Abstract
Recently, two proteins have been localized in the arcuate nucleus (ARC) and implicated in the regulation of food intake: the serine-threonine-kinase mammalian target of rapamycin (mTOR) as part of the TOR signaling complex 1 (TORC1), and nesfatin-1 derived from the precursor protein nucleobindin2. However, the exact cell types are not well described. Therefore, we performed double-labeling studies for NPY, CART, nesfatin-1 and pmTOR in the ARC. In this study, we showed that nesfatin-1 is not only intracellularly co-localized with cocaine- and amphetamine-regulated transcript (CART) peptide as reported before, but also with phospho-mTOR (pmTOR) and neuropeptide Y (NPY) in ARC neurons. Quantification revealed that 59 ± 5% of the pmTOR-immunoreactive (ir) neurons were immunoreactive for nesfatin-1. Moreover, double labeling for nesfatin-1 and NPY exhibited that 19 ± 5% of the NPY positive cells were also immunoreactive for nesfatin-1. Furthermore, we could also confirm results from previous studies, showing that the majority of nesfatin-1 neurons are also positive for CART peptide, whereas most of the pmTOR is co-localized with NPY and only to a lesser extent with CART.
Keywords: Arcuate nucleus, Rat, Nesfatin-1, Phospho-mTOR, CART, NPY
1. Introduction
The arcuate nucleus (ARC) of the hypothalamus plays a crucial role in the regulation of energy homeostasis and food intake in the mammalian brain. Two “first-order” populations of neurons are particularly important: a group of neuropeptide Y (NPY) and agouti-related peptide (AgRP) neurons located in the ventromedial part and another group of cocaine- and amphetamine-regulated transcript (CART) and pro-opiomelanocortin (POMC) neurons in the lateral part of the arcuate nucleus [6,7,18,31]. 90% of the NPY neurons co-express AgRP, likewise, 90% of CART neurons are co-localized with POMC [2,6,13]. Intracerebroventricular (icv) injections of NPY and AgRP stimulate food intake in rodents, whereas POMC/CART neurons exert an inhibitory effect on rodent food ingestion [3,5,19–21,27,30].
Recently, two other proteins have been localized in ARC neurons that were suggested to play a regulatory role in the control of feeding: the serine–threonine-kinase mammalian target of rapamycin (mTOR) as part of the TOR signaling complex 1 (TORC1), and the protein nesfatin-1/nucleobindin2 (NUCB2) [4,24]. Recently, Cota et al. found that 90% of phosphorylated mTOR (phospho-mTOR, pmTOR) in ARC neurons are co-localized with NPY, and only to a small extent with POMC/CART [4]. Also, their studies showed that pmTOR expression in ARC neurons is reduced in fasted compared to ad libitum fed animals [4]. This supports the hypothesis that mTOR is involved in the mediation of satiety signaling.
Nesfatin-1 is formed by post-translational cleavage from the precursor protein NUCB2 [22,24]. The enzyme prohormone convertase (1/3) splits NUCB2 into three fragments: the N-terminal nesfatin-1, nesfatin-2 and the C-terminal peptide fragment nesfatin-3 [24]. Acute icv or intraperitoneal (ip) injection of nesfatin-1 potently inhibits food intake in rats and mice, and similarly, chronic third ventricular administration induces a loss in body weight, whereas nesfatin-2 and -3 have no effect [24,28]. Conversely, chronic central infusion of a NUCB2 antisense oligonucleotide over a period of 10 days increased food intake as well as body weight gain in rats pointing toward a physiological role of nesfatin-1/NUCB2 as modulator of food intake [24]. In the same way, it has been shown that the expression of NUCB2 mRNA as well as the activation of nesfatin-1 neurons in the paraventricular nucleus (PVN) of the hypothalamus is influenced by fasting and re-feeding [17,24]. Interestingly, in the ARC, 64% of the nesfatin-1/NUCB2 neurons are co-localized with CART, but not with NPY [24]. The activation of this population of neurons may inhibit food intake. This is in accordance with the observation made in vitro using whole cell current clamps of ARC brain sections that the application of nesfatin-1 leads to an electrophysiological inhibition of NPY/AgRP neurons [26]. This population of neurons is known to be involved in the initiation of food intake. For instance, peripheral ghrelin exerts its stimulatory effect on food intake via NPY/AgRP neurons in the ARC [32].
