Abstract
Adverse conditions in utero can have transgenerational effects, in the absence of a subsequent insult. We aimed to investigate the contribution of the maternal pregnancy environment vs. germ line effects in mediating alterations to cardiorenal and metabolic physiology in offspring from mothers born small. Uteroplacental insufficiency was induced by bilateral uterine artery and vein ligation (Restricted group) or sham surgery (Control group) in Wistar-Kyoto rats. Restricted and control female offspring (F1) were mated with either breeder males (embryo donor) or vasectomized males (embryo recipient). Embryo transfer was performed at embryonic day (E) 1, whereby second-generation (F2) embryos gestated (donor-in-recipient) in either a control (Cont-in-Cont, Rest-in-Cont) or restricted (Cont-in-Rest, Rest-in-Rest) mother. In male and female offspring, glomerular number and size were measured at postnatal day (PN) 35, and systolic blood pressure, glucose control, insulin sensitivity, and pancreatic β-cell mass were measured in separate sibling cohorts at 6 mo. Rest-in-Rest offspring were hypothesized to have similar characteristics (reduced growth, altered metabolic control, and hypertension) to non-embryo-transferred Rest, such that embryo transfer would not be a confounding experimental influence. However, embryo-transferred Rest-in-Rest offspring underwent accelerated growth during the peripubertal phase, followed by slowed growth between 2 and 3 mo of age compared with non-embryo-transferred Rest groups. Furthermore, renal function and insulin response to a glucose load were different to respective non-embryo-transferred groups. Our data demonstrate the long-term effects of in vitro embryo manipulation, which confounded the utility of this approach in delineating between the maternal pregnancy environment and germ line effects that drive transgenerational outcomes.
Keywords: blood pressure, glucose tolerance, kidney function, low birth weight
adverse exposures during fetal life, including poor maternal nutrition and/or uteroplacental insufficiency are commonly associated with low birth weight and increased susceptibility to the development of cardiovascular disease, chronic kidney disease, and Type 2 diabetes in adulthood (1, 19, 31, 32). Many human and animal studies have provided further evidence for programmed effects that are transmitted to subsequent generations in the absence of continued pregnancy interventions (28). Pathways for transmission are yet to be elucidated but may involve adverse pregnancy adaptations by the prenatally exposed first-generation (F1) female (maternal constraint), a direct insult to developing germ cells that give rise to the second generation (F2) and/or alterations to F1 body tissues (paternal and/or maternal) that induce changes to germ cells prior to conception of the F2 generation; the latter two constituting germ line effects that occur irrespective of the maternal pregnancy environment (15).
Uteroplacental insufficiency is the major cause of low birth weight in Westernized nations and is characterized by reduced nutrient and oxygen delivery to the developing fetus (43). Others have demonstrated that, in a rat model of uteroplacental insufficiency, gestational diabetes and obesity in F1 females were associated with macrosomic F2 offspring with impaired glucose tolerance and insulin resistance in adult life (3). Recently, we have shown that nonobese, growth-restricted F1 females exhibited some loss of glucose tolerance during pregnancy and her F2 male offspring (although born of normal body weight) developed hypertension, blunted first-phase insulin response and deficits in nephron number and pancreatic β-cell mass (14, 16, 37). Similarly, a maternal low-protein diet has been shown to program hypertension in two generations of male and female rat offspring (5, 34, 35), which was associated with resistance artery dysfunction in the pregnant, prenatally protein-deprived F1 female (35). Although these studies demonstrated adverse pregnancy adaptations that may be associated with the F2 phenotype, germ line effects that are independent of the maternal pregnancy environment could not be eliminated.
Calorie restriction during prenatal and early postnatal development in female rats was associated with insulin resistance and fasting hyperglycemia in offspring that gestated in surrogate F1 mothers that had never been nutrient-deprived (33). This suggests that modifications made to the germ line, during F1 prenatal and/or postnatal exposure to reduced nutrition, contributed to the transgenerational metabolic phenotype that a “normal” pregnancy environment could not prevent. However, the potential for adverse effects to be a mere consequence of “mismatched” environments, rather than the F1 donors' nutritional history per se is unknown. Furthermore, whether there are additive effects from gestating in a protein-deprived female and whether a similar metabolic phenotype manifested in non-embryo-transferred, naturally gestated progeny were not investigated. Indeed, in vitro embryo manipulation has the potential to halt processes that would normally take place during the preimplantation period and go on to affect long-term offspring health (6). Thus, it is of critical importance that observations made in embryo-transferred offspring, which are used to delineate between the maternal pregnancy environment and germ line effects in contributing to disease, are directly compared with naturally gestated progeny.
The aim of this study was to use embryo transfer techniques in a well-established rat model of uteroplacental insufficiency to determine the contribution of the maternal pregnancy environment vs. the germ line in mediating transgenerational effects. We hypothesized that Cont-in-Cont and Rest-in-Rest offspring would have similar characteristics in terms of growth and cardiorenal and metabolic health to their respective non-embryo-transferred counterpart (Control and Restricted). This would ensure that embryo transfer was not a confounding influence and allow separation between maternal and germ line (gamete) drivers of transgenerational outcomes. If the maternal environment dominates, we would expect Cont-in-Rest offspring to be similar to non-transferred Restricted, while a dominant embryo effect would be evident through changes in Rest-in-Cont offspring.
MATERIALS AND METHODS
Animals
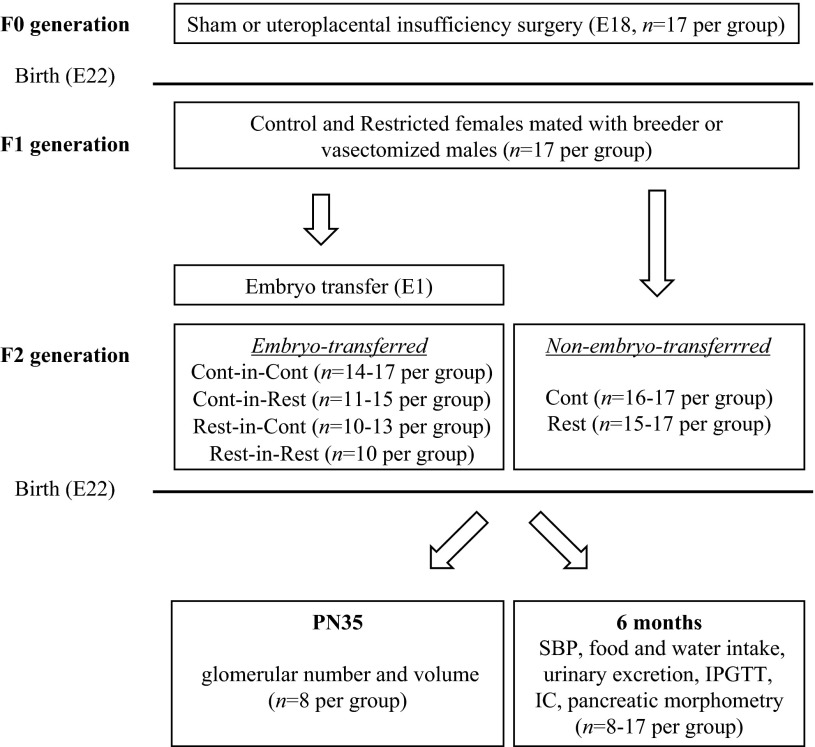
All experiments were approved by The University of Melbourne Animal Ethics Committee prior to commencement and were conducted in accordance with accepted standards of humane animal care. Wistar-Kyoto rats (16–18 wk of age) were housed in an environmentally controlled room (constant temperature 22°C), with a 12:12-h light-dark cycle and had access to food and tap water ad libitum. Figure 1 illustrates the generation of animal groups. Briefly, F0 female rats were mated with healthy breeder males and randomly allocated to uteroplacental insufficiency (offspring termed F1 Restricted) or sham (offspring termed F1 Control) surgery at E18 (n = 17 per group). Under isoflurane anesthesia, the uteroplacental insufficiency group underwent bilateral uterine vessel (artery and vein) ligation, and the sham group was exposed to identical conditions, but the uterine vessels were not ligated (24, 25, 42). The F0 females delivered naturally at term (E22), and F1 pups remained with their mothers until natural weaning by postnatal day 35 (PN35). F1 female body weights were measured at PN1, PN7, PN14, and PN35 and at 2, 3 and 4 mo, and systolic blood pressure was measured prior to mating. At 17–23 wk of age, F1 control and restricted females (one female randomly selected per litter) were mated with healthy breeder males and embryo transfer performed at E1 (offspring termed embryo-transferred) (7–9). The F1 females delivered naturally at E22, and F2 pups remained with their mothers until natural weaning at PN35. An additional cohort of F2 offspring were identically managed, but embryo transfer was not performed during the F1 pregnancy (n = 17 per group). This previously published F2 non-embryo-transferred cohort has been included in this article for direct comparisons with F2 embryo-transferred groups (14, 37). F2 offspring were studied at PN35 and at 6 mo of age (one male and one female for each age studied were randomly selected from each litter; n = 8–17 per group).
Fig. 1.
