Abstract
Background
Sleep quality may be an important, yet relatively neglected, predictor of treatment outcome in cognitive-behavioral therapy (CBT) for anxiety disorders. Specifically, poor sleep quality may impair memory consolidation of in-session extinction learning. We therefore examined sleep quality as a predictor of treatment outcome in CBT for social anxiety disorder and the impact of d-cycloserine (DCS) on this relationship.
Methods
One hundred sixty-nine participants with a primary diagnosis of DSM-IV generalized social anxiety disorder were recruited across three sites. Participants were enrolled in 12 weeks of group CBT. Participants randomly received 50 mg of DCS (n = 87) or pill placebo (n = 82) 1 hr prior to sessions 3–7. Participants completed a baseline measure of self-reported sleep quality and daily diaries recording subjective feelings of being rested upon wakening. Outcome measures including social anxiety symptoms and global severity scores were assessed at each session.
Results
Poorer baseline sleep quality was associated with slower improvement and higher posttreatment social anxiety symptom and severity scores. Moreover, patients who felt more “rested” after sleeping the night following a treatment session had lower levels of symptoms and global severity at the next session, controlling for their symptoms and severity scores the previous session. Neither of these effects were moderated by DCS condition.
Conclusions
Our findings suggest that poor sleep quality diminishes the effects of CBT for social anxiety disorder and this relation is not attenuated by DCS administration. Therapeutic attention to sleep quality prior to initiation of CBT and during the acute treatment phase may be clinically indicated.
Keywords: sleep quality, cognitive behavioral therapy, psychotherapy, social anxiety disorder, social phobia, d-cycloserine
INTRODUCTION
Sleep difficulties are common in patients with anxiety disorders; thus, improvements in sleep quality have frequently been examined as an outcome of cognitive behavioral therapy for anxiety disorders.[1] However, it is possible that the reverse relationship holds such that sleep quality is predictive of treatment outcome in cognitive-behavioral therapy (CBT) for anxiety disorders. Sleep has been shown to be important for memory and learning processes.[2] In exposure-based treatments in particular, poor sleep quality may impair memory consolidation of extinction learning, which is thought to be crucial for treatment gains.[3]
Several studies using experimental fear conditioning paradigms with healthy subjects have shown that sleep deprivation is associated with impaired extinction learning,[4] poorer recall of fear extinction,[5, 6] and reduced generalization of extinction to novel stimuli.[7] These findings suggest that in the context of treatment, poor sleep quality may be associated with inferior treatment outcome due to poor in-session extinction learning, poor between-session recall of extinction learning, and reduced generalization of treatment exposures to stimuli encountered in vivo. Although these analog studies provide initial evidence that sleep affects extinction learning, they do not indicate whether the effect of sleep on extinction learning ultimately results in inferior CBT treatment outcome among individuals with clinical disorders. One study showed that among women with significant spider fears (established by self-report cutoffs) who were given simulated exposure therapy for spider phobia, sleep deprivation resulted in extinction loss, and poorer generalization of extinction to novel stimuli.[8] Another recent study showed that individuals who were asked to take a 90-min nap after a one-session virtual reality exposure for spider phobia showed better reductions in self-reported fear and catastrophic spider-related cognitions during approach to a live spider than individuals who took no nap.[9] These studies suggest that experimental manipulation of sleep impacts treatment outcome for anxiety disorders; however, it remains unclear whether naturally occurring differences in sleep quality predict treatment outcome.
If poor sleep quality does, in fact, cause reduced extinction learning and poorer treatment outcome, an important question is how to overcome these deficits. One possibility is the use of pharmacological agents that promote fear learning. D-cycloserine (DCS), an analog of D-alanine and a partial agonist at the NMDA receptor has been shown to augment fear learning[10] and enhance treatment outcomes of CBT for anxiety including social anxiety disorder.[11–13] Moreover, research shows that administration of DCS to sleep deprived animals partially reverses the negative effects of sleep on extinction learning.[4] These findings suggest that DCS may be able to mitigate any negative effects of sleep disturbance on treatment outcome by counteracting loss of memory consolidation due to sleep disruption. However, it is unclear whether the DCS effects will simply be additive to the effects of sleep disturbance (i.e. equally improving the results of those with and without sleep disturbance), or whether DCS might be particularly beneficial for those with sleep disturbance, thus moderating the effect of sleep disturbance on outcomes. This latter effect would be evidenced by an interaction between DCS and sleep disturbance.
We sought to address an important gap in the literature by examining sleep quality as a prognostic indicator of CBT for social anxiety disorder. Moreover, we sought to explore whether DCS augmentation of CBT impacts the effect of sleep quality on treatment outcome. We analyzed data from a randomized controlled trial of DCS augmentation of CBT for social anxiety disorder. This study showed that individuals receiving DCS had a faster rate of symptom improvement during the course of the 12-week treatment compared to those receiving placebo.[14] In this study, we measured baseline levels of perceived sleep quality as measured by self-report and weekly ratings of the subjective sense of nonrestorative sleep or how “rested” participants felt the morning after each therapy session, reflecting the night of sleep after the therapy session. Data from two nationally representative samples show that the presence of social anxiety disorder is associated with disturbance in both of these indicators of sleep quality. Specifically, individuals with social anxiety disorder reported higher levels of difficulty initiating sleep, difficulty maintaining sleep, early morning awakening, and nonrestorative sleep compared to individuals without social anxiety disorder.[15, 16] Moreover, evidence suggests that nonrestorative sleep is more consistently associated with role impairment than other sleep problems.[15] Our primary hypotheses were (1) that pretreatment self-reported sleep quality would predict treatment outcome such that those with poorer sleep quality would evidence fewer treatment gains and higher endpoint severity, and (2) that feeling more rested the morning after a therapy session (session X) would be related to better outcome at the next session (session X + 1) when controlling for outcome at previous session (session X). We also investigated whether DCS moderated the relationship between perceived sleep quality and treatment outcome such that the relationship between sleep quality and treatment outcome would be weaker in the group receiving DCS compared to those receiving placebo.
MATERIALS AND METHODS
PARTICIPANTS
Participants (N = 169) with a primary diagnosis of DSM-IV generalized social anxiety disorder were recruited across three study sites (Boston University [BU], Massachusetts General Hospital [MGH], and Southern Methodist University [SMU]). To be eligible, participants had to be 18 years of age or older, have a primary diagnosis of generalized social anxiety disorder, and a total score on the Liebowitz Social Anxiety Scale of 60 or greater. Participants were excluded if they had a lifetime history of bipolar disorder, schizophrenia, psychosis, delusional disorders, obsessive-compulsive disorder, seizures, or significant head trauma; had an eating disorder, posttraumatic stress disorder, or a history of substance or alcohol abuse or dependence in the last 6 months; reported significant suicidal ideation; were on psychotropic medications (including sleep medications) or isoniazid; were engaged in concurrent psychotherapy initiated within 3 months of baseline or ongoing psychotherapy directed specifically toward the treatment of social anxiety disorder; had previously not responded to adequately delivered exposure treatment; had a serious medical illness or instability for which hospitalization may be likely the following year; or were pregnant or lactating. The integrity and reliability of the diagnostic assessments, efficacy evaluations, and treatment intervention were established through training and cross-site supervision by the senior clinicians.[14]
PROCEDURE
All three sites used identical study protocols. The protocol was approved by the Institutional Review Boards of BU, MGH, and SMU and all patients provided written informed consent after the study procedures were explained. Participants were recruited through advertisements and referrals from local clinical facilities and programs. Participants completed an initial telephone screen to ensure basic eligibility criteria were met. Potential participants then completed an eligibility session consisting of a psychiatric assessment, medical history, and physical examination. Eligible participants were then enrolled into a CBT protocol consisting of 12 weekly, 2.5-hr sessions conducted in a group format with four to six patients and two therapists per group. Briefly, the treatment protocol included exposure to fear cues with the goal of fear extinction (sessions 3–7), cognitive restructuring (sessions 8–12), and in vivo exposure practice to challenge maladaptive cognitions (sessions 8–12). At session 3, participants were randomly assigned (stratified by baseline social anxiety disorder severity) to oral administration of either 50 mg of d-cycloserine (n = 87) or pill placebo (n = 82) 1-hr prior to sessions 3–7. Detailed methodology of the parent RCT are available elsewhere.[14]
MEASURES
Covariates
Several covariates were examined based on previous research on this dataset indicating that they were related to treatment outcome.[14, 17] Demographic covariates included gender, ethnicity, and cohabitation status (binary variable). Covariates reflecting baseline severity included number of comorbid disorders, neuroticism based on the 60-item NEO Five-Factor Inventory,[18] and baseline depression symptoms based on the Montgomery-Åsberg Depression Scale (MADRS).[19]
Perceived Sleep Quality
Two self-report measures evaluated perceived sleep quality. Baseline sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI[20]). The PSQI is a well-validated self-report instrument that assesses sleep quality in the past month. The measure consists of seven component items (range 0–3): subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. These component items are summed to create a global PSQI score with higher scores reflecting poorer sleep quality (range 0–21). In the current study, participants reported an average baseline global sleep quality score of 6.42 (SD = 3.20, range 0–17). A score of 5 has been shown to differentiate between “good” sleepers (lower scores) and “poor” sleepers (higher scores); using this cutoff, 56% of the current sample were “poor” sleepers and 44% of the sample were “good” sleepers.[20] Of note, there were no significant differences in baseline PSQI scores for individuals in the DCS (M = 6.67, SD = 3.53) versus placebo group (M = 6.11, SD = 2.87, P = .27). Perceived sleep quality over the course of the intervention was assessed with weekly diary ratings of how rested participants felt in the morning after each therapy session (restedness). Participants responded to the prompt “This morning I feel rested” using a 5-point Likert-type scale from “Not at all” to “Extremely”; thus, higher ratings reflect feelings of being better rested. These data reflect perceived sleep quality for the night in which memory consolidation of in-session learning was occurring.
Treatment Outcome
Two measures were used to assess treatment outcome, the Liebowitz Social Anxiety Scale (LSAS[21]) and the Clinical Global Impression—Severity Scale (CGI-S[22]). The LSAS is a 48-item clinician-rated scale that assesses severity of social anxiety symptoms by asking participants about their level of fear and avoidance in social and performance situations. The CGI-S is a single-item clinician-rated measure that reflects overall clinical severity on a 1 (“normal, not at all ill”) to 7 (“extremely ill”) scale. The LSAS and CGI-S were assessed at weeks 0, 2, 3, 4, 5, 6, 7, 8, 10, 12, and at posttreatment by clinicians blind to treatment condition.
DATA ANALYSIS PLAN
Multilevel models (MLM) were used to analyze the data. Level 1 of the MLM was comprised of the repeated assessments of the outcomes (LSAS and CGI-S) over sessions, which were nested within subjects (level 2). In all of our MLM analyses, we controlled for the following variables at baseline to help insure that the effects of sleep quality were not due to these potential confounding variables: social phobia symptoms (LSAS), global severity (CGI-S), baseline depression symptoms (MADRS), number of comorbid disorders, neuroticism, gender, ethnicity, and cohabitation status. These variables were included as covariates because previous research on this dataset indicated that they were related to outcome.[17] In addition, treatment condition (DCS versus Placebo) was included in all analyses.
Two sets of analyses were performed. In the first set, we examined the impact of baseline sleep quality measured by the PSQI on posttreatment levels of outcome (LSAS and CGI-S) as well as on the slope of improvement in outcome over the course of acute treatment (through posttreatment). Previous analyses of these outcome data indicated that the slope of change in these outcomes over time was linear[14]; therefore, time was modeled as linear (in weeks from baseline) in the current analyses, but was “centered” at week 13 (post-treatment) so that the intercept would reflect the effect of baseline sleep quality on posttreatment levels of the outcome. Previous analyses also indicated that the slope was related to treatment condition, ethnicity, cohabitation status, and initial severity, so the interactions between each of these predictors and time were also included in these analyses. To test the relation between baseline sleep quality and outcome, PSQI score was included as a level-2 predictor of the slope over time. To investigate whether DCS treatment might moderate the relation between baseline sleep quality and outcome, we also included the three-way interaction between baseline sleep quality, time, and condition and the two-way interaction between baseline sleep quality and condition (the other two-way interactions among these three variables were already included in the model [see above]). We hypothesized that baseline sleep quality would moderate the slope of improvement in outcome over time (a baseline sleep quality × time interaction) such that participants with poorer sleep quality would demonstrate slower rates of decline in symptom severity over time. We also hypothesized the participants with poorer baseline sleep quality would have higher levels of symptom severity at posttreatment.
