SUMMARY
Tip-growing pollen tubes achieve rapid elongation while maintaining cell wall integrity by balancing local expansion, controlled by local changes in wall viscosity, against exocytosis, influenced by the activity of the actin cytoskeleton, cellular energetics, and calcium and proton physiology.
Key words: cell expansion, cell walls, cytoskeleton dynamics, polarity, pollen development.
Abstract
In this review, we address the question of how the tip-growing pollen tube achieves its rapid rate of elongation while maintaining an intact cell wall. Although turgor is essential for growth to occur, the local expansion rate is controlled by local changes in the viscosity of the apical wall. We focus on several different structures and underlying processes that are thought to be major participants including exocytosis, the organization and activity of the actin cytoskeleton, calcium and proton physiology, and cellular energetics. We think that the actin cytoskeleton, in particular the apical cortical actin fringe, directs the flow of vesicles to the apical domain, where they fuse with the plasma membrane and contribute their contents to the expanding cell wall. While pH gradients, as generated by a proton-ATPase located on the plasma membrane along the side of the clear zone, may regulate rapid actin turnover and new polymerization in the fringe, the tip-focused calcium gradient biases secretion towards the polar axis. The recent data showing that exocytosis of new wall material precedes and predicts the process of cell elongation provide support for the idea that the intussusception of newly secreted pectin contributes to decreases in apical wall viscosity and to cell expansion. Other prime factors will be the localization and activity of the enzyme pectin methyl-esterase, and the chelation of calcium by pectic acids. Finally, we acknowledge a role for reactive oxygen species in the control of wall viscosity.
INTRODUCTION
The growing pollen tube delivers the sperm cells to the ovule in higher plants and thus is central to the process of fertilization and sexual reproduction. It is also of enormous importance to food production given that approximately 80% of the plant material we eat, namely fruits, nuts, seeds, and grains, requires a preceding fertilization by a pollen tube. Owing to its accessibility and ease of observation, the growing pollen tube further captures the interests of scientists who aim to elucidate fundamental issues of growth and development (Steer and Steer, 1989; Hepler et al., 2001). Thus, we know that pollen tubes grow exclusively at their extreme apex, and that they reach rates of elongation (1 cm h–1) that are faster than any other plant cell (Bedinger et al., 1994). We have also learned a great deal about the underlying structures and physiological processes (Hepler et al., 2001; Cole and Fowler, 2006; Campanoni and Blatt, 2007; Chebli and Geitmann, 2007; Krichevsky et al., 2007; Qin and Yang, 2011). It will be the purpose of this review to consider the mechanism of pollen tube growth in angiosperms, where we focus mainly on the processes that locally change cell wall viscosity and thus alter the rate of expansion in the pollen tube apex. We also consider related topics including vesicular trafficking, the actin cytoskeleton, ion fluxes and gradients (calcium and protons), and the production of energy. Companion articles in this issue of Molecular Plant cover several important and related topics; the reader is directed to those articles for further information pertaining to mechanism of pollen tube growth.
OVERVIEW OF POLLEN TUBE GROWTH
General Cytology
The pollen tube represents a remarkable example of highly polarized cell expansion. In lily, by the time the pollen tube tip reaches the ovule, its length to width ratio exceeds 5000:1. Because lily pollen grains, as well as those from several other species, will germinate and grow in vitro in restricted microscope slide chambers, the resulting pollen tube can be observed in great detail by a variety of microscopic methods (Hepler et al., 2001; Holdaway-Clarke and Hepler, 2003; Chebli and Geitmann, 2007). Overall, pollen tubes are characterized by a spheroidal tip, connected by a smooth transition to an ever-elongating tubular-shaped shank (Figure 1, DIC). A quick microscopic inspection reveals a distinct zonation in the apical cytoplasmic domain. The extreme apex possesses a ‘clear zone’, so named because the amyloplasts, with their highly refractile starch grains, and the vacuoles are prevented from moving into the apical 20 μm or so of the tube (Heslop-Harrison and Heslop-Harrison, 1990; Lovy-Wheeler et al., 2007) (Figure 1, DIC). The lack of starch grains gives the apical cytoplasm a smooth appearance; however, careful inspection, with Nomarski DIC optics, dark field microscopy or especially with electron microscopy (Figure 2), reveals that many different organelles are contained therein including mitochondria, elements of the endoplasmic reticulum, Golgi dictyosomes, and numerous vesicles (Lancelle and Hepler, 1992). Indeed, the latter usually become organized into a ‘V’-shaped aggregate, called an inverted cone, in which the base of the cone abuts the apical plasma membrane at the tip.
Figure 1.
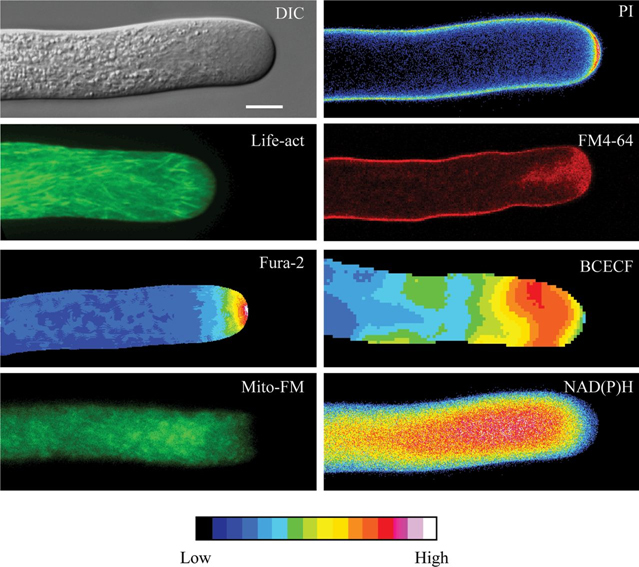
Images of Living Lily Pollen Tubes Showing Different Structures or Activities.
DIC: When examined with Nomarski differential interference contrast optics, the internal structure of the tube is depicted clearly. A clear zone in the apex stands apart from the shank, which contains numerous refractive amyloplasts. The marker is 10 μm and applies to all the images. PI: A tube stained with propidium iodide (PI) displays greater staining in the cell wall at the extreme apex indicating a thickened cell wall. Life-act: The pollen tube has been transiently transformed with Lifeact–GFP, which stains F-actin. Note the apical fringe. FM4-64: This dye vitally stains the plasma membrane and the inverted cone of vesicles. Fura-2: This cell has been injected with Fura-2-dextran, which allows us to image the free Ca2+ throughout the cell. Note the steep tip-focused gradient of Ca2+ at the extreme apex. Typically, the [Ca2+] is above 1 μM (colors of red to white on the LUT), while, 20 μm back from the tip, the concentration is 100–200nM (color blue on the LUT). BCECF: This cell has been injected with BCECF-dextran, a pH-sensitive dye. The image shows the prominent alkaline band in the clear zone, in which the pH reaches 7.5 (color orange to red on the LUT). The tip is slightly acidic at approximately pH 6.8 (color green to blue on the LUT). Mito-FM: The fluorescent dye Mitotracker-FM stains mitochondria, and shows that these organelles accumulate towards the base of the clear zone. NAD(P)H: The endogenous reduced co-factor, NAD(P)H possess fluorescence and thus can be imaged directly. The strongest signal localizes to the region of accumulated mitochondria, seen to the left. This figure is from Rounds et al. (2011b).
Figure 2.
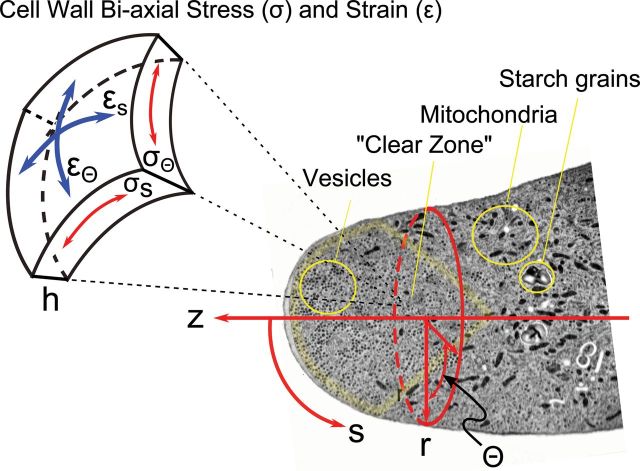
Coordinate Systems for Pollen Tube Growth Models.
An electron micrograph (Lancelle and Hepler, 1992), labeled to show the location of mitochondria, starch grains, secretory vesicles, and the ‘clear zone’ is overlain with a cylindrical coordinate system in which elongation is measured along the z-axis while the variable r(z) specifies the location of the cell wall along z and thus cell shape. Since the cell is assumed to be axisymmetric around z, cell shape is also fully described by Кs(S), curvature along the meridian (μm–1). Cell wall properties and dynamics are specified in a unit cell in which tensile stress in the wall (σ, MPa) is followed along two orthogonal directions, circumferential (σθ), and meridional (σs), and the corresponding rates of strain (s–1) are termed έr and έs. Wall thickness (h, μm) is a balance between loss of material by viscous flow and secretion of new wall material by vesicle fusion with the cell membrane. The respective values of the two components of biaxial stress at any point on the surface of the cell depend upon internal hydrostatic pressure, wall thickness, local wall curvature, and the degree of flow coupling (Dumais et al., 2004).
Another important feature of pollen tube growth that has helped us understand underlying processes in greater detail is the observation that the growth rate oscillates (Pierson et al., 1996; Holdaway-Clarke and Hepler, 2003; Chebli and Geitmann, 2007). In lily pollen tubes, changes in growth rate can vary from 100 nm s–1 to over 500 nm s–1 during a period of 15–50 s (Cárdenas et al., 2008). The associated processes also usually oscillate either in activity or position, but, while they show the same periodicity as the growth rate, they usually do not show the same phase. To determine the phase relationship, namely whether the process occurs before or after growth, we use cross-correlation analysis (Brillinger, 1981). In this procedure, two oscillatory, time series processes, such as growth rate and intracellular [Ca2+], which have been simultaneously collected, are systematically compared with one another. The analysis permits the investigator to determine both the strength of correlation and the lag between these two processes (Brillinger, 1981). These data allow us to establish tentative temporal hierarchies in the underlying physiological events (Figure 3) (Holdaway-Clarke et al., 1997; Holdaway-Clarke and Hepler, 2003; Chebli and Geitmann, 2007). In the sections below, we will make reference to particular oscillatory data as they have contributed to our understanding of the growth process.
Figure 3.
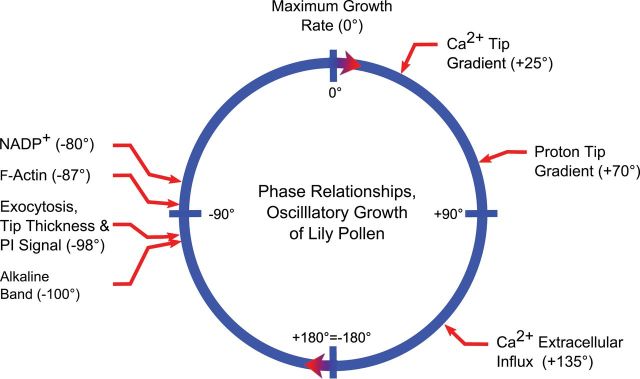
Phase Relationships in Oscillatory Growth.
This diagram shows the phase relationship between several different processes and oscillatory pollen tube growth. The positive sign (+) indicates that the increase in the particular event follows the increase in growth rate, whereas the negative sign (–) indicates that the increase in the event precedes the increase in growth rate. It has come as a surprise that the increase in the intracellular Ca2+ follows in the increase in growth rate. In addition, the increase in intracellular Ca2+ is out of phase with the increase in extracellular Ca2+ influx, and even more out of phase with the process of exocytosis. A series of events including the increase in NAD(P)+, F-actin, exocytosis, build-up of wall material at the tip, and alkalinity all precede and thus anticipate the increase in growth rate. These events deserve further attention as potential stimulators of the growth rate.
Vesicles: The Delivery of New Cell Wall Material
Pectin Delivery and Composition
As it progresses forward, the growing pollen tube continually deposits new cell wall material (Steer and Steer, 1989). This wall, especially at the tube apex, consists mainly of pectin, which is synthesized largely as methoxylated esters (O’Neill et al., 1990) and packaged into vesicles in the Golgi dictyosomes (Staehelin and Moore, 1995; Bosch and Hepler, 2005). The enzyme pectin methyl-esterase (PME), perhaps also secreted in the same vesicles (Li et al., 2002), de-methoxylates the pectin exposing carboxyl residues; when these are crosslinked by Ca2+, the pectin backbone gains considerable strength (Willats et al., 2001a; Bosch and Hepler, 2005; Palin and Geitmann, 2012; Wolf and Greiner, 2012). The balance between secretion and PME activity thus yields a gradient at the pollen tube apex with highly esterified pectins at the extreme apex and a progressively less esterified mix of polymers at distances removed from the apex. This condition has been observed by antibody binding in pollen tubes of Nicotiana (Bosch et al., 2005), Solanum (Parre and Geitmann, 2005), and Arabidopsis (Dardelle et al., 2010; Chebli et al., 2012). Plant PME de-methoxylates pectin in a specific block-wise pattern, which is crucial for the co-operative formation of long stretches (eight or more residues) of Ca2+ crosslinks (Grant et al., 1973; Catoire et al., 1998; Willats et al., 2001b). Micro-indentation studies provide direct evidence for changes in cell wall stiffness due presumably to these reactions outlined above. Thus, the extreme apical wall is ‘softer’, that is, less viscous than the cell wall back from the tip, and, during oscillatory growth, an episode of rapid growth is preceded by a local softening of the apical wall (Zerzour et al., 2009). Still further work reveals that the gradient in mechanical properties of the apical cell wall fits closely with the pattern of de-esterification (Fayant et al., 2010).