Generation of animals for embryo- and non-embryo-transferred groups and experimental design. F0 generation females were mated and surgery performed at embyronic day 18 (E18; sham or uteroplacental insufficiency; n = 17 per group), followed by natural delivery at E22. F1 generation control and restricted female offspring (1 randomly selected female per litter) were mated with healthy breeder (embryo donor) or vasectomized (embryo recipient) males and embryo-transfer performed at E1 (embryo-transferred cohort), followed by natural delivery at E22 (n = 10–17 per group). An additional cohort of F2 generation offspring was identically managed by mating F1 control and restricted female offspring with breeder males, but embryo transfer was not performed during the F1 pregnancy (n = 17 per group; this previously published non-embryo-transferred cohort has been included in this manuscript for direct comparisons to embryo-transferred groups) (14, 37). F2 offspring were studied at PN35 (glomerular number and volume) or at 6 mo (SBP, food and water intake, urinary excretion, IPGTT, IC, and pancreatic morphometry) of age (1 male and 1 female randomly selected for each study age from each litter; n = 8–17 per group). E, embryonic day; F0, initial generation; F1, first generation; F2, second generation; IC, insulin challenge; IPGTT, intraperitoneal glucose tolerance test; PN, postnatal day; SBP, systolic blood pressure.
Embryo Transfer Procedure
F1 Control and Restricted females were randomly allocated (without using siblings) to one of two groups, donor or recipient. Donor females were mated with a healthy breeder male overnight. Recipient females were simultaneously mated with a vasectomized male to initiate pseudopregnancy, which made them receptive to transplanted embryos. At E1, after confirmation of sperm in a vaginal smear, donor females (Control and Restricted) were euthanized with an intraperitoneal injection of ketamine (100 mg/kg body wt) and Ilium Xylazil-20 (30 mg/kg body wt), and embryos of similar morphological quality (∼10–12 embryos recovered) were harvested from both oviducts. The oviducts were cut open, and embryos surrounded by cumulus cells were flushed out and placed in a small drop (50 μl) of culture media (GMOPS, Vitrolife AB, Goteborg, Sweden) on a warming plate at 37°C for 20 min, while recipient females were prepared. Recipient females (Control and Restricted) were anesthetized with isoflurane, and all embryos were transferred into one oviduct by inserting a transfer pipette into the ampular opening. The recipient female recovered and delivered naturally at term (E22). F2 pups remained with their mothers until weaning at PN35. Four embryo-transferred groups were generated (donor-in-recipient): Cont-in-Cont, Cont-in-Rest, Rest-in-Cont, and Rest-in-Rest.
Blood Pressure, Insulin Challenge, and Intraperitoneal Glucose Tolerance Test
Systolic blood pressure was measured in the morning by tail cuff in F2 offspring that were acclimatized to the restraint procedure at 2, 3, 4, and 6 mo (14, 16, 23, 24, 42). One week after the last blood pressure measurement at 6 mo, F2 male and female offspring were fasted overnight before an insulin challenge (IC). A tail vein blood sample (300 μl) was taken prior to and following a subcutaneous bolus injection of insulin (Actrapid, Novo Nordisk Pharmaceuticals, North Rocks, NSW, Australia; 1 U/kg body wt), and tail vein blood samples were collected at 20, 40, and 60 min (30, 36, 37). Two weeks after the IC, F2 male and female offspring were administered a single dose of analgesic (carprofen: 0.1 mg/kg body wt) and under isoflurane anesthesia, the right carotid artery was isolated and catheterized. Catheter patency was maintained by daily flushing with heparinized saline. An intraperitoneal glucose tolerance test (IPGTT) was performed 4 days after the catheterization surgery following an overnight fast. Blood samples via the arterial catheter were taken prior to (10 and 5 min before) and following an intraperitoneal injection of glucose (1 g/kg body wt) at 5, 10, 20, 30, 45, 60, 90, and 120 min (30, 37, 38). At completion of the IC or IPGTT, animals were allowed access to food and water ad libitum.
Food and Water Intake and Urinary Excretion
At 6 mo of age, F2 animals were weighed and placed individually in metabolic cages for 24-h measurements of food and water intake and urine production (13, 14, 16, 24). Rats were acclimatized to the metabolic cages by placing them in them for short daylight periods on two separate occasions and once overnight. Concentrations of urine sodium, chloride, creatinine, and total protein were performed using commercially available kits, according to the manufacturer's instructions (Cobas Integra 400; Roche Diagnostics, Burgess Hill, West Sussex, UK). Plasma samples were collected upon removal from metabolic cages at postmortem and analyzed for creatinine and sodium to determine creatinine clearance [urinary creatinine (μmol/l) × 24 h urine production (ml)]/[plasma creatinine (μmol/l) × 1,440 (min)] and fractional sodium excretion, respectively.
Postmortem Blood and Tissue Collection
At 6 mo of age, nonfasted F2 male and female rats were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg body wt) and Ilium Xylazil-20 (30 mg/kg body wt), and a postmortem was performed. Blood was collected by cardiac puncture for determination of plasma triglycerides, adiponectin, and leptin concentrations. Heart, kidneys, pancreas, and dorsal fat were rapidly excised and weighed. A piece of pancreatic tissue (∼1 cm3) from the hepatic end of F2 6-mo-old rats was fixed in 10% neutral buffered formalin for histological analysis (4, 21, 29, 37). The right kidney was collected following perfusion fixation (4% paraformaldehyde) in a separate cohort of F2 rats culled at PN35 and used for stereological analyses (2, 14, 16, 24, 42).
Plasma Metabolite Analyses
Plasma glucose concentrations were measured in duplicate using a scaled-down version of the enzymatic fluorometric analysis (16, 36, 37). Plasma insulin, adiponectin, and leptin concentrations were measured in duplicate using a commercially available rat insulin radioimmunoassay or ELISA kit (Merck Millipore, Billerica, MA) (16, 21, 30, 38). Plasma triglyceride concentrations were measured in duplicate using an automated centrifugal analyzer (Cobas Integra 400; Roche Diagnostics, Burgess Hill, West Sussex, UK). Homeostatic model of assessment for insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin values obtained prior to the glucose bolus: HOMA-IR = fasting plasma insulin (μU/ml) × fasting plasma glucose (mmol/l)/22.5 (21, 30, 36, 37).
Pancreatic Morphometry, Glomerular Number, and Volume
Three pancreas sections of equal distance apart from the hepatic end were selected and immunostained for insulin-positive β-cells (1:200 dilution; Dako, Kingsgrove, NSW, Australia) (n = 6–9 per group). Pancreatic β-cell mass was calculated, as described previously (16, 21, 36, 37). Glomerular number and individual glomerular volume were determined in the right kidney of F2 male and female rats at PN35 using the Cavalieri and physical dissector method (n = 6–9 per group) (2, 14, 16, 24, 42).
Statistical Analyses
Values are expressed as means ± SE, with n representing the number of animals in each group. For F1 maternal characteristics, a Student's unpaired t-test was performed between F2 Control and Restricted rats allocated to non-embryo-transferred groups and a two-way ANOVA performed for those allocated to embryo transfer; F1 group (Control vs. Restricted), and mating allocation (donor vs. recipient). A two-way ANOVA was performed for some cardiorenal and metabolic parameters to determine main effects of embryo transfer (non-embryo-transferred vs. embryo-transferred) and group (Control vs. Restricted). Student's unpaired t-test was performed following the observation of a significant interaction. Comparisons between F2 non-embryo-transferred Control and Restricted groups were made using a Student's unpaired t-test, as previously published (14, 37). A one-way ANOVA and Student-Newman-Keuls (SNK) post hoc analyses were used to determine differences across all F2 groups (Control, Restricted, Cont-in-Cont, Cont-in-Rest, Rest-in-Cont, and Rest-in-Rest). P < 0.05 was considered statistically significant.
RESULTS
F1 Maternal Characteristics
Uteroplacental insufficiency surgery reduced the total number of viable pups (Restricted: 6.1 ± 0.6 vs. Control: 8.3 ± 0.6) and their body weight (P < 0.05, Table 1) at PN1. F1 Restricted female rats remained lighter than those in the Control group at all ages (P < 0.05), but percentage weight gain during pregnancy was not different (Table 1). Systolic blood pressure was not different between F1 rats in the Control and Restricted groups, but it was 3–7 mmHg lower in recipient vs. donor females prior to mating (P < 0.05, Table 1). Maternal age at mating was, on average, 18.9 to 21.7 wk and did not differ between groups.
Table 1.
