, Travassos PE, Pinheiro PB, Hazin FHV, Tolotti MT, Barbose TM. 2011. Diet of the blue marlin (Makaira nigricans, Lacepede 1802) (Perciformes: Istiophoridae) of the Southwestern equatorial Atlantic ocean. Braz. J. Aqua. Sci. Technol. 15, 65–70. ( 10.14210/bjast.v15n1.p65-70) [DOI] [Google Scholar]
- 6.Wisner RL. 1958. Is the spear of istiophorid fishes used in feeding? Pac. Sci. 12, 60–70. [Google Scholar]
- 7.Aleev YG. 1969. Function and gross morphology in fish . [Transl. from the Russian by M. Raveh] Jerusalem: Keter Press. [Google Scholar]
- 8.Walker JA, Ghalambor CK, Griset OL, McKenney D, Reznick DN. 2005. Do faster starts increase the probability of evading predators? Funct. Ecol. 19, 808–815. ( 10.1111/j.1365-2435.2005.01033.x) [DOI] [Google Scholar]
- 9.Lanczos C. 1956. Applied analysis. Englewood cliffs, NJ: Prentice Hall. [Google Scholar]
- 10.Domenici P, Batty RS, Simila T, Ogam E. 2000. Killer whales (Orcinus orca) feeding on schooling herring (Clupea harengus) using underwater tail-slaps: kinematic analyses of field observations. J. Exp. Biol. 203, 283–294. [DOI] [PubMed] [Google Scholar]
- 11.Domenici P. 2001. The scaling of locomotor performance in predator–prey encounters: from fish to killer whales. Comp. Biochem. Physiol. A. Comp. Physiol. 131, 169–182. ( 10.1016/S1095-6433(01)00465-2) [DOI] [PubMed] [Google Scholar]
- 12.Vogel S. 2008. Modes and scaling in aquatic locomotion. Integr. Comp. Biol. 48, 702–712. ( 10.1093/icb/icn014) [DOI] [PubMed] [Google Scholar]
- 13.Oliver SP, Turner JR, Gann K, Silvosa M, D'Urban Jackson T. 2013. Thresher sharks use tail-slaps as a hunting strategy. PLoS ONE 8, e67380 ( 10.1371/journal.pone.0067380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iosilevskii G, Weihs D. 2008. Speed limits on swimming of fishes and cetaceans. J. R. Soc. Interface 5, 329–338. ( 10.1098/rsif.2007.1073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block BA, Booth D. 1992. Direct meausement of swimming speed and depth of blue marlin. J. Exp. Biol. 166, 267–284. [Google Scholar]
- 16.Webb PW, Debuffrenil V. 1990. Locomotion in the biology of large aquatic vertebrates. Trans. Am. Fish. Soc. 119, 629–641. () [DOI] [Google Scholar]
- 17.Wueringer BE, Squire L, Kajiura SM, Hart NS, Collin SP. 2012. The function of the sawfish's saw. Curr. Biol. 22, R150–R151. ( 10.1016/j.cub.2012.01.055) [DOI] [PubMed] [Google Scholar]
- 18.Neiman AB, Russell DF. 2004. Two distinct types of noisy oscillators in electroreceptors of paddlefish. J. Neurophysiol. 92, 492–509. ( 10.1152/jn.00742.2003) [DOI] [PubMed] [Google Scholar]
- 19.Aalbers SA, Bernal D, Sepulveda CA. 2010. The functional role of the caudal fin in the feeding ecology of the common thresher shark Alopias vulpinus. J. Fish. Biol. 76, 1863–1868. ( 10.1111/j.1095-8649.2010.02616.x) [DOI] [PubMed] [Google Scholar]
- 20.Nowacek DP, Behaviour V. 2002. Sequential foraging behaviour of bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, FL. Behaviour 139, 1125–1145. ( 10.1163/15685390260437290) [DOI] [Google Scholar]
- 21.Similä T, Ugarte F. 1993. Surface and underwater observations of cooperatively feeding killer whales in northern Norway. Can. J. Zool. 71, 1494–1499. ( 10.1139/z93-210) [DOI] [Google Scholar]
- 22.Dill LM. 1974. The escape response of zebra danio (Brachydanio rerio) I. The stimulus for escape. Anim. Behav. 22, 711–722. ( 10.1016/S0003-3472(74)80022-9) [DOI] [Google Scholar]
- 23.Domenici P. 2002. The visually mediated escape response in fish: predicting prey responsiveness and the locomotor behaviour of predators and prey. Mar. Freshw. Behav. Physiol. 35, 87–110. ( 10.1080/10236240290025635) [DOI] [Google Scholar]
- 24.Webb PW. 1986. Effect of body form and response threshold on the vulnerability of four species of teleost prey attacked by largemouth bass (Micropterus salmoides). Can. J. Fish. Aquat. Sci. 43, 763–771. ( 10.1139/f86-094) [DOI] [Google Scholar]
- 25.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Krause J, Butlin R, Peuhkuri N, Pritchard VL. 2000. The social organisation of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol. Rev. (Camb) 75, 477–501. ( 10.1111/j.1469-185X.2000.tb00052.x) [DOI] [PubMed] [Google Scholar]
- 27.Klimley AP, Holloway CF. 1999. School fidelity and homing synchronicity of yellowfin tuna, Thunnus albacares. Mar. Biol. 133, 307–317. ( 10.1007/s002270050469) [DOI] [Google Scholar]
- 28.Lauder GV, Drucker EG. 2004. Morphology and experimental hydrodynamics of fish fin control surfaces. IEEE J. Ocean Eng. 29, 556–571. ( 10.1109/JOE.2004.833219) [DOI] [Google Scholar]
- 29.Webb PW. 2002. Control of posture, depth, and swimming trajectories of fishes. Integr. Comp. Biol. 42, 94–101. ( 10.1093/icb/42.1.94) [DOI] [PubMed] [Google Scholar]
- 30.Schultz O. 1987. Taxonomische Neugruppierung der Überfamilie Xiphioidea (Pisces, Osteichthyes). Ann. Naturhist. Mus. Wien 89, 95–202. [Google Scholar]
- 31.Fierstine HL, Voigt NL. 1996. Use of rostral characters for identifying adult billfishes (Teleostei: Perciformes: Istiophoridae and Xiphiidae). Copeia 1996, 148–161. ( 10.2307/1446950) [DOI] [Google Scholar]