Recently, we showed that peripheral injection of ghrelin activates a proportion of nesfatin-1 immunoreactive neurons in the ARC [14]. These results were interesting in light of previous studies showing that ghrelin primarily activates NPY neurons in the ARC [15,23,29]. Therefore, nesfatin-1 may not only be co-localized with POMC/CART neurons, but also with NPY. To investigate these hypotheses double-labeling studies were performed for NPY, CART, nesfatin-1 and also pmTOR in the ARC.
2. Methods
2.1. Animals
Male Sprague–Dawley rats (Harlan-Winkelmann Co., Borchen, Germany) weighing 250–300 g were housed in a group of four rats under conditions of controlled illumination (12:12 h light/dark cycle), humidity, and temperature (22 + 2 °C) for at least 21 days prior to the experiments. Animals were fed with a standard rat diet (Altromin®, Lage, Germany) and tap water ad libitum. To minimize the stress response, all rats were accustomed to the experimental conditions for a period of 14 days by handling them daily and putting them in a supine position to mimic the procedure of intraperitoneal (ip) injection of the anesthetic immediately before carrying out the transcardial perfusion. The handling was carried out between 9:00 and 11:00 h at the beginning of the light phase. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the state authority for animal research conduct.
2.2. Experimental protocol
Ad libitum fed rats were sacrificed during the light phase between 9:00 and 11:00 am. Rats (n = 4) were deeply anesthetized with 100 mg/kg ketamine (Ketanest®, Curamed, Karlsruhe, Germany) and 10 mg/kg xylazine (Rompun® 2%, Bayer, Leverkusen, Germany) and heparinized with 2500 IU heparin ip (Liquemin®, Hoffmann-La Roche, Grenzach-Whylen, Germany). Transcardial perfusion was performed as described before [16].
2.3. Double labeling immunohistochemistry for nesfatin-1, pmTOR, CART, and NPY in the arcuate nucleus of the hypothalamus
Brain sections (25 μm) were pre-treated with a 1% (w/v) sodium borohydride solution for 15 min. After rinsing sections in PBS for three times, sections were incubated with 3% (v/v) H2O2 in PBS to block peroxidase activity in tissue. After rinsing in TNT wash buffer (0.1 M Trizma hydrochloride, 0.15 M NaCl and 0.05% (v/v) Tween 20, pH 7.5) for three times, sections were incubated in TNT blocking buffer (0.1 M Trizma hydrochloride, 0.15 M NaCl and 1% (w/v) donkey normal serum, pH 7.5) for 1 h. Afterwards, the diluted primary antibody solution (rabbit anti-CART; Phoenix-Pharmaceuticals, Inc., 1:2000; or rabbit anti-Phospho-mTOR (pmTOR); Cell Signaling Technology, Inc., Danvers, MA, USA; 1:100; or rabbit anti-NPY; Phoenix-Pharmaceuticals, Inc., Burlingame, CA, USA, 1:500 in TNT blocking buffer) was applied for 48 h at room temperature. After rinsing the sections in TNT wash buffer three times, and incubation in a solution containing 3% (w/v) goat serum and 0.1% (v/v) sodium azide in TNT buffer for 1 h, sections were incubated with the secondary antibody goat biotin-SP-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 12 h at room temperature (1:2000 in 3% (w/v) goat normal serum and 0.1% (v/v) sodium azide in TNT buffer).
After rinsing in TNT wash buffer three times (sodium azide free), sections were incubated in avidin-biotin peroxidase complex (ABC; 1:1000; Vector Laboratories, UK) in TNT buffer for 5 h. Subsequently, sections were rinsed in TNT wash buffer three times again, and then incubated in TSA™ fluorescein or tetramethyl rhodamine tyramide in amplification solution (PerkinElmer, Waltham, MA, USA) for 10 min at room temperature. After rinsing sections in TNT wash buffer three times, sections were incubated with the second primary antibody solution (rabbit anti-Nesfatin-1; Phoenix-Pharmaceuticals, Inc., 1:500, or rabbit anti-CART; Phoenix-Pharmaceuticals, Inc., 1:400; or rabbit anti-NPY; Phoenix-Pharmaceuticals, Inc., 1:500 in PBS containing 1% (w/v) donkey normal serum) for 48 h at room temperature.
