Abstract
The temporomandibular joint (TMJ) disc lacks functional replacement after discectomy. We investigated tissue-engineered bilayer polylactide (PLA) discs and autologous adipose stem cells (ASCs) as a potential replacement for the TMJ disc. These ASC discs were pre-cultured either in control or in differentiation medium, including transforming growth factor (TGF)-β1 for one week. Prior to implantation, expression of fibrocartilaginous genes was measured by qRT-PCR. The control and differentiated ASC discs were implanted, respectively, in the right and left TMJs of rabbits for six (n = 5) and 12 months (n = 5). Thereafter, the excised TMJ areas were examined with cone beam computed tomography (CBCT) and histology. No signs of infection, inflammation or foreign body reactions were detected at histology, whereas chronic arthrosis and considerable condylar hypertrophy were observed in all operated joints at CBCT. The left condyle treated with the differentiated ASC discs appeared consistently smoother and more sclerotic than the right condyle. The ASC disc replacement resulted in dislocation and morphological changes in the rabbit TMJ. The ASC discs pre-treated with TGF-β1 enhanced the condylar integrity. While adverse tissue reactions were not shown, the authors suggest that with improved attachment and design, the PLA disc and biomaterial itself would hold potential for TMJ disc replacement.
Keywords: temporomandibular joint disc, biodegradable replacement, discectomy, tissue engineering, polylactide, adipose stem cell
1. Introduction
1.1. Background
Internal derangement of the temporomandibular joint (TMJ) frequently involves displacement of the TMJ disc, the most common TMJ arthropathy [1,2]. The displaced or otherwise damaged TMJ disc is exposed to morphological changes and degeneration [2–4]. Similar to articular cartilage, the TMJ disc lacks an intrinsic regenerative capacity. Surgery is considered for patients suffering from significant joint dysfunction and persistent pain [1,5,6]. Discectomy is required when other treatment options are unsatisfactory or the TMJ disc has become morphologically too altered to be repositioned or repaired by other therapies [1,5–7]. After discectomy, the joint surfaces are exposed to direct mechanical loading and abrasion. This predisposes the joint to arthritic deformation, which may ultimately necessitate total joint replacement [1,8]. The development of a functional replacement for the TMJ disc is warranted.
The TMJ disc is composed of specialized fibrocartilaginous tissue differing from both hyaline and meniscal cartilage [9]. The cell population in the TMJ disc is referred to as fibrochondrocytes, because cells display both fibroblast and chondrocyte-like morphology [10,11]. The structural framework of the TMJ disc consists of collagens, predominantly type I collagen [4,10,12,13] and proteoglycans rich in chondroitin and dermatan sulphates [13–16]. The number and distribution of extracellular matrix (ECM) components in the TMJ disc are anisotropic, and dependent on the species and age-dependent variations [12–14].
Different materials, including autologous tissue grafts, natural and synthetic biomaterials, have been proposed for replacement the excised TMJ disc. Autografts from various sources such as auricular cartilage [17–19], dermis [20–22], fat [23], fascia and temporal muscle [24–26] have been used. In addition to morbidity caused by harvest, autografts have not been successful in treatment of the TMJ disorders [17–20,27,28]. Xenogenic ECM prepared from porcine urinary bladder has recently reported to support the formation of functional tissue resembling that of the native TMJ disc without signs of pathologic changes in the articulating surfaces [29].
The failure and detrimental effects of synthetic implants based on silicone and polytetrafluoroethylene [30–32] have directed interest towards biodegradable materials. The first attempt to engineer the TMJ disc was performed using type I collagen mesh [33]. Subsequently, synthetic polyglycolide (PGA) has been the most extensively studied biodegradable polymer used for tissue engineering of the TMJ disc [34–36]. PGA has good cytocompatibility with TMJ disc-derived cells, but exhibits too rapid resorption in order to sustain the mechanical integrity and open porosity as well supporting of the construct [34,36–38]. Polylactide (PLA) has a longer degradation time; and is therefore promising as a synthetic replacement for the TMJ disc [39,40].
Various cell sources have been examined for TMJ disc engineering. TMJ disc-derived fibrochondrocytes appear a natural choice [33,37,41–43]. These cells may be harvested during arthroscopic examination. Nevertheless, the use of the patient's own TMJ disc-derived cells is compromised by the degenerative changes in the TMJ disc [42]. Moreover, TMJ disc-derived fibrochondrocytes tend to change their phenotype during expansion in the laboratory [10,37,40,44]. Alternatively, chondrocytes from other cartilage types [36,37,45,46] or dermal fibroblasts [43] have been examined for TMJ disc engineering. The disadvantage of autologous cartilage is its limited availability owing to donor site scarcity and morbidity. These factors have increased interest towards the use of stem cells for the TMJ disc replacement [44,45,47,48]. Adipose tissue is an expendable, and abundant source of autologous adult stem cells capable of undergoing differentiation towards cells of mesenchymal origin, including the fibroblasts and chondrocytes found in the TMJ disc [40,49,50].
Tissue engineering aims to provide sustainable regeneration of damaged tissues by using live cells to produce new tissue possessing similar properties to the original tissue. This is achieved in an appropriate stimulatory environment in a three-dimensional culture system created with biodegradable scaffolds [42,51] or by cell self-assembly in a scaffold-free approach [43,45,52]. Ideally, the tissue-engineered TMJ disc would provide a structurally and functionally analogous equivalent to the native TMJ disc. Only few studies on TMJ disc engineering have been performed in vivo [27,36]. However, cell-based solutions have not so far been used for replacement of the TMJ disc in an animal model.
According to anatomical features, pig is suggested as the most suitable model for TMJ disc studies [2,37,53,54], but is far too expensive and laborious to handle in the primary experiments. With respect to the TMJ disc, the rabbit model also has many similarities to the human TMJ disc, including biochemical composition [53], morphology with biconcave parasagittal profile, internal collagen fibre organization [55] and attachments of the TMJ disc [56]. In addition, the rabbit TMJ exhibits significant lateral and anteroposterior movements in comparison with other mammals [57].
Our group has previously reported the potential of ASC-seeded non-woven PLA discs for TMJ disc engineering in vitro [40]. The aim of this study was to evaluate novel ASC-seeded PLA discs as a tissue-engineered replacement of the TMJ disc in vivo. Our hypothesis was that the PLA disc would provide a suitable basis for the formation of fibrous-like replacement tissue, and ASCs would further improve the fibrocartilaginous TMJ disc-like tissue formation. The study was performed with adult rabbits for extended follow-ups in order to better reflect the long-term effect of the implant in the adult patient population. Furthermore, because the dimensions of the implanted PLA discs were equal, the possible unbalance owing to bilateral effects remained negligible at the starting point. The treated sides differed only in the pre-culture conditions for the ASC discs which were compared with respect to TMJ disc regeneration.