F1 body weights and premating systolic blood pressure
| Control |
Restricted |
Two-Way ANOVA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 Group | Non-Embryo Transferred Cont |
Donors |
Recipients |
Non-Embryo-Transferred |
Donors |
Recipients |
F1 Group | Mating Allocation | ||||
| F2 Group Generated | (n = 17) | Cont-in- Cont (n = 20) | Cont-in- Rest (n = 13) | Cont-in- Cont (n = 17) | Rest-in- Cont (n = 13) | Rest (n = 16) | Rest-in-Cont (n = 13) | Rest-in-Rest (n = 11) | Cont-in-Rest (n = 13) | Rest-in-Rest (n = 11) | ||
| Body weight, g | ||||||||||||
| PN1 | 4.3 ± 0.0 | 4.2 ± 0.1 | 4.3 ± 0.0 | 4.2 ± 0.1 | 4.3 ± 0.1 | 3.3 ± 0.1* | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.1 | P < 0.05 | NS |
| PN7 | 10.2 ± 0.3 | 10.2 ± 0.2 | 10.8 ± 0.4 | 10.0 ± 0.4 | 10.0 ± 0.4 | 7.0 ± 0.3* | 8.0 ± 0.4 | 8.7 ± 0.5 | 8.4 ± 0.5 | 7.9 ± 0.5 | P < 0.05 | NS |
| PN14 | 23.0 ± 0.4 | 22.6 ± 0.4 | 23.7 ± 1.1 | 22.3 ± 0.6 | 20.8 ± 1.0 | 16.9 ± 0.7* | 18.9 ± 0.9 | 20.9 ± 0.7 | 20.0 ± 0.9 | 19.4 ± 1.2 | P < 0.05 | NS |
| PN35 | 75 ± 1 | 72 ± 1 | 74 ± 2 | 72 ± 1 | 72 ± 2 | 61 ± 2* | 65 ± 2 | 69 ± 3 | 66 ± 2 | 67 ± 3 | P < 0.05 | NS |
| 2 mo | 158 ± 3 | 156 ± 3 | 160 ± 2 | 155 ± 3 | 158 ± 3 | 147 ± 4* | 143 ± 4 | 148 ± 4 | 144 ± 3 | 145 ± 6 | P < 0.05 | NS |
| 3 mo | 209 ± 3 | 204 ± 3 | 209 ± 4 | 202 ± 3 | 202 ± 3 | 187 ± 3* | 187 ± 4 | 195 ± 4 | 192 ± 4 | 190 ± 4 | P < 0.05 | NS |
| Mating | 240 ± 3 | 239 ± 3 | 234 ± 4 | 233 ± 3 | 230 ± 3 | 211 ± 4* | 221 ± 5 | 222 ± 6 | 219 ± 5 | 221 ± 5 | P < 0.05 | NS |
| Delivery | 276 ± 3 | nm | nm | 270 ± 4 | 268 ± 4 | 241 ± 4* | nm | nm | 253 ± 7 | 257 ± 5 | P < 0.05 | nm |
| % weight gain | 15.1 ± 0.8 | nm | nm | 16.2 ± 1.3 | 17.0 ± 1.6 | 13.9 ± 1.1 | nm | nm | 16.5 ± 1.0 | 16.6 ± 0.7 | NS | nm |
| SBP, mmHg | 138 ± 3 | 136 ± 3 | 136 ± 3 | 131 ± 2 | 133 ± 2 | 136 ± 3 | 137 ± 2 | 133 ± 3 | 130 ± 4 | 130 ± 2 | NS | P < 0.05 |
All data are expressed as means ± SE.
Right column represents results from two-way ANOVA for embryo-transferred groups (no significant interactions were observed). F1 body weights and premating systolic blood pressure. Body weights were measured at PN1, PN7, PN14, PN35, and at 2 and 3 mo and at mating. Where relevant, body weights were measured on the day of delivery (E22), and the percentage weight gain was calculated.
Significant difference (P < 0.05) between non-embryo-transferred Control and Restricted groups (Student's unpaired t-test). Systolic blood pressure (SBP) was measured a week prior to mating. 1 female randomly selected per litter, n = 11–20 per group. Data show the F1 female (Control and Restricted) allocated to non-embryo transferred, donor or recipient. nm indicates no measures for donor females (not pregnant). E, embryonic day; F1, first generation; F2, second generation; PN, postnatal day; NS, not significant.
Embryo Transfer Success Rate
The number of embryos transferred at E1 was not different between groups (Cont-in-Cont: 11.0 ± 0.4, Cont-in-Rest: 9.9 ± 0.5, Rest-in-Cont: 10.1 ± 0.4, and Rest-in-Rest: 9.9 ± 0.6). Embryo transfer success rate, defined as the number of viable pups at birth as a percentage of the total number of embryos transferred, was similar across F2 groups (Cont-in-Cont: 56 ± 5, Cont-in-Rest: 59 ± 4, Rest-in-Cont: 61 ± 5, and Rest-in-Rest: 68 ± 3%). Compared with naturally gestated Control and Restricted groups, however, litter size of embryo-transferred groups was reduced (P < 0.05), which was attributed to a reduced number of female, but not male, pups (P < 0.05, Table 2).
Table 2.
F2 litter size and body weights
| Non-Embryo-Transferred |
Embryo-Transferred |
|||||
|---|---|---|---|---|---|---|
| Cont | Rest | Cont | Cont-in-Rest | Rest-in-Cont | Rest-in-Rest | |
| Litter size | ||||||
| Total | 9.31 ± 0.34b | 8.00 ± 0.57b | 5.94 ± 0.49a | 5.92 ± 0.42a | 6.27 ± 0.52a | 6.44 ± 0.48a |
| Males | 4.13 ± 0.36 | 3.36 ± 0.34 | 3.18 ± 0.46 | 3.08 ± 0.31 | 3.73 ± 0.69 | 2.67 ± 0.44 |
| Females | 5.19 ± 0.34 | 4.64 ± 0.46 | 2.76 ± 0.43 | 2.83 ± 0.37 | 2.64 ± 0.41 | 4.00 ± 0.41 |
| Body weights, g | ||||||
| Males | (n = 16) | (n = 15) | (n = 17) | (n = 15) | (n = 13) | (n = 10) |
| PN1 | 4.63 ± 0.05 | 4.64 ± 0.05 | 4.78 ± 0.07 | 4.61 ± 0.07 | 4.86 ± 0.12 | 4.76 ± 0.07 |
| PN7 | 11.0 ± 0.2 | 10.8 ± 0.3 | 11.5 ± 0.3 | 11.1 ± 0.3 | 11.4 ± 0.5 | 12 ± 0.3 |
| PN14 | 23.0 ± 0.5a | 23.2 ± 0.6a,b | 24.1 ± 0.6a,b | 23.3 ± 0.5a,b | 24.2 ± 0.9a,b | 25.2 ± 0.6b |
| PN35 | 81 ± 1a,b | 78 ± 2a | 82 ± 1a,b | 82 ± 1a,b | 86 ± 2b | 91 ± 1c |
| 2 mo | 217 ± 3b | 194 ± 6*a | 217 ± 4a,b | 220 ± 4a,b | 231 ± 6b | 239 ± 3c |
| 3 mo | 300 ± 2 | 296 ± 6 | 306 ± 5 | 304 ± 5 | 313 ± 8 | 320 ± 6 |
| 4 mo | 351 ± 2 | 351 ± 9 | 355 ± 6 | 351 ± 5 | 360 ± 7 | 367 ± 7 |
| 6 mo | 404 ± 4 | 406 ± 9 | 414 ± 6 | 410 ± 6 | 407 ± 6 | 421 ± 6 |
| Female | (n = 17) | (n = 17) | (n = 14) | (n = 11) | (n = 10) | (n = 10) |
| PN1 | 4.39 ± 0.06 | 4.41 ± 0.07 | 4.52 ± 0.07 | 4.40 ± 0.08 | 4.55 ± 0.11 | 4.40 ± 0.07 |
| PN7 | 10.3 ± 0.2a | 10.6 ± 0.3a,b | 11.0 ± 0.2a,b | 10.9 ± 0.3a,b | 11.5 ± 0.4b | 11.2 ± 0.5a,b |
| PN14 | 23.0 ± 0.4a | 22.7 ± 0.5a,b | 23.9 ± 0.5b | 23.9 ± 0.5b | 24.0 ± 0.9b | 23.3 ± 1.2b |
| PN35 | 74 ± 1a,b | 72 ± 1a | 77 ± 1a,b | 76 ± 2a,b | 78 ± 2b | 78 ± 2b |
| 2 mo | 155 ± 2a,b | 148 ± 2a | 153 ± 3a,b | 156 ± 4a,b | 162 ± 2b | 161 ± 2b |
| 3 mo | 203 ± 3 | 204 ± 2 | 207 ± 3 | 204 ± 3 | 209 ± 4 | 204 ± 4 |
| 4 mo | 225 ± 2 | 226 ± 1 | 226 ± 3 | 224 ± 4 | 224 ± 4 | 221 ± 5 |
| 6 mo | 255 ± 3 | 254 ± 3 | 253 ± 4 | 254 ± 4 | 255 ± 4 | 248 ± 5 |
All data are expressed as means ± SE. Litter size (total and male and female given separately) and body weights were measured at PN1, PN7, PN14, PN35 and at 2, 3, 4 and 6 mo of age.
Significant difference (P < 0.05) between non-embryo-transferred Control and Restricted (Student's unpaired t-test).
Significant differences across the six groups, as indicated by letters that differ [one-way ANOVA with Student-Newman-Keuls (SNK) post hoc analysis]. One male and one female were randomly selected per litter; n = 8–17 per group.
Embryo Transfer Effects on F2 Growth and Physiology
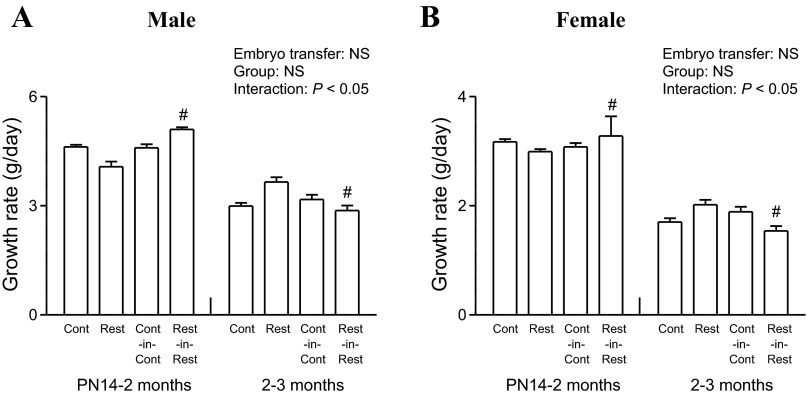
A two-way ANOVA was performed to determine main effects of embryo transfer (non-embryo-transferred and embryo-transferred) and group (Control and Restricted). F2 male litter size was not different between non-embryo-transferred and embryo-transferred groups (Table 2), but a significant reduction was observed in F2 females (P < 0.05, Table 2). Embryo transfer resulted in increased F2 male (P < 0.05), but not female, body weight at PN1 (Table 2). Growth rates were accelerated from PN14 to 2 mo and slowed from 2–3 mo in F2 Rest-in-Rest males and females compared with F2 Rest counterparts (P < 0.05, Fig. 2, A and B).
Fig. 2.
Effect of embryo transfer and maternal birth weight on F2 growth rates. Growth rates measured between PN14–2 mo and 2–3 mo (A and B) in F2 male and female offspring. All data are expressed as means ± SE; 1 male and 1 female randomly selected per litter, n = 7–17 per group. Two-way ANOVA was performed to determine main effects of embryo transfer (non-embryo-transferred and embryo-transferred) and group (Control and Restricted). Growth rates at PN14–2 mo and 2–3 mo were analyzed separately and plotted on the same graph within each sex. #P < 0.05 vs. non-embryo-transferred counterpart (Student's unpaired t-test following observations of significant interaction). PN, postnatal day; NS, not significant.
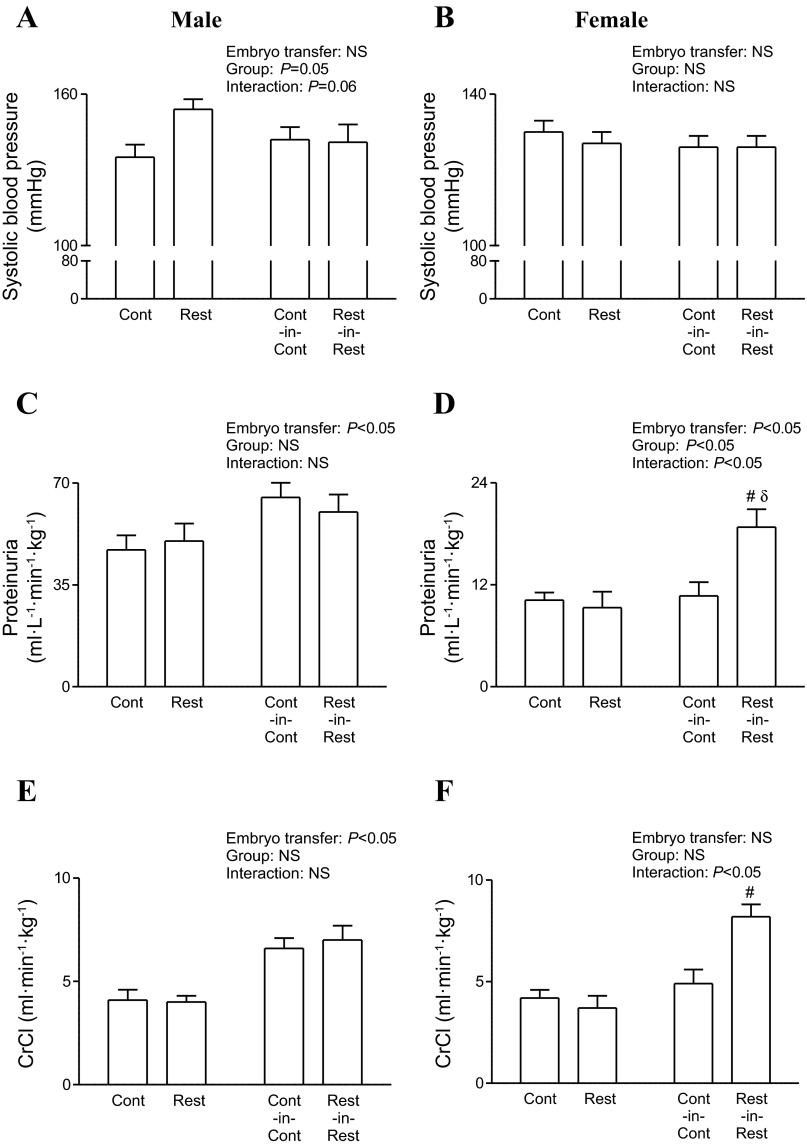
Systolic blood pressure was comparable between F2 non-embryo-transferred and embryo-transferred males and females (Fig. 3, A and B). Total protein excretion (Fig. 3C) and creatinine clearance (Fig. 3E) were elevated in embryo-transferred vs. non-embryo-transferred F2 males regardless of maternal birth weight (P < 0.05). In F2 females, increased total protein excretion and creatinine clearance were observed in F2 Rest-in-Rest offspring only compared with non-embryo-transferred Restricted (P < 0.05, Fig. 3, D and F).
Fig. 3.
Effect of embryo transfer and maternal birth weight on F2 cardiorenal physiology at 6 mo of age. Systolic blood pressure (A and B), total protein excretion (C and D), and creatinine clearance rates (E and F) in F2 male and female offspring. All data are expressed as means ± SE; 1 male and 1 female were randomly selected per litter; n = 7–17 per group. Two-way ANOVA was performed to determine main effects of embryo transfer (non-embryo-transferred and embryo-transferred) and group (Control and Restricted). #P < 0.05 vs. non-embryo-transferred counterpart (Student's unpaired t-test following observation of significant interaction), δP < 0.05 vs. group counterpart (Student's unpaired t-test following observation of significant interaction). CrCl, creatinine clearance; F2, second generation.
Area under the glucose curve (AUGC) from an IPGTT was comparable between F2 non-embryo-transferred and embryo-transferred males and females (data not shown). First-phase area under the insulin curve (AUIC) in response to a glucose load was greater in F2 male (P < 0.05, Table 5), but not F2 female, embryo-transferred offspring compared with non-embryo-transferred (data not shown). AUGC in response to an IC was comparable between non-embryo-transferred and embryo-transferred F2 males and females (data not shown).
Table 5.
F2 metabolic control, insulin sensitivity, and plasma metabolite concentrations at 6 mo of age
| Non-Embryo-Transferred |
Embryo-Transferred |
|||||
|---|---|---|---|---|---|---|
| Cont | Rest | Cont-in-Cont | Cont-in-Rest | Rest-in-Cont | Rest-in-Rest | |
| Males | (n = 10) | (n = 10) | (n = 9–10) | (n = 8–10) | (n = 9–10) | (n = 4–8) |
| Fasting glucose, mmol/l | 7.6 ± 0.4 | 7.1 ± 0.3 | 7.5 ± 0.4 | 7.6 ± 0.7 | 7.5 ± 0.5 | 8.0 ± 0.4 |
| Fasting insulin, ng/ml | 0.48 ± 0.11 | 0.38 ± 0.07 | 0.72 ± 0.18 | 0.61 ± 0.16 | 0.46 ± 0.10 | 0.69 ± 0.22 |
| Fasting insulin:glucose | 0.063 ± 0.015 | 0.052 ± 0.010 | 0.094 ± 0.027 | 0.073 ± 0.017 | 0.059 ± 0.009 | 0.083 ± 0.024 |
| HOMA-IR | 3.8 ± 0.9 | 3.0 ± 0.6 | 5.9 ± 1.6 | 5.7 ± 2.3 | 3.9 ± 1.1 | 6.2 ± 2.2 |
| AUGC from IPGTT, mmol·l−1·min−1 | 1647 ± 97 | 1727 ± 95 | 1605 ± 82 | 1687 ± 131 | 1613 ± 97 | 1788 ± 192 |
| First-phase insulin AUC, ng·ml−1·min−1 | 5.9 ± 0.6 | 4.3 ± 0.8* | 8.4 ± 1.5 | 7.4 ± 2.2 | 4.7 ± 1.0 | 12.5 ± 3.1 |
| Second-phase insulin AUC, ng·ml−1·min−1 | 98 ± 15 | 80 ± 6 | 131 ± 10 | 136 ± 31 | 128 ± 13 | 175 ± 32 |
| AUIC:AUGC | 0.069 ± 0.010 | 0.051 ± 0.006 | 0.089 ± 0.008 | 0.089 ± 0.019 | 0.083 ± 0.007 | 0.108 ± 0.022 |
| AUGC from IC, mmol·l−1·min−1 | 265 ± 22a,b | 228 ± 9a | 252 ± 18a,b | 263 ± 12a,b | 290 ± 21a,b | 281 ± 12b |
| Triglycerides, mmol/l | 0.792 ± 0.057 | 1.063 ± 0.086* | 1.154 ± 0.091 | 1.266 ± 0.087 | 1.116 ± 0.082 | 1.031 ± 0.097 |
| Leptin, ng/ml | 7541 ± 789a,b | 6331 ± 397a | 11168 ± 1381b,c | 8605 ± 897a,b,c | 12023 ± 1354c | 11091 ± 1290b,c |
| Adiponectin, μg/ml | 19.5 ± 1.6 | 20.6 ± 1.7 | 22.2 ± 1.4 | 22.7 ± 0.8 | 22.7 ± 1.1 | 24.7 ± 1.9 |
| Pancreatic β-cell mass, mg1 | 4.6 ± 0.3a,b | 3.2 ± 0.2*a | 4.8 ± 0.9a,b | 4.6 ± 0.7a,b | 6.4 ± 0.5b | 5.0 ± 1.1a,b |
| Females | (n = 10) | (n = 9) | (n = 8–10) | (n = 7–10) | (n = 4–10) | (n = 7–10) |
| Fasting glucose, mmol/l | 6.7 ± 0.3 | 6.5 ± 0.3 | 6.5 ± 0.3 | 7.5 ± 0.5 | 7.1 ± 0.7 | 6.2 ± 0.4 |
| Fasting insulin, ng/ml | 0.24 ± 0.04 | 0.25 ± 0.03 | 0.36 ± 0.09 | 0.48 ± 0.12 | 0.56 ± 0.14 | 0.42 ± 0.10 |
| Fasting insulin:glucose | 0.034 ± 0.005 | 0.041 ± 0.007 | 0.053 ± 0.012 | 0.061 ± 0.012 | 0.078 ± 0.017 | 0.065 ± 0.012 |
| HOMA-IR | 1.8 ± 0.4 | 1.7 ± 0.2 | 2.6 ± 0.7 | 4.2 ± 1.2 | 4.4 ± 1.3 | 3.0 ± 0.9 |
| AUGC from IPGTT, mmol·l−1·min−1 | 1666 ± 51 | 1476 ± 88 | 1691 ± 41 | 1609 ± 163 | 1748 ± 26 | 1884 ± 206 |
| First-phase insulin AUC, ng·ml−1·min−1 | 4.6 ± 0.7 | 2.9 ± 0.5* | 4.1 ± 1 | 6.8 ± 1.4 | 5.8 ± 1.8 | 4.6 ± 0.9 |
| Second-phase insulin AUC, ng·ml−1·min−1 | 79 ± 10 | 88 ± 10 | 78 ± 9 | 112 ± 22 | 116 ± 14 | 83 ± 5 |
| AUIC:AUGC | 0.049 ± 0.007 | 0.062 ± 0.006 | 0.050 ± 0.007 | 0.085 ± 0.023 | 0.070 ± 0.010 | 0.050 ± 0.008 |
| AUGC from IC, mmol·l−1·min−1 | 223 ± 11 | 193 ± 4 | 241 ± 16 | 223 ± 10 | 241 ± 8 | 260 ± 11 |
| Triglycerides, mmol/l−1 | 0.607 ± 0.057 | 0.563 ± 0.044 | 0.878 ± 0.115 | 0.931 ± 0.093 | 0.711 ± 0.050 | 0.684 ± 0.056 |
| Leptin, ng/ml | 3738 ± 441 | 3510 ± 418 | 4525 ± 477 | 4969 ± 463 | 3809 ± 441 | 4157 ± 291 |
| Adiponectin, μg/ml | 34.3 ± 2.0 | 37.0 ± 3.2 | 37.4 ± 1.9 | 34.8 ± 2.4 | 32.9 ± 2.5 | 32.6 ± 1.8 |
| Pancreatic β-cell mass, mg1 | 2.4 ± 0.8a,b | 3.6 ± 1.4*a,b | 2.0 ± 0.4a | 3.8 ± 0.5a,b | 4.4 ± 0.6a,b | 5.0 ± 1.3b |
All data are expressed as means ± SE. Fasting plasma glucose and insulin were measured prior to a bolus of glucose from an intraperitonal glucose tolerance test (IPGTT), while plasma adiponection, leptin, and triglycerides were measured from blood collected at postmortem. Fasting insulin:glucose and homeostatic model assessment of insulin resistance (HOMA-IR) were calculated from fasting plasma glucose and insulin concentrations. First- and second-phase insulin AUC were calculated from the IPGTT. One male and one female were randomly selected per litter; n = 4–10 per group.
Significant difference (P < 0.05) between non-embryo-transferred Control and Restricted (Student's unpaired t-test).
Significant differences across the six groups, as indicated by letters that differ (one-way ANOVA with SNK post hoc analysis).
For pancreatic β-cell mass analyses, litter size is n = 6–9 per group. F2 metabolic control, insulin sensitivity and plasma metabolite concentrations at 6 mo of age.
AUC, area under the curve; AUGC, area under the glucose curve; AUIC, area under the insulin curve; F2, second generation; HOMA-IR, homeostatic model assessment of insulin resistance; IC, insulin challenge.
Comparison Between Non-Embryo Transferred Groups (Naturally Gestated Progeny)
F2 growth and organ weights.
There were no differences in body weight at PN1 between F2 Control and Restricted offspring (Table 2). However, at 2 mo of age, F2 Restricted males were lighter than Control (P < 0.05), with no differences in F2 females (Table 2). This was attributed to a reduced growth rate between PN14 and 2 mo (P < 0.05, data not shown), followed by accelerated growth between 2 and 3 mo (P < 0.05, data not shown), at which time body weight was no longer different between F2 Control and Restricted (Table 2). Pancreas weight was reduced in F2 Restricted vs. Control males and females, and left ventricular weight was reduced in F2 Restricted females only (P < 0.05, Table 3). There were no differences in whole heart, kidney, or dorsal fat weight between these two groups (Table 3).
Table 3.
F2 organ weights at 6 mo of age
| Non-Embryo-Transferred |
Embryo-Transferred |
|||||
|---|---|---|---|---|---|---|
| Cont | Rest | Cont-in-Cont | Cont-in-Rest | Rest-in-Cont | Rest-in-Rest | |
| Male, % body wt | (n = 14) | (n = 12) | (n = 15) | (n = 13) | (n = 10) | (n = 8) |
| Heart | 0.334 ± 0.006 | 0.333 ± 0.006 | 0.329 ± 0.005 | 0.332 ± 0.004 | 0.334 ± 0.004 | 0.342 ± 0.011 |
| Left ventricle | 0.239 ± 0.003 | 0.240 ± 0.004 | 0.232 ± 0.003 | 0.242 ± 0.003 | 0.240 ± 0.003 | 0.248 ± 0.006 |
| Kidney | 0.605 ± 007a,b | 0.624 ± 0.014a | 0.584 ± 0.007b | 0.608 ± 0.008a,b | 0.596 ± 0.007a,b | 0.590 ± 0.009a,b |
| Pancreas | 0.215 ± 0.011 | 0.182 ± 0.008* | 0.205 ± 0.007 | 0.213 ± 0.007 | 0.202 ± 0.007 | 0.213 ± 0.007 |
| Dorsal fat | 1.28 ± 0.09a | 1.28 ± 0.09a | 1.55 ± 0.06a,b | 1.45 ± 0.10a,b | 1.70 ± 0.05b | 1.71 ± 0.09b |
| Female, % body wt | (n = 15) | (n = 14) | (n = 14) | (n = 11) | (n = 10) | (n = 10) |
| Heart | 0.379 ± 0.004a | 0.368 ± 0.004a | 0.367 ± 0.004a | 0.406 ± 0.010b | 0.358 ± 0.005a | 0.367 ± 0.007a |
| Left ventricle | 0.273 ± 0.003a,b | 0.264 ± 0.004*a | 0.266 ± 0.003a | 0.285 ± 0.004b | 0.268 ± 0.005a | 0.273 ± 0.004a,b |
| Kidney | 0.652 ± 0.009b | 0.663 ± 0.008a,b | 0.618 ± 0.003a | 0.620 ± 0.006a | 0.634 ± 0.010a,b | 0.633 ± 0.007a,b |
| Pancreas | 0.315 ± 0.012 | 0.277 ± 0.016* | 0.285 ± 0.013 | 0.314 ± 0.015 | 0.320 ± 0.020 | 0.338 ± 0.025 |
| Dorsal fat | 1.01 ± 0.05 | 1.05 ± 0.03 | 1.15 ± 0.05 | 1.13 ± 0.05 | 1.12 ± 0.07 | 1.07 ± 0.07 |
All data are expressed as means ± SE. F2 organ weights at 6 mo of age. Weights of heart, left ventricle, kidney (combined left and right), pancreas, and dorsal fat were measured at postmortem and are expressed relative to body weights. 1 male and 1 female randomly selected per litter, n = 8–15 per group.
Significant difference (P < 0.05) between non-embryo-transferred Control and Restricted (Student's unpaired t-test).
Significant differences across the six groups, as indicated by letters that differ (one-way ANOVA with SNK post hoc analysis).
F2 systolic blood pressure, food and water intake, and kidney function.
At 2, 3 and 4 mo of age, systolic blood pressure was not different between F2 Control and Restricted male and female offspring (data not shown). However, at 6 mo of age, systolic blood pressure was elevated in F2 Restricted vs. Control males (P < 0.05), but not females (Table 4). There were no differences in food and water intake or any of the renal parameters measured between F2 Control and Restricted males or females (Table 4).
Table 4.
F2 systolic blood pressure, food and water intake, and renal parameters at PN35 or 6 mo of age
| Non-Embryo-Transferred |
Embryo-Transferred |
|||||
|---|---|---|---|---|---|---|
| Cont | Rest | Cont-in-Cont | Cont-in-Rest | Rest-in-Cont | Rest-in-Rest | |
| Males | (n = 14) | (n = 10–12) | (n = 14–15) | (n = 14–15) | (n = 10–11) | (n = 9–10) |
| Systolic blood pressure, mmHg | 135 ± 5 | 154 ± 4* | 142 ± 5 | 150 ± 6 | 141 ± 4 | 141 ± 7 |
| Food, g·24 h−1·kg−1 | 58 ± 1b | 58 ± 2b | 55 ± 2a,b | 59 ± 2b | 51 ± 2a | 49 ± 1a |
| Water, ml·24 h−1·kg−1 | 100 ± 7b | 98 ± 8b | 86 ± 5a,b | 88 ± 4a,b | 77 ± 4a,b | 73 ± 6a |
| Urine flow, l·24 h−1·kg−1 | 0.045 ± 0.005 | 0.050 ± 0.004 | 0.044 ± 0.003 | 0.039 ± 0.003 | 0.039 ± 0.003 | 0.040 ± 0.004 |
| Na+, mmol·l−1 24 h −1·kg−1 | 2.2 ± 0.2 | 2.4 ± 0.2 | 3.0 ± 0.3 | 2.5 ± 0.3 | 2.7 ± 0.3 | 2.5 ± 0.2 |
| Cl−·mg·l−1·24 h−1·kg−1 | 3.7 ± 0.3 | 4.0 ± 0.3 | 5.2 ± 0.5 | 4.4 ± 0.5 | 5.3 ± 0.5 | 4.6 ± 0.3 |
| TP, ml·l−1min−1·kg−1 | 47 ± 5 | 50 ± 6 | 65 ± 5 | 59 ± 6 | 74 ± 8 | 60 ± 6 |
| CrCl, ml·min−1·kg−1 | 4.1 ± 0.5a | 4.0 ± 0.3a | 6.6 ± 0.5b | 5.6 ± 0.6a,b | 7.7 ± 0.8b | 7.0 ± 0.7b |
| FENa+, % | 0.311 ± 0.033c | 0.298 ± 0.028b,c | 0.240 ± 0.024a,b,c | 0.236 ± 0.027a,b,c | 0.197 ± 0.022a,b | 0.188 ± 0.015a |
| Glomerular number | 24,415 ± 1740 | 23,035 ± 750 | 24,470 ± 812 | 23,134 ± 841 | 21,971 ± 609 | 25,003 ± 662 |
| Individual glomerular volume, ×10−4 mm3 | 4.1 ± 0.2 | 4.4 ± 0.2 | 4.2 ± 0.2 | 4.6 ± 0.2 | 4.3 ± 0.2 | 4.1 ± 0.2 |
| Females | (n = 16) | (n = 12–14) | (n = 12–13) | (n = 10–11) | (n = 7–10) | (n = 11–13) |
| Systolic blood pressure, mmHg | 130 ± 3 | 127 ± 3 | 126 ± 3 | 127 ± 4 | 121 ± 5 | 126 ± 3 |
| Food, g·24 h−1·kg−1 | 78 ± 4b | 72 ± 1a,b | 76 ± 3b | 76 ± 4b | 69 ± 2a,b | 62 ± 4a |
| Water, ml·24 h−1·kg−1 | 135 ± 6b | 146 ± 7b | 147 ± 6b | 139 ± 5b | 135 ± 7b | 110 ± 5a |
| Urine flow, l·24 h−1·kg−1 | 0.068 ± 0.006 | 0.082 ± 0.004 | 0.075 ± 0.006 | 0.081 ± 0.008 | 0.078 ± 0.008 | 0.059 ± 0.003 |
| Na+, mmol·l−1·24 h−1·kg−1 | 4.3 ± 0.5 | 4.2 ± 0.5 | 3.9 ± 0.2 | 5.3 ± 0.3 | 4.7 ± 0.7 | 4.1 ± 0.3 |
| Cl−, mg·l−1·24 h−1·kg−1 | 5.5 ± 0.4 | 6.2 ± 0.7 | 6.0 ± 0.4 | 7.7 ± 0.5 | 7.4 ± 0.8 | 6.7 ± 0.3 |
| TP, ml·l−1·min−1·kg−1 | 10.2 ± 0.9 | 9.3 ± 1.9 | 10.7 ± 1.6 | 19.0 ± 2.7 | 10.2 ± 0.8 | 18.8 ± 2.1 |
| CrCl, ml·min−1·kg−1 | 4.2 ± 0.4a | 3.7 ± 0.6a | 4.9 ± 0.7a | 5.9 ± 0.7a | 6.1 ± 0.7a | 8.2 ± 0.6b |
| FENa+, % | 0.534 ± 0.071 | 0.505 ± 0.030 | 0.437 ± 0.033 | 0.475 ± 0.044 | 0.389 ± 0.032 | 0.257 ± 0.021 |
| Glomerular number1 | 23,238 ± 1,600 | 22,531 ± 928 | 23,409 ± 803 | 22,551 ± 1,377 | 24,656 ± 1,209 | 22,285 ± 564 |
| Individual glomerular volume, ×10−4 mm3 | 4.4 ± 0.4 | 4.5 ± 0.2 | 3.8 ± 0.2 | 3.7 ± 0.2 | 3.9 ± 0.4 | 3.9 ± 0.2 |
All data are expressed as means ± SE. F2 Systolic blood pressure, food and water intake and renal parameters were measured at 6 mo of age. Glomerular number and individual glomerular volume were measured in separate sibling cohort at PN35. One male and one female were randomly selected per litter for each study age; n = 8–17 per group.
Significant difference (P < 0.05) between non-embryo-transferred Control and Restricted (Student's unpaired t-test).
Significant differences across the six groups, as indicated by letters that differ (one-way ANOVA with SNK post hoc analysis).
For glomerular number and individual glomerular volume analyses, litter size is n = 8 per group.
Cl−, chloride; CrCl, creatinine clearance; FENa+, fractional sodium excretion; F2, second generation; Na+, sodium; TP, total protein.
F2 metabolic control, insulin sensitivity, and plasma metabolite concentrations.
There were no differences in fasting plasma glucose and insulin concentrations, insulin sensitivity [homeostatic model of assessment for insulin resistance (HOMA-IR) index, AUIC:AUGC, AUGC from IC], glucose tolerance (AUGC from IPGTT), or second-phase insulin response to a glucose bolus between F2 Control and Restricted males or females (Table 5). Glucose-stimulated first-phase insulin secretion, however, was reduced in both male and female F2 Restricted vs. Control offspring (P < 0.05, Table 5). Plasma triglyceride concentrations were elevated in F2 Restricted vs. Control males (P < 0.05), but there were no differences in females, nor in plasma leptin or adiponectin concentrations in either sex (Table 5). Male and female Restricted offspring had reduced (−29%) and elevated (twofold) pancreatic β-cell mass, respectively, compared with Control counterparts (P < 0.05, Table 5).
Comparison of All Groups (Naturally Gestated Progeny and Embryo-Transferred Groups)
F2 growth and organ weights.
At PN35 and 2 mo of age, F2 Rest-in-Rest males were heavier than all other groups, and Rest-in-Cont were heavier than F2 Restricted group (P < 0.05, Table 2). At PN7, F2 Rest-in-Cont females were heavier than F2 Control and by PN14, all embryo-transferred females were heavier than female rats in the Control group (P < 0.05, Table 2). At PN35 and 2 mo, F2 Rest-in-Cont and Rest-in-Rest females were heavier than F2 Rest offspring (P < 0.05, Table 2). In males, F2 Cont-in-Cont offspring had increased kidney weight compared with F2 Restricted offspring (P < 0.05), while all other groups remained intermediate (Table 3). Dorsal fat mass was greater in F2 Rest-in-Cont and Rest-in-Rest males compared with non-embryo-transferred F2 Cont and Rest (P < 0.05, Table 3). Whole heart, left ventricle, and pancreas weights were not different between groups of F2 male offspring (Table 3). In females, heart weight was increased in F2 Cont-in-Rest compared with other groups (P < 0.05, Table 3). This was associated with increased left ventricular weight compared with F2 Rest, Cont-in-Cont, and Rest-in-Cont (P < 0.05, Table 3). Kidney weight was reduced in F2 Cont-in-Cont and Cont-in-Rest females compared with non-embryo-transferred F2 Control (P < 0.05, Table 3). Pancreas and dorsal fat mass were not different between groups in female offspring (Table 3). Body weights were comparable within all viable F2 male and female groups at PN1 and from 3 mo of age (Table 2).
F2 systolic blood pressure, food and water intake, and kidney function.
At 3 mo, female F2 Rest-in-Rest had lower systolic blood pressure compared with Rest-in-Cont and non-embryo-transferred groups (P < 0.05, data not shown). F2 Rest-in-Rest and Rest-in-Cont males consumed less food than non-embryo-transferred groups and F2 Cont-in-Rest (P < 0.05, Table 4). Water intake was also reduced in F2 Rest-in-Rest males compared with Control and Restricted (P < 0.05, Table 4). In females, food intake was reduced in F2 Rest-in-Rest compared with F2 Control, Cont-in-Cont, and Cont-in-Rest groups (P < 0.05, Table 4). Water consumption was reduced in F2 Rest-in-Rest females compared with all other groups (P < 0.05, Table 4). Creatinine clearance was elevated in F2 Rest-in-Rest, Rest-in-Cont, and Cont-in-Cont males compared with non-embryo-transferred groups (P < 0.05, Table 4). In females, however, this increase in creatinine clearance occurred only in F2 Rest-in-Rest compared with all other groups (P < 0.05, Table 4). In males, fractional excretion of sodium was reduced in F2 Rest-in-Rest compared with non-embryo-transferred groups and reduced in F2 Rest-in-Cont compared with F2 Cont (P < 0.05, Table 4). There were no differences in systolic blood pressure at 2, 4 (data not shown), and 6 (Table 4) mo of age in F2 male and female offspring. Urine flow rate and excretion of sodium, chloride, and total protein were not different between groups in F2 males and females (Table 4). There were no differences in fractional excretion of sodium in F2 females (Table 4).
F2 metabolic control, insulin sensitivity, and plasma metabolite concentrations.
F2 Rest-in-Rest males, but not females, had elevated first- and second-phase insulin area under the curve (AUC) compared with non-embryo-transferred offspring (P < 0.05), while other groups remained intermediate between the two (Table 5). F2 Rest-in-Rest males, but not females, had greater AUGC from IC compared with non-embryo-transferred F2 Restricted rats (P < 0.05, Table 5). Plasma leptin concentrations were elevated in F2 Cont-in-Cont, Rest-in-Cont, and Rest-in-Rest compared with F2 Restricted male offspring only (P < 0.05, Table 5). Plasma leptin concentrations were also greater in F2 Rest-in-Cont compared with non-embryo-transferred F2 Control male offspring (P < 0.05, Table 5). Pancreatic β-cell mass was elevated in F2 Rest-in-Cont compared with F2 Rest male offspring (P < 0.05, Table 5). In females, F2 Rest-in-Rest had increased β-cell mass compared with F2 Cont-in-Cont (P < 0.05, Table 5). Across the six groups, there were no differences in plasma glucose, insulin, triglyceride and adiponectin levels, glucose tolerance, or pancreatic β-cell mass in F2 males or females (Table 5).
DISCUSSION
Previously, we reported that low maternal birth weight, induced by uteroplacental insufficiency in rats, was associated with a range of growth, cardiorenal, and metabolic alterations in second-generation male offspring (14, 37). This included slowed growth during the peripubertal period (PN14 to 2 mo of age) followed by accelerated growth until 3 mo, and blunted first-phase insulin response to a glucose load, reduced pancreatic β-cell mass, and transient hypertension in adulthood (14, 37). However, the contribution of an adverse maternal pregnancy environment (maternal constraint) vs. intrinsic germ line effects in driving these transgenerational outcomes remains unknown. Thus, the aim of this study was to delineate between these two factors in mediating the observed changes to growth and cardiorenal and metabolic health in F2 progeny from low-birthweight mothers.
The reduced litter size and increased birth weight in the embryo-transferred groups may suggest enhanced nutrient delivery during gestation that continues throughout lactation compared with non-embryo-transferred groups. However, in the current study, litter size was not equalized between the groups. We have previously shown that reducing litter size from sham-operated dams impairs maternal mammary morphology, lactation, and subsequent postnatal growth and health of the offspring (25, 38). F2 Rest-in-Rest embryo-transferred offspring underwent accelerated growth during the peripubertal phase followed by slowed growth between 2 and 3 mo compared with non-embryo-transferred, naturally gestated F2 Restricted offspring. Given that growth trajectory in early childhood is a known determinant of adulthood risk for chronic disease (11), differences in growth profiles between embryo-transferred and non-embryo-transferred Restricted groups may predict differences in their future health status. For F2 Cont-in-Cont and/or Rest-in-Rest animals, body weight, growth profiles, renal function, and insulin response to a glucose load were different to respective non-embryo-transferred groups. Importantly, this would not have been identified in the absence of a well-controlled study design, which included F2 Cont-in-Cont and Rest-in-Rest groups that served as controls for embryo transfer. Therefore, we suggest that embryo transfer procedures are limited in their ability to delineate between maternal constraints and germ line effects that are relevant to natural pregnancies.
Factors Inherent to Embryo Transfer Procedures
Embryo manipulations for commercial livestock are associated with a phenomenon, known as “large offspring syndrome” (39, 45). A recent study in humans also reported increased risk for a number of neonatal complications due to assisted reproductive technologies (22). These procedures usually involve in vitro fertilization with embryo culture in serum for about 1 wk and asynchronous transfer into an advanced gestational-stage recipient. Importantly, our procedures involved in vivo fertilization, with embryo culture restricted to <20 min within 1 day of synchronous mating, and gestational length was unaltered. Thus, increased PN1 body weight in embryo-transferred Restricted males is unlikely to be attributed to these factors. Transferred embryos in the current study were briefly exposed to anesthesia at E1 of pregnancy (recipient placement). While this early gestational exposure to anesthetic is unlikely to result in neurodevelopmental impairments seen with mid-gestation to late-gestation isoflurane exposure (26, 40), embryo implantation and subsequent pregnancy success rate may have been compromised.
The culture media used in the current study were formulated to best match conditions of a normal mammalian oviduct environment (17). Future studies, however, may benefit from comprehensive analyses of oviduct fluid in pregnant females from different experimental groups (i.e., a Control and Restricted pregnant female) to help formulate culture media that best mimics each environment. An alternative explanation for the observed effects of oviduct embryo transfer comes from the work of Xie et al. (44), who demonstrated that the very act of pipetting embryos creates a shear force on the embryo itself. Such forces are sufficient to induce an up-regulation of stress-activated protein kinases, which, in turn, can have downstream effects on embryo gene expression and development.
During early embryonic development, environmental cues induce epigenetic, heritable modifications that manifest into functional consequences. These include chromatin remodeling (DNA methylation and histone modification) and noncoding RNA and microRNA modifications that are usually set early in development but may occur in response to environmental cues later in life (10, 20). In particular, the expression of certain genes, several of which may be associated with growth, can be modulated during embryo culture. Additionally, the maternal intrauterine and/or systemic pregnancy adaptations (cardiovascular, renal, metabolic, and endocrine) may induce epigenetic alterations in the pretransferred and posttransferred embryo, thus influenced by both the donor and recipient maternal environment. Indeed, we cannot be certain the maternal adaptation to pregnancy in recipient females matched that of our naturally pregnant females (16). Signals released by the “unmatched” embryo or fetus may have initiated a unique maternal response with subsequent programming influences that are completely independent of transgenerational effects. Although we recorded blood pressure prior to mating, no physiological measurements were performed during pregnancy, so that we could minimize the known, confounding influence of maternal stress exposure on F2 outcomes (16).
Donor and Recipient Synchrony
The stage of estrous cycle and maternal age of donor and recipient female must match for embryo survival (18). For all animals in the current study, mating was in proestrus, and we were stringent in ensuring that females were closely matched in terms of mating age between all groups, as well as body weights within Control and Restricted groups. Recipient females that are further along in pregnancy than donor females carry heavier ovine fetuses (45), probably due to advanced uterine secretory signals. Although donor and recipient females were mated simultaneously overnight, this could have occurred at any time within the 18-h period for which they were housed with the male breeders. This potential mismatch, between donor and recipient gestational stage, may have had an impact on pregnancy success rate and F2 outcomes compared with naturally gestated offspring, albeit unlikely biased to a specific group.
The reason some (body weight, growth profile, total protein excretion, creatinine clearance, and insulin response to a glucose load), but not all (glomerular number and volume, blood pressure, glucose and insulin AUC, insulin sensitivity, and β-cell mass), parameters were affected by embryo transfer may be because there are different “critical periods” for developing systems. The majority of differences observed with embryo transfer compared with non-embryo-transferred groups remained consistent between F2 Cont-in-Cont and Rest-in-Rest. However, F2 Rest-in-Rest males and females had different growth rates compared with F2 Restricted counterparts, while F2 Controls remained similar. This was similarly observed for F2 Rest-in-Rest, but not F2 Cont-in-Cont, females who had increased total protein excretion and creatinine clearance compared with their non-embryo-transferred counterparts. Increased insulin response to glucose was also observed in F2 Rest-in-Rest, but not F2 Cont-in-Cont males, compared with their respective non-embryo-transferred counterparts. These data indicate that changes to renal function and insulin response to glucose were not due solely to an embryo transfer procedure but dependent on the embryo and/or maternal (donor and recipient) environment. These complex interactions between the fetus and maternal environment prevented identification of a clear pathway for transgenerational effects that occurred in the natural pregnancies.
Future Directions
Embryo-transfer procedures are ingrained with a number of confounding elements that limit its utility in separating between maternal and germ line influences in the transgenerational programming of disease. While an F2 phenotype via the paternal line eliminates direct maternal influences, males born to preeclamptic mothers are more likely to have female partners that develop preeclampsia (12, 46). This paternal link suggests a Restricted father has the potential to influence his partner's adaptation to pregnancy, and via this indirect pathway, the F2 phenotype may be programmed.
To eliminate a confounding influence of in vitro embryo exposure, a sham surgery in the non-embryo-transferred group (embryos harvested and returned to the same uterus) would be of benefit. However, this does not eliminate various factors associated with the procedure itself. Thus an, ovarian cross-transplantation may be considered for future studies. Studies in rats and rabbits have reported preserved hormonal and fertile functions with intact ovarian transplantation (27, 41). In our rat model, programmed phenotypes in F2 offspring, which could be generated from ovaries from Restricted rats transplanted into a Control or Restricted F1 mother, would suggest germ line effects that are independent of the maternal environment. These effects, however, may stem from either a multigenerational impact (insult to the pregnant F0 directly affecting F1 gametes that give rise to the F2) or F1 somatic influences (prenatal or postnatal) to gametes prior to transplantation. Persistence of disease to the F3 does not separate these given that both multigenerational and F1 somatic influences can induce epigenetic alterations in the F2 that are inherited and transmissible to the F3. What remains of clinical interest is whether programmed effects are mediated by specific pregnancy adaptations, independent of the germ line that can be therapeutically targeted. If future studies identify a predominant role for the germ line, interventions may be resolved to ensuring an optimal and healthy lifestyle to minimize the risk of disease.
Perspectives and Significance
Transgenerational studies have provided evidence for transmission of diseases to subsequent generations; however, the pathway responsible remains to be elucidated. This study aimed to separate the role of the maternal environment vs. germ line effects in mediating transgenerational outcomes associated with uteroplacental insufficiency. However, embryo transferred Cont-in-Cont and Rest-in-Rest offspring were not similar in terms of growth, cardiorenal, and metabolic phenotypes to their naturally gestated counterparts (Control and Restricted). We were, therefore, unable to translate findings from embryo-transferred offspring to those from natural pregnancies. Because of a number of confounding factors associated with embryo transfer, our studies were not able to identify the primary driving force behind transgenerational disease outcomes, maternal constraint in a growth-restricted mother, or germ line effects that are independent of the maternal pregnancy environment.
GRANTS
This study was supported by the National Health and Medical Research Council of Australia (Grant 400004), Heart Foundation of Australia (G 08M 3698), and the March of Dimes Births Defect Foundation, USA (Grant 6-FY08-269). L. A. Gallo was supported by a Heart Foundation Biomedical Scholarship and The University of Melbourne Faculty Research Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.T., L.A.G., L.A.C.-M., K.M.M., and M.E.W. conception and design of research; M.T., L.A.G., A.N.H., A.J.J., and K.T.W. performed experiments; M.T., L.A.G., A.N.H., K.M.M., and M.E.W. analyzed data; M.T., L.A.G., A.N.H., D.K.G., K.M.M., and M.E.W. interpreted results of experiments; M.T. and L.A.G. prepared figures; M.T. and L.A.G. drafted manuscript; M.T., L.A.G., A.N.H., A.J.J., K.T.W., L.A.C.-M., D.K.G., K.M.M., and M.E.W. edited and revised manuscript; M.T., L.A.G., A.N.H., A.J.J., K.T.W., L.A.C.-M., D.K.G., K.M.M., and M.E.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Current addresses: M. Tran, Department of Biochemistry and Molecular Biology, Monash University, Clayton, Australia; L. A. Gallo, Mater Research Institute-University of Queensland, Translational Research Institute, Woolloongabba, Australia; A. N. Hanvey, Murdoch Children's Research Institute, The Royal Children's Hospital, Parkville, Australia; K. T. Westcott, Ludwig Institute for Cancer Research Ltd., Austin Hospital, Heidelberg, Australia.
REFERENCES
- 1.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol 161: 111–172, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes 51: 1499–1506, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S. β-cell turnover: Its assessment and implications. Diabetes 50: S20–S24, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res 54: 83–90, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Calle A, Fernandez-Gonzalez R, Ramos-Ibeas P, Laguna-Barraza R, Perez-Cerezales S, Bermejo-Alvarez P, Ramirez MA, Gutierrez-Adan A. Long-term and transgenerational effects of in vitro culture on mouse embryos. Theriogenology 77: 785–793, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Di Nicolantonio R, Koutsis K, Westcott KT, Wlodek ME. Lack of evidence for a role for either the in utero or suckling periods in the exaggerated salt preference of the spontaneously hypertensive rats. Physiol Behav 86: 500–507, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Di Nicolantonio R, Koutsis K, Westcott KT, Wlodek ME. Relative contribution of the prenatal versus postnatal period on development of hypertension and growth rate of the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol 33: 9–16, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Di Nicolantonio R, Koutsis K, Wlodek ME. Fetal versus maternal determinants of the reduced fetal and placental growth in spontaneously hypertensive rats. J Hypertens 18: 45–50, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Drake AJ, Liu L. Intergenerational transmission of programmed effects: public health consequences. Trends Endocrinol Metab 21: 206–213, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. Br Med J 322: 948–953, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, Varner MW. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med 344: 867–872, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Gallo LA, Denton KM, Moritz KM, Tare M, Parkington HC, Davies M, Tran M, Jefferies AJ, Wlodek ME. Long-term alteration in maternal blood pressure and renal function after pregnancy in normal and growth restricted rats. Hypertension 60: 206–213, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Gallo LA, Tran M, Cullen-McEwen LA, Denton KM, Jefferies AJ, Moritz KM, Wlodek ME. Transgenerational programming of fetal nephron deficits and sex-specific adult hyptertension in rats. Reprod Fertil Dev In press [DOI] [PubMed] [Google Scholar]
- 15.Gallo LA, Tran M, Master JS, Mortiz KM, Wlodek ME. Maternal adaptations and inheritance in the transgenerational programming of adult disease. Cell Tissue Res 349: 863–880, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Gallo LA, Tran M, Moritz KM, Mazzuca MQ, Parry LJ, Westcott KT, Jefferies AJ, Cullen-McEwen LA, Wlodek ME. Cardio-renal and metabolic adaptations during pregnancy in female rats born small: implications for maternal health and second-generation fetal growth. J Physiol 590: 617–630, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner DK, Lane M. Towards a single embryo transfer. Reprod Biomed Online 6: 470–481, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Geisert RD, Morgan GL, Zavy MT, Blair RM, Gries LK, Cox A, Yellin T. Effect of asynchronous transfer and oestrogen administration on survival and development of porcine embryos. J Reprod Fertil 93: 475–481, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. Br Med J 303: 1019–1022, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol 84: 131–176, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD, McConell GK. Short-term exercise training early in life restores deficits in pancreatic β-cell mass associated with growth restriction in adult male rats. Am J Physiol Endocrinol Metab 301: E931–E940, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Marino JL, Moore VM, Willson KJ, Rumbold A, Whitrow MJ, Giles LC, Davies MJ. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One 9: e80398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzuca MQ, Tare M, Parkington HC, Dragomir NM, Parry LJ, Wlodek ME. Uteroplacental insufficiency programmes vascular dysfunction in non-pregnant rats: compensatory adaptations in pregnancy. J Physiol 590: 3375–3388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol 587: 2635–2646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Dowd R, Kent JC, Moseley JM, Wlodek ME. Effects of uteroplacental insufficiency and reducing litter size on maternal mammary function and postnatal offspring growth. Am J Physiol Regul Integr Comp Physiol 294: R539–R548, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Palanisamy A. Maternal anesthesia and fetal neurodevelopment. Int J Obstet Anesth 21: 152–162, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Petroianu A, Alberti LR, Vasconcellos LS. Morphologic, endocrinologic and natural pregnancy assessment of allogeneic ovarian orthotopic transplantation without a vascular pedicle in rabbits. Eur J Obstet Gynecol Reprod Biol 133: 70–75, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Roseboom TJ, Watson ED. The next generation of disease risk: Are the effects of prenatal nutrition transmitted across generations? Evidence from animal and human studies. Placenta 33: 40–44, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Siebel AL, Gallo LA, Guan TC, Owens JA, Wlodek ME. Cross-fostering and improved lactation ameliorates deficits in endocrine pancreatic morphology in growth-restricted adult male rat offspring. J Dev Origins Health Dis 1: 234–244, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Siebel AL, Mibus A, De Blasio MJ, Westcott KT, Morris MJ, Prior L, Owens JA, Wlodek ME. Improved lactational nutrition and postnatal growth ameliorates impairment of glucose tolerance by uteroplacental insufficiency in male rat offspring. Endocrinology 149: 3067–3076, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Styrud J, Eriksson UJ, Grill V, Swenne I. Experimental intrauterine growth retardation in the rat causes a reduction of pancreatic β-cell mass, which persists into adulthood. Biol Neonate 88: 122–128, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Sangiorgi F, Lee PWN, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 292: E1270–E1279, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension 47: 982–987, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Torrens C, Poston L, Hanson MA. Transmission of raised blood pressure and endothelial dysfunction to the F2 generation induced by maternal protein restriction in the F0, in the absence of dietary challenge in the F1 generation. Br J Nutr 100: 760–766, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Tran M, Gallo LA, Wadley GD, Moritz KM, Wlodek ME. Effect of pregnancy for females born small on later life metabolic disease risk. PLoS One 7: e45188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran M, Gallo LA, Jefferies AJ, Moritz KM, Wlodek ME. Transgenerational metabolic outcomes associated with uteroplacental insufficiency. J Endocrinol 217: 105–118, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Wadley GD, Siebel AL, Cooney GJ, McConell GK, Wlodek ME, Owens JA. Uteroplacental insufficiency and reducing litter size alters skeletal muscle mitochondrial biogenesis in a sex specific manner in the adult rat. Am J Physiol Endocrinol Metab 294: E861–E869, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Walker SK, Hartwich KM, Seamark RF. The production of unusually large offspring following embryo manipulation: concept and challenges. Theriogenology 45: 111–120, 1996 [Google Scholar]
- 40.Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H. Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res 66: 435–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Bilolo KK, Qi S, Xu D, Jiang W, Vu MD, Chen H. Restoration of fertility in oophorectomized rats after tubo-ovarian transplantation. Microsurgery 22: 30–33, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18: 1688–1696, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84: 2316–2337, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev 74: 1287–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod 3: 155–163, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Zusterzeel PL, te MR, Raijmakers MT, Roes EM, Peters WH, Steegers EA. Paternal contribution to the risk for pre-eclampsia. J Med Genet 39: 44–45, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]