After washing in PBS for three times, brain sections were treated for 12 h with the secondary antibody (fluorescein isothiocyanate (FITC) labeled donkey anti-rabbit IgG; 1:200 or with tetramethyl rhodamine isothiocyanate (TRITC) labeled donkey anti-rabbit IgG, 1:200; Jackson ImmunoResearch Laboratories Inc. in 1% (w/v) goat normal serum, and 0.1% (v/v) sodium azide in PBS) at room temperature. After washing in PBS for three times, sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 min to counterstain cell chromatin. Sections were rinsed in PBS three times again, then embedded in 10 μl antifading solution (100 mg/ml 1,4-diazabicyclo [2.2.2] octane (Sigma-Aldrich Corp., St. Louis, MO, USA) in 90% (v/v) glycerine, 10% (v/v) PBS, pH 7.4), and analyzed using a confocal laser scanning microscope (cLSM 510 Meta, Carl Zeiss, Jena, Germany).
2.4. Data analysis
One out of eight of all consecutive coronal 25 μm sections were counted for nesfatin-1-, phospho-mTOR-, NPY-, and CART-ir staining bilaterally in the ARC (n = 4 rats; Bregma −2.12 to −3.60 mm) according to the coordinates by Paxinos and Watson's rat brain atlas [25]. Quantitative assessment of nesfatin-1-, CART-, NPY- and phospho-mTOR-ir was achieved by counting the total number of immunoreactive neurons, and double labeled neurons (CART + pmTOR; nesfatin-1 + CART; pmTOR + nesfatin-1; NPY + -nesfatin-1; NPY + pmTOR) in the ARC. All data are presented as mean ± SE.
3. Results
On average, we found 53 ± 10 CART-ir (n = 4 rats) neurons per section (∼23% of the counted neurons), 78 ± 18 phospho-mTOR-ir (n = 4 rats) neurons/section (∼34% of the counted neurons), 97 ± 23 nesfatin-1-ir (n = 4 rats) neurons/section (∼42% of the counted neurons) and 65 ± 22 NPY-ir (n = 4 rats) neurons/section (∼27% of the counted neurons) in the ARC. CART immunoreactive neurons were located predominantly in the lateral part of the ARC, whereas NPY-ir cells were found mostly in the ventral part of the ARC. PmTOR neurons were evenly distributed in the ARC, whereas more nesfatin-1 immunoreactive neurons were found in the lateral part.