In order to maintain the appropriate level of viscosity in the apical cell wall, two related mechanisms have arisen that provide control over the activity of PME, effectively preventing it from either too quickly or too extensively de-esterifying the methoxyl esters. First, the sequence of a prominent PME from tobacco pollen tubes reveals that the protein has two domains: an N-terminal pro-region, followed by the C-terminal enzymatic domain (Bosch et al., 2005), in which the pro-region acts as an endogenous inhibitor of the enzymatic domain. However, when the full-length protein is secreted, it will be acted upon by proteases in the wall, thereby releasing the pro-region from the enzymatic domain, thus permitting the enzyme to catalyze de-esterification. These steps will take some time, meaning that the methoxy esters will not immediately be de-esterified following exocytosis. The second mechanism involves the synthesis of a separate protein, pectin methyl-esterase inhibitor (PMEI) (Wolf et al., 2003; Röckel et al., 2008). Not surprisingly, PMEI bears similarity to the pro-region of PME itself. In studies of tobacco pollen tubes, Röckel et al. (2008) find that, while a YFP fusion of the Arabidopsis pollen-specific PME (AtPPME1) occurs throughout the cell wall including tip and shank, a YFP fusion of PMEI (AtPME1-2) only appears in the cell apex. Further studies in which AtPMEI-2:YFP appears together with the endocytosis marker dye FM4-64 in brefeldin A-induced aggregates provide support for the idea that PMEI is retrieved through endocytosis (Röckel et al., 2008). These authors suggest that endocytosis maintains PMEI in the apical cell wall and prevents it from extending down the shank of the tube. Maintaining PMEI at the tube apex will retard de-esterification reactions and prevent an excessive crosslinking of the pectin chains so that the apical cell wall retains the ability to expand and grow.
Exocytosis: Spatial Analysis
The vesicles containing cell wall matter accumulate in the inverted cone (Lancelle and Hepler, 1992; Bove et al., 2008). They are transported along actin cables, presumably by a myosin XI, to the apical domain, where it has been suggested that those vesicles most proximal to the apical plasma membrane fuse and contribute their contents to the cell wall (Lancelle and Hepler, 1992). As new wall material is added at the extreme apex of the tube, it progressively replaces the previously deposited material, which is moved away from the tip by expansion (Rojas et al., 2011). From these considerations, it has seemed likely that vesicles enter the inverted cone and then are secreted at the apical plasma membrane, close to the polar axis of the tube. However, recent studies question this conclusion, providing evidence that vesicles may actually fuse and secrete their contents, not at the polar axis, but in an annulus outside of the polar axis (Bove et al., 2008; Zonia and Munnik, 2008; Geitmann and Dumais, 2009; Geitmann, 2010). Based on staining with two related but different dyes, FM4-64 and FM1-43, Zonia and Munnik (2008) argue that secretion in tobacco pollen tubes occurs 6–10 µm from the pole, along the flanks. Given the small size of the tobacco pollen tube, especially when compared to lily, these distances put the site of secretion a significant distance from the polar axis, indeed along the shoulder and even shank of the tube. Bove et al. (2008), in studies of lily using spectral imaging, also support an off-axis delivery of vesicles, as do Geitmann and Dumais (2009). Finally, we draw attention to the studies of Certal et al. (2008), which show that the H+-ATPase in tobacco pollen tubes is unequivocally deposited some distance from the apical polar axis. Of course, this enzyme is not a major cell wall component, and may be responding to selective factors that target its secretory location. But it does show that pollen tubes have the ability to secrete in different locations.
Despite the several studies above, which provide evidence for an off-axis or annular deposition of new wall material in growing pollen tubes, some recent observations strongly support the idea that deposition occurs along, or reasonably close to, the polar axis. For example, in an examination of PME–GFP secretion in tobacco pollen tubes, we observe that the apical arc of extracellular fluorescence, while covering the dome, is centered along the apical axis and does not extend onto the shank of the tube (Bosch et al., 2005; McKenna et al., 2009). A second example comes from Lee et al. (2008), also in studies of exocytosis in tobacco pollen tubes, in which they have overexpressed a tobacco pollen receptor like kinase (RLK) fused to GFP. First, they show that the RLK is localized to the plasma membrane in the tip and proximal regions of the shank of the pollen tube. However, when the RLK is subjected to fluorescence recovery after photobleaching (FRAP), they show that fluorescence recovery is fastest and most evident at the apical polar axis, indicating that vesicle delivery of membrane proteins occurs mainly at the pollen tube tip (Lee et al., 2008). Finally, Rojas et al. (2011), using particles applied to the cell surface of lily pollen tubes, demonstrate that the maximal local expansion rate of the cell wall occurs at or very close to the polar axis, and drops off sharply at points away from the polar axis (Figure 4C, redrawn from Rojas et al. (2011) showing local expansion rates).
Figure 4.
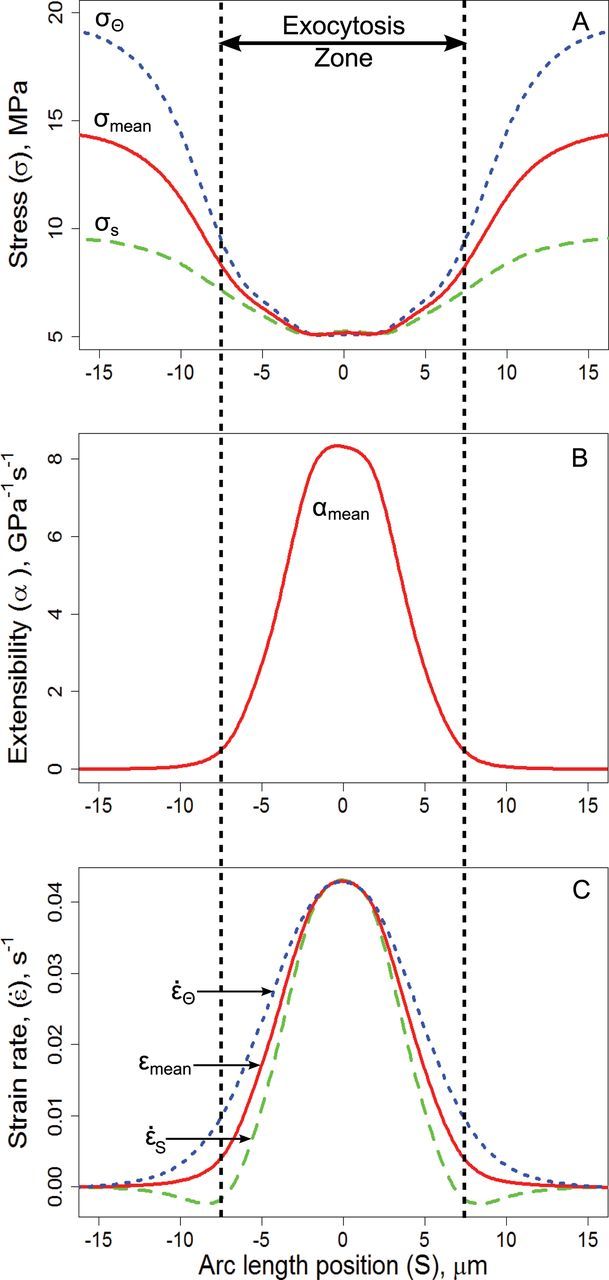
Pollen Tube Rheology Derived from Empirical Measurements of Cell Shape and Surface Expansion Rates (Redrawn from Rojas et al. (2011) with Calculations of Extensibility as Derived by Bernal et al. (2007) and Dumais et al. (2004)).
(A) illustrates tensile stress along the meridian (σs) and the circumference (σθ) and the average stress (σmean = (σs + σθ)/2) from the tip (S = 0 μm) to the shank (S = 15 μm). Stress is minimum at the tip (~5 MPa), the point of maximum curvature in this cell (Кs = Кθ = 0.2 μm–1, Figure 4B in Rojas et al. (2011)), equal in all directions, and is calculated as σs = P/(2·h·Кs) constant turgor (0.2 MPa) impinging on a thin shell of constant thickness (0.1 μm) of smoothly changing curvature (note that the axis values on this plot differ from Rojas et al. (2011) and are recalculated from data in the same paper). Meridional and circumferential stress both increase rapidly towards the shank, at which point Кs disappears and Кθ becomes 1/r (cell radius, r = 5 μm) so that σθ becomes 2·P·r/h ~ 20 MPa (hoop stress of a cylinder) while σs = P·r/h ~ 10 MPa (axial stress of a cylinder). (C) plots the local strain rate along S (έs, s–1) and θ (έθ, s–1) as measured (Rojas et al., 2011) by following the trajectory of fluorescent beads. Mean strain rate (έmean, s–1) is calculated as (έs + έθ)/2. For (B), mean stress and strain were used to estimate the extensibility (αmean = έmean/σmean, GPa–1 s–1) of the wall material along the meridian. In the absence of a more complex set of constitutive equations and information about values for flow coupling and possible material anisotropy (crucial factors in determining cell shape (Dumais et al., 2006)) and wall thickness, we choose extensibility to illustrate the spatial pattern of wall rheology required to give rise to the observed patterns of shape and growth rates rather than attempt to calculate viscosity.
Taken together, these observations show that the greatest flux density of wall material (e.g. number of vesicles per second per square micron) occurs in a region centered at the polar axis. Some confusion may have arisen because the greatest overall flux of vesicle secretion may be in an annulus a few microns from the tip. Thus, when examining a 3-D volume, the total rate of vesicle flux towards the cell edge might well appear to be higher in a circumpolar annulus than at the tip, even though the rate of vesicle fusion per unit wall area will be less. Indeed, a miss-match between the rates of local expansion and of local vesicle fusion might be part of the oscillatory mechanism and part of the steering mechanism for chemotropism.
Exocytosis: Temporal Analysis
A temporal analysis of secretion has been made using different methods (McKenna et al., 2009). The first employs direct measurements of changes in wall thickness from DIC images; due to the limits of resolution, this procedure is restricted to pollen tubes like lily with reasonably thick cell walls. The second method takes advantage of the fluorescent dye propidium iodide (PI), which robustly stains plant cell walls including those of pollen tubes (Figure 1, PI). Indeed, a detailed examination reveals that PI binds to pectin where it competes with Ca2+ (Rounds et al., 2011a). Changes in PI fluorescence are monitored during pollen tube growth and can be made with both lily and tobacco pollen tubes. A third method exploits a clone of PME linked to GFP, which has been expressed in tobacco pollen tubes, and which allows us to examine exocytosis directly (McKenna et al., 2009). In the analysis below, we focus mainly on the results using PI fluorescence because the method works with both lily and tobacco pollen tubes and because it provides better sensitivity than the other methods.
The results indicate that, as the pollen tube growth rate oscillates, so too does the exocytosis of pectin with the same period, but with a clearly different phase. An analysis of the phase relationship using cross-correlation reveals that pectin is deposited in advance of the increase in growth rate (Figure 3), with values of –98° in lily and –124° in tobacco (McKenna et al., 2009). Confirmation comes from the studies of PME–GFP expression, in which the data indicate that PME in tobacco pollen tubes is secreted in advance of growth by –128° (McKenna et al., 2009). While there is difference in the magnitude of the values obtained, with tobacco being more advanced than lily, all methods indicate that exocytosis temporally precedes growth in pollen tubes. Further support comes from the work of Lee et al. (2008), who took advantage of atRabA4d, a pollen-specific homolog of atRabA4b, which has been tied to vesicle trafficking in root hairs. Although exocytosis was not directly observed, they nevertheless were able to document and quantify a forward movement of membrane components thought to anticipate secretion (Lee et al., 2008). Their data indicate for Arabidopsis pollen tubes that YFP–RabA4d fluorescence oscillates and that it accumulates in the clear zone in advance of an increase in growth by –53.1° (Lee et al., 2008).
A further important observation derives from the comparison of the exocytosis and the growth rate data for both lily and tobacco pollen tubes. Just from visual inspection, there appeared to be a correlation between the preceding wall thickness or PI fluorescence change with the following change in growth rate. Subsequently, when these data were subjected to a detailed statistical analysis, the results revealed that the preceding exocytosis can account for over 90% of the variation in the rate and extent of growth (McKenna et al., 2009). In brief, there is a remarkable correlation between exocytosis and growth, in which the anticipatory exocytotic event predicts the subsequent rate and magnitude of growth. These observations invite speculation concerning a possible mechanism for local reduction in wall viscosity; this idea is developed later in the article.
It is important to add that, while oscillatory growth is dramatic in pollen tube, it is not the only tip-growing cell that shows such growth behavior. For example, root hairs of Arabidopsis show oscillations in growth rate (Monshausen et al., 2007). More recently, evidence from PI staining of the apical root hair wall indicates that the oscillatory increase in fluorescence precedes the changes in the growth rate by an advance of –112° (Rounds et al., 2011a). Among the fungi, López-Franco et al. (1994) described pulsed or oscillatory growth in somatic hyphae of several different species. Some of these examples, such as the Zygomycete, Gilbertella persicaria and the Ascomycete, Neurospora crassa show rates of growth and rapidity of oscillations that are comparable to those of lily pollen tubes. Furthermore, these authors suggest that the oscillations in growth rate are generated by oscillations in the delivery and discharge of secretory vesicles (López-Franco et al., 1994). It seems possible that oscillatory growth may be common among tip-growing cells, and further that an anticipatory secretory event, which has been documented for pollen tubes and root hairs, may underlie the changes in growth rate.
Endocytosis
While the emphasis above has been on exocytosis, it is important to note that endocytosis is also a major contributor to pollen tube growth (Parton et al., 2001; Moscatelli and Idilli, 2009; Onelli and Moscatelli, 2013). Based on the amount of material needed to support the ever-expanding cell wall, it becomes evident that a considerable excess of plasma membrane is delivered during the secretory process. This excess membrane is retrieved through endocytosis, and calculations show that it can be up to 80% of the membrane originally secreted (Picton and Steer, 1983b; Steer and Steer, 1989; Onelli and Moscatelli, 2013). Direct observation of endocytosis from ultrastructural investigations reveal numerous clathrin coated vesicles 6–15 μm back from the tip in tobacco pollen tubes (Derksen et al., 1995). Moscatelli et al. (2007) used positively and negatively charged nanogold coupled with electron microscopic analysis to document the events of endocytosis. Their results indicate considerable complexity within tobacco pollen tubes; for example, regions of the plasma membrane to which the positively charged nanogold became bound were reused in the secretory pathway through the Golgi system. By contrast, apical plasma membrane domains that bound negatively charged nanogold appeared to associate with degradative pathways (Moscatelli et al., 2007).
Aside from the relatively few studies noted above using methods that unambiguously target endocytosis, several studies on pollen tubes and root hairs have used the FM dyes, notably FM4-64 and FM1-43, coupled with fluorescence microscopy (Šamaj et al., 2006). These dyes are extremely easy to use and provide lovely images, in which the plasma membrane together with the inverted cone of vesicles is selectively stained (Figure 1, FM4-64). Initial studies emphasized their potential in following the process of endocytosis (Bolte et al., 2004). However, a more detailed analysis, at least in pollen tubes, reveals that the FM4-64 signal is a composite of both endocytotic and exocytotic events (Šamaj et al., 2006). Parton et al. (2001) clearly showed how components of the inverted cone, which seemingly arise from an endocytotic event at the cell apex and flow basipetally, quickly reverse and enter the pool of forward-flowing vesicles, becoming poised to undergo exocytosis. In the study by McKenna et al. (2009) on exocytosis, we had wanted to include an equivalent analysis of endocytosis. We therefore gave considerable effort to the measurement of fluorescence from FM4-64, and changes therein during oscillatory growth. Like Parton et al. (2001), we recorded oscillatory fluorescence in the FM4-64-stained inverted cone in lily pollen tubes. However, when these data were subjected to cross-correlation analysis, they failed to provide a clear result (McKenna, Kunkel, and Hepler, unpublished observations). Thus, it was impossible for us to confidently determine a phase relationship with growth; as a consequence, we discontinued this project. However, the role of endocytosis in the control of pollen tube growth deserves close attention, as it will likely provide key insights into the growth mechanism.
Actin Structure and Function
The actin cytoskeleton is a major component of the pollen tube, where it participates in cytoplasmic streaming and other events such as polarized growth. We have noted that vesicles move along actin microfilaments, but it is important to emphasize that all the organelles and inclusions in the pollen tube cytoplasm are in constant motion (Lovy-Wheeler et al., 2007; Cai and Cresti, 2009), with these too being driven largely by myosin along actin microfilaments. However, deciphering actin structure has proved difficult due to the sensitivity of these filaments to the processes of fixation and staining. Nevertheless, certain procedures appear successful in preserving actin microfilaments, with confidence arising from the confirmation of certain complexes such as the cortical actin fringe, which has been observed in both fixed and live samples. Thus, when lily pollen tubes are fixed by rapid freezing, then substituted, and subsequently rehydrated and stained with an actin antibody, they are seen to possess a fringe or collar of actin in the apical domain (Lovy-Wheeler et al., 2005). This fringe, which is composed of longitudinally oriented microfilaments, begins a few μm behind the extreme apex and extends basally for 5–10 μm. Of note, all the microfilaments of the fringe are concentrated in the cortical cytoplasm, close to the plasma membrane.
Important recent evidence using heavy meromyosin labeling shows that reverse fountain cytoplasmic streaming most likely derives from a spatially different orientation of actin microfilaments: thus, those actin microfilaments along the edge of the cell are oriented with their plus or barbed ends towards the cell apex, while those microfilaments more centrally located have their barbed ends oriented towards the pollen grain (Lenartowska and Michalska, 2008). Because myosin XI is a plus-end-directed motor (Tominaga et al., 2003), the different orientations of the actin microfilaments account for the characteristic reverse fountain streaming pattern (Iwanami, 1956). Further insight into the ability of the apical actin cytoskeleton to organize itself in a way that promotes ‘reverse fountain’ streaming comes from the studies using Jasplakinolide to stimulate actin polymerization, where Cárdenas and co-workers (2005) found that this reagent blocked pollen tube growth and inhibited cytoplasmic streaming in the shank of the tube. However, it promoted the formation of swollen tube tips containing a toroid of cytoplasmic motion in which the motion next to the plasma membrane moved forward while that in the central core was basally directed. Because the pattern of streaming resembled that of ‘reverse fountain’ streaming, Cárdenas et al. (2005) concluded that promotion of actin polymerization contributes to this aspect of motility characteristic of pollen tubes.
The cortical fringe in normal pollen tubes has now been observed both through rapid freeze fixation, and also by room-temperature fixation in which the crosslinking reagent ethylene glycol bis[sulfosuccinimidyl succinate (sulfo-EGS) has been used to stabilize the structure (Lovy-Wheeler et al., 2005). Most recently, it has been observed in living cells using Lifeact, a 17-amino-acid fragment of yeast actin binding protein 140, which has been linked to GFP and expressed transiently in both lily and tobacco pollen tubes (Vidali et al., 2009) (Figure 1, Lifeact). Through comparison with other probes, including GFP–mTalin, GFP–actin depolymerizing factor (ADF), and GFP–fimbrin (Wilsen et al., 2006), Lifeact is superior in showing the cortical actin fringe in the tube apex and prominent cables of actin throughout the shank. Lifeact also does not appear to affect actin dynamics or pollen tube growth (Vidali et al., 2009).
We repeatedly find that the cortical actin fringe is dynamic, with rapid turnover being necessary to allow it to keep pace with growth. These dynamic properties almost certainly arise from the activity of different actin binding proteins. While a thorough discussion of this topic is beyond the scope of this review, we do mention a few interacting proteins that seem particularly important. ADF, which, together with actin interacting protein (AIP), localizes to the actin fringe, likely is modulated in activity by the local pH (Gungabissoon et al., 1998; Allwood et al., 2002; Chen et al., 2002; Lovy-Wheeler et al., 2006); this topic is discussed later under the section on proton flux. Another family of proteins intimately involved with microfilament polymerization are the formins (Cheung and Wu, 2004; Van Gisbergen and Bezanilla, 2013), and recent evidence shows that at least one member of this family (FH5) localizes to the apical fringe or mesh in tobacco pollen tubes (Cheung et al., 2010). Together with formins, one naturally suspects that profilin, through its ability to deliver actin–GTP monomers to the polymerization site, is an important cofactor in the growth of new actin microfilaments (Vidali and Hepler, 2001). Finally, we mention villin/gelsolin, as a protein that might participate in the degradation of the actin fringe. When stimulated by elevated levels of Ca2+, this protein fragments actin microfilaments (Zhang et al., 2010). Given the high level of Ca2+ at the pollen tube apex, together with villin/gelsolin, it is easy to understand why actin microfilaments are not seen therein.
We think that the cortical fringe contributes to cell elongation and to the unique spatial distribution of cytoplasmic components in the pollen tube apex because treatment with latrunculin B, at low concentrations (2nM) that do not stop cytoplasmic streaming, nevertheless block growth of the pollen tube and lead to the occlusion of the apical clear zone. Thus, large organelles, such as amyloplasts and vacuoles, which here-to-fore had been restricted from motion into the clear zone, now move in freely (Vidali et al., 2001). Studies on living tubes expressing Lifeact reveal that these treatments rapidly destroy the cortical fringe, but not the longitudinal actin cables throughout the shank of the tube (Rounds, unpublished). We conclude from these observations that the fringe is extremely labile, and that it likely turns over rapidly as the pollen tube grows. Not only does it, by some unknown mechanism, contribute to the polarized distribution of the organelles, but it directly contributes to the growth process itself.
The spatial organization of actin microfilaments in the pollen tube apex raises interesting questions concerning the accumulation of vesicles in the apex. While a dense palisade of microfilaments occupies the apical cortex in lily pollen tubes (Lovy-Wheeler et al., 2005), relatively few microfilaments are observed in the inverted cone. Addressing this issue, Kroeger and co-workers (Kroeger et al., 2009; Kroeger and Geitmann, 2012a) suggest that vesicles move forward along the cortex by the actin fringe and are released into the inverted cone. From this point, vesicle motion may be controlled by diffusion, with pulling forces being directed both acropetally and basepitally. On the one hand, exocytosis provides a path that pulls vesicles acropetally toward the apical plasma membrane, while, on the other hand, the centrally positioned actin microfilaments at the base on the inverted cone, which are plus-end-directed towards the pollen grain, pull vesicles basepitally (Lenartowska and Michalska, 2008). These ideas help account for the chaotic motion of vesicles in the apex, and for the lack of organized actin microfilaments within the inverted cone.
To test for a role for the cortical fringe in the focused delivery and secretion of vesicles, Bou Daher and Geitmann (2011) studied pollen tubes of Camellia grown perpendicular to controlled electrical fields. They first noted that a significant percentage of pollen tubes when stimulated by the field responded with a growth reorientation. By applying a very low concentration of latrunculin (1nM), they demonstrated a significant reduction in the percentage of tubes that reoriented in the electric field, as well as an increase in the response time (Bou Daher and Geitmann, 2011). Further evidence to support a role for the actin fringe in growth polarity came from studies in which the position of the actin fringe was observed to extend more on the outside of curving pollen tube and less on the inside (Bou Daher and Geitmann, 2011). Dong et al. (2012) observed a similar change in the position of the cortical fringe simply in pollen tubes of lily that were naturally reorienting without a particular stimulus. They additionally report that, in general, the actin fringe associates with rapid growth in lily pollen tubes, and moreover that the density of the fringe oscillates as the growth rate oscillates (Dong et al., 2012).
The issue of actin oscillation received attention in earlier studies, first by Fu et al. (2001) and later by Hwang et al. (2005), in which GFP–mTalin was used as the actin reporter. The results showed that the actin intensity in the apical domain of tobacco pollen tubes oscillated, with the increase in fluorescence anticipating by about –90° the increase in growth rate (Hwang et al., 2005). However, given the problems with GFP–mTalin as a reliable reporter for F-actin (Wilsen et al., 2006), as well as its negative effect on pollen tube growth (Hwang et al., 2005), there is a need for further verification. A different approach for observing the oscillation of actin involved the use of fluorescently tagged DNase, which binds avidly to G-actin, and provides a clear signal at concentrations that have no effect on growth rate (Cárdenas et al., 2008). Studies on lily pollen tubes reveal distinct oscillations in DNase fluorescence, which are interpreted to indicate changes in G-actin. When subjected to cross-correlation analysis, the peaks (G-actin) follow the increase in growth rate by +87°. However, the troughs in the DNase signal, which bear an equally strong relationship to the growth rate as the peaks, are seen to precede growth by –87°. We interpret the troughs to indicate reduced G-actin, or increased F-actin, and thus conclude that an increase in actin polymerization precedes the increase in growth rate by –87° (Cárdenas et al., 2008). In summary, two different methods on pollen tubes from two different species indicate that an increase in F-actin anticipates an increase in growth rate by about a quarter of a cycle.
While the discussion above has focused on the role of actin in exocytosis, recent studies indicate that actin also plays a role in endocytosis (Moscatelli et al., 2012). Using low concentrations of latrunculin B, which did not inhibit growth during 15-min time intervals, Moscatelli et al. (2012) find that the inhibition of actin polymerization prevented the endocytosis of plasma membrane along the shank of tobacco pollen tubes. However, these same conditions had no effect on the endocytosis from the apical region, as detected using negatively charged nanogold as the marker. Data are also emerging from non-pollen tube systems, showing a pronounced effect of latrunculin B on endocytosis (Baluška et al., 2002), suggesting that actin plays a widespread role in the process of membrane retrieval. A further interesting observation reported by Baluška et al. (2002) concerns the endocytotic uptake of pectin in maize root cells. To the best of our knowledge, similar observations have not been made on pollen tubes; however, if such a system operates, it could potentially play a major role in fine tuning the deposition of this crucial cell wall component during apical growth.
In summary, actin plays a pivotal role in the control of pollen tube growth. That the actin cytoskeleton participates in cytoplasmic streaming has been appreciated for years. More recently, evidence has emerged that actin, and perhaps particularly the apical cortical fringe, associates with rapid growth and with changes in growth direction. Preliminary evidence thus supports the idea that the cortical fringe contributes to the polarity of cell growth through the targeted delivery of vesicles. More attention on this interesting and important topic is warranted.
Ion Physiology
Given the speed of growth and the amplified processes that support it, one easily appreciates that several different ions and changes therein will play important roles (Holdaway-Clarke and Hepler, 2003; Campanoni and Blatt, 2007; Hepler and Winship, 2010; Konrad et al., 2011). Indeed, modeling of pollen tube growth has often focused on the flux and status of these ions where they are perceived to play primary regulatory roles (Kroeger et al., 2008; Michard et al., 2009; Liu et al., 2010; Tavares et al., 2011). Because of the attention this topic has received, we focus only briefly on calcium and protons.
Ca2+
It has been known for decades that Ca2+ is essential for pollen tube growth (Brewbaker and Kwack, 1963). Understanding its role has been complicated by the realization that the ion has a two-fold contribution to cell viability and growth (Holdaway-Clarke and Hepler, 2003; Hepler and Winship, 2010). Within the cytoplasm, Ca2+ plays a pivotal role as a signaling agent or second messenger. Here, the Ca2+ resides at a basal concentration of 100–200nM. Various conditions or stimuli can cause the [Ca2+] to sharply rise, that is, to 1 μM or higher, thereby activating or inhibiting a reaction (Kudla et al., 2010). In the extracellular environment, Ca2+ participates in the crosslinking of acidic residues of pectin in the cell wall; its local concentration generally resides between 10 μM and 10mM (Picton and Steer, 1983a). When the [Ca2+] is below 10 μM, the cell bursts, presumably because the wall has too few Ca2+ crosslinks and becomes too weak, whereas when the [Ca2+] is above 10mM, the cell stops growing, presumably because the acidic residues are so extensively crosslinked that the cell wall can no longer expand (Picton and Steer, 1983a).
Cytosolic Ca2+
First, we draw attention to the presence of the standing gradient of free Ca2+ in the pollen tube, which is focused at the inner surface of the apical plasma membrane (Rathore et al., 1991; Miller et al., 1992), and referred to as the ‘tip-focused’ gradient. While most studies have been made on in vitro grown pollen tubes, it is important to note that these Ca2+ gradients have also been observed in Arabidopsis pollen tubes grown in vivo (Iwano et al., 2004, 2009). In lily pollen tubes, the [Ca2+] reaches well above 1 μM at the inner surface of the plasma membrane, and then drops to basal levels of 100–200nM within 20 μm (Figure 1, Fura-2). Because elevated Ca2+ stimulates secretion in both plant and animal cells (Zorec and Tester, 1992; Battey et al., 1999; Sutter et al., 2000), it seems likely that the tip-focused gradient promotes vesicle fusion and exocytosis, specifically in the pollen tube apex. Strong support for this idea comes from the work of Malhó and Trewavas (1996), who show that experimental modulation of the strength and position of the apical Ca2+ gradient regulates the direction of pollen tube growth, presumably by controlling the location of secretion.
When it was discovered that the high point of the tip-focused gradient oscillates with the same period as the growth rate (Pierson et al., 1996), it was initially attractive to imagine that the changes in [Ca2+] fueled the changes in secretion, which was necessary for growth (Kroeger et al., 2008). However, studies from oscillating pollen tubes reveal that the Ca2+ oscillations do not show the same phase relationship as growth rate, or of the process of secretion (McKenna et al., 2009). Cross-correlation analysis of the oscillatory growth rate and intracellular Ca2+ data indicates that the increase in Ca2+ slightly follows (+10°–40°), rather than leads, the increase in growth rate (Messerli et al., 2000; Cárdenas et al., 2008); thus, the displacement of Ca2+ from secretion can be as much as 140° (Figure 2). Although we recognize that there may be complex feedback processes (Kroeger et al., 2008, 2011), we nevertheless take these results to indicate that the oscillatory Ca2+ signal does not control the oscillations in secretion or in the rate of growth. Indeed, recent studies on Arabidopsis and Nicotiana pollen tubes grown in vivo fail to observe regular oscillations in the apical [Ca2+] (Iwano et al., 2009). However, these data do not rule out a role for Ca2+ in secretion. During the oscillation of the tip-focused gradient, the high values of 3–10 μM (Messerli et al., 2000) only drop to 750nM at the lowest point (Pierson et al., 1996). In brief, there is always a Ca2+ gradient at the pollen tube apex. Furthermore, given the relative sensitivity of secretion to Ca2+ (Sutter et al., 2000), the low point of the gradient in the pollen tube remains capable of stimulating secretion (for details, see Hepler and Winship, 2010). To summarize, whereas Ca2+ appears to be essential for secretion in the pollen tube apex, the oscillations in [Ca2+] do not control the oscillations in secretion or in the growth rate.
Although the change in Ca2+ may not control the growth rate, the increase in growth may stimulate the increase in Ca2+. A tentative explanation suggests that the increase in growth deforms the plasma membrane, which opens stretch-activated Ca2+ channels, allowing an influx that fuels the tip-focused gradient (Dutta and Robinson, 2004). The elevated Ca2+ might then cause a decrease in growth rate through the activation of villin/gelsolin, an actin binding protein that fragments actin microfilaments in the vicinity of the apex (Zhang et al., 2010). These events would slow the delivery of new vesicles and thus growth (Cárdenas et al., 2008). Other ideas about how Ca2+ and actin might interact involves the small G-protein, ROP1, and its stimulation of two downstream complexes, RIC3 and RIC4 (Gu et al., 2005; Yan et al., 2009), where RIC is an acronym for ROP-interactive CRIB-containing proteins, and CRIB is an acronym for Cdc42/Rac interacting binding motif. Gu et al. (2005) suggest that the ROP1:RIC3 complex activates Ca2+ signaling, whereas ROP1:RIC4 promotes actin assembly. In more recent work, this group suggests that a balance between these two complexes plays a central role in controlling the Ca2+/actin status and the oscillatory nature of pollen tube growth (Yan et al., 2009). For more information on this topic, see the article in this issue by Yang and co-workers.
Addressing briefly the matter of Ca2+ entry into the pollen tube, we already drew attention to stretch-activated channels for which Dutta and Robinson (2004) provide experimental support. However, there are data in support of other types of Ca2+ channels (Hepler et al., 2011; Konrad et al., 2011); of particular interest are those characterized as cyclic nucleotide gated channels (CNGCs) (Frietsch et al., 2007), and those identified as glutamate receptor channels (GLR) (Michard et al., 2011). For each, there is evidence for 20 such channels in the Arabidopsis genome; importantly, some CNGCs are specific to pollen tubes. The bottom line is that there is a rich array of mechanisms controlling the entry of Ca2+ into pollen tubes. For details, see Hepler et al. (2011) and Konrad et al. (2011).
Extracellular Ca2+ Influx
Use of the non-invasive extracellular, ion-selective electrode reveals that there is an extracellular influx of Ca2+ focused at the tip of the pollen tube (Kühtreiber and Jaffe, 1990). In addition, this influx oscillates in magnitude with the same period as the oscillation in growth rate. However, it is delayed in phase from growth by +130°–140° and, in addition, it is phase-delayed from the oscillation in the gradient of intracellular Ca2+ by +100° or more (Figure 2) (Holdaway-Clarke et al., 1997; Messerli et al., 1999). Although the extracellular influx, when first discovered, had been assumed to directly drive the changes in intracellular Ca2+ (Kühtreiber and Jaffe, 1990), the lack of phase relationship requires an alternate explanation. We think that the explanation lies with the changing ion-binding properties of the growing cell wall. As noted above, the cell wall at the apex is largely composed of pectin, which is initially secreted as methoxyl esters, and then subsequently de-esterified, to expose acidic residues, which bind Ca2+ (Bosch and Hepler, 2005). Indeed, the phase delay evident in the extracellular influx data probably stems from the time needed for the PME full-length protein to be cleaved and for the active PME domain to diffuse to the site of action and de-esterify the methoxy esters. That the wall binding of Ca2+ accounts for the extracellular influx derives from the calculations of the amount of wall material and the changes in de-esterification that must occur during growth (Hepler and Winship, 2010). Calculations indicate that wall binding of Ca2+ accounts for virtually all of the extracellular influx that is observed; by contrast, an assessment of the amount of influx needed to support the intracellular tip-focused gradient indicates that it would be virtually too low to be detected by the extracellular electrode (Hepler and Winship, 2010).
Protons (H+)
While much of the emphasis on ions has been directed at Ca2+, arguably, protons occupy an equally central place in our quest to decipher the mechanism of pollen tube growth (Feijó et al., 1999; Felle, 2001; Certal et al., 2008). It is thought that the pollen tube, like virtually all plant cells, uses the H+-ATPase to generate gradients that play a pivotal role in the control of the membrane potential and ion transport across membranes (Feijó et al., 1999; Palmgren, 2001). Although more difficult to image than Ca2+ because of their mobility, H+ activity and spatial gradients nevertheless have been detected in growing pollen tubes. Thus, lily pollen tubes possess a pH gradient at the apex, in which the tip most region is slightly acidic (pH 6.8), with an alkaline band (pH 7.5) a few microns behind (Feijó et al., 1999) (Figure 1, BCECF). Like so many physiological processes, the pH gradient oscillates during oscillatory growth, with the changes in acidity at the tip following growth, similar to that of intracellular Ca2+ (Figure 2). However, the alkaline band oscillates in such a manner that the increase in pH leads and thus anticipates the increase in growth rate by about –100° (Lovy-Wheeler et al., 2006).
Studies on the activity and localization of a pollen-specific H+-ATPase fused to GFP (Nt AHA–GFP) in tobacco provide data that agree well with our knowledge of pH gradients. Thus, the plasma membrane-localized enzyme does not occur at the extreme apex, but rather is secreted back from the tip (Certal et al., 2008), in keeping with the location of the acidic domain at the extreme apex and the alkaline band along the sides of the clear zone (Feijó et al., 1999). A slightly earlier study on another H+-ATPase (NpPMA5) in Nicotiana plumbaginifolia (Lefebvre et al., 2005) similarly showed that the protein was localized to the plasma membrane along the shank but not at the apical dome. In further studies, Certal et al. (2008) used fluorescent redistribution after bleaching and revealed that recovery of the fluorescent signal proceeds from the flanks towards the tip. These data are consistent with the insertion of the H+-ATPase along the flanks of the apical domes, and not at the polar axis (Certal et al., 2008). However, it needs to be noted that, while Nt AHA–GFP does not stain the apical plasma membrane, it does appear in the inverted cone region of the clear zone and thus immediately underlies the apical plasma membrane. An explanation for these localization patterns is not established. Perhaps the accumulation of Nt AHA–GFP in the inverted cone reflects endocytotic retrieval of enzyme rather than a prelude to exocytosis.
The distribution of the H+-ATPase together with its activity as expressed in the apical pH gradient stimulate our thinking about if and how this enzyme might contribute to polarized pollen tube growth. Different factors intersect in an interesting and possibly meaningful way. For example, the alkaline band occupies a similar position to that of the cortical actin fringe (Lovy-Wheeler et al., 2006). Some additional factors include two actin binding proteins, namely ADF and AIP, which also localize to the region of the actin fringe, and which are sensitive to changes in pH (Gungabissoon et al., 1998; Allwood et al., 2002; Chen et al., 2002; Lovy-Wheeler et al., 2006). In more recent work, Bou Daher and Geitmann (2011) further tease apart the contribution of different members of the ADF family, where they show that ADF7, but not ADF10, localizes to the cortical actin fringe. Taken together, an interaction between pH, the actin fringe, and the delivery of vesicles may underlie a central process in growth regulation. Specifically, elevated pH, which, in the alkaline band, anticipates the increase in growth, may achieve its affect by activating ADF in the presence of AIP to fragment actin and stimulate new actin polymerization. The increased actin polymerization in the fringe may then stimulate growth through an enhanced delivery of vesicles, which in turn provides new wall material.
Energy Production during Pollen Tube Growth
Given the extremely rapid rates of growth together with the massive production of new wall material, it will be obvious that substantial amounts of energy are required to support these activities. It has been noted that the pollen tube exhibits the highest rate of respiration for any known higher plant tissue (Itoh and Sekiya, 1994; Tadege and Kuhlemeier, 1997; Rounds et al., 2011b). The pollen tube received early attention as a model for the study of energy production. In the sixties, Dickinson (1965) showed that lily pollen had three stages of respiration. Shortly before germination, respiration was high, followed by a slightly reduced period of oxygen use as the pollen tube began growing (~40% of the first phase). Finally, as the pollen tube began growing in earnest, oxygen utilization achieved its highest level—nearly twice the original rate (Dickinson, 1965). In keeping with these data, Dickinson (1966) subsequently showed that oligomycin, an inhibitor of the mitochondrial F0-ATPase, could drastically reduce growth of lily pollen tubes.
These observations, together with those showing that mitochondria aggregated towards the apex (Figure 1, Mito-FM) (Lovy-Wheeler et al., 2007), fueled the idea that aerobic respiration was necessary for growth in pollen tubes. However, several lines of evidence suggest that growth can be maintained in the absence of rapid respiration. Recent work by Rounds et al. (2010) using oligomycin showed that, after a brief period of growth inhibition, pollen tubes begin growing again using aerobic fermentation, and producing alcohol to regenerate NAD+ for glycolysis. Kuhlemeier and colleagues (Tadege et al., 1999; Mellema et al., 2002; Gass et al., 2005) demonstrated that petunia pollen has evolved a pyruvate dehydrogenase (PDH) bypass that uses pyruvate decarboxylase to shunt some pyruvate into the fermentative pathway and then into acetyl-CoA giving an extra metabolic boost to these pollen tubes.
Because NAD(P)H has stronger autofluorescence than NAD(P)+, the amount in the cell can be monitored without the addition of dyes or other markers (Cárdenas et al., 2006) (Figure 1, NAD(P)H). Rounds et al. (2010) showed that treatment with oligomycin dramatically increased the NAD(P)H signal, revealing that the signal indeed is due to respiratory NAD(P)H utilization. In an earlier work, Cárdenas et al. (2006) showed that the NAD(P)H signal oscillates and used cross-correlation analysis to show that the oxidized form precedes growth. This led to the hypothesis that respiratory ATP production might drive rapid growth and the associated oscillations. Surprisingly, when lily tubes grow in the presence of oligomycin, they produce ethanol (Rounds et al., 2010). Under these conditions, mitochondria no longer stay close to the tip as they do in aerobic respiratory growth and, as a consequence, the NAD(P)H signal at the tip is much lower and no longer oscillates. Despite these changes in the distribution and activity of the mitochondria, pollen tube growth continues, although slower than under control conditions. Also noteworthy, the growth rate continues to oscillate but with a slightly longer period. These data suggest that aerobic respiration increases the growth rate and either directly or indirectly affects the period of growth oscillations. However, aerobic respiration is not necessary for oscillatory growth to occur (Rounds et al., 2010).
FACTORS THAT MODIFY LOCAL CELL WALL EXPANSION RATES
Pollen tube growth comes about by cell wall expansion balanced with pectin deposition, with concomitant uptake of water and osmotic adjustment. We often think of pollen tube growth rates as the distance traveled by the cell tip over time, that is, along the z-axis in Figure 2. This makes sense, given that the ultimate criterion for pollen tube success is the fastest delivery of the sperm nuclei down the long tube to the ovule. However, when thinking about the mechanisms that support and regulate tip extension, it is more useful to define growth as an irreversible, local expansion of the pollen tube cell wall, balanced by local deposition of new wall material to maintain wall thickness and integrity. Under these circumstances, it is the difference in expansion rate caused by changes in cell wall viscosity across the tube tip that results in changes in directions of pollen tube elongation and different cell shapes (Campàs and Mahadevan, 2009). We see differential expansion at the tip controlling the rate and direction of growth in several kinds of situations, including chemotaxis, recovery from osmotic or chemical disturbance, and growth rate oscillation.
Turgor
Typical for all plant cells, including pollen tubes, turgor provides the force that drives cell growth (Taiz, 1984; Cosgrove, 1999; Harold, 2002; Winship et al., 2010; Hill et al., 2012; Kroeger and Geitmann, 2012a, 2012b). In recent years, Zonia and Munnik (2007, 2009) have promoted the idea that oscillatory changes in turgor account for the oscillatory changes in pollen tube growth rate. Those ideas can be rejected on the grounds that internal hydrostatic pressure does not change in growing lily pollen tubes (Benkert et al., 1997; Zerzour et al., 2009), that, being expressed in all directions, turgor does not provide a vector for polarized growth (Winship et al., 2010), that pollen tubes with their high hydraulic conductance could not possibly build a pressure gradient (Sommer et al., 2008), and that the time required to change the water potential in order to change the internal hydrostatic pressure could not account for the rapid changes in oscillatory growth rate observed in pollen tubes (Winship et al., 2010). Thus, the key to understanding the rapid, oscillatory, and highly polarized growth of the pollen tube rests with the spatial and temporal patterns of viscosity in the apical cell wall, leading to differential yielding without bursting (Harold, 2002; Dumais et al., 2006).
Wall Yielding
Pectin Associated Enzymes
We have repeatedly emphasized that the pollen tube wall consists mainly of pectin (Bosch and Hepler, 2005; Chebli and Geitmann, 2007). However, it is important to recognize that other wall components are also present, although in much lower concentrations than pectin. For example, some arabinogalactan proteins are present (Roy et al., 1998; Nguema-Ona et al., 2012); inhibition of their activity with Yariv phenylglycoside significantly destabilizes the normal deposition and intercalation of new wall material (Roy et al., 1998). Regarding cellulose, there is disagreement about its presence, particularly at the extreme apex where growth occurs. Ferguson et al. (1998), using TEM analysis of gold-labeled cellobiohydrolase, find cellulose first appearing at 5–15 μm back from the tip, but not at the growing point per se. However, more recent studies build an argument that, while cellulose may not be a major component, it nevertheless is present. For example, the use of enzymes (cellulase) (Aouar et al., 2010) or inhibitors of cellulose synthesis (2,6-dichlorobenzonitrile (DCB)) (Anderson et al., 2002) led to the formation of enlarged apical regions and in some instances tube bursting. Even more recently, the cellulose synthase complexes have been imaged close to the pollen tube apex in tobacco (Cai et al., 2011) and Arabidopsis (Chebli et al., 2012). Other stains including calcofluor and CBM3a (cellulose binding module 3a) provide further support for the presence of cellulose. Finally, the application of scanning electron microscopy provides evidence for fibers, presumably cellulose, that are oriented parallel to the long axis of the pollen tube (Chebli et al., 2012). When taken together, a persuasive case is made for cellulose being present, including in the apex of the pollen tube where it may contribute to the stabilization of the apical region. However, we nevertheless assert that the relative amount is likely very low, especially in lily pollen tubes, which failed to provide a signal for linearly aligned elements in the Pol Scope (Lovy-Wheeler, Hepler, and Oldenbourg, unpublished observations), a very sensitive polarized light microscope that can detect small amounts of birefringent material (Oldenbourg, 1996). We submit that pectin continues to emerge as the cell wall component at the apex whose deposition and modification control pollen tube growth.
Given the central importance of pectin and its state of esterification, it follows that PME levels could have a profound effect on pollen tube growth (Bosch and Hepler, 2005; Fayant et al., 2010; Palin and Geitmann, 2012). Thus, exogenously applied PME blocks growth in both lily and tobacco pollen tubes (Bosch et al., 2005; Parre and Geitmann, 2005). But even more subtle modification of PMEs can have a profound effect on pollen tube growth. For example, silencing of one of the prominent PME genes in tobacco showed that, while overall PME activity was not reduced, the pollen from the silenced plants displayed significantly slower growth than controls (Bosch and Hepler, 2006), suggesting that a proper balance of existing PMEs is necessary for optimal growth. Studies on the VANGUARD1 (VGD1) locus in Arabidopsis also strongly support a role for PME in controlling pollen tube growth. First, Jiang et al. (2005) show that VGD1 encodes a PME. Subsequently, they find that functional interruption of VGD1 reduces PME activity by 18% and significantly retards pollen tube growth. That the mutant pollen tubes when grown in vitro often burst suggests the presence of a weakened apical wall. However, in vivo, there is evidence that the repression of growth in the mutant may arise from an interaction of the pollen tube with the stylar tissue (Jiang et al., 2005). Similar results have been obtained by Tian et al. (2006), who produced a knockout of a pollen-specific PME gene (AtPPME1) in Arabidopsis, which displayed a 20% reduction in enzyme activity. Visual inspection revealed that the mutant pollen tubes, when compared with wild-type controls, were shorter as well as curved and misshaped. Despite their obvious problems, the mutant pollen tubes were able to achieve fertilization at levels comparable to those from wild-type plants.
A final thought for this specific topic is the need to keep in mind that PME action is two-sided. While de-esterification exposes carboxyl groups that can crosslink with Ca2+, and stiffen the cell wall, this same reaction also yields a proton, which can locally acidify the wall and lead to events that can loosen the wall. For example, acidification can activate hydrolases, which break wall bonds, and also de-activate PME. Taken together, these observations further emphasize the central importance of PMEs in controlling optimal pollen tube growth.
Ca2+ Chelation
Given the importance of Ca2+ as a crosslinker of acidic residues to provide tensile strength in the pollen tube tip, it follows that both the concentration and availability of Ca2+ might affect tip growth (Bosch and Hepler, 2005; Palin and Geitmann, 2012; Wolf and Greiner, 2012). Studies by Picton and Steer (1983a) on Tradescantia pollen revealed the tubes grew maximally in medium in which the [Ca2+] was between 0.1 and 1mM; above or below these concentrations, the growth rate was sharply reduced. Above 1mM, tube growth slowed and, at 10mM, it stopped, with electron micrographs showing that the tip cell wall had become markedly thickened. Below 0.1mM, and especially below 10 μM, pollen tube tips became swollen, with bursting often seen (Picton and Steer, 1983a). A likely explanation is that, at 10mM, the concentration of free Ca2+ in the cell wall free space is so high that the chemical equilibrium between bound and unbound Ca2+ is shifted towards the crosslinked state, rendering the apical wall especially viscous and unable to yield to the existing turgor. However, below 10 μM, the supply of Ca2+ is diffusion limited so that the demand for Ca2+ by newly de-methoxylated pectins exceeds supply and too few carboxyl residues are crosslinked. The insufficiently strengthened wall then bursts in response to the internal hydrostatic pressure.
These studies indicate that modulation of the [Ca2+] and the degree of pectin esterification can have a profound effect on the yielding of the pollen tube apical cell wall. How might these factors work together to control growth? Examining not pollen tubes, but internode cells of the green alga Chara, Boyer (2009) and Proseus and Boyer (2012) have developed the idea that pectin, acting as a Ca2+ chelator, is a crucial regulator of cell wall expansion and growth. In Chara, pectin is also a major component of the cell wall; however, in contrast to pollen tubes, it is secreted as the free acid rather than the methoxylated ester. In their model, newly secreted acidic pectin competes for Ca2+, which may already be bound to the existing pectin of the wall (Proseus and Boyer, 2012). However, because the existing pectin in the wall is under strain imposed by turgor, the Ca2+ crosslinks will be distorted and weakened. The recently secreted pectin, which is not yet part of the wall matrix, will compete away some of the Ca2+ and thus remove existing wall bonds. The net effect will be a relaxation of the wall tensile strength, which will allow turgor-driven expansion. As the wall stretches, newly secreted pectin inserts into the wall matrix, which, together with an influx of Ca2+ from the medium, will ensure that structural stability is maintained (Proseus and Boyer, 2012).
Conceivably, the Ca2+/pectate cycle could contribute to the oscillatory growth of the pollen tube. In this scheme, it is possible that the secretion of new pectin together with PME creates the acidic residues, in the correct block-wise spatial pattern, which then compete for the wall-bound Ca2+. Under these circumstances, the existing pectin matrix becomes distorted and weakened, as suggested by Proseus and Boyer (2012). The newly secreted pectin would compete away Ca2+ from the existing matrix, further weakening the wall and allowing turgor-driven expansion. Influx of Ca2+ from the medium would finally stabilize the newly formed wall. However, direct measurement with the extracellular electrode indicates that peak influx is significantly delayed (by +130°–140°) from the oscillation in growth (Holdaway-Clarke et al., 1997). To account for this offset, it is conceivable that the peak demand for Ca2+ binding by newly secreted pectin could be delayed relative to growth if freshly secreted PME takes time to act.
Delayed Ca2+ demand and hence Ca2+ binding relative to maximum growth could also explain the sharp decrease in relative expansion rates from tip to flank as observed in the strain pattern studies of Rojas et al. (2011), the only study of pollen tubes in which direct measurements of strain rates can be combined with geometry to estimate wall extensibility. We have redrawn their data and calculated extensibility in Figure 4. In their model, peak expansion rates occur at the tip of the cell (Figure 4C), even though wall stress (Figure 4A) is least at the more curved tip. Thus, stress anisotropy alone is not sufficient to explain cell shape; the wall must vary in a controlled manner from tip to shank. The wall is presumably least viscous, resulting in the highest extensibility, at the tip (Figure 4B) due to the lowest proportion of de-methoxylated and Ca2+ crosslinked pectins. The wall material deposited and incorporated at the tip, during maximum growth (defined as 0º), then takes the time required to become the shoulder of the tip, which is about 1/3 of a cycle. Because, at this moment, it is maximally de-methoxylated, this portion of the cell wall generates the greatest demand for Ca2+, which causes a rapid decrease in viscosity and local expansion rates, and a marked increase in Ca2+ uptake and chelation. We suggest that the zone of exocytosis must correspond to the zone of maximum expansion. In conclusion, although there are several important details that need to be resolved, it is entirely plausible that the Ca2+/pectate cycle plays an important role in the control of pollen tube growth.
Expansins
Studies of wall yielding in cells undergoing diffuse growth have focused considerable attention on expansins—a unique class of proteins that non-enzymatically weaken the bonds within the cell wall matrix and cellulose, and allow controlled loosening so that turgor-driven cell growth can occur (Cosgrove, 1999; Lee et al., 2001; Cosgrove et al., 2002). While a class of expansins, namely β-expansins, has been identified in grasses and is associated with pollen therein, the role of these proteins appears to be in loosening the walls of cells in the stigma and style, in order to allow penetration of the pollen tube (Cosgrove et al., 1997; Valdivia et al., 2009; Tabuchi et al., 2011). There is no evidence that β-expansins, or other expansins, actually affect the growth of the pollen tube per se.
Reactive Oxygen Species (ROS)
A consequence of aerobic metabolism is the production of reactive oxygen species (ROS). While we usually think of these as deleterious to life, emerging evidence indicates that ROS may play a key signaling role in controlling growth, including tip growth of root hairs and pollen tubes. In diffusely growing plant cells, there is evidence that ROS can stimulate the scission of polysaccharide bonds and thus cause stress relaxation (Schopfer, 2001). Particularly pertinent are the studies of Fry (1998), which show that pectins can be cleaved by a hydroxyl radical produced in the apoplast.
An influential study by Foreman et al. (2003) revealed that ROS, generated by an NAD(P)H oxidase, participated in the generation of the tip high Ca2+ gradient essential for root hair growth. More recent work indicates that ROS may also participate in pollen tube tip growth. Potocký et al. (2007) find that ROS, based on staining with chloromethyl dichlorodihydrofluorescein diacetate and nitroblue tetrazolium, are enriched at the tip of tobacco pollen tubes. In addition, they find that ROS scavengers and the inhibitor diphenylene iodonium chloride inhibit growth, whereas application of hydrogen peroxide can promote growth. Finally, Potocký et al. (2007) report that exogenously applied Ca2+ increases NAD(P)H oxidase activity. Similar results have emerged from studies of germinating and growing kiwifruit pollen where Speranza et al. (2011) show an involvement of ROS. Still more recently, Potocký et al. (2012) provide further evidence showing that Ca2+, together with signaling phospholipids, such as phosphatidic acid, and phosphatidylinositol 4,5, bisphosphate activate NAD(P)H oxidase activity. There thus appear to be different feedback loops in which ROS can simulate Ca2+, and in which Ca2+ can stimulate an increase in ROS. More work is needed to sort out the details about how these pathways operate, especially in vivo.
Intussusception
In addition to Ca2+/pectate as a modifier of cell wall viscosity, we suggest that the process of exocytosis itself contributes fundamentally to the bulk properties of the wall modulating the rate of turgor-driven expansion. It has been recognized for years from studies of diffusely growing cells that at the least the synthesis and secretion of new wall material must keep pace with growth, or else the cell wall would become too thin and break (Taiz, 1984; Cosgrove, 1999). What is perhaps unique about oscillating pollen tubes is that these processes of secretion and growth are temporally separated from one another. Further, the studies of both lily and tobacco pollen tubes tell us that the amount of new wall material predicts to a high degree the magnitude and extent of the growth pulse that follows (McKenna et al., 2009). From these observations, we suggest that newly secreted pectin becomes inserted into the wall matrix where it loosens the bonds between the existing pectate linkages causing a reduction in bulk viscosity. In this way, increased rates of exocytosis and intussusception act as a local accelerator of the strain rate, while increased Ca2+/pectate crosslinking acts as a brake. Internal hydrostatic pressure then extends the wall in a controlled fashion, giving rise to the observed increase in growth. In this argument, we reach back to the idea of ‘intussusception’ as a mechanism for loosening cell wall bonds and allowing growth (Ray, 1987, 1992). The basis for this process has been put forth by Ray (1992), who stated: ‘The ability of newly introduced polymers to bind to the existing wall indicates that, when introduced, their bonding capabilities are not yet satisfied. When juxtaposed with pre-existing polymer chains engaged in bonding to others, the new molecules most plausibly would bind by being able to substitute (by exchange) their bonding capabilities for those of a pre-existing chain’s partner. Since the previously existing intermolecular bonds would be under tensile stress due to the load carried by the wall structure, while newly formed bonds would not, this exchange will be exergonic, should be spontaneous, and would relax stress in the wall and allow it to extend, i.e., loosen it.’
As emphasized by Ray (1987, 1992), in order for intussusception to work, the newly secreted material must be inserted into the matrix of the wall; mere application to the inner wall surface will strengthen the existing structure but will not cause stress relaxation of existing wall bonds. Because the cell wall has a certain degree of porosity, macromolecules of modest size (20–30 kDa) can simply diffuse into the matrix (Baron-Epel et al., 1988; Fleischer et al., 1999). But, beyond this, a more active mechanism for intercalation of new wall material comes from the recent work of Proseus and Boyer (2005, 2006). Using isolated cell walls of internode cells of Chara, they showed that fluorescent dextrans up to 70 kDa could be forced through the cell wall matrix by applying pressures similar to turgor from an oil-filled microcapillary (Proseus and Boyer, 2005). Extending their ideas to an intact cell, they argue that new wall material is initially secreted into a thin periplasmic space between the outer edge of the plasma membrane and the inner surface of the cell wall. Once in this space, the new, but disorganized, wall components are subjected to internal hydrostatic pressure, which forces these molecules into the wall matrix.
Alternatively, as pointed out by Ray (1992), the existing wall bonds, because they withstand the forces generated by turgor, are stretched into a higher energy state. The introduction of a new, unloaded polymer chain into this environment would result in the formation of new bonds, which would spread the energy of the system over the new structure in an exergonic reaction, and effectively pull the new chain into the polymeric matrix. We also note that the binding of the new polymer does not require fully developed Ca2+ binding zones, merely the existence of such hydrogen and ionic bonds as will hold together a loose pectin gel before crosslinking.
In addition to the work of Proseus and Boyer (2005) showing that fluorescent tagged dextrans could be induced to permeate the Chara cell wall, much earlier, Ray (1967), using high-resolution autoradiography, had shown that, while cellulose was deposited on the inner surface of growing oat coleoptile walls, hemicellulose became inserted throughout the thickness of the cell wall. Evidence therefore exists supporting the argument that newly synthesized wall matrix components can become intercalated into and throughout the existing wall structure. It follows, therefore, given the energetics of the stressed wall, that the newly arrived, and unstressed, molecules would become incorporated into the existing wall structure, providing a stress relaxation that would permit turgor-driven expansion.
Cell Wall Porosity and Pollen Tube Polarity
It is well recognized that the cell wall is a hydrated and porous entity through which ions and small molecules can move freely. But, as we have noted earlier, the pores are of such a size that even macromolecules of 20–30 kDa can penetrate the wall thickness. Direct evidence of the presence of large pores in tobacco pollen tubes comes from a recent study using transmission electron microscopy and cryo-field emission scanning electron microscopy (Derksen et al., 2011). The electron micrographs reveal that the cell wall, including that at the apex, consists of a lattice work of polymers with 40–50-nm-sized pores, which should be sufficient to allow moderate-sized macromolecules to pass through. Proseus and Boyer (2005) have further shown that, under pressure equivalent to that generated by turgor, macromolecular dextrans up to 70 kD could be forced through the isolated Chara walls, whereas much larger ones (e.g. 580 kD) could not. Given these conditions, it is apparent that the pores are sufficient to permit a variety of cell wall precursors to move through the wall and become intercalated into the matrix. However, these pores are adjustable and this property could contribute importantly to determining which parts of the wall will expand and which will not. For example, it is known that, as homogalacturonans become de-esterified and crosslinked with Ca2+ and rhamnogalacturonans II become crosslinked with borate, the pore size diminishes (Baron-Epel et al., 1988; Fleischer et al., 1999). These processes, which will be manifest in the older parts of the wall, including in particular the shank where it starts to curve towards the apical dome, will ensure that the insertion of new wall material will be restricted to the extreme apex. Collectively, these events will contribute to the maintenance of polarity in pollen tube growth. The pollen tube apical cell wall thus possesses properties of self-assembly and structural modification that propagates its own polarity and growth form.
SUMMARY STATEMENT
Studies of pollen tubes have identified many different factors that contribute to the growth and polarity of this unique cell. The ER/Golgi complex synthesizes the components of the cell wall, which are packaged into vesicles and driven from the shank of the tube towards the apex by the acto-myosin cytoskeleton. The vesicles collect in the ‘inverted cone’ in the pollen tube apex. Although there continues to be discussion about exactly where these vesicles deposit their cargo, different factors, notably the presence of the tip-focused Ca2+ gradient, will bias the process of secretion towards the polar axis of the pollen tube.
It is thought that the actin cytoskeleton, in particular the apical actin fringe, which resides in the cortex a short distance back from the extreme tip, plays a pivotal role in the targeted delivery of vesicles. The actin fringe is highly labile, and turns over quickly—a property that may owe much to the position of the alkaline band and the nearby association of two related actin binding proteins, ADF and AIP. Under the local alkaline conditions generated by the H+-ATPase, ADF in the presence of AIP will fragment microfilaments of the actin fringe, while promoting polymerization of new microfilaments. The H+-ATPase thus becomes a key contributor to polarized growth.
The cell wall also emerges as an active participant in the regulation of growth. It has the crucial task of remaining structurally intact while yielding to turgor. Within the cell wall, pectin attracts our attention as the component, which, when crosslinked with Ca2+, creates a wall with increased viscosity. But, through a subtle balance between the de-esterification of its methoxy esters by PME and Ca2+ crosslinking, the wall achieves the balance point that permits rapid growth without bursting. There appear to be different factors or processes that contribute to wall yielding at the tip. We think it is clear that Ca2+ together with the enzymes that control de-esterification plays a key role. Another factor may be ROS produced by NAD(P)H oxidase. Finally, we draw attention to the synthesis and intercalation of new wall material by the process of intussusception as being a major contributor to cell growth.
FUNDING
We thank the NSF, Grant No. MCB-0847876, for support during the preparation of this review.
ACKNOWLEDGMENTS
P.K.H. thanks Prof. Peter M. Ray for insightful comments and criticisms on our studies of exocytosis in pollen tubes, which are important in our evaluation herein of the factors that control cell growth. We also thank two anonymous reviewers who made several helpful criticisms; we believe that our responses to their comments have greatly improved this article. We additionally thank our colleagues at UMass for helpful discussions during the preparation of the manuscript. No conflict of interest declared.
REFERENCES
- Allwood E.G., Anthony R.G., Smertenko A.P., Reichelt S., Drøbak B.K., Doonan J.H., Weeds A.G., Hussey P.J. (2002). Regulation of the pollen-specific actin-depolymerizing factor LIADF1. Plant Cell. 14, 2915–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.R., Barnes W.S., Bedinger P. (2002). 2,6-dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J. Plant Physiol. 159, 61–67 [Google Scholar]
- Aouar L., Chebli Y., Geitmann A. (2010). Morphogenesis of complex plant cell shapes: the mechanical role of crystalline cellulose in growing pollen tubes. Sex. Plant Reprod. 23, 15–27 [DOI] [PubMed] [Google Scholar]
- Baluška F., Hlavacka A., Šamaj J., Palme K., Robinson D.G., Matoh T., McCurdy D.W., Menzel D., Volkmann D. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells: insights from brefeldin A-induced compartments. Plant Physiol. 130, 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Epel O., Gharyal P.K., Schindler M. (1988). Pectins as mediators of wall porosity in soybean cells. Planta. 175, 389–395 [DOI] [PubMed] [Google Scholar]
- Battey N.H., James N.C., Greenland A.J., Brownlee C. (1999). Exocytosis and endocytosis. Plant Cell. 11, 643–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger P.A., Hardeman K.J., Loukides C.A. (1994). Travelling in style: the cell biology of pollen. Trends Cell Biol. 4, 132–138 [DOI] [PubMed] [Google Scholar]
- Benkert R., Obermeyer G., Bentrup F.W. (1997). The turgor pressure of growing lily pollen tubes. Protoplasma. 198, 1–8 [Google Scholar]
- Bernal R., Rojas E., Dumais J. (2007). The mechanics of top growth morphogenesis: what we have learned from rubber balloons. J. Mech. Mater. Struct. 2, 1157–1168 [Google Scholar]
- Bolte S., Talbot C., Boutte Y., Catrice O., Read N.D., Satiat-Jeunemaitre B. (2004). FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 214, 159–173 [DOI] [PubMed] [Google Scholar]
- Bosch M., Hepler P.K. (2005). Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell. 17, 3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Hepler P.K. (2006). Silencing of the tobacco pollen pectin methylesterase NtPPME1 results in retarded in vivo pollen tube growth. Planta. 223, 736–745 [DOI] [PubMed] [Google Scholar]
- Bosch M., Cheung A.Y., Hepler P.K. (2005). Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 138, 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Daher F., Geitmann A. (2011). Actin is involved in pollen tube tropism through redefining the spatial targeting of secretory vesicles. Traffic. 12, 1537–1551 [DOI] [PubMed] [Google Scholar]
- Bove J., Vaillancourt B., Kroeger J., Hepler P.K., Wiseman P.W., Geitmann A. (2008). Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol. 147, 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J.S. (2009). Cell wall biosynthesis and the molecular mechanism of plant enlargement. Funct. Plant Biol. 36, 383–394 [DOI] [PubMed] [Google Scholar]
- Brewbaker J.L., Kwack B.H. (1963). The essential role of calcium ion in pollen germination and pollen tube growth. Amer. J. Bot. 50, 859–865 [Google Scholar]
- Brillinger D. (1981). Time Series: Data Analysis and Theory (San Francisco, CA: Holden-Day; ). [Google Scholar]
- Cai G., Cresti M. (2009). Organelle motility in the pollen tube: a tale of 20 years. J. Exp. Bot. 60, 495–508 [DOI] [PubMed] [Google Scholar]
- Cai G., Faleri C., Del Casino C., Emons A.M.C., Cresti M. (2011). Distribution of callose synthase, cellulose synthase, and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol. 155, 1169–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanoni P., Blatt M.R. (2007). Membrane trafficking and polar growth in root hairs and pollen tubes. J. Exp. Bot. 58, 65–74 [DOI] [PubMed] [Google Scholar]
- Campàs O., Mahadevan L. (2009). Shape and dynamics of tip-growing cells. Curr. Biol. 19, 2102–2107 [DOI] [PubMed] [Google Scholar]
- Cárdenas L., Lovy-Wheeler A., Kunkel J.G., Hepler P.K. (2008). Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 146, 1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L., Lovy-Wheeler A., Wilsen K.L., Hepler P.K. (2005). Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil. Cytoskel. 61, 112–127 [DOI] [PubMed] [Google Scholar]
- Cárdenas L., McKenna S.T., Kunkel J.G., Hepler P.K. (2006). NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol. 142, 1460–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoire L., Pierron M., Morvan C., du Penhoat C.H., Goldberg R. (1998). Investigation of the action patterns of pectin methylesterase isoforms through kinetic analyses and NMR spectroscopy: implications in cell wall expansion. J. Biol. Chem. 273, 33150–33156 [DOI] [PubMed] [Google Scholar]
- Certal A.C., Almeida R.B., Carvalho L.M., Wong E., Moreno N., Michard E., Carneiro J., Rodriguez-Leon J., Wu H.M., et al. (2008). Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell. 20, 614–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y., Geitmann A. (2007). Mechanical principles governing pollen tube growth. Floricult. Ornamen. Biotech. 1, 232–245 [Google Scholar]
- Chebli Y., Kaneda M., Zerzour R., Geitmann A. (2012). The cell wall of the Arabidopsis pollen tube-spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol. 160, 1940–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Wong E.I., Vidali L., Estavillo A., Hepler P.K., Wu H.M., Cheung A.Y. (2002). The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell. 14, 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.Y., Wu H.-M. (2004). Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell. 16, 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.Y., Niroomand S., Wu H.-M. (2010). A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc. Natl Acad. Sci. U S A. 107, 16390–16395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R.A., Fowler J.E. (2006). Polarized growth: maintaining focus on the tip. Curr. Opin. Plant Biol. 9, 579–588 [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J. (1999). Enzymes and other agents that enhance cell wall extensibility. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 391–417 [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J., Bedinger P., Durachko D.M. (1997). Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl Acad. Sci. U S A. 94, 6559–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J., Li L.C., Cho H.T., Hoffmann-Benning S., Moore R.C., Blecker D. (2002). The growing world of expansins. Plant Cell Physiol. 43, 1436–1444 [DOI] [PubMed] [Google Scholar]
- Dardelle F., Lehner A., Ramdani Y., Bardor M., Lerouge P., Driouich A., Mollet J-C. (2010). Biochemical and immunocytological characterization of Arabidopsis pollen tube cell wall. Plant Physiol. 153, 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J., Janssen G.-J., Wolter-Arts M., Lichtscheidl I., Adlassnig W., Ovecka M., Doris F., Steer M. (2011). Wall architecture with high porosity is established at the tip and maintained in growing pollen tubes of Nicotiana tabacum . Plant J. 68, 495–506 [DOI] [PubMed] [Google Scholar]
- Derksen J., Rutten T., Lichtscheidl I.K., de Win A.H.N., Pierson E.S., Rongen G. (1995). Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma. 188, 267–276 [Google Scholar]
- Dickinson D.B. (1965). Germination of lily pollen: respiration and tube growth. Science. 150, 1818–1819 [DOI] [PubMed] [Google Scholar]
- Dickinson D.B. (1966). Inhibition of pollen respiration by oligomycin. Nature. 210, 1362–1363 [Google Scholar]
- Dong H., Pei W., Ren H.R. (2012). Actin fringe is correlated with tip growth velocity of pollen tubes. Mol. Plant. 5, 1160–1162 [DOI] [PubMed] [Google Scholar]
- Dumais J., Long S.R., Shaw S.L. (2004). The mechanics of surface expansion anisotropy in Medicago trunculata root hairs. Plant Phys. 136, 3266–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais J., Shaw S.L., Steele C.R., Long S.R., Ray P.M. (2006). An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. Int. J. Dev. Biol. 50, 209–222 [DOI] [PubMed] [Google Scholar]
- Dutta R., Robinson K.R. (2004). Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 135, 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayant P., Girlanda O., Chebli Y., Aubin C.E., Villemure I., Geitmann A. (2010). Finite element model of polar growth in pollen tubes. Plant Cell. 22, 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó J.A., Sainhas J., Hackett G.R., Kunkel J.G., Hepler P.K. (1999). Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J. Cell Biol. 144, 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H.H. (2001). pH: signal and messenger in plant cells. Plant Biol. 3, 577–591 [Google Scholar]
- Ferguson C., Teeri T.T., Siika-aho M., Read S.M., Bacic A. (1998). Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum . Planta. 206, 452–460 [Google Scholar]
- Fleischer A., O’Neill M.A., Ehwald R. (1999). The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 121, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J., Demidchik V., Bothwell J.H.F., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D.G., et al. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 422, 442–446 [DOI] [PubMed] [Google Scholar]
- Frietsch S., Wang Y.F., Sladek C., Poulsen L.R., Romanowsky S.M., Schroeder J.I., Harper J.F. (2007). A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl Acad. Sci. U S A. 104, 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S.C. (1998). Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 332, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Wu G., Yang Z. (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 152, 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass N., Glagotskaia T., Mellema S., Stuurman J., Barone M., Mandel T., Roessner-Tunali U., Kuhlemeier C. (2005). Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell. 17, 2355–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann A. (2010). How to shape a cylinder: pollen tube as a model system for the generation of complex cellular geometry. Sex. Plant Reprod. 23, 63–71 [DOI] [PubMed] [Google Scholar]
- Geitmann A., Dumais J. (2009). Not-so-tip-growth. Plant Signal. Behav. 4, 136–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G.T., Morris E.R., Rees D.A., Smith P.J.C., Thom D. (1973). Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 32, 195–198 [Google Scholar]
- Gu Y., Fu Y., Dowd P., Li S.D., Vernoud V., Gilroy S., Yang Z.B. (2005). A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169, 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungabissoon R.A., Jiang C.J., Drøbak B.K., Maciver S.K., Hussey P.J. (1998). Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J. 16, 689–696 [Google Scholar]
- Harold F.M. (2002). Force and compliance: rethinking morphogenesis in walled cells. Fungal Genet. Biol. 37, 271–282 [DOI] [PubMed] [Google Scholar]
- Hepler P.K., Winship L.J. (2010). Calcium at the cell wall-cytoplast interface. J. Integr. Plant Biol. 52, 147–160 [DOI] [PubMed] [Google Scholar]
- Hepler P.K., Kunkel J.G., Rounds C.M., Winship L.J. (2011). Calcium entry into pollen tubes. Trends Plant Sci. 17, 32–38 [DOI] [PubMed] [Google Scholar]
- Hepler P.K., Vidali L., Cheung A.Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17, 159–187 [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. (1990). Dynamic aspects of apical zonation in the angiosperm pollen tube. Sex. Plant Reprod. 3, 187–194 [Google Scholar]
- Hill A.E., Shachar-Hill B., Skepper J.N., Powell J., Shachar-Hill Y. (2012). An osmotic model of growing pollen tube. PloS One. 7, e36585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Hepler P.K. (2003). Control of pollen tube growth: role of ion gradients and fluxes. New Phytol. 159, 539–563 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Feijó J.A., Hackett G.R., Kunkel J.G., Hepler P.K. (1997). Pollen tube growth and the intracellular cytostolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 9, 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.U., Gu Y., Lee Y.J., Yang Z. (2005). Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell. 16, 5385–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A., Sekiya J. (1994). Tissue specificity of mitochondrial F0F1-ATPase activity of Lilium longiflorum plant. FEBS Lett. 356, 229–232 [DOI] [PubMed] [Google Scholar]
- Iwanami Y. (1956). Protoplasmic movement in pollen grains and tubes. Phytomorphol. 6, 288–295 [Google Scholar]
- Iwano M., Entani T., Shiba H., Kakita M., Nagai T., Mizuno H., Miyawaki A., Shoji T., Kubo K., Isogai A., et al. (2009). Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 150, 1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M., Shiba H., Miwa T., Che F.S., Takayama S., Nagai T., Miyawaki A., Isogai A. (2004). Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis . Plant Physiol. 136, 3562–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Yang S.L., Xie L.F., Puah C.S., Zhang X.Q., Yang W.C., Sundaresan V., Ye D. (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell. 17, 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K.R., Wudick M.M., Feijó J.A. (2011). Calcium regulation of tip growth: new genes for old mechanisms. Curr. Opin. Plant Biol. 14, 721–730 [DOI] [PubMed] [Google Scholar]
- Krichevsky A., Kozlovsky S.V., Tian G.W., Chen M.H., Zaltsman A., Citovsky V. (2007). How pollen tubes grow. Dev. Biol. 303, 405–420 [DOI] [PubMed] [Google Scholar]
- Kroeger J.H., Geitmann A. (2012a). Pollen tube growth: getting a grip on cell biology through modeling. Mech. Res. Comm. 42, 32–39 [Google Scholar]
- Kroeger J.H., Geitmann A. (2012b). The pollen tube paradigm revisited. Curr. Opin. Plant Biol. 15, 618–624 [DOI] [PubMed] [Google Scholar]
- Kroeger J.H., Bou Daher R., Grant M., Geitmann A. (2009). Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophys. J. 97, 1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger J.H., Geitmann A., Grant M. (2008). Model for calcium dependent oscillatory growth in pollen tubes. J. Theor. Biol. 253, 363–374 [DOI] [PubMed] [Google Scholar]
- Kroeger J.H., Zerzour R., Geitmann A. (2011). Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. PLoS One. 6, e18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J., Batistic O., Hashimoto K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell. 22, 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühtreiber W.M., Jaffe L.F. (1990). Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J. Cell Biol. 110, 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle S.A., Hepler P.K. (1992). Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum . Protoplasma. 167, 215–230 [Google Scholar]
- Lee Y., Choi D., Kende H. (2001). Expansins: ever-expanding numbers and functions. Curr. Opin. Plant Biol. 4, 527–532 [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Szumlanski A., Nielsen E., Yang Z. (2008). Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 181, 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B., Arango M., Oufattole M., Crouzet J., Purnelle B., Boutry M. (2005). Identification of a Nicotiana plumbaginifolia plasma membrane H+-ATPase gene expressed in the pollen tube. Plant Mol. Biol. 58, 775–787 [DOI] [PubMed] [Google Scholar]
- Lenartowska M., Michalska A. (2008). Actin filament organization and polarity in pollen tubes revealed by myosin II subfragment 1 decoration. Planta. 228, 891–896 [DOI] [PubMed] [Google Scholar]
- Li Y.Q., Mareck A., Faleri C., Moscatelli A., Liu Q., Cresti M. (2002). Detection and localization of pectin methylesterase isoforms in pollen tubes of Nicotiana tabacum L. Planta. 214, 734–740 [DOI] [PubMed] [Google Scholar]
- Liu J., Piette B.M., Deeks M.J., Franklin-Tong V.E., Hussey P.J. (2010). A compartmental model analysis of integrative and self-regulatory ion dynamics in pollen tube growth. PLoS One. 5, e13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Franco R., Bartnicki-Garcia S., Bracker C.E. (1994). Pulsed growth of fungal hyphal tips. Proc. Natl Acad. Sci. U S A. 91, 12228–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A., Cárdenas L., Kunkel J.G., Hepler P.K. (2007). Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motil. Cytoskel. 64, 217–232 [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A., Kunkel J.G., Allwood E.G., Hussey P.J., Hepler P.K. (2006). Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell. 18, 2182–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A., Wilsen K.L., Baskin T.I., Hepler P.K. (2005). Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 221, 95–104 [DOI] [PubMed] [Google Scholar]
- Malhó R., Trewavas A.J. (1996). Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 8, 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna S.T., Kunkel J.G., Bosch M., Rounds C.M., Vidali L., Winship L.J., Hepler P.K. (2009). Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell. 21, 3026–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellema S., Eichenberger W., Rawyler A., Suter M., Tadege M., Kuhlemeier C. (2002). The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. Plant J. 30, 329–336 [DOI] [PubMed] [Google Scholar]
- Messerli M.A., Creton R., Jaffe L.F., Robinson K.R. (2000). Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev. Biol. 222, 84–98 [DOI] [PubMed] [Google Scholar]
- Messerli M.A., Danuser G., Robinson K.R. (1999). Pulsatile influxes of H+, K+ and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. J. Cell Sci. 112, 1497–1509 [DOI] [PubMed] [Google Scholar]
- Michard E., Alves F., Feijó J.A. (2009). The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int. J. Dev. Biol. 53, 1609–1622 [DOI] [PubMed] [Google Scholar]
- Michard E., Lima P.T., Borges F., Silva A.C., Portes M.T., Carvalho J.E., Gilliham M., Liu L.H., Obermeyer G., Feijó J.A. (2011). Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science. 332, 434–437 [DOI] [PubMed] [Google Scholar]
- Miller D.D., Callaham D.A., Gross D.J., Hepler P.K. (1992). Free Ca2+ gradient in growing pollen tubes of Lilium . J. Cell Sci. 101, 7–12 [Google Scholar]
- Monshausen G.B., Bibikova T.N., Messerli M.A., Shi C., Gilroy S. (2007). Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc. Natl Acad. Sci. U S A. 104, 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli A., Idilli A.I. (2009). Pollen tube growth: a delicate equilibrium between secretory and endocytic pathways. J. Integr. Plant Biol. 51, 727–739 [DOI] [PubMed] [Google Scholar]
- Moscatelli A., Ciampolini F., Rodighiero S., Onelli E., Cresti M., Santo N., Idilli A. (2007). Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J. Cell Sci. 120, 3804–3819 [DOI] [PubMed] [Google Scholar]
- Moscatelli A., Idilli A.I., Rodighiero S., Caccianiga M. (2012). Inhibition of actin polymerisation by low concentration Latrunculin B affects endocytosis and alters exocytosis in shank and tip of tobacco pollen tubes. Plant Biol. 14, 770–782 [DOI] [PubMed] [Google Scholar]
- Nguema-Ona E., Coimbra S., Vicre-Gibouin M., Mollet J.C., Driouich A. (2012). Arabinogalactan proteins in root and pollen-tube cells: distribution and functional aspects. Ann. Bot. 110, 383–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M.A., Albersheim P., Darvill A. (1990). The pectic polysaccharides of primary cell walls. In Methods in Plant Biochemistry, Carbohydrates, Dey P.M., Harborne J.B, eds (London: Academic Press; ), pp. 415–441 [Google Scholar]
- Oldenbourg R. (1996). A new view on polarization microscopy. Nature. 381, 811–812 [DOI] [PubMed] [Google Scholar]
- Onelli E., Moscatelli A. (2013). Endocytic pathways and recycling in growing pollen tubes. Plants. 2, 211–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin R., Geitmann A. (2012). The role of pectin in plant morphogenesis. Biosystems. 109, 397–402 [DOI] [PubMed] [Google Scholar]
- Palmgren M.G. (2001). Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 817–845 [DOI] [PubMed] [Google Scholar]
- Parre E., Geitmann A. (2005). Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense . Planta. 220, 582–592 [DOI] [PubMed] [Google Scholar]
- Parton R.M., Fischer-Parton S., Watahiki M.K., Trewavas A.J. (2001). Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J.Cell Sci. 114, 2685–2695 [DOI] [PubMed] [Google Scholar]
- Picton J.M., Steer M.W. (1983a). Evidence for the role of Ca2+ ions in tip extension in pollen tubes. Protoplasma. 115, 11–17 [Google Scholar]
- Picton J.M., Steer M.W. (1983b). Membrane recycling and the control of secretory activity in pollen tubes. J. Cell Sci. 63, 303–310 [DOI] [PubMed] [Google Scholar]
- Pierson E.S., Miller D.D., Callaham D.A., van Aken J., Hackett G., Hepler P.K. (1996). Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol. 174, 160–173 [DOI] [PubMed] [Google Scholar]
- Potocký M., Jones M.A., Bezvoda R., Smirnoff N., Žárský V. (2007). Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 174, 742–751 [DOI] [PubMed] [Google Scholar]
- Potocký M., Pejchar P., Gutkowska M., Jimenez-Quesada M.J., Potocka A., Alche Jde D., Kost B., Žárský V. (2012). NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J. Plant Physiol. 169, 1654–1663 [DOI] [PubMed] [Google Scholar]
- Proseus T.E., Boyer J.S. (2005). Turgor pressure moves polysaccharides into growing cell walls of Chara corallina . Ann. Bot. (Lond.). 95, 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proseus T.E., Boyer J.S. (2006). Identifying cytoplasmic input to the cell wall of growing Chara corallina . J. Exp. Bot. 57, 3231–3242 [DOI] [PubMed] [Google Scholar]
- Proseus T.E., Boyer J.S. (2012). Calcium deprivation disrupts enlargement of Chara corallina cells: further evidence for the calcium pectate cycle. J. Exp. Bot. 63, 3953–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Yang Z. (2011). Rapid tip growth: insights from pollen tubes. Semin. Cell Dev. Biol. 22, 816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore K.S., Cork R.J., Robinson K.R. (1991). A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev. Biol. 148, 612–619 [DOI] [PubMed] [Google Scholar]
- Ray P.M. (1967). Radioautographic study of cell wall deposition in growing plant cells. J. Cell Biol. 35, 659–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P.M. (1987). Principles of plant cell expansion. In Physiology of Cell Expansion during Plant Growth, Cosgrove D.J., Knievel D.P, eds (Washington, DC: American Society of Plant Physiology; ), pp. 1–17 [Google Scholar]
- Ray P.M. (1992). Mechanisms of wall loosening for cell growth. Curr. Top. Plant Biochem. Physiol. 11, 18–41 [Google Scholar]
- Röckel N., Wolf S., Kost B., Rausch T., Greiner S. (2008). Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 53, 133–143 [DOI] [PubMed] [Google Scholar]
- Rojas E.R., Hotton S., Dumais J. (2011). Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys. J. 101, 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds C.M., Hepler P.K., Fuller S.J., Winship L.J. (2010). Oscillatory growth in lily pollen tubes does not require aerobic energy metabolism. Plant Physiol. 152, 736–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds C.M., Lubeck E., Hepler P.K., Winship L.J. 2011a. Propidium iodide competes with Ca2+ to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiol. 157, 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds C.M., Winship L.J., Hepler P.K. (2011b). Pollen tube energetics: respiration, fermentation and the race to the ovule. Ann. Bot. Plants., doi:10.1093/aobpla/plr019, first published online 4 August 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Jauh G.Y., Hepler P.K., Lord E.M. (1998). Effects of Yariv phenylglycoside on cell wall assembly in the lily pollen tube. Planta. 204, 450–458 [DOI] [PubMed] [Google Scholar]
- Šamaj J., Muller J., Beck M., Bohm N., Menzel D. (2006). Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends Plant Sci. 11, 594–600 [DOI] [PubMed] [Google Scholar]
- Schopfer P. (2001). Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 28, 679–688 [DOI] [PubMed] [Google Scholar]
- Sommer A., Geist B., Da Ines O., Gehwolf R., Schaffner A.R., Obermeyer G. (2008). Ectopic expression of Arabidopsis thaliana plasma membrane intrinsic protein 2 aquaporins in lily pollen increases the plasma membrane water permeability of grain but not of tube protoplasts. New Phytol. 180, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza A., Crinelli R., Scoccianti V., Geitmann A. (2011). Reactive oxygen species are involved in pollen tube initiation in kiwifruit. Plant Biol. 14, 64–76 [DOI] [PubMed] [Google Scholar]
- Staehelin L.A., Moore I. (1995). The plant Golgi apparatus: structure, functional organization and trafficking mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 261–288 [Google Scholar]
- Steer M.W., Steer J.M. (1989). Pollen tube tip growth. New Phytol. 111, 323–358 [DOI] [PubMed] [Google Scholar]
- Sutter J.U., Homann U., Thiel G. (2000). Ca2+-stimulated exocytosis in maize coleoptile cells. Plant Cell. 12, 1127–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A., Li L.C., Cosgrove D.J. (2011). Matrix solubilization and cell wall weakening by beta-expansin (group-1 allergen) from maize pollen. Plant J. 68, 546–559 [DOI] [PubMed] [Google Scholar]
- Tadege M., Kuhlemeier C. (1997). Aerobic fermentation during tobacco pollen development. Plant Mol. Biol. 35, 343–354 [DOI] [PubMed] [Google Scholar]
- Tadege M., Dupuis I.I., Kuhlemeier C. (1999). Ethanolic fermentation: new functions for an old pathway. Trends Plant Sci. 4, 320–325 [DOI] [PubMed] [Google Scholar]
- Taiz L. (1984). Plant cell expansion: regulation of cell wall mechanical properties. Annu. Rev. Plant Physiol. 35, 585–657 [Google Scholar]
- Tavares B., Domingos P., Dias P.N., Feijó J.A., Bicho A. (2011). The essential role of anionic transport in plant cells: the pollen tube as a case study. J. Exp. Bot. 62, 2273–2298 [DOI] [PubMed] [Google Scholar]
- Tian G.-W., Chen M.-H., Zaltsman A., Citovsky V. (2006). Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 294, 83–91 [DOI] [PubMed] [Google Scholar]
- Tominaga M., Kojima H., Yokota E., Orii H., Nakamori R., Katayama E., Anson M., Shimmen T., Oiwa K. (2003). Higher plant myosin XI moves processively on actin with 35nm steps at high velocity. EMBO J. 22, 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia E.R., Stephenson A.G., Durachko D.M., Cosgrove D. (2009). Class B beta-expansins are needed for pollen separation and stigma penetration. Sex. Plant Reprod. 22, 141–152 [DOI] [PubMed] [Google Scholar]
- Van Gisbergen P.A.C., Bezanilla M. (2013). Plant formins: membrane anchors for actin polymerization. Trends Cell Biol. [DOI] [PubMed] [Google Scholar]
- Vidali L., Hepler P.K. (2001). Actin and pollen tube growth. Protoplasma. 215, 64–76 [DOI] [PubMed] [Google Scholar]
- Vidali L., McKenna S.T., Hepler P.K. (2001). Actin polymerization is necessary for pollen tube growth. Mol. Biol. Cell. 12, 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Rounds C.M., Hepler P.K., Bezanilla M. (2009). Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS One. 4, e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats W.G., McCartney L., Mackie W., Knox J.P. (2001a). Pectin: cell biology and prospects for functional analysis. Plant Mol. Biol. 47, 9–27 [PubMed] [Google Scholar]
- Willats W.G., Orfila C., Limberg G., Buchholt H.C., van Alebeek G.J., Voragen A.G., Marcus S.E., Christensen T.M., Mikkelsen J.D., Murray B.S., et al. (2001b). Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls: implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 276, 19404–19413 [DOI] [PubMed] [Google Scholar]
- Wilsen K.L., Lovy-Wheeler A., Voigt B., Menzel D., Kunkel J.G., Hepler P.K. (2006). Imaging the actin cytoskeleton in growing pollen tubes. Sex. Plant Reprod. 19, 51–62 [Google Scholar]
- Winship L.J., Obermeyer G., Geitmann A., Hepler P.K. (2010). Under pressure, cell walls set the pace. Trends Plant Sci. 15, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Greiner S. (2012). Growth control by cell wall pectins. Protoplasma. 249 Suppl. 2, S169–S175 [DOI] [PubMed] [Google Scholar]
- Wolf S., Grsic-Rausch S., Rausch T., Greiner S. (2003). Identification of pollen-expressed pectin methylesterase inhibitors in Arabidopsis . Febs Lett. 555, 551–555 [DOI] [PubMed] [Google Scholar]
- Yan A., Xu G., Yang Z.B. (2009). Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc. Natl Acad. Sci. U S A. 106, 22002–22007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerzour R., Kroeger J., Geitmann A. (2009). Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev. Biol. 334, 437–446 [DOI] [PubMed] [Google Scholar]
- Zhang H., Qu X., Bao C., Khurana P., Wang Q., Xie Y., Zheng Y., Chen N., Blanchoin L., Staiger C.J., et al. (2010). Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell. 22, 2749–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L., Munnik T. (2007). Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci. 12, 90–97 [DOI] [PubMed] [Google Scholar]
- Zonia L., Munnik T. (2008). Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J. Exp. Bot. 59, 861–873 [DOI] [PubMed] [Google Scholar]
- Zonia L., Munnik T. (2009). Uncovering hidden treasures in pollen tube growth mechanics. Trends Plant Sci. 14, 318–327 [DOI] [PubMed] [Google Scholar]
- Zorec R., Tester M. (1992). Cytoplasmic calcium stimulates exocytosis in a plant secretory cell. Biophys. J. 63, 864–867 [DOI] [PMC free article] [PubMed] [Google Scholar]