2. Material and methods
2.1. Preparation of the polylactide disc
The biomaterial disc designed to replace the rabbit TMJ disc was composed of two layers: an upper layer of non-woven mat of poly(L/D)lactide (P(L/D)LA) 96/4 was sealed from bottom side with a P(L/DL)LA 70/30 smooth foil to hold seeded cells inside the disc. Medical grade P(L/D)LA 96/4 with an intrinsic viscosity of 3.5 dl g−1 (Purac Biochem b.v., Gorinchem, The Netherlands) and medical grade P(L/DL)LA 70/30 with an intrinsic viscosity of 6.1 dl g−1 (LR 708, Boehringer, Ingelheim, Germany) were used to prepare the non-woven mat and the foil, respectively. The PLA disc was prepared according to a previously described protocol [40] to the dimensions of 7 × 5 × 1.2 cm.
2.2. Harvest and culture of cells
Subcutaneous adipose tissue was harvested from the neck area of 10 adult (2.5- to 3-year-old) female New Zealand White rabbits under anaesthesia. The mean weight of rabbits was 3.7 kg. ASC isolation was performed according to the previously described protocol [40]. ASCs were expanded in control medium containing Dulbecco's modified Eagle's medium (Sigma-Aldrich Chemie GmbH, Steinheim, Germany); 10 per cent foetal bovine serum (FBS; Gibco, Invitrogen Life Technologies, Paisley, UK); 1 per cent antibiotic/antimycotic (100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 250 ng ml−1 amphotericin B; Gibco, Invitrogen); and 1 per cent l-glutamine (Gibco, Invitrogen) for two weeks. After expansion, ASCs were seeded as a suspension at a concentration of 105 cells ml−1 in a volume of 40 μl into the PLA discs. ASCs were allowed to adhere to the fibres of the PLA disc for 3 h in a cell incubator in humidified atmosphere at 37°C in 5 per cent CO2 prior to media addition. One half of the ASC discs were maintained in control medium and the other half in chondrogenic medium comprising of DMEM/F12 supplemented with 1 per cent FBS, 1 per cent antibiotic/antimycotic, 1 per cent l-glutamine, 6.25 μg ml−1 insulin (Sigma, St Louis, MO), 50 nM ascorbic acid (Sigma) and 10 ng ml−1 transforming growth factor (TGF)-β1 (Sigma). Chondrogenic medium was supplemented with TGF-β1 in the first media exchange after 24 h of culture. The medium was changed twice during one week culture prior to discectomy operation. ASCs at passage 5 were used for implantation. There were two parallel samples of the ASC discs representing each of the rabbits in both culture media. One of the samples was taken for implantation, and the other was used for qRT-PCR to measure gene expression at the time of implantation.
2.3. Implantation and excision of the adipose stem cell discs
All of the 10 test animals underwent bilateral discectomy of the TMJ disc without pre-operative fasting. Subcutaneous injection of 60 000 IU kg−1 benzylpenicillin procaine/benzathine–penicillin (Duplocillin LA, Intervet International, the Netherlands) was given prior to the surgery as a prophylaxis for infection. The operation was performed under general anaesthesia using 0.5 mg kg−1 medetomide (Domitor 1.0 mg ml−1, Orion Inc., Espoo, Finland) and 25 mg kg−1 ketamine hydrochloride (Ketalar 50 mg ml−1, Pfizer Inc., Espoo, Finland) which were administered subcutaneously (s.c.). In addition, 50 ml of saline was injected s.c. to prevent dehydration during surgery. The operation site was shaved and then scrubbed thoroughly with chlorhexidine gluconate solution (Klorhexol 5 mg ml−1, Leiras Inc., Finland). A vertical incision of 2 cm was made through the skin about 1 cm distally of the lateral canthus of the eye. Soft tissues were dissected to the bony surface, and the upper and lateral parts of the zygomatic arch were exposed. The lateral part of zygomatic arch was saved to maintain the anatomic structure as original as possible and to provide some lateral support for the implant in order to reduce the chance for the implant to dislocate. The TMJ capsule was then opened with a scalpel in order to expose the condyle head. The fibrous part of the TMJ disc was removed in both joints for subtotal discectomy (90%). The ASC discs were inserted into the joint space in front of the condyle. Photographs of the surgical protocol are shown in figure 1. The right and left TMJ joints were operated on the control and the differentiated ASC disc, respectively. The surgical procedure was exactly the same in order to eliminate the effect of different operation techniques to the results. Each animal was treated with autologous ASCs. The ASC disc was secured with a mattress suture (5/0 Monocryl, Johnson & Johnson Int., Belgium) around the zygomatic arch, and free movement of the lower jaw was verified. As the size of TMJ is very limited, it was not possible to stabilize the implant by suturing it to the periphery of the joint capsule or to use fixation in the medial area of the joint space. The joint capsule and the access incision were then closed in layers with multiple sutures (5/0 Vicryl rapid, Johnson & Johnson Int.). The 10 test animals were divided into two test groups of five animals each for follow-up periods of six and 12 months.
Figure 1.
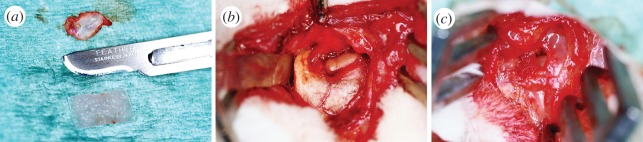
(a) The excised TMJ disc next to the ASC disc implant. (b) Zygomatic arch and condyle exposed after TMJ disc removal and the ASC disc in its position between the condyle and zygomatic arch after implantation. (Online version in colour.)
For post-operative analgesia buprenorfin, 0.03 mg kg−1 (Temgesic 0.3 mg ml−1, Schering–Plough Europe, Brussels, Belgium) s.c. was used. The analgesic medication was administered every 12 h for 2 days post-operatively. After the operation, all test animals had free access to their ordinary food: dry pellets, fresh vegetables, dry hay and water. All animals were housed individually in single cages. After the follow-up periods of six and 12 months, the animals were sacrificed by an intravenous overdose of pentobarbital sodium (Mebunat vet 60 mg ml−1, Orion Inc., Finland). The heads of the rabbits were removed and placed in 70 per cent ethanol.
2.4. Limited cone beam computed tomography
Radiological examination was performed using limited CBCT (dental micro CT/CBCT) with 3DX Accu-I-Tomo device (Accuitomo, J. Morita MFG, Kyoto Japan) after the follow-up of six and 12 months. CBCT examination was performed with 1 mm slice thickness in 80 kV and 1.5 mA using voxel size of 80 µm. A radiation field of 80 × 80 mm was used to obtain both condyles into the same view.
2.5. Histology
After imaging, the TMJ area was examined histologically. For histological examination, the specimens were dehydrated by increasing series of ethanol and embedded into methylmethacrylate. The specimen blocks were cut in half sagittally through the whole condyle. The slices of 5 µm in thickness were prepared and stained with haematoxylin–eosin and Masson's trichrome.
2.6. qRT-PCR
After the pre-culture, one of the parallel ASC discs including both control and differentiation treatment was analysed for messenger RNA expressions in order to measure the gene expression of the fibrocartilage ECM components at the time of implantation. Total RNA was isolated from the ASC discs using Trizol-reagent (Invitrogen, Paisley, UK) according to the manufacturer's instructions. First-strand cDNA was reverse transcribed from total RNA using the high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA). The mRNA level of aggrecan, types I and II collagen were analysed by qRT-PCR method. The sequences and AC numbers of the primers (Oligomer Oy, Helsinki, Finland) are presented in table 1. The qRT-PCR protocol followed the manufacturer's instruction The parameters and calculations used are described elsewhere [40].
Table 1.
Primer sequences for qRT-PCR.
| gene | accession number | primer sequence | product size (bp) |
|---|---|---|---|
| GAPDH | L23961 | forward: 5′-GGG TGG TGG ACC TCA TGG T-3′ reverse: 5′-CGG TGG TTT GAG GGC TCT TA-3′ |
57 |
| aggrecan | L38480 | forward: 5′-GGG ACG TGT GCG CAT CA-3′ reverse: 5′-GTA GTT GGG CAG CGA GAC CTT-3′ |
54 |
| type I collagen | D49399 | forward: 5′-GGG ACA CAA CGG ATT GCA A-3′ reverse: 5′-GCA CCT TGA TCA CCA TGT TGA C-3′ |
59 |
| type II collagen | D83229 | forward: 5′-CCC CGT CTG CCC TAC TGA-3′ reverse: 5′-GTT CTC CTT TCT GCC CCT TTG-3′ |
67 |
2.7. Statistical analyses
All data are presented as median and range. Data were analysed using GraphPad Prism v. 5.01 software. The Kruskal–Wallis test was used to assess differences between control and treated ASC discs at six and 12 months (n = 5), and Dunn's test was performed for pairwise multiple comparison. Two-tailed p-values of less than 0.05 were considered significant.
3. Results
3.1. Results of qRT-PCR
Relative changes in the expression of aggrecan and types I and II collagen genes were measured from the ASC discs after one week of pre-culture at the time of implantation (figure 2). The differentiation of ASC discs seemed to increase the relative expression of aggrecan to seven- and 14-fold in six- and 12-months' group, respectively. Minor decrease and increase were observed in type I collagen expression. In the 12-month group, the differentiation of the ASC discs seemed to induce the relative expression of all the measured genes but only the fivefold increase in type II collagen expression was statistically significant (p < 0.05) compared with the control ASC discs. Donor-dependent differences were observed in the gene expressions of the rabbits.
Figure 2.
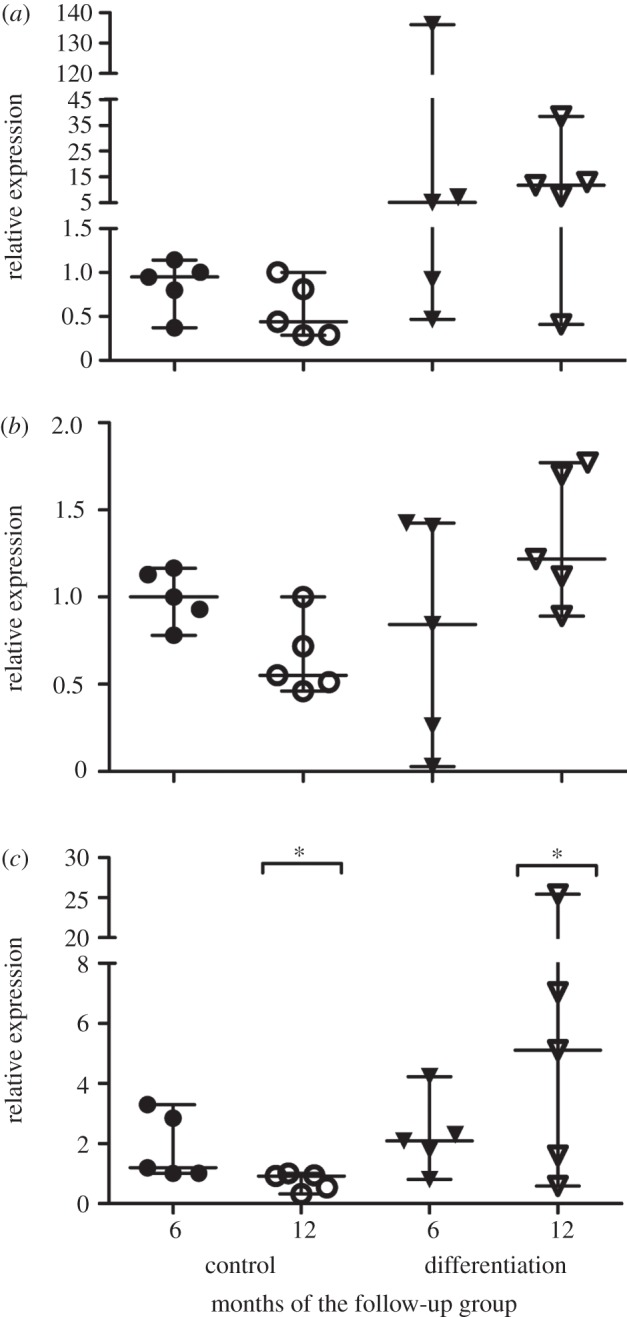
The relative expression levels of (a) aggrecan, (b) type I and (c) II collagen of the ASC discs after one week pre-culture prior to implantation measured by qRT-PCR. The six and 12 months denote the division of the rabbits in the subsequent follow-up groups. The difference in type II collagen expression between the control and differentiated ASC discs in the 12-month group was found statistically significant and is denoted by an asterisk (p < 0.05).
3.2. Animal tests
All test animals healed uneventfully, and no adverse effects were noticed during follow-up period of six and 12 months. All animals maintained their weight as well. In both the six- and 12-month groups, the implanted ASC discs were found to be dislocated either anteriorly or posteriorly of the condylar head in the joint space.
3.3. Findings on cone beam computed tomography
Chronic arthrosis was visible in all the operated joints (figure 3); however, the morphological findings differed between the joint sides. The common feature was considerable hypertrophy of the condyles. In the six-month group, the shape of the right condyle treated with control ASC disc seemed consistently more irregular than that of the left condyle. The left condyle treated differentiated ASC discs appeared smooth and more calcified near the condylar surface. More microcysts were detected in the right than left joint. The bone in the temporal articulation surface was more radio-opaque in the TMJs treated with differentiated ASC disc than with the control ACS disc. The findings were similar in the 12-month study group, where the hypertrophy of the condyles was further increased. The temporal bone medially to the condyle showed thinning as well.
Figure 3.
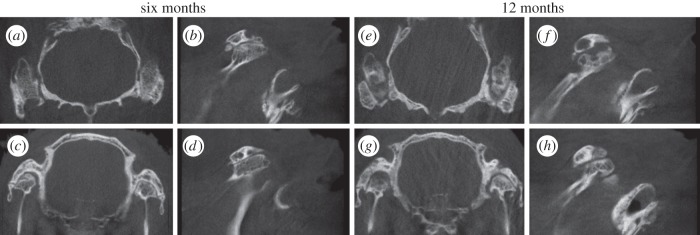
CBCT images of the operated TMJ joints in the six- and 12-month groups represented as (a,e) axial and (c,g) coronal views as well as sagittal views of the (b,f) right and (d,h) left TMJs treated with control and differentiated ASC discs, respectively.