Double labeling for nesfatin-1 (102 ± 33 neurons/sections) and pmTOR (81 ± 17 neurons/section) revealed that 47 ± 10 neurons/section were immunoreactive for both peptides (Figs. 1A and 2A). Consequently, 59 ± 5% of the pmTOR-ir neurons were immunoreactive for nesfatin-1. Moreover, double labeling for nesfatin-1 (117 ± 14 neurons/sections) and NPY (46 ± 6 neurons/section) exhibited that 19 ± 5% of the NPY positive cells were also immunoreactive for nesfatin-1 (9 ± 3 neurons/section, Figs. 1B and 2B). Immunostaining of nesfatin-1 (92 ± 8 neurons/section) and CART (60 ± 10 neurons/section) revealed 36 ± 6 neurons/section to be positive for both peptides (Figs. 1C and 3A), corresponding to 60 ± 10% of CART-ir neurons being co-localized with nesfatin-1.
Fig. 1.
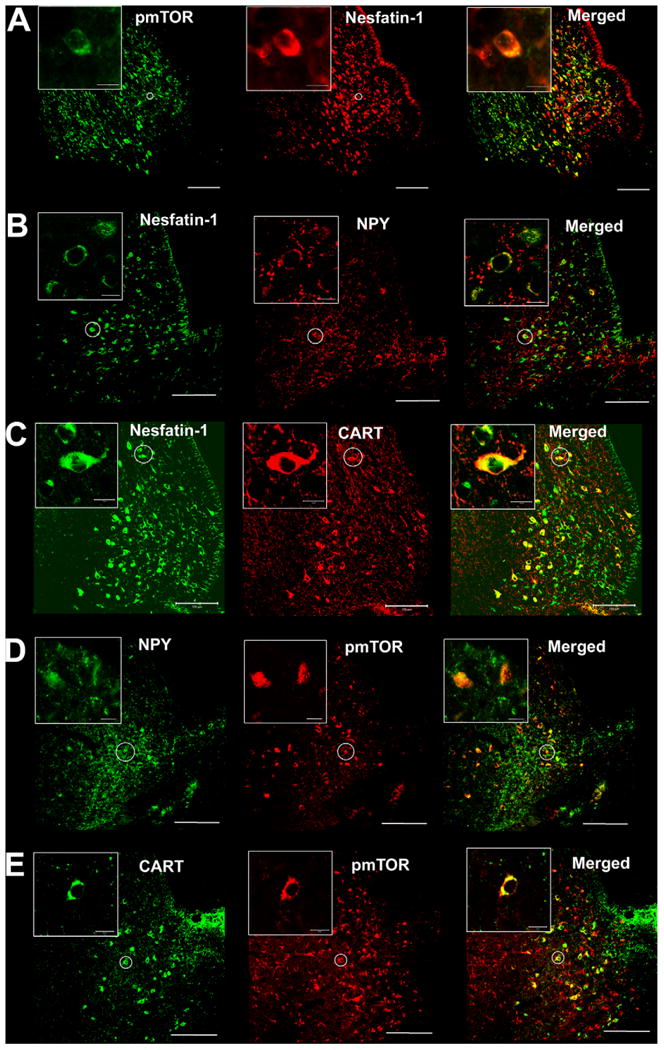
Confocal microscopy of double labeled neurons in the rat arcuate nucleus of the hypothalamus (ARC) with antibodies against nesfatin-1, pmTOR, NPY and CART. The major portions of the pmTOR-ir (green) neurons were also immunoreactive for nesfatin-1 (red) (A). A proportion of NPY neurons (red) were also immunopositive for nesfatin-1 (green) (B). Most of the CART neurons (red) displayed also nesfatin-1 immunoreactivity (green) (C). A considerable portion of NPY neurons (green) showed also pmTOR immunoreactivity (red) (D). Moreover, a portion of CART neurons (green) co-labeled with pmTOR (red) (E). The inserts display a zoom-in of double labeled neurons. The white scale bar represents 100 and 10 μm in the inserts. (For interpretation of the references to color in this figure caption, the reader is referred to the web version of the article.)
Fig. 2.
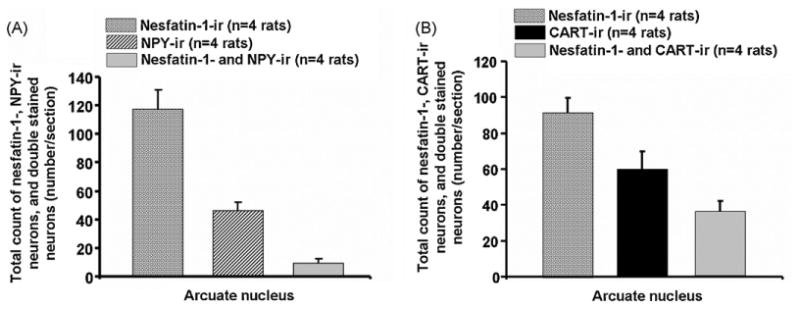
Total number of neurons immunopositive for nesfatin-1 and NPY (A) or nesfatin-1 and CART (B) as well as number of double labeled neurons in the ARC for the respective maker (A and B). Data are expressed as mean and SE of the number of rats indicated in parentheses.
Fig. 3.
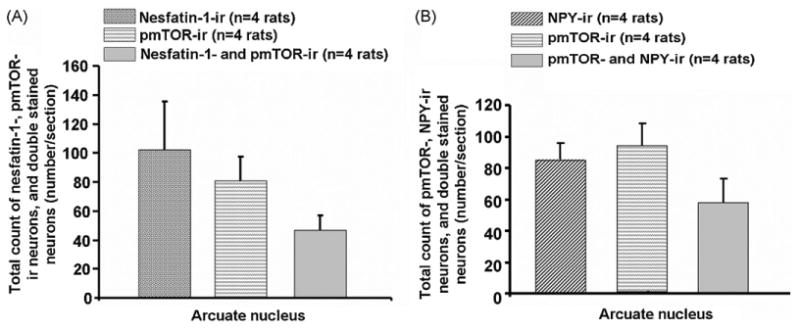
Total number of neurons immunoreactive for nesfatin-1 and pmTOR (A) or NPY and pmTOR (B) as well as number of double labeled neurons in the ARC for the respective maker (A and B). Data are expressed as mean and SE of the number of rats indicated in parentheses.
Additionally, 58 ± 15 neurons/section displayed co-localization for NPY (84 ± 10 neurons/section) and pmTOR (93 ± 15 neurons/section) corresponding to 70 ± 17% of the NPY neurons being also positive for pmTOR (Figs. 1D and 3B). Staining for CART and pmTOR revealed that 38 ± 5% (17 ± 2 neurons/section) of CART neurons (46 ± 5 neurons/section) were also immunoreactive for pmTOR (61 ± 1 neurons/section; Figs. 1E and 4).
Fig. 4.
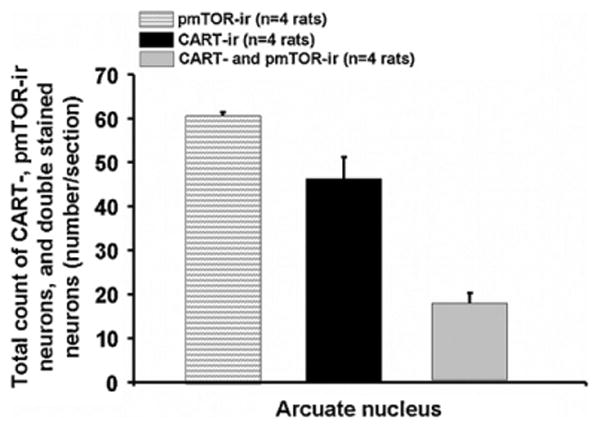
Total number of pmTOR-, CART- and double labeled neurons in the ARC. Data are expressed as mean and SE of the number of rats indicated in parentheses.
4. Discussion
In this investigation, we studied the distribution of nesfatin-1, pmTOR, CART and NPY in neurons in the ARC by means of immunohistochemical double labeling. We observed nesfatin-1 being co-localized with pmTOR neurons, and, to a lesser extent, with NPY neurons. These observations – co-expression of nesfatin-1 and pmTOR as well as the co-localization of nesfatin-1 with NPY in ARC neurons – have been described for the first time in the present study.
Recent studies showed, that NPY/AgRP and POMC/CART neurons in the ARC are co-localized with two other regulators of food intake, the above-mentioned peptides nesfatin-1 and mTOR [4,24]. It has been reported, that ∼90% of the NPY neurons in the ARC are co–localized with the phosphorylated, i.e. activated form of the serine–threonine–kinase mTOR (pmTOR) [4]. This enzyme plays a role in the integration of peptidergic signals regulating food intake behavior [4]. An increased activity of mTOR suppresses food intake when energy supply is sufficient [4]. Accordingly, if only few high energy substrates are available to fasted animals, mTOR activity in the ARC is decreased, thus stimulating food intake [4]. A decreased mTOR activity was found to lead to increased NPY expression, thus presumably stimulating food intake in test animals [4]. In the present study we could show the co-localization of a high proportion of pmTOR neurons with NPY or CART in the ARC. However, in contrast to NPY, it remains to be established whether mTOR influences CART expression in the ARC.
In the present study, we were able to show that ∼64% of the nesfatin-1-ir neurons are co-localized with CART. Nesfatin-1 immunoreactivity could be detected in several brain nuclei of the hypothalamus such as the supraoptic nucleus (SON), the paraventricular nucleus of the hypothalamus (PVN), and others including the ARC [1,8-10,12,17,24]. Additionally, co-localization of nesfatin-1 in ARC neurons has been also reported for neurotensin, GHRH, and α-MSH [8].
Nesfatin-1 exerts an inhibitory effect on food intake after icv or ip injection [24,28]. Chronic icv administration of nesfatin-1 induces a loss in body weight in rodents [24]. The expression of NUCB2 mRNA in the PVN in fasted rats is decreased. Consequently, the expression of NUCB2 mRNA in this brain nucleus was found to increase after re-feeding [24]. The adaptive expression pattern of the NUCB2 mRNA shows the potentially regulative impact of this peptide on food intake in mammals. Presumably, the expression of NUCB2 mRNA is also regulated by the neuropeptide α-MSH [24]. However, electrophysiological studies performed on brain sections showed that nesfatin-1 inhibits orexigenic NPY neurons [26]. Taking this into account, it appears feasible that neighboring nesfatin-1 neurons in the ARC induce an inhibition of NPY neurons which in turn leads to a reduction in food intake [26].
To date, it is not known, how intracellular regulation of nesfatin-1 expression is controlled. It is an interesting observation from our present study that ∼50% of the nesfatin-1 immunoreactive neurons in the ARC are co-localized with pmTOR. Furthermore, we found, as previously described [24], ∼60% of the CART-ir neurons in the ARC to be also positive for nesfatin-1. Taking into account that CART and nesfatin-1 both inhibit food intake after icv injection and that a great number of CART-ir neurons in the ARC is co-localized with nesfatin-1, suggests a potentially synergetic effect on the inhibition of food intake. Nevertheless, the physiological role of the co-localization of pmTOR, CART and nesfatin-1, which all have anorexigenic functions, remains to be further evaluated. It is feasible that pmTOR may modulate the activity of CART and nesfatin-1 in these cells, or they could act in concert to inhibit food intake.