Our second set of analyses examined the effect of session-by-session feelings of restedness on outcome. In these MLM analyses, restedness ratings following sleep the night after each weekly therapy session was used to predict symptoms and global severity (LSAS and CGI-S) at the beginning of the next therapy session. To control for the possibility that restedness might be related to next week’s symptom severity simply because it was related to this week’s symptom severity, we also controlled for the current week’s symptom severity. In addition, the interaction of DCS condition and restedness was included as a predictor of next week’s symptom severity to investigate whether the effect of restedness on severity would be moderated by condition. We hypothesized that greater restedness would predict lower symptom severity the next week.
In all analyses, we used the Satterthwaite approximation[23] to calculate the degrees of freedom for the significance tests for the regression coefficients, which yields different degrees of freedom for each t-test.
RESULTS
Means, standard deviations, and correlations among the study variables (at baseline) are presented in Table 1. Poorer baseline sleep quality was associated with higher baseline LSAS (r = .19, P = .013), CGI-S (r = .17, P = .026), MADRS (r = .50, P < .001), neuroticism (r = .31, P < .001), and a greater number of comorbid disorders (r = .31, P < .001). Baseline sleep quality was not related to sex, ethinicity, or cohabitation (Ps > .141).
TABLE 1.
Baseline means, SDs, and correlations for primary study variables
| Mean | SD | PSQI | Rested | LSAS | |
|---|---|---|---|---|---|
| PSQI | 6.40 | 3.23 | |||
| Rested | 1.74 | 0.93 | − 0.17* | ||
| LSAS | 81.57 | 16.07 | 0.19* | −0.13 | |
| CGI-S | 5.29 | 0.84 | 0.17* | −0.11 | 0.76** |
Note. PSQI, Pittsburgh Sleep Quality Index; Rested, how “rested” participants felt the morning after the session, LSAS, Liebowitz Social Anxiety Scale, CGI-S, Clinical Global Impression—Severity Scale.
P < .05,
P < .01.
THE RELATION BETWEEN BASELINE SLEEP QUALITY AND TREATMENT OUTCOME
Initial analyses showed that condition (DCS, PLA) did not moderate the relation between baseline sleep quality and outcome, neither as indexed by severity at posttreatment (P = .701 for LSAS and P = .907 for CGI-S) nor the slope of improvement in symptoms over time (P = .505 for LSAS and P = .656 for CGI-S). Thus, these interaction terms were dropped and the models recomputed. Results indicated that poorer baseline sleep quality predicted higher levels of social anxiety symptoms and global severity at posttreatment, b = 4.25, t(122) = 2.86, P = .005 for LSAS and b = .20, t(160) = 1.98, P = .049 for CGI-S (see Figs. 1 and 2). Similarly, those with poorer baseline sleep quality also showed significantly slower improvement in symptoms and severity over time, b = 0.26, t(232) = 2.52, P = .012 for the baseline sleep quality × time interaction for LSAS, and b = 0.02, t(147) = 2.34, P = .021 for the baseline sleep quality × time interaction for CGI-S.
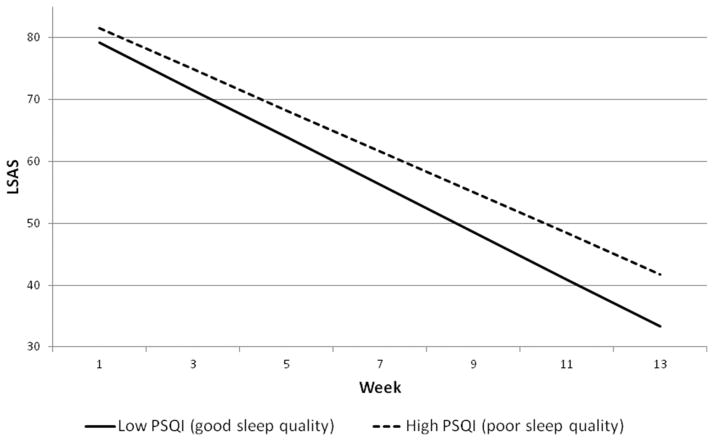
Figure 1.
Slope of change in LSAS for participants high or low in baseline sleep quality. High PSQI represents 1 standard deviation above the mean. Low PSQI represents 1 standard deviation below the mean.
Figure 2.
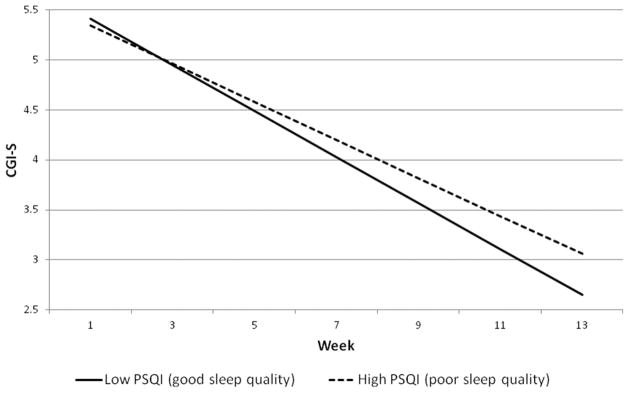
Slope of change in CGI-S for participants high or low in baseline sleep quality. High PSQI represents 1 standard deviation above the mean. Low PSQI represents 1 standard deviation below the mean.
We used the approach suggested by Aiken and West[24] to probe the nature of these baseline sleep quality × time interactions. Following Aiken and West, we plotted the change in outcome over time for participants with low (1 SD below the mean) or high (1 SD above the mean) PSQI scores (see Figs. 1 and 2). This technique uses data from all participants to calculate expected growth curves for participants with specified levels of PSQI (in our case, we calculated the growth curves for participants whose PSQI was 1 SD below the mean and for participants whose PSQI was 1 SD above the mean; see Figs. 1 and 2). The slope of improvement in outcome was faster for participants low on baseline PSQI (1 SD below the mean; PSQI = 3.22, reflecting lower sleep disturbance) compared to those high on baseline PSQI (1 SD above the mean; PSQI = 9.62, reflecting greater sleep disturbance); for LSAS, low PSQI: b = −3.84, t(240) = 25.89, P < .001 versus high PSQI: b = −3.31, t(227) = 22.92, P < .001, and for CGI-S, low PSQI: b = −0.23, t(151) = 16.10, P < .001 versus high PSQI: b = −0.19, t(145) = 13.24, P < .001. The net effect of these slope differentials at posttreatment between those low and high in baseline PSQI is 8.49 points on the LSAS (33.29 versus 41.79), and 0.41 points on the CGI-S (2.65 versus 3.06).
THE RELATION BETWEEN RESTEDNESS AND NEXT SESSION OUTCOME
The next set of analyses examined the relation between restedness the morning after each therapy session and severity at the beginning of the next session, controlling for symptom severity at the concurrent therapy session (as well as all the other control variables detailed above). Initial analyses showed that DCS treatment condition did not moderate the relation between restedness and severity at the next session (P = .754 for LSAS, P = .728 for CGI-S). Therefore, the interaction was dropped and the analyses were recomputed. The results showed that participants who reported greater restedness after therapy (i.e. those who felt more rested after sleep the night of the therapy session X) reported lower symptom and severity scores at the next session (session X+1), b = −0.53, t(428) = 2.06, P = .040 for LSAS and b = −0.04, t(295) = −2.20, P = .028 for CGI-S, controlling for their symptoms and severity scores the previous session (session X). Thus, the per-week difference between those who felt “not at all” rested (0) and those who felt “extremely” rested (4) was 2.12 points on the LSAS scale and 0.16 points on the CGI-S. A 1 point difference on the restedness scale every week translated into a 6.4 point difference on LSAS and a .48 point difference on CGI-S at posttreatment.
DISCUSSION
To our knowledge, this is the first published study to examine perceived sleep quality as a predictor of treatment outcome in CBT for social anxiety disorder. Our findings are consistent with the hypothesis that sleep quality has a significant impact on the course and outcome of CBT for social anxiety disorder. Specifically, poorer baseline sleep quality was associated with slower improvement over time and poorer endpoint treatment outcome. How “rested” individuals were after sleeping the night of their treatment session predicted subsequent symptom and global severity scores after accounting for prior symptom and severity levels. These analyses controlled for several indicators of severity, including baseline severity, baseline depressive symptoms, and number of comorbid disorders, suggesting that sleep quality is not merely an indicator of general distress, but rather an independent predictor of treatment outcome.
We also investigated whether treatment condition would moderate the relationship between perceived sleep quality and treatment outcome such that individuals who received DCS would show a diminished association between sleep quality and treatment outcome. DCS did not appear to moderate the negative effect of poor sleep quality. These findings suggest that although DCS and sleep quality both impact the rate of improvement during treatment, these effects are independent. This could mean that DCS impacts memory consolidation through a different mechanism than does sleep quality or that DCS facilitates fear extinction during wake, but not during sleep.[25] It is also possible that the effect of sleep quality affects treatment outcome through mechanisms other than memory consolidation. For example, sleep may impact emotion regulation,[26] which is involved in the maintenance of anxiety pathology.[27]Further research aimed at understanding the processes by which sleep quality is associated with treatment outcome would improve our ability to mitigate the negative impact of poor sleep on CBT.
Regardless of the specific mechanism of action, our findings suggest that clinicians should consider addressing sleep quality prior to initiating CBT for social anxiety disorder to achieve optimal treatment benefits. One case study showed that five sessions of CBT for insomnia was associated with improvements in sleep quality in an individual with comorbid insomnia and social anxiety disorder.[28] It is possible that even more minimal sleep interventions may be useful prior to initiating CBT for social anxiety disorder, such as starting with modules on sleep hygiene, relaxation, and/or cognitive restructuring of sleep-related beliefs among individuals who report poor sleep quality. Though pharmacological interventions such as hypnotics may also improve sleep, these medications have been shown to reduce rapid eye movement sleep,[29]which is thought to be particularly important for memory consolidation of extinction learning.[30] Similarly, certain psychosocial interventions for sleep, such as sleep restriction, may actually exacerbate anxiety and sleep disturbance.[31] Thus, care should be taken in selecting clinical interventions that improve sleep quality and further research should be done to identify how to improve sleep quality in a way that will most effectively and efficiently improve CBT outcomes.
Most anxiety disorders are associated with sleep impairment[15] and CBT for anxiety relies on similar treatment principles across disorders. Thus, it is possible that our findings and the clinical implications may apply to CBT treatment for anxiety disorders other than social anxiety disorder. Further research examining the relationship between sleep quality and treatment outcome for other anxiety disorders would be beneficial in determining whether sleep quality is a general prognostic indicator for CBT for anxiety disorders.
Several limitations should be considered when interpreting our results. Although we statistically controlled for several indicators of severity, it is possible that sleep quality captures some aspect of severity that is not assessed by the other covariates. Moreover, this study relied exclusively on self-report measures of perceived sleep quality. Objective measures of sleep quality would lend more confidence to our findings that sleep quality is prospectively associated with treatment outcome. Experimental manipulation of sleep is needed to definitively establish causality between sleep disturbance and treatment outcome. However, we sought to examine how naturally occurring sleep is linked to treatment outcome for individuals with anxiety disorders. Finally, it is important to note that the group treatment sessions were 2.5 hr in length. It is possible that the effect of sleep quality on treatment outcome may be stronger for treatments with longer sessions compared to treatments with shorter sessions. The relationship between sleep quality and treatment outcome should be examined in treatment studies with sessions of a more standard length to more closely reflect the potential impact of sleep quality on treatment outcome across clinical practice.
CONCLUSIONS
Our findings showed that poorer perceived sleep quality was associated with slower improvement and poorer treatment outcome in CBT for social anxiety disorder. We examined this relationship prospectively, controlling for prior social anxiety symptoms and general clinical severity scores as well as other indicators of severity and possible confounds. These findings suggest that clinicians should assess and treat poor sleep quality prior to initiating CBT for social anxiety disorder to achieve maximum therapeutic benefit. Further examination of the mechanisms by which sleep quality affects CBT outcomes and generalization of these findings to CBT for other anxiety disorders is warranted.
Acknowledgments
Contract grant sponsor: NIH; contract grant numbers: R01MH078308 (Hofmann) and R01MH075889 (Pollack).
This study was funded by NIH grants R01MH078308 (Hofmann) and R01MH075889 (Pollack) from the National Institute of Mental Health. The sponsor (NIH) had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
The authors would like to thank Stephen Wisniewski, Ph.D. for providing free statistical consultation during the design development and Richard G. Heimberg, Ph.D. for providing a 1-day training and consultation workshop in cognitive behavioral group therapy prior to participant enrollment. He received compensation for this contribution. We would also like to thank Ashley Witt, B.A., and Shelley Capozzoli, B.A. at Boston University for their help with the data management and data entry. We also thank the following individuals for serving as therapists or clinical assessors in the study: Allison Applebaum, Ph.D., Anu Asnaani, M.A. Jacqueline Bullis, M.A., Cassidy A. Gutner, M.A., John A. Richey, Ph.D., Alice T. Sawyer, M.A., Maria Steenkamp, Ph.D. (at Boston University); Lindsey DeBoer, M.A., Deborah Corbitt-Shindler, M.A., Katherine Croft, M.A., Catherine Dodson, M.A., Pamela Handelsman, B.A., Grant Holland, M.A., Kristin Julian, M.A., Erica Simon, M.A., Anne Miller, M.A., Matthew Leahy, Ph.D. (at Southern Methodist University); and Ryan Jacoby, B.A., Meghan Keogh, Ph.D., Libby Marks, B.A., Laura Morris, B.A., Don Robinaugh, M.A., Sharon Sung, Ph.D., (at Massachusetts General Hospital). These individuals received financial contribution for their institutional efforts.
Footnotes
Trial Registration: http://www.ClinicalTrials.gov, ID#NCT006339 84, http://www.clinicaltrials.gov/ct2/show/NCT00633984
Conflict of Interest: Dr. Zalta declares no conflicts of interest. Dr. Dowd reported receiving grant support from NIMH, Neuronetics, Cervel Neurotech, Otsuka, and the Research Foundation for Mental Hygeine and is a consultant for Cervel Neurotech. Dr. Rosen-field reported receiving grant funding from NIMH and consulting for the University of Miami. Dr. Smits reported receiving royalties from various book publishers unrelated to this study. Dr. Otto noted serving as a consultant for MicroTransponder Inc. and reported receiving royalties from multiple publishers, including Routledge, the publisher of the CBT manual used in this study. Dr. Simon reported receiving grant support from the American Foundation for Suicide Prevention, Forest Laboratories, NIMH, and the Department of Defense; consulting for the Massachusetts General Hospital Psychiatry Academy; and having stock options in Elan, Dandreon, G Zero, and Gatekeeper. Dr. Meuret reported receiving grant support from NIH and serving as a consultant for Palo Alto Health Sciences Inc. Dr. Marques reported consulting for the Massachusetts General Hospital Psychiatry Academy and receiving payment for manuscript preparation for Hazelden Publishing and Harvard Health Publications. Dr. Hofmann reported serving as a consultant for Merck-Schering/Plough, receiving grant support from NIMH, and receiving royalties from multiple publishers, including Routledge, the publisher of the CBT manual used in this study. Dr. Pollack noted the following disclosures for the preceding 36 months: Advisory Boards and Consultation: Corcept, Eli Lilly, Johnson and Johnson, Ironwood Pharmaceuticals, Medavante, Merck, Otsuka, Targia Phamaceuticals, and Transcept Research; Grants and Grants Pending: NIDA, NIMH, NCCAM, Bristol Myers Squibb, Euthymics, Forest Laboratories, GlaxoSmithKline, and Eli Lilly; Equity: Doyen Medical, Medavante, Mensante Corporation, Mindsite, Targia Pharmaceuticals; Royalty/patent: SIGH-A, SAFER interviews.
References
- 1.Belleville G, Cousineau H, Levrier K, et al. The impact of cognitive-behavior therapy for anxiety disorders on concomitant sleep disturbances: a meta-analysis. J Anxiety Disord. 2010;24:379–386. doi: 10.1016/j.janxdis.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 3.Craske MG, Kircanski K, Zelikowsky M, et al. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri AJ, Root DH. Effects of REM deprivation and an NMDA agonist on the extinction of conditioned fear. Physiol Behav. 2008;93:274–281. doi: 10.1016/j.physbeh.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Spoormaker VI, Schröter MS, Andrade KC, et al. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp. 2012;33:2362–2376. doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spoormaker VI, Sturm A, Andrade KC, et al. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J Psychiatr Res. 2010;44:1121–1128. doi: 10.1016/j.jpsychires.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Pace-Schott EF, Milad MR, Orr SP, et al. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Pace-Schott EF, Verga PW, Bennett TS, Spencer RMC. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res. 2012;46:1036–1044. doi: 10.1016/j.jpsychires.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margraf J, Kleim B, Wilhelm F, et al. Sleep enhances exposure therapy. Paper presented at the 46th Annual Meeting of the Association for Behavioral and Cognitive Therapies; National Harbor, MD. 2012. [Google Scholar]
- 10.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 11.Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of d-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann SG, Sawyer AT, Asnaani A. D-cycloserine as an augmentation strategy for cognitive behavioral therapy for anxiety disorders: an update. Curr Pharm Des. 2012;18:5659–5662. doi: 10.2174/138161212803530916. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann SG, Meuret AE, Smits JAJ, et al. Augmentation of exposure therapy for social anxiety disorder with d-cycloserine. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann SG, Smits JAJ, Rosenfield D, et al. D-cycloserine as an augmentation strategy of cognitive behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170:751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth T, Jaeger S, Jin R, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60:1364–1371. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsawh HJ, Stein MB, Belik S-L, et al. Relationship of anxiety disorders, sleep quality, and functional impairment in a community sample. J Psychiatr Res. 2009;43:926–933. doi: 10.1016/j.jpsychires.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Smits JAJ, Hofmann SG, Rosenfield D, et al. D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: prognostic and prescriptive variables. J Consult Clin Psychol. 2013 doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa PTJ, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 19.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 20.Buysee DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Heimberg RG, Horner KJ, Juster HR, et al. Psychometric properties of the Liebowitz social anxiety scale. Psychol Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- 22.Guy W. Assessment Manual for Psychpharmacology. Washington, DC: U.S. Government Printing Office; 1976. [Google Scholar]
- 23.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- 24.Aiken L, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, London: Sage; 1991. [Google Scholar]
- 25.Kuriyama K, Honman M, Yoshiike T, Kim Y. Valproic acid but not d-cycloserine facilitates sleep-dependent offline learning of extinction and habituation of conditioned fear in humans. Neuropharmacology. 2013;64:424–431. doi: 10.1016/j.neuropharm.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 27.Amstadter AB. Emotion regulation and anxiety disorders. J Anxiety Disord. 2008;22:211–221. doi: 10.1016/j.janxdis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang NK. Brief CBT-I for insomnia comorbid with social phobia: a case study. Behav Cogn Psychother. 2010;38:113–122. doi: 10.1017/S1352465809990488. [DOI] [PubMed] [Google Scholar]
- 29.Mednick SC, McDevitt EA, Walsh JK, et al. The critical role of sleep spindles in hippocampal-dependent memroy: a pharmacology study. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta S, O’Malley MW. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J Neurosci. 2013;33:4561–4569. doi: 10.1523/JNEUROSCI.5525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]