Calcified loose bodies were detected in the joint space in all the treatment groups. The size and shape of the loose bodies varied from 0.3 to 2 mm depending on the direction measurements were taken. The number of particles in a joint also varied ranging from 0 to 10. Particles were usually located superiorly of the condyle behind the zygomatic arc and secondly anteriorly of the zygomatic arch. The fragments seemed more numerous on the medial and lateral sides of the right condyle than on the left side. No signs of infection, inflammation or foreign body reaction were observed in any of the treated joints in either of the follow-up groups on the basis of CBCT findings.
3.4. Histological findings
The signs of chronic arthrosis were also observable in the histological sections. The hyaline cartilage on the joint surfaces seemed smooth but the cartilage–bone interface appeared irregular, especially in the 12-month group (figure 4). The superficial cartilage of the left joint treated with differentiated ASC discs appeared more regular than in the right joint. In some areas, hypertrophy of the cartilage seemed considerable (figure 4c). The formation of microcysts was confirmed (figure 4d,e). Calcified loose bodies, also detected in the CBCT, were covered with cartilaginous tissue (figure 4f). The remnants of the dislocated PLA disc were visible six and still after 12 months after implantation (figure 5). They were detected more frequently at the anterior part of the joint capsule than the posterior part of the joint capsule. PLA fibres of the dislocated ASC discs were surrounded by the fibrous tissue, but no adverse tissue reactions, such as foreign body reactions, were observed. As a biomaterial, PLA did not cause irritation and allowed the normal regeneration of adjacent tissue in the joint.
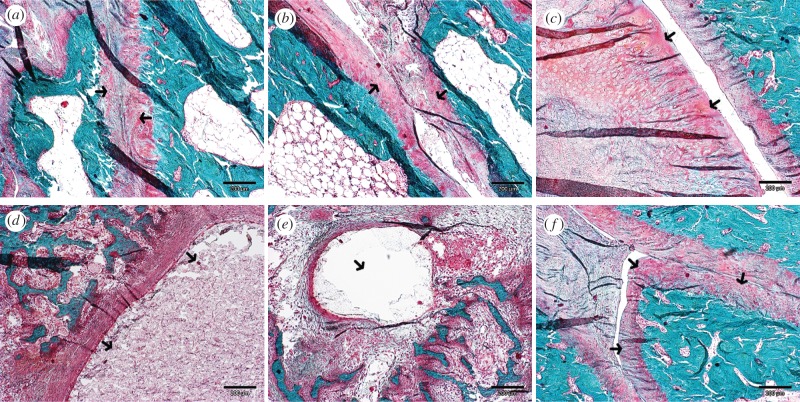
Figure 4.
The sagittal histological sections of the TMJ area stained with Masson's trichrome. (a) The TMJ joint space of the right treated with control ASC disc and (b) the left side treated with differentiated ASC disc in the six-month group. Hyaline cartilage covering the joint surfaces are pointed by arrows. (c) The substantial hypertrophy of condylar cartilage developed in the control ASC disc treated side of the TMJ at 12 months of the follow-up. (d) Microcysts developed in the condylar and (e) temporal bone sides and (f) calcified loose body covered with hyaline-like cartilage are shown in the control ASC disc treated joints after 12 months. The described details are indicated by arrows. Scale bar, 200 µm. (Online version in colour.)
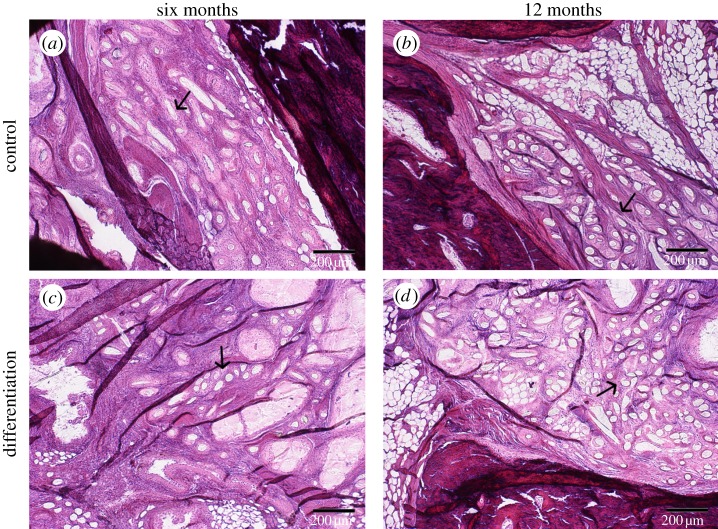
Figure 5.
(a,b) The remnants of the ASC discs stained with haematoxylin–eosin of the control and (c,d) differentiated ASCs discs after (a,c) six and (b,d) 12 months of implantation. Some of the PLA fibres are indicated by arrows. The scale bar is 200 µm. (Online version in colour.)
4. Discussion
There is ample evidence that discectomy will in many instances improve the condition for the patient on a short follow-up. The mouth opening will increase and thereby the function will improve and a number of patients have less pain and discomfort [6,58–61]. Yet, the underlying problem has not been solved by discectomy alone and the patient will be left with increasing condylar head degeneration, crepitus and in time, an increasing restriction of function. Therefore, there is room for improvement, and tissue engineering has the potential for long-term cure, unlike the situation with discectomy only. This cure might include a tissue-engineered condyle as well [1,2]. In this study, we evaluated a novel construct composed of ASCs and the bilayer biodegradable PLA discs as a potential replacement of the TMJ disc after discectomy in rabbit animal model. To the best of our knowledge, this is the first in vivo study of a tissue-engineered TMJ construct consisting of both cells and biomaterial scaffolds.
The test group of aged rabbits was chosen to better reflect the effects of the ASC discs replacement on an actual patient population. Laboratory animals are often juvenile and tissue regeneration after surgery accordingly outperforms that in older animals. Moreover, the follow-up points of six and 12 months were considered long enough to better distinguish the reactions actually related to ASC discs tissue from those occurring normally in the healing tissue after surgery. All test animals recovered well from surgery and maintained good health for the six- and 12-month follow-up periods.
The PLA disc and ASCs used here demonstrated the potential of the TMJ disc engineering in vitro [40]. The non-woven fibrous mat was designed to roughly resemble the ECM framework in the native TMJ disc. The fibrous format of a scaffold was demonstrably beneficial with respect to cell number maintenance and ECM formation in fibrochondrogenic cultures [34]. In addition, one week pre-culture, as used here, has previously shown to be a suitable time for the adhesion and retention of rabbit perichondrial cells on the porous PLA scaffolds in vitro [62].
The stable positioning of ASC discs on the implantation site proved challenging. All the implanted ASC discs appeared to be displaced from the intended location in both six- and 12-month study groups. Accordingly, the condition in the operated TMJ partly resembled the situation seen after discectomy that lead to changes in the condylar morphology [1,8,63]. Similar difficulties in anchoring any graft or biomaterial replacement to remaining tissues (retrodiscal tissue remnants, lateral pterygoid muscle attachments or the lateral pole of the condylar head) are known to be a technical challenge in TMJ surgery [20,23,24,64]. In this study, we aimed to secure fixation of the implant to the unmoving zygomal part of the TMJ and we also placed the smooth foil inferiorly against condyle to in order to reduce the effects of mastication movements which may also predispose the implant to dislocation. In this system, the ASCs in the non-woven mat would also have a direct contact with zygomatic hyalinous cartilage that could facilitate cell ingrowth and stimuli for ASCs to differentiate. Brown et al. [65] succeeded in securing the TMJ disc implant in its location suturing the implant via its marginal zones against the temporal fossa and joint capsule in canine animal model. In the rabbit model, used here, small holes could have been drilled through the zygomatic bone for additional stabilizing sutures. The stability of the PLA disc could also be improved by moulding it into a rounded shape with either integrated sutures or reinforced periphery area for these sutures. With such suturing the implant would be stabilized better against lateromedial and anterodistal displacement. Nonetheless, according to the histological findings, the PLA fibres of the displaced ASC discs did not cause adverse tissue reactions in the TMJ. Instead, it appeared that the PLA fibres were able to directly bond to bone located in the TMJ space. In this respect, as a biomaterial, PLA might offer good biocompatibility for TMJ surgery.
Given our results, progressive chronic arthrosis was evident in all operated joints. Histological findings similar to those of our study, including deformation and decrease in the morphological integrity of condylar cartilage, have previously been described in osteoarthritic TMJs [66]. Emergence of loose calcified bodies and erosion of the condyle has also been observed with TMJ disc transplants [19]. The considerable hypertrophy of the condyles was visible on both treated sides. Similar findings of condylar hypertrophy have been reported in conjunction with deformation occurring after disc displacement [67,68]. Surgically induced disc displacement in rabbits has resulted in progressive enlargement of the condyle. Moreover, similar to our study, extensive hyperplasia of the condylar cartilage has been reported [67].
The presence of ASCs may have further facilitated the condylar hypertrophy. The potential of ASCs for bone regeneration has previously been clinically demonstrated in the reconstruction of a hemimaxillectomy patient [69], in large reconstruction of the mandible [70] and in the treatment of the large cranial defects [71] as well as cell culture in vitro [72–74]. In vivo findings of ASC induced osteogenesis have been contradictory [74–77].
Although condylar hypertrophy was apparent in both joints, the morphological findings differed between the right and left sides. The right TMJ, treated with the control ASC disc, was more irregular and microcystic than the left TMJ, which was visibly smoother and more sclerotic. The differences between the right and left sides increased from the six to 12-month follow-up groups. Because the dimensions of the ASC disc and surgical procedure were the same for all the samples, it can be assumed that possible bilateral effects between the TMJ sides remained negligible at the starting point. Accordingly, the detected side differences were mainly caused by different pre-culture conditions. An increase in both bone and cartilage formation in vivo has likewise been reported after local articular administration of TGF-β1 [78,79]. TGF-β1 is known either to promote or inhibit joint destruction in vivo depending on type, differentiation stage and health status of target cells, and on the environment [80].
TGF-β1 is a traditional differentiation factor used in chondrogenic cultures [81] and is also advantageous in fibrochondrogenic cultures of mature TMJ disc-derived fibrochondrocytes [39,45] as well as ASCs aiming at TMJ disc reconstruction [40]. There were some donor-dependent differences between autologous ASC disc implants at the time of implantation. The observed differences between the treatment groups after pre-culture remained mainly insignificant. The differentiation medium supplemented with TGF-β1 showed a tendency to increase the gene expression in most of the cases but only type II collagen expression showed a significant difference to control one in the 12-month group. Although type II collagen is the major type in hyaline cartilage, in the TMJ disc, it is found in lesser amounts [10,13,82]. The pre-culture parameters can further be optimized with respect to cell seeding density, time of culture and supplements in the medium [34]. Here, the volume and cell concentration for scaffold loading was chosen on the basis of the most homogeneous and dense cell distribution attained in the preliminary pilot cultures. High cell density is essential for chondrogenic differentiation to occur in cell culture [83,84]. This is also demonstrated in fibrochondrogenic ECM formation in TMJ disc engineering [35]. Recently, different co-cultures of stem cells and mature fibrochondrogenic cells have been suggested to have potential route for the production of fibrocartilaginous tissue in vitro [45,48].
5. Conclusion
Chronic arthrosis was discernible in all operated joints, which may have been caused by the implanted ASC disc or its displacement or both. The presence of ASCs probably enhanced the significant hypertrophy of the condyles on both sides. The use of ASC in the TMJ disc engineering may be useful but an improved system for cell differentiation or utilization of additional cell source needs to be considered. The cell labelling would also ease estimation of the actual cell effects on the TMJ regeneration. Apparently, differentiated ASC discs treated with TGF-β1 lead to more regular morphology and increased calcification of the condyle in the TMJ. Nonetheless, PLA fibres were well tolerated in the TMJ space, no signs of inflammatory reaction appeared. Accordingly as a biomaterial, PLA has potential in TMJ surgery. In further studies the design of the PLA disc needs to be improved in order to ensure a better fit and stability of the PLA disc in the correct anatomical position. The mechanical properties of the ASC need to be measured as well as the performance evaluated against unseeded PLA disc and empty discectomy control.
Acknowledgements
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committees of the University of Helsinki.
The authors thank Ms Miia Juntunen, Mrs Hilkka Mäkinen and Mrs Mirja Hyppönen for expert technical assistance. Histola Research Limited is acknowledged for preparing the histological sections and staining. The work was financially supported by the competitive research funding of the Pirkanmaa Hospital District (9J069, 9M058), Helsinki University Central Hospital (TYH 2010232), the University of Tampere and the Finnish Cultural Foundation and National Doctoral Programme of Musculoskeletal Disorders and Biomaterials.
References
- 1.Ingawale S, Goswami T. 2009. Temporomandibular joint: disorders, treatments, and biomechanics. Ann. Biomed. Eng. 37, 976–996 10.1007/s10439-009-9659-4 (doi:10.1007/s10439-009-9659-4) [DOI] [PubMed] [Google Scholar]
- 2.Detamore MS, Athanasiou KA. 2003. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 9, 1065–1087 10.1089/10763270360727991 (doi:10.1089/10763270360727991) [DOI] [PubMed] [Google Scholar]
- 3.Ali AM, Sharawy M. 1996. Histochemical and immunohistochemical studies of the effects of experimental anterior disc displacement on sulfated glycosaminoglycans, hyaluronic acid, and link protein of the rabbit craniomandibular joint. J. Oral Maxillofac. Surg. 54, 992–1004 10.1016/S0278-2391(96)90399-7 (doi:10.1016/S0278-2391(96)90399-7) [DOI] [PubMed] [Google Scholar]
- 4.Ali AM, Sharawy MM. 1996. An immunohistochemical study of collagen types III, VI and IX in rabbit craniomandibular joint tissues following surgical induction of anterior disk displacement. J. Oral Pathol. Med. 25, 78–85 10.1111/j.1600-0714.1996.tb00197.x (doi:10.1111/j.1600-0714.1996.tb00197.x) [DOI] [PubMed] [Google Scholar]
- 5.Dolwick MF. 1997. The role of temporomandibular joint surgery in the treatment of patients with internal derangement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 83, 150–155 10.1016/S1079-2104(97)90106-2 (doi:10.1016/S1079-2104(97)90106-2) [DOI] [PubMed] [Google Scholar]
- 6.Dolwick MF. 2007. Temporomandibular joint surgery for internal derangement. Dent. Clin. North Am. 51, 195–208 10.1016/j.cden.2006.10.003 (doi:10.1016/j.cden.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 7.Eriksson L, Westesson PL. 2001. Discectomy as an effective treatment for painful temporomandibular joint internal derangement: a 5-year clinical and radiographic follow-up. J. Oral Maxillofac. Surg. 59, 750–758; discussion 8–9 10.1053/joms.2001.24288 (doi:10.1053/joms.2001.24288) [DOI] [PubMed] [Google Scholar]
- 8.Mercuri LG, Edibam NR, Giobbie-Hurder A. 2007. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J. Oral Maxillofac. Surg. 65, 1140–1148 10.1016/j.joms.2006.10.006 (doi:10.1016/j.joms.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 9.Almarza AJ, Athanasiou KA. 2004. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 32, 2–17 10.1023/B:ABME.0000007786.37957.65 (doi:10.1023/B:ABME.0000007786.37957.65) [DOI] [PubMed] [Google Scholar]
- 10.Landesberg R, Takeuchi E, Puzas JE. 1996. Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Arch. Oral Biol. 41, 761–767 10.1016/S0003-9969(96)00068-4 (doi:10.1016/S0003-9969(96)00068-4) [DOI] [PubMed] [Google Scholar]
- 11.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, Athanasiou KA. 2006. Cell type and distribution in the porcine temporomandibular joint disc. J. Oral Maxillofac. Surg. 64, 243–248 10.1016/j.joms.2005.10.009 (doi:10.1016/j.joms.2005.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minarelli AM, Liberti EA. 1997. A microscopic survey of the human temporomandibular joint disc. J. Oral Rehabil. 24, 835–840 10.1046/j.1365-2842.1997.00595.x (doi:10.1046/j.1365-2842.1997.00595.x) [DOI] [PubMed] [Google Scholar]
- 13.Mills DK, Fiandaca DJ, Scapino RP. 1994. Morphologic, microscopic, and immunohistochemical investigations into the function of the primate TMJ disc. J. Orofac. Pain 8, 136–154 [PubMed] [Google Scholar]
- 14.Nakano T, Scott PG. 1996. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch. Oral Biol. 41, 845–853 10.1016/S0003-9969(96)00040-4 (doi:10.1016/S0003-9969(96)00040-4) [DOI] [PubMed] [Google Scholar]
- 15.Nakano T, Scott PG. 1989. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch. Oral Biol. 34, 749–757 10.1016/0003-9969(89)90082-4 (doi:10.1016/0003-9969(89)90082-4) [DOI] [PubMed] [Google Scholar]
- 16.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. 2005. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 24, 45–57 10.1016/j.matbio.2004.11.006 (doi:10.1016/j.matbio.2004.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker MR, Spagnoli DB. 1996. Autogenous dermal and auricular cartilage grafts for temporomandibular joint repair. Atlas Oral Maxillofac. Surg. Clin. North Am. 4, 75–92 [PubMed] [Google Scholar]
- 18.Takatsuka S, Narinobou M, Nakagawa K, Yamamoto E. 1996. Histologic evaluation of auricular cartilage grafts after discectomy in the rabbit craniomandibular joint. J. Oral Maxillofac. Surg. 54, 1216–1225; discussion 25–26 10.1016/S0278-2391(96)90355-9 (doi:10.1016/S0278-2391(96)90355-9) [DOI] [PubMed] [Google Scholar]
- 19.Sandler NA, Macmillan C, Buckley MJ, Barnes L. 1997. Histologic and histochemical changes in failed auricular cartilage grafts used for a temporomandibular joint disc replacement: a report of three cases and review of the literature. J. Oral Maxillofac. Surg. 55, 1014–1019 10.1016/S0278-2391(97)90082-3 (doi:10.1016/S0278-2391(97)90082-3) [DOI] [PubMed] [Google Scholar]
- 20.Dimitroulis G. 2005. The use of dermis grafts after discectomy for internal derangement of the temporomandibular joint. J. Oral Maxillofac. Surg. 63, 173–178 10.1016/j.joms.2004.06.051 (doi:10.1016/j.joms.2004.06.051) [DOI] [PubMed] [Google Scholar]
- 21.Meyer RA. 1988. The autogenous dermal graft in temporomandibular joint disc surgery. J. Oral Maxillofac. Surg. 46, 948–954 10.1016/0278-2391(88)90332-1 (doi:10.1016/0278-2391(88)90332-1) [DOI] [PubMed] [Google Scholar]
- 22.Tucker MR, Jacoway JR, White RP., Jr 1986. Autogenous dermal grafts for repair of temporomandibular joint disc perforations. J. Oral Maxillofac. Surg. 44, 781–789 10.1016/0278-2391(86)90153-9 (doi:10.1016/0278-2391(86)90153-9) [DOI] [PubMed] [Google Scholar]
- 23.Dimitroulis G. 2011. Condylar morphology after temporomandibular joint discectomy with interpositional abdominal dermis-fat graft. J. Oral Maxillofac. Surg. 69, 439–446 10.1016/j.joms.2010.07.021 (doi:10.1016/j.joms.2010.07.021) [DOI] [PubMed] [Google Scholar]
- 24.Thyne GM, Yoon JH, Luyk NH, McMillan MD. 1992. Temporalis muscle as a disc replacement in the temporomandibular joint of sheep. J. Oral Maxillofac. Surg. 50, 979–988 10.1016/0278-2391(92)90059-9 (doi:10.1016/0278-2391(92)90059-9) [DOI] [PubMed] [Google Scholar]
- 25.Su-Gwan K. 2001. Treatment of temporomandibular joint ankylosis with temporalis muscle and fascia flap. Int. J. Oral Maxillofac. Surg. 30, 189–193 10.1054/ijom.2001.0047 (doi:10.1054/ijom.2001.0047) [DOI] [PubMed] [Google Scholar]
- 26.Pogrel MA, Kaban LB. 1990. The role of a temporalis fascia and muscle flap in temporomandibular joint surgery. J. Oral Maxillofac. Surg. 48, 14–19 10.1016/0278-2391(90)90173-Y (doi:10.1016/0278-2391(90)90173-Y) [DOI] [PubMed] [Google Scholar]
- 27.Feinberg SE, McDonnell EJ. 1995. The use of a collagen sheet as a disc replacement in the rabbit temporomandibular joint. J. Oral Maxillofac. Surg. 53, 535–542; discussion 43 10.1016/0278-2391(95)90066-7 (doi:10.1016/0278-2391(95)90066-7) [DOI] [PubMed] [Google Scholar]
- 28.Dimitroulis G. 2011. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int. J. Oral Maxillofac. Surg. 40, 561–568 10.1016/j.ijom.2010.11.020 (doi:10.1016/j.ijom.2010.11.020) [DOI] [PubMed] [Google Scholar]
- 29.Brown BN, Chung WL, Pavlick M, Reppas S, Ochs MW, Russell AJ, Badylak SF. 2011. Extracellular matrix as an inductive template for temporomandibular joint meniscus reconstruction: a pilot study. J. Oral Maxillofac. Surg. 69, e488–e505 10.1016/j.joms.2011.02.130 (doi:10.1016/j.joms.2011.02.130) [DOI] [PubMed] [Google Scholar]
- 30.Estabrooks LN, Fairbanks CE, Collett RJ, Miller L. 1990. A retrospective evaluation of 301 TMJ Proplast-Teflon implants. Oral Surg. Oral Med. Oral Pathol. 70, 381–386 10.1016/0030-4220(90)90164-N (doi:10.1016/0030-4220(90)90164-N) [DOI] [PubMed] [Google Scholar]
- 31.Mercuri LG, Giobbie-Hurder A. 2004. Long-term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J. Oral Maxillofac. Surg. 62, 1088–1096 10.1016/j.joms.2003.10.012 (doi:10.1016/j.joms.2003.10.012) [DOI] [PubMed] [Google Scholar]
- 32.Westesson PL, Eriksson L, Lindstrom C. 1987. Destructive lesions of the mandibular condyle following diskectomy with temporary silicone implant. Oral Surg. Oral Med. Oral Pathol. 63, 143–150 10.1016/0030-4220(87)90302-1 (doi:10.1016/0030-4220(87)90302-1) [DOI] [PubMed] [Google Scholar]
- 33.Thomas M, Grande D, Haug RH. 1991. Development of an in vitro temporomandibular joint cartilage analog. J. Oral Maxillofac. Surg. 49, 854–857 10.1016/0278-2391(91)90015-E (doi:10.1016/0278-2391(91)90015-E) [DOI] [PubMed] [Google Scholar]
- 34.Almarza AJ, Athanasiou KA. 2004. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 10, 1787–1795 10.1089/ten.2004.10.1787 (doi:10.1089/ten.2004.10.1787) [DOI] [PubMed] [Google Scholar]
- 35.Almarza AJ, Athanasiou KA. 2005. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann. Biomed. Eng. 33, 943–950 10.1007/s10439-005-3311-8 (doi:10.1007/s10439-005-3311-8) [DOI] [PubMed] [Google Scholar]
- 36.Puelacher WC, Wisser J, Vacanti CA, Ferraro NF, Jaramillo D, Vacanti JP. 1994. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J. Oral Maxillofac. Surg. 52, 1172–1178 10.1016/0278-2391(94)90538-X (doi:10.1016/0278-2391(94)90538-X) [DOI] [PubMed] [Google Scholar]
- 37.Springer IN, Fleiner B, Jepsen S, Acil Y. 2001. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials 22, 2569–2577 10.1016/S0142-9612(01)00148-X (doi:10.1016/S0142-9612(01)00148-X) [DOI] [PubMed] [Google Scholar]
- 38.Detamore MS, Athanasiou KA. 2005. Evaluation of three growth factors for TMJ disc tissue engineering. Ann. Biomed. Eng. 33, 383–390 10.1007/s10439-005-1741-y (doi:10.1007/s10439-005-1741-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen KD, Athanasiou KA. 2008. Scaffold and growth factor selection in temporomandibular joint disc engineering. J. Dent Res. 87, 180–185 10.1177/154405910808700205 (doi:10.1177/154405910808700205) [DOI] [PubMed] [Google Scholar]
- 40.Mäenpää K, Ellä V, Mauno J, Kellomäki M, Suuronen R, Ylikomi T, Miettinen S. 2010. Use of adipose stem cells and polylactide discs for tissue engineering of the temporomandibular joint disc. J. R. Soc. Interface 7, 177–188 10.1098/rsif.2009.0117 (doi:10.1098/rsif.2009.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johns DE, Athanasiou KA. 2007. Improving culture conditions for temporomandibular joint disc tissue engineering. Cells, Tissues, Organs 185, 246–257 10.1159/000102173 (doi:10.1159/000102173) [DOI] [PubMed] [Google Scholar]
- 42.Allen KD, Athanasiou KA. 2006. Tissue engineering of the TMJ disc: a review. Tissue Eng. 12, 1183–1196 10.1089/ten.2006.12.1183 (doi:10.1089/ten.2006.12.1183) [DOI] [PubMed] [Google Scholar]
- 43.Johns DE, Wong ME, Athanasiou KA. 2008. Clinically relevant cell sources for TMJ disc engineering. J. Dent Res. 87, 548–552 10.1177/154405910808700609 (doi:10.1177/154405910808700609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen KD, Athanasiou KA. 2007. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue Eng. 13, 101–110 10.1089/ten.2006.0094 (doi:10.1089/ten.2006.0094) [DOI] [PubMed] [Google Scholar]
- 45.Kalpakci KN, Kim EJ, Athanasiou KA. 2011. Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering. Acta Biomater. 7, 1710–1718 10.1016/j.actbio.2010.12.015 (doi:10.1016/j.actbio.2010.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson DE, Athanasiou KA. 2008. Passaged goat costal chondrocytes provide a feasible cell source for temporomandibular joint tissue engineering. Ann. Biomed. Eng. 36, 1992–2001 10.1007/s10439-008-9572-2 (doi:10.1007/s10439-008-9572-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoben GM, Koay EJ, Athanasiou KA. 2008. Fibrochondrogenesis in two embryonic stem cell lines: effects of differentiation timelines. Stem Cells 26, 422–430 10.1634/stemcells.2007-0641 (doi:10.1634/stemcells.2007-0641) [DOI] [PubMed] [Google Scholar]
- 48.Hoben GM, Willard VP, Athanasiou KA. 2009. Fibrochondrogenesis of hESCs: growth factor combinations and co-cultures. Stem Cells Dev. 18, 79–89 10.1089/scd.2008.0024 (doi:10.1089/scd.2008.0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. 2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 10.1089/107632701300062859 (doi:10.1089/107632701300062859) [DOI] [PubMed] [Google Scholar]
- 50.Hildner F, Albrecht C, Gabriel C, Redl H, van Griensven M. 2011. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J. Tissue Eng. Regen. Med. 5, e36–e51 10.1002/term.386 (doi:10.1002/term.386) [DOI] [PubMed] [Google Scholar]
- 51.Langer R, Vacanti JP. 1993. Tissue engineering. Science 260, 920–926 10.1126/science.8493529 (doi:10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- 52.Hu JC, Athanasiou KA. 2006. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 12, 969–979 10.1089/ten.2006.12.969 (doi:10.1089/ten.2006.12.969) [DOI] [PubMed] [Google Scholar]
- 53.Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. 2011. An interspecies comparison of the temporomandibular joint disc. J. Dent Res. 90, 193–198 10.1177/0022034510381501 (doi:10.1177/0022034510381501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bermejo A, Gonzalez O, Gonzalez JM. 1993. The pig as an animal model for experimentation on the temporomandibular articular complex. Oral Surg. Oral Med. Oral Pathol. 75, 18–23 10.1016/0030-4220(93)90399-O (doi:10.1016/0030-4220(93)90399-O) [DOI] [PubMed] [Google Scholar]
- 55.Mills DK, Daniel JC, Herzog S, Scapino RP. 1994. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J. Oral Maxillofac. Surg. 52, 1279–1292 10.1016/0278-2391(94)90051-5 (doi:10.1016/0278-2391(94)90051-5) [DOI] [PubMed] [Google Scholar]
- 56.Savalle WP, Weijs WA, James J, Everts V. 1990. Elastic and collagenous fibers in the temporomandibular joint capsule of the rabbit and their functional relevance. Anat. Rec. 227, 159–166 10.1002/ar.1092270204 (doi:10.1002/ar.1092270204) [DOI] [PubMed] [Google Scholar]
- 57.Weijs WA, Dantuma R. 1980. Functional anatomy of the masticatory apparatus in the rabbit (Oryctolagus cuniculus L.). Neth. J. Zool. 31, 99–147 10.1163/002829680X00212 (doi:10.1163/002829680X00212) [DOI] [Google Scholar]
- 58.Tolvanen M, Oikarinen VJ, Wolf J. 1988. A 30-year follow-up study of temporomandibular joint meniscectomies: a report on five patients. Br. J. Oral Maxillofac. Surg. 26, 311–316 10.1016/0266-4356(88)90049-6 (doi:10.1016/0266-4356(88)90049-6) [DOI] [PubMed] [Google Scholar]
- 59.Wilkes CH. 1991. Surgical treatment of internal derangements of the temporomandibular joint. A long-term study. Arch. Otolaryngol. Head Neck Surg. 117, 64–72 10.1001/archotol.1991.01870130070019 (doi:10.1001/archotol.1991.01870130070019) [DOI] [PubMed] [Google Scholar]
- 60.Miloro M, Henriksen B. 2010. Discectomy as the primary surgical option for internal derangement of the temporomandibular joint. J. Oral Maxillofac. Surg. 68, 782–789 10.1016/j.joms.2009.09.091 (doi:10.1016/j.joms.2009.09.091) [DOI] [PubMed] [Google Scholar]
- 61.Takaku S, Toyoda T. 1994. Long-term evaluation of discectomy of the temporomandibular joint. J. Oral Maxillofac. Surg. 52, 722–728 10.1016/0278-2391(94)90486-3 (doi:10.1016/0278-2391(94)90486-3) [DOI] [PubMed] [Google Scholar]
- 62.Giurea A, Klein TJ, Chen AC, Goomer RS, Coutts RD, Akeson WH, Amiel D, Sah RL. 2003. Adhesion of perichondrial cells to a polylactic acid scaffold. J. Orthop. Res. 21, 584–589 10.1016/S0736-0266(02)00263-2 (doi:10.1016/S0736-0266(02)00263-2) [DOI] [PubMed] [Google Scholar]
- 63.Eriksson L, Westesson PL. 1985. Long-term evaluation of meniscectomy of the temporomandibular joint. J. Oral Maxillofac. Surg. 43, 263–269 10.1016/0278-2391(85)90284-8 (doi:10.1016/0278-2391(85)90284-8) [DOI] [PubMed] [Google Scholar]
- 64.Dimitroulis G, Slavin J. 2006. Histological evaluation of full thickness skin as an interpositional graft in the rabbit craniomandibular joint. J. Oral Maxillofac. Surg. 64, 1075–1080 10.1016/j.joms.2006.03.011 (doi:10.1016/j.joms.2006.03.011) [DOI] [PubMed] [Google Scholar]
- 65.Brown BN, Chung WL, Almarza AJ, Pavlick MD, Reppas SN, Ochs MW, Russell AJ, Badylak SF. 2012. Inductive, scaffold-based, regenerative medicine approach to reconstruction of the temporomandibular joint disk. J. Oral Maxillofac. Surg. 70, 2656–2668 10.1016/j.joms.2011.12.030 (doi:10.1016/j.joms.2011.12.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Axelsson S, Holmlund A, Hjerpe A. 1992. An experimental model of osteoarthrosis in the temporomandibular joint of the rabbit. Acta Odontol. Scand. 50, 273–280 10.3109/00016359209012773 (doi:10.3109/00016359209012773) [DOI] [PubMed] [Google Scholar]
- 67.Ali AM, Sharawy M. 1995. Enlargement of the rabbit mandibular condyle after experimental induction of anterior disc displacement: a histomorphometric study. J. Oral Maxillofac. Surg. 53, 544–560 10.1016/0278-2391(95)90068-3 (doi:10.1016/0278-2391(95)90068-3) [DOI] [PubMed] [Google Scholar]
- 68.Hinton RJ. 1992. Alterations in rat condylar cartilage following discectomy. J. Dent. Res. 71, 1292–1297 10.1177/00220345920710060501 (doi:10.1177/00220345920710060501) [DOI] [PubMed] [Google Scholar]
- 69.Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. 2009. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 38, 201–209 10.1016/j.ijom.2009.01.001 (doi:10.1016/j.ijom.2009.01.001) [DOI] [PubMed] [Google Scholar]
- 70.Sándor GK, et al. 2013. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J. Oral Maxillofac. Surg. 71, 938–950 10.1016/j.joms.2012.11.014 (doi:10.1016/j.joms.2012.11.014) [DOI] [PubMed] [Google Scholar]
- 71.Thesleff T, Lehtimäki K, Niskakangas T, Mannerström B, Miettinen S, Suuronen R, Öhman J. 2011. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery 68, 1535–1540 10.1227/NEU.0b013e31820ee24e (doi:10.1227/NEU.0b013e31820ee24e) [DOI] [PubMed] [Google Scholar]
- 72.Haimi S, et al. 2009. Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng. A 15, 1473–1480 10.1089/ten.tea.2008.0241 (doi:10.1089/ten.tea.2008.0241) [DOI] [PubMed] [Google Scholar]
- 73.Supronowicz P, Gill E, Trujillo A, Thula T, Zhukauskas R, Ramos T, Cobb RR. 2011. Human adipose-derived side population stem cells cultured on demineralized bone matrix for bone tissue engineering. Tissue Eng. A 17, 789–798 10.1089/ten.tea.2010.0357 (doi:10.1089/ten.tea.2010.0357) [DOI] [PubMed] [Google Scholar]
- 74.Hattori H, Masuoka K, Sato M, Ishihara M, Asazuma T, Takase B, Kikuchi M, Nemoto K. 2006. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 76, 230–239 [DOI] [PