Abstract
Background
This study's objective was to explore relationships between patient role preferences during the cancer treatment decision-making process and quality of life (QOL).
Methods
As part of a larger survey conducted by the American Cancer Society, 1-year cancer survivors completed a survey in 2000. This paper is based upon respondents from Minnesota (response rate 37.4%). Standardized measures included the Profile of Mood States (scores converted to have a range, 0-100; 100 is the best mood), the Short Form (SF)-36 (standardized scores), and the Control Preferences Scale. Patients' actual and preferred role preference distributions and concordance between the roles were compared to QOL scores using two-sample t-test methodology.
Results
Survivors (n=594) actual role in cancer care was 33% active, 50% collaborative and 17% passive. Their preferred role was 35% active, 53% collaborative, and 13% passive. 88% of survivors had concordant preferred and actual roles. Survivors with concordant roles had higher SF-36 Physical Component Scores (PCS) (p<0.01), higher vitality (p=0.01), less fatigue (p<0.01), less confusion (p=0.01), less anger (p=0.046) and better overall mood (p=0.01). These results were similar in both the female and the younger (age <60) cohorts. Survivors with active actual roles had higher PCS (p<0.01), less tension (p=0.04), and higher vitality (p=0.04) than those being collaborative or passive. No differences existed in QOL scores by preferred role.
Conclusions
Survivors who experienced discordance between their actual role and preferred role reported substantial QOL deficits in both physical and emotional domains. These results are indicative of the need to support patient preferences.
Keywords: quality of life, decision analysis, quality of care, control preferences, cancer survivors
Introduction
The evolution of modern interdisciplinary cancer care and the emergence of personalized medicine have increased the presence and importance of patient-centered communication.1 Research has demonstrated that two-way communication, shared understanding and trust between patients and health care providers are paramount to the success of treatment.2,3,4 Such research has also shown patients desire information regarding medical condition and available treatment options so medical decision making can occur.5-9 In response, cancer care in the United States has made a concerted effort to become more patient-focused and collaborative.3,10
Much has been written about how to provide information and engage patients in making decisions. The optimal approach depends upon the extent of participation (active, collaborative, or passive) the patient wants or needs.7,8,11-15 Various studies have explored the degree of involvement patients want. In 1996, Beaver et al, reported the majority (52%) of newly diagnosed breast cancer patients preferred a passive role.16 This finding was challenged in 2006 as Hack et al. reported only 22% of breast cancer patients preferred a passive role.17 Late stage disease and tumors of the reproductive system, especially among males, tend to be associated with a more passive approach by patients.15,18
A large, multi-sample study involving cancer patients with a variety of tumors found that roughly 25% of patients prefer to have an active role in treatment decision making, 50% prefer a collaborative role, and 25% prefer to have doctors make decisions for them.19 Thus it is known that patients' preferred role in medical decision making varies among individuals15,20 , but is relatively trait-like, meaning it doesn't change, over time.15,18,19 Coulter summarized that “desire for participation has been found to vary according to age, educational status, disease severity and cultural background.”5
Past studies with cancer survivor participants have reported associations between Quality of Life (QOL) and satisfaction with control or involvement in health care.21,22 Griggs et al (2007) reported treatment satisfaction was associated with an increase in mental health.23 This report uses population-based data to describe decision making preferences, demographics, and QOL of cancer survivors. Moreover, our aim is to determine if QOL is impacted by patient concordance or discordance in preferred and actual decision making roles.
Methods
Eligibility and Recruitment
The American Cancer Society's (ACS) Study of Cancer Survivors (SCS) is a longitudinal, population-based study of adult cancer survivors designed to examine physical and psychosocial adjustment to cancer, and changes in QOL. The cancer survivors were drawn from 11 state cancer registries. The study had the approval of the Institutional Review Board (IRB) of Emory University. Additional IRB approvals were obtained in each state. Details of the complete study design and analysis have been previously reported.24 This paper reports on results of Minnesota survivors using the SCS survey as administered at 1 year post-diagnosis, conducted by the ACS, the Minnesota Cancer Surveillance System (Minnesota's cancer registry), and the Mayo Clinic (IRB 0-2462-01).
Newly diagnosed cancer patients were selected from the state cancer registry and screened for eligibility. To be eligible to participate, patients had to be at least 18 years of age, diagnosed with one of the ten most common cancers (prostate, female breast, lung, colorectal, bladder, Non-Hodgkin's lymphoma, skin melanoma, kidney, ovarian and uterine) and have stage I-IV at the time of diagnosis. Patients were ineligible if they were identified as being mentally incompetent by their physicians, or were institutionalized or incarcerated at the time of recruitment. Physicians identified in the state cancer registry were notified that their patient had been sampled for this study and given an opportunity to update patient eligibility. Subsequently, survivors were consented and surveyed via mail and telephone. The overall response rate in Minnesota, including physician notification and survivor recruitment, was 37.4%. The methodological implications of this low response rate were discussed in detail by Smith et al (2007).24
Measures
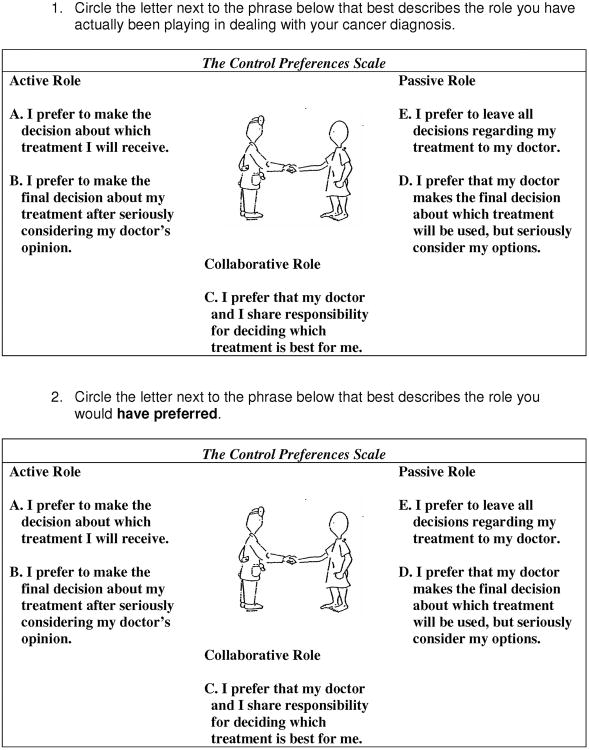
Patient decision making preferences were measured by the Control Preference Scale (CPS) (Figure 1).25 The two-item tool allows the patient to record their actual (item 1) and preferred (item 2) roles in decision making. The goal of the development of this tool was to allow clinicians to assess patient role preferences and experiences in order to facilitate communication.
Figure 1.
Control Preferences Scale.
Patient health related QOL was measured by the 36 item SF-36 Health Survey which is a validated self-reported tool composed of questions regarding health status, feelings and ability to do usual activities as recollected during the last 4 weeks.26,27 The SF-36 measure is comprised of 2 summary scales. The Physical Component Scale (PCS) is composed of physical functioning, physical role functioning, bodily pain and general health indexes. The Mental Component Scale (MCS) is composed of vitality, social functioning, emotional role functioning and mental health indexes. The summary scales and indexes are standardized and age-adjusted.
Patient mood was measured by the Profile of Mood States (POMS).28 The POMS consists of 37 items, each rated on a scale from 0-5 with 0 meaning ‘no’ and 5 meaning ‘always’. Each item asks the patient if he or she has experienced a particular feeling in last week. Sample items are “unhappy”, “lively”, “worn-out”, “tense”, “angry”, and “unable to concentrate”. The POMS produces an overall score and a score for each of 6 subscales: anger-hostility (A/H), confusion-bewilderment (C/B), depression-dejection (D/D), fatigue-inertia (F/I), tension-anxiety (T/A), and vigor-activity (V/A).
Statistical Considerations
All assessments were scored using the appropriate algorithms. In order to improve interpretability and comparability, the POMS total and subscale scores were converted to a 0-100 point scale, where 100 represented the best possible mood or QOL; so, a higher number means better mood or QOL. Both PCS and MCS are norm-based with a mean of 50 and standard deviation of 10. Higher scores are indicative of better health status.27 Responses to the CPS were used to categorize patients actual and preferred roles as active, collaborative, or passive.15 Further, actual and preferred CPS responses were compared. If actual role and preferred role were the same, the patient's role agreement was categorized as being concordant. If they were not the same, the patient's role agreement was categorized as being discordant.
Role preference distributions were compared to demographic categories and QOL scores using Fisher's exact, two-sample t-test and Kruskall-Wallis methodology, as appropriate. General linear modeling techniques were employed to determine any relationships between QOL scores of PCS, MCS and POMS mood as dependent variables with baseline characteristics and CPS scores as independent variables.
With 594 observations, with approximately 7.4 times as many subjects in the Concordance group than in the Discordance group, a two-sample t-test (with a 0.05 two-tailed test of significance) has 80% power to detect a mean difference that equals 0.36 times the pooled standard deviation of the two groups), suggesting a standardized effect size of 0.36.29
Results
Patient Population
There were 594 eligible patients. They completed the CPS and at least one of the POMS or SF-36 questionnaires approximately 1 year after diagnosis. Baseline characteristics indicate the survivors were predominantly over the age of 60 (54%), were female (54%), had breast (23%) or prostate (21%) cancers, and had treatment of chemotherapy (32%), radiation therapy (30%) or surgery-resection (66%) (Table 1). The majority (59%) of patients had at least one comorbidity. Asthma (9%), depression (8%), diabetes (7%), heart condition (8%), high blood pressure (31%), or ‘other’ (12%) were most frequently reported.
Table 1. Patient Baseline Demographics (N=594).
| Total | Total | ||
|---|---|---|---|
| Age Group | Gender | ||
| Under 60 | 273 (46.0%) | Female | 330 (55.6%) |
| 60 & Over | 321 (54.0%) | Male | 264 (44.4%) |
| Cancer Type | Cancer Treatment | ||
| Bladder | 19 (3.2%) | Surgery - Resection | 392 (66.0%) |
| Breast | 134 (22.6%) | Chemotherapy | 188 (31.6%) |
| Colorectal | 90 (15.2%) | Radiation | 179 (30.1%) |
| Kidney | 27 (4.5%) | Bone Marrow Transplant | 4 (0.7%) |
| Lung | 78 (13.1%) | Hormone Therapy | 73 (12.3%) |
| Non-Hodgkins | 31 (5.2%) | Immunotherapy | 14 (2.4%) |
| Ovarian | 30 (5.1%) | ||
| Prostate | 126 (21.2%) | Race | |
| Skin/Melanoma | 23 (3.9%) | White | 578 (97.5%) |
| Uterine | 36 (6.1%) | Black | 3 (0.5%) |
| Other | 12 (0.8% |
CPS Responses
CPS results indicate 33% of survivors actually played an active role in treatment decision-making, 50% played a collaborative role, and 17% played a passive role (Table 2). The role preferred had approximately the same distribution. The patient population had a distribution of role agreement of 523 (88%) concordant and 71 (12%) discordant. The Kappa statistic indicates in this sample we would conclude that there is 80% more agreement than we would expect by chance alone. Of the patients reporting discordance, 52 (73%) played a role that was more active than preferred and 19 (27%) played a role that was less active than preferred. There were no demographic differences between concordant patients and discordant patients.
Table 2. Patient Control Preferences Scale Distribution (N=594).
| Control Preferences Scale Summary | |
|---|---|
| Actual Role Played | |
| Active | 196 (33%) |
| Collaborative | 299 (50%) |
| Passive | 99 (17%) |
| Role Preferred | |
| Active | 208 (35%) |
| Collaborative | 312 (52.5%) |
| Passive | 74 (12.5%) |
| Role Agreement | |
| Discordance | 71 (12%) |
| Concordance | 523 (88%) |
| Discordant Category | |
| Role played was more active than preferred | 52 (73%) |
| Role played was less active than preferred | 19 (27%) |
CPS Results and QOL Scores
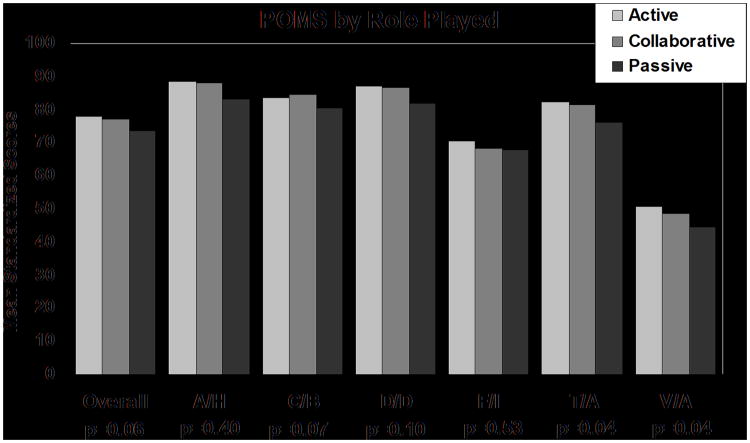
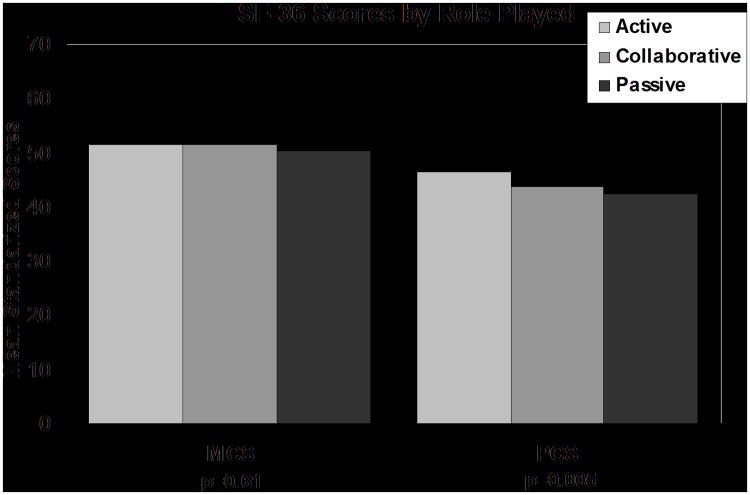
Kruskall-Wallis analysis showed patients reporting an actual active role had higher SF-36 PCS scores (mean of 46.5 vs.43.7 for collaborative and 42.5 for passive, p<0.01), higher SF-36 Physical Function index (mean of 51.3 vs. 49.5 for collaborative and 47.9 for passive, p=0.04), higher POMS tension/anxiety scores (mean of 82.8 vs.81.4 for collaborative and 76.3 for passive, p=0.04) and higher POMS vitality scores (mean of 50.8 vs.48.6 for collaborative and 44.4 for passive, p=0.04) (Figures 2,3). Survivors' actual role was not related to SF-36 MCS scores, POMS total score, or other POMS subscale scores. Survivors preferred role was not related to any mean QOL scores.
Figure 2. Profile of Mood States Scores by Role Played.
Statistically significant differences occurred for Tension/Anxiety and Vigor/Activity. P-values are based on the Kruskal-Wallis nonparametric test.
Figure 3. SF-36 Scores by Role Played.
The Physical Component score was statistically significantly different between roles played. P-values are based on the Kruskal-Wallis nonparametric test.
The primary aim of this study was to determine whether patient concordance or discordance of preferred and actual roles had an impact on QOL. Survivors with concordant roles reported better QOL scores than those with discordant roles. Specifically, they had higher mean SF-36 PCS (45.0 vs. 40.5, p<0.001) score and better mean POMS mood score (77.4 vs. 73.1, p=0.01) (Table 3). SF-36 index analyses indicated better scores in concordant patients for physical functioning (50.2 vs. 47.5, p=0.02), bodily pain (51.0 vs. 47.9, p=0.01), general health (50.5 vs. 45.6, p<0.01) and vitality (49.1 vs. 45.4, p=0.01). POMS subscales indicate concordant patients indicated less anger (88.0 vs. 84.9, p=0.046), higher vitality (49.5 vs. 42.3, p=0.01), less fatigue (70.2 vs. 60.0, p<0.01), less confusion (84.1 vs. 79.5, p=0.01) and better overall mood (POMS total) In a subset analysis, younger concordant patients (age less than 60) and female concordant patients had similar results compared to their discordant counter parts. Older discordant patients only had significantly lower PCS (38.9 vs. 43.0, p=0.03) and vitality (41.6 vs. 51.3, p=0.02) scores as compared to older concordant patients and male discordant patients only had worse fatigue as compared to male concordant patients (62.1 vs. 72.4, p=0.02). There were no differences in any QOL scores within the discordant group when comparing those who played a role that was more active than preferred (n=52) vs. those who played a role that was less active than preferred (n=19).
Table 3. Concordance/Discordance in Preferred and Actual Control Preferences Scale Roles with Quality of Life Scores (Mean (SD)).
| Concordance (N=523) | Discordance (N=71) | Total (N=594) | p value | |
|---|---|---|---|---|
| SF-36 | ||||
| Physical Component Score | 45.0 (11.0) | 40.5 (10.7) | 44.5 (11.1) | 0.001 |
| Physical Functioning Index | 50.2 (9.6) | 47.5 (10.1) | 49.8 (9.7) | 0.02 |
| Physical Role Functioning | 47.9 (10.8) | 45.2 (11.2) | 47.5 (10.9) | 0.05 |
| Bodily Pain | 51.0 (9.3) | 47.9 (9.1) | 50.6 (9.3) | 0.01 |
| General Health | 50.5 (10.4) | 45.6 (10.8) | 49.9 (10.6) | <0.01 |
| Mental Component Score | 51.4 (10.0) | 51.1 (9.9) | 51.4 (10.0) | 0.65 |
| Vitality | 49.1 (10.0) | 45.4 (10.8) | 48.6 (10.1) | 0.01 |
| Social Functioning | 50.6 (9.2) | 48.1 (10.3) | 50.3 (9.4) | 0.07 |
| Emotional Role Functioning | 49.0 (10.7) | 49.2 (10.0) | 49.0 (10.6) | 0.80 |
| Mental Health | 51.1 (8.9) | 50.5 (9.4) | 51.0 (9.0) | 0.74 |
| POMS | ||||
| Total POMS | 77.4 (14.9) | 73.1 (15.0) | 76.9 (15.0) | 0.01 |
| Anger/Hostility Subscale | 88.0 (14.8) | 84.9 (15.9) | 87.6 (15.0) | 0.046 |
| Confusion/Bewilderment Subscale | 84.1 (14.7) | 79.5 (16.6) | 83.6 (15.0) | 0.01 |
| Depression/Dejection Subscale | 86.5 (17.1) | 85.3 (17.0) | 86.4 (17.1) | 0.28 |
| Fatigue/Inertia Subscale | 70.2 (23.1) | 60.0 (26.6) | 69.0 (23.8) | 0.002 |
| Tension/Anxiety Subscale | 81.3 (17.8) | 79.3 (19.1) | 81.0 (18.0) | 0.47 |
| Vigor/Activity Subscale | 49.5 (22.2) | 42.3 (23.8) | 48.7 (22.5) | 0.01 |
SD= Standard Deviation
Generalized Linear Modeling techniques were employed to determine if baseline characteristics (age, gender, race, cancer treatment) and CPS defined concordance were predictive of PCS, MCS and mood scores. Results indicate that age is statistically significant in each model and concordance is significant in the PCS and POMS models. The amount of variance accounted for by age and concordance was small (<5%) with R2 values less than 0.08, indicating that QOL scores were highly individualistic relative to the demographics that were recorded. Hence, little variation in the set of predictors explained the variation in the outcomes.
Discussion
This study of cancer survivors who had completed assessments within 1 year post-diagnosis confirmed previously posited relationships between the decision-making role and QOL domains. Discordance between actual and preferred roles resulted in poorer physical health and poorer mood (anger, confusion, fatigue and vigor) but did not result in worse mental well-being. Similar results were found within the younger patient and female subsets. Survivors whose actual role was active, had less tension and more vigor. Poorer QOL for discordant survivors was not dependent upon the direction of the discordance. Previous research has shown that that treatment satisfaction and QOL are related.4,30,31 Our findings go beyond this to support the hypothesis that role satisfaction, not just treatment satisfaction, impacts patient QOL.
Dow et al and both Hodgkinson et al. studies (2007)7,13,14 have indicated that making decisions regarding health care was an important need of patients. Patients wished to have some control,7,13 and most frequent unmet goals were in the domain of existential survivorship, as indicated on the Cancer Survivors Unmet Needs measure, which included items such as ‘cope with changes to my beliefs’; and ‘make decisions about my life in context of uncertainty.’13 Breast cancer survivors report that being informed regarding medical decisions is one of the most important pieces of advice to give newly diagnosed patients, thus confirming patient preference to play an active role in decision making.11 A recent systematic review found an increasing trend in patient preferences for shared decision making; in 50% of studies published prior to 2000 the majority of patients preferred to share decisions, this increased to 71% of studies published in 2000 or later.32 These studies did not, however, have any comparisons between the decision making and QOL.
Yet satisfaction with treatment and care patterns has been associated with QOL. In particular, Griggs (2007) reported a relationship between satisfaction of information and vitality, mental health and distress.23 Satisfaction likely has several components to it including patient-physician interaction, access to care, quality of care and outcomes. One important component of satisfaction in patient-physician interaction during cancer treatment is the degree to which a patient plays his or her preferred role in cancer treatment decision making. Our study found better physical and emotional QOL in patients who played an active role and in those with concordance between preferred and actual role played. Similar to Griggs et al, our study found concordance of roles was associated with higher vitality (POMS V/A) and better overall mood (POMS total score). Unlike Griggs (2007) results, mental health was not associated with an actual or preferred decisional role in our study.
Regardless of the role preferred, survivors in our study who experienced their preferred level of input into the decision making process reported better QOL and associated outcomes. Survivors with concordant actual and preferred roles may be more adherent to treatment protocols, therefore, resulting in higher QOL scores in some areas. Or, it could be that concordant roles led to improved knowledge, self-efficacy, and/or self-management in these patients, thus improving QOL.
A recent meta-analysis found that information needs were greatest among those who preferred an active role in treatment decisions.33 Information satisfaction has been related to global QOL, physical well-being, social well-being, emotional well-being, and functional well-being in descriptive studies.34 Arora et al. (2002) found that access to information was associated with better well-being and higher perceptions of health competence.35 Patients playing their preferred role may have more appropriate access to information, which may lead to better QOL. Other speculations include those playing a preferred role might find the experience less distressing. People who prefer active or collaborative roles and do not attain them might become frustrated. Those who prefer a more passive role may become overwhelmed when physicians ask them to engage in decision making.
This study recorded control preferences at 1 year post treatment. A reasonable question is to ask if the findings would have been different had the CPS had been recorded at various time points. We have seen CPS scores change very little in the short term (within 6 months). However, there are very limited data on the longitudinal nature of control preferences. One study (Hack (2005)) reports a 48% concordance in baseline role assumed vs role preferred 3 years later. The CPS would seem to be more trait-like than state-like.
When considering the clinical implications of our results, we considered minimally important differences. Although there are many methods for establishing and many factors influencing minimally important differences, previous work has often found differences of a half standard deviation in HRQOL measures to be clinically important36; variability in thresholds is underscored by a study suggesting that differences as low as 0.1 standard deviation could be important in certain situations37. In our study, statistically significant differences between concordant and discordant patients ranged in size from roughly one half standard deviation for SF-36 PCS to a little less than a third of a standard deviation for the POMS Total Mood score. This amounts to a 5 point difference in the SF-36 PCS (which reports a minimum clinically important difference in the range typically of 3-5 points) relative to a population norm of 50 and a 4 point difference in the POMS relative to a population norm of 75. Results such as these suggest that clinicians should encourage patients who are discordant to pursue a preferred role in order to improve QOL.
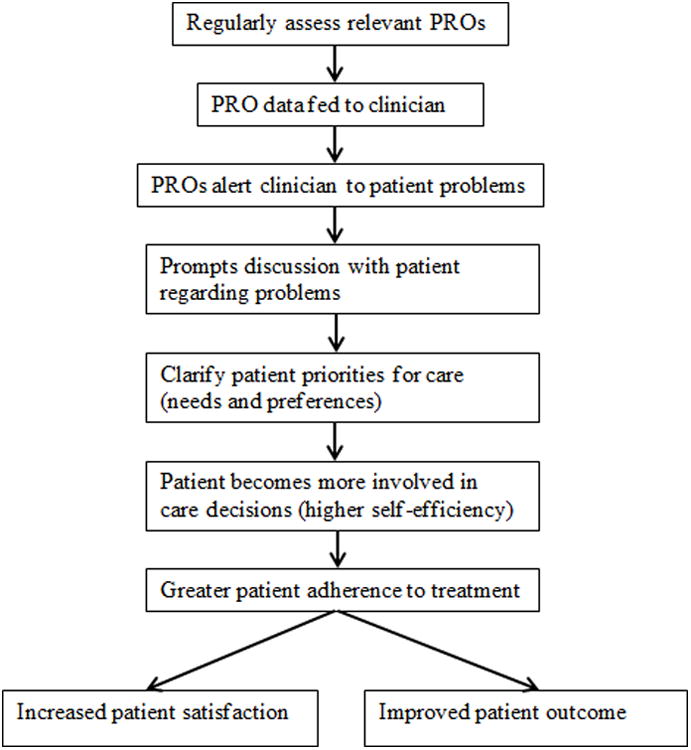
Current endeavors in augmenting patient-centered care, which involves systematic evidence based structures aimed at integrating the patient's perspective more formally into the decision making process, involves the use of patient-reported outcomes (PROs) in a paradigm of treatment and referral (Figure 4). Our results support the use of this paradigm. The model in Figure 4 indicates an integrated flow of information and decision making processes between the patient and the health care team which will promote improved care, QOL, and patient outcomes. PROs are assessed regularly at each patient clinical appointment and results are communicated to the clinical team. Within our context, patient-centric care considers that patients playing a preferred role may receive a wide array of benefits. The entire health care system is moving towards patient-centric care which means our results are particularly timely. This is relevant to the Patient-Centered Outcomes Research Institute (PCORI) and its recent mandate to generate standardized methods and applications to improve patient-centered care.38 Our data suggest that the benefits hypothesized by PCORI's focus on patient-centered care are empirically demonstrable.
Figure 4. Use of Patient Reported Outcomes to Enhance Patient-Centered Care.
There were methodological limitations of the ACS survey documented by Smith (2007) that mandate caution in broad interpretation of our findings. In particular, the relatively low response rate puts into question the generalizability of our results to the general adult cancer population. Further the results were recorded at 1 year post diagnosis which prevents a longitudinal profile from being constructed and examined for differences in our findings over time. Finally our data are derived strictly from a data set comprised of patients from the upper mid-west.
Conclusions
Patients having discordance between their preferred and actual roles in cancer care reported substantial QOL deficits in both physical and emotional domains. Improved patient satisfaction with care and improved QOL may be achieved by meeting patient expectations with respect to the amount of input they have in making treatment decisions.
Acknowledgments
The American Cancer Society (ACS) Studies of Cancer Survivors were funded as an intramural program of research conducted by the ACS Behavioral Research Center. We wish to acknowledge the cooperation and efforts of the Minnesota Cancer Surveillance System (Minnesota's cancer registry), and the cancer survivors, their physicians, and their loved ones who contributed to the collection of these data. The authors assume full responsibility for analyses and interpretation of these data.
This work was supported through grants from the NIH CA 25224 and CA 37404. JAS is supported by research grants from the National Institutes of Arthritis, Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Aging (NIA), National Cancer Institute (NCI) and the Agency for Health Quality and Research Center for Education and Research on Therapeutics (CERTs). JAS is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
Footnotes
Informed Consent: Informed consent was obtained from all subjects participating in this investigation.
Financial disclosures: JAS has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Ardea, Regeneron, Allergan, URL pharmaceuticals and Novartis. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology's Guidelines Subcommittee of the Quality of Care Committee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee.
References
- 1.Epstein RM, Street RL., Jr . Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. National Cancer Institute, NIH Publication No. 07-6225; Bethesda, MD: 2007. [Google Scholar]
- 2.Thorne SE, Robinson CA. Health care relationships: The chronic illness perspective. Res Nurs Health. 1988;11(5):293–300. doi: 10.1002/nur.4770110504. [DOI] [PubMed] [Google Scholar]
- 3.Street RL, Jr, Gordon HS, Ward MM, Krupat E, Kravitz RL. Patient participation in medical consultations: why some patients are more involved than others. Med Care. 2005;43:960–969. doi: 10.1097/01.mlr.0000178172.40344.70. [DOI] [PubMed] [Google Scholar]
- 4.Street RL, Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Education and Counseling. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Coulter A. Patient information and shared decision-making in cancer care. Br J Cancer. 2003;89:S15–S16. doi: 10.1038/sj.bjc.6601080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luker KA, Beaver K, Leinster SJ, Owens RG, Degner LF, Sloan JA. The information needs of women newly diagnosed with breast cancer. J Adv Nurs. 2003;22:134–141. doi: 10.1046/j.1365-2648.1995.22010134.x. [DOI] [PubMed] [Google Scholar]
- 7.Dow KH, Ferrell BR, Haberman MR, Eaton L. The meaning of quality of life in cancer survivorship. Oncol Nurs Forum. 1999;26(3):519–528. [PubMed] [Google Scholar]
- 8.Robb C, Haley WE, Balducci L, et al. Impact of breast cancer survivorship on quality of life in older women. Crit Rev Oncol Hematol. 2007;62(1):84–91. doi: 10.1016/j.critrevonc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Woolf SH, Chan EC, Harris R, et al. Promoting informed choice: transforming health care to dispense knowledge for decision making. Ann Intern Med. 2005;143:293–300. doi: 10.7326/0003-4819-143-4-200508160-00010. [DOI] [PubMed] [Google Scholar]
- 10.Anderson R, Rice R, et al. Changing the US Health Care System. J Wiley & Sons; New York: 2007. [Google Scholar]
- 11.Ferrell BR, Grant M, Funk B, Otis-Green S, Garcia N. Quality of life in breast cancer. Cancer Pract. 1996;4:331–340. [PubMed] [Google Scholar]
- 12.Dubenske LL, Gustafson DH, Shaw BR, Cleary JF. Web-Based Cancer Communication and Decision Making Systems: Connecting Patients, Caregivers, and Clinicians for Improved Health Outcomes. JF Med Decis Making. 2010;30(6):732–744. doi: 10.1177/0272989X10386382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Breast cancer survivors' supportive care needs 2-10 years after diagnosis. Support Care Cancer. 2007;15(5):515–523. doi: 10.1007/s00520-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkinson K, Butow P, Fuchs A, et al. Long-term survival from gynecologic cancer: psychosocial outcomes, supportive care needs and positive outcomes. Gynecol Oncol. 2007;104(2):381–389. doi: 10.1016/j.ygyno.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Degner LF, Kristjanson LJ, Bowman D, et al. Informational needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–1492. [PubMed] [Google Scholar]
- 16.Beaver K, Luker KA, Owens RG, Leinster SJ, Degner LF, Sloan JA. Treatment decision making in women newly diagnosed with breast cancer. Cancer Nursing. 1996;19(1):8–19. doi: 10.1097/00002820-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hack TF, Degner LF, Watson P, Sinha L. Do patient benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15:9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- 18.Beaver K, Booth K. Information needs and decision-making preferences: Comparing findings for gynaecological, breast and colorectal cancer. Eur J Oncol Nurs. 2007;11(5):409–416. doi: 10.1016/j.ejon.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Singh JA, Sloan JA, Atherton PJ, et al. Preferred Roles in Treatment Decision Making Among Patients With Cancer: A Pooled Analysis of Studies Using the Control Preferences Scale. Am J Manag Care. 2010;16(9):688–696. [PMC free article] [PubMed] [Google Scholar]
- 20.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 21.Blank TO, Bellizzi KM. After prostate cancer: predictors of well-being among long-term prostate cancer survivors. Cancer. 2006;106(10):2128–2135. doi: 10.1002/cncr.21865. [DOI] [PubMed] [Google Scholar]
- 22.Bowman KF, Rose JH, Deimling GT. Appraisal of the cancer experience by family members and survivors in long-term survivorship. Psychooncology. 2006;15(9):834–845. doi: 10.1002/pon.1039. [DOI] [PubMed] [Google Scholar]
- 23.Griggs JJ, Sorbero ME, Mallinger JB, et al. Vitality, mental health, and satisfaction with information after breast cancer. Patient Educ Couns. 2007;66(1):58–66. doi: 10.1016/j.pec.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Smith T, Stein KD, Mehta CC, et al. The rationale, design, and implementation of the American Cancer Society's studies of cancer survivors. Cancer. 2007;109(1):1–12. doi: 10.1002/cncr.22387. [DOI] [PubMed] [Google Scholar]
- 25.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 26.Ware JJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 27.Ware JJ, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project J Clin Epidemiol. 1998;51(11):903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 28.McNair DM, Lorr M, Droppelman LF. Edits Manual: Profile of Mood States. San Diego: Educational and Testing Service; 1971. [Google Scholar]
- 29.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14(1):73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 30.Molenaar S, Sprangers MA, Rutgers EJ, et al. Decision Support for Patients With Early-Stage Breast Cancer: Effects of an Interactive Breast Cancer CDROM on Treatment Decision, Satisfaction, and Quality of Life. J Clin Oncol. 2001 Mar;19(6):1676–1687. doi: 10.1200/JCO.2001.19.6.1676. [DOI] [PubMed] [Google Scholar]
- 31.Husson O, Mols F, van de Poll-Franse VL. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol. 2011;22(4):761–772. doi: 10.1093/annonc/mdq413. Epub 2010 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: A systematic review. Patient Education and Counseling. 2012;86(1):9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ankem K. Factors influencing information needs among cancer patients: A meta-analysis. Library & Information Science Res. 2006;28:7–23. [Google Scholar]
- 34.Davies NJ, Kinman G, Thomas RJ, Bailey T. Information satisfaction in breast and prostate cancer patients: implications for quality of life. Psychooncology. 2008;17:1048–1052. doi: 10.1002/pon.1305. [DOI] [PubMed] [Google Scholar]
- 35.Arora NK, Johnson P, Gustafson DH, McTavish F, Hawkins RP, Pingree S. Barriers to information access, perceived health competence, and psychosocial health outcomes: test of a mediation model in a breast cancer sample. Patient Educ Couns. 2002;47:37–46. doi: 10.1016/s0738-3991(01)00170-7. [DOI] [PubMed] [Google Scholar]
- 36.Sloan JA, Symonds T, Vargas-Chanes D, Fridley B. Practical Guidelines for Assessing the Clinical Significance of Health-Related Quality of Life Changes within Clinical Trials. Drug Information Journal. 2003;37:23–31. [Google Scholar]
- 37.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. New Engl J Med. 1996;28:835–40. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 38.Gabriel SE, Normand ST. Getting the Methods Right — The Foundation of Patient-Centered Outcomes Research. N Engl J Med. 2012;367:787–790. doi: 10.1056/NEJMp1207437. [DOI] [PubMed] [Google Scholar]