We observed in this study that ∼19% of the NPY neurons in the ARC are immunoreactive for nesfatin-1. The physiological role of this co-localization is still unknown. It is conceivable that nesfatin-1 has other functions that have not yet been identified as suggested by previous report that nesfatin-1 immunoreactive neurons are activated by restraint stress [11]. Moreover, nesfatin-1 could play a modulatory role one the release of NPY thereby modulating food intake in an inhibitory manner. This speculation is supported by the observation of nesfatin-1 in the neuronal bodies but not in fibers [8,10].
In conclusion, we showed that a portion of the NPY neurons in the ARC is immunoreactive for the satiety peptide nesfatin-1. The physiological role of the intracellular co-localization remains to be investigated. Additionally, we found that a number of pmTOR neurons are also nesfatin-1 positive. We could also confirm the results from earlier studies demonstrating the intracellular co-localization of pmTOR/NPY, pmTOR/CART and CART/nesfatin-1.
Acknowledgments
This work was supported by grants from the German Research Foundation to P.K. (DFG KO 3864/2-1), A.S. (DFG STE 1765/1-1), M.G. (GO 1718/1-1), and from Charité-Universitätsmedizin Berlin to P.K. (UFF 09/41730 and 09/42458) as well as Veterans Administration Research Career Scientist Award (Y.T.), Veterans Administration Merit Award (Y.T.), NIHDK 33061 (Y.T.), Center Grant DK-41301 (Animal Core) (Y.T.).
References
- 1.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, et al. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–94. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- 2.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998;95:15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–9. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 4.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 5.Edwards CM, Abbott CR, Sunter D, Kim M, Dakin CL, Murphy KG, et al. Cocaine- and amphetamine-regulated transcript, glucagon-like peptide-1 and corticotrophin releasing factor inhibit feeding via agouti-related protein independent pathways in the rat. Brain Res. 2000;866:128–34. doi: 10.1016/s0006-8993(00)02257-5. [DOI] [PubMed] [Google Scholar]
- 6.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–85. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 7.Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, et al. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 8.Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–79. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, et al. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–81. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Goebel M, Stengel A, Wang L, Lambrecht NW, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–6. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–24. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandt D, Siewert J, Sieburg B, al Tai O, Schimiczek M, Goebell H, et al. Peptide YY inhibits exocrine pancreatic secretion in isolated perfused rat pancreas by Y1 receptors. Pancreas. 1995;10:180–6. doi: 10.1097/00006676-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–2. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 14.Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–68. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agoutirelated protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–43. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 16.Kobelt P, Tebbe JJ, Tjandra I, Bae HG, Rüter J, Klapp BF, et al. Two immunocytochemical protocols for immunofluorescent detection of c-Fos positive neurons in the rat brain. Brain Res Brain Res Protoc. 2004;13:45–52. doi: 10.1016/j.brainresprot.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, et al. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 18.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamineregulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–32. [PubMed] [Google Scholar]
- 19.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 20.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–8. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Larsen PJ, Vrang N, Petersen PC, Kristensen P. Chronic intracerebroventricular administration of recombinant CART(42-89) peptide inhibits and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes Res. 2000;8:590–6. doi: 10.1038/oby.2000.76. [DOI] [PubMed] [Google Scholar]
- 22.Miura K, Titani K, Kurosawa Y, Kanai Y. Molecular cloning of nucleobindin, a novel DNA-binding protein that contains both a signal peptide and a leucine zipper structure. Biochem Biophys Res Commun. 1992;187:375–80. doi: 10.1016/s0006-291x(05)81503-7. [DOI] [PubMed] [Google Scholar]
- 23.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 24.Oh I, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 26.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, et al. A C–terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu H, Oh I, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–71. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 29.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–32. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 30.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA. 1985;82:3940–3. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrang N, Larsen PJ, Clausen JT, Kristensen P. Neurochemical characterization of hypothalamic cocaine-amphetamine-regulated transcript neurons. J Neurosci. 1999;19:RC5. doi: 10.1523/JNEUROSCI.19-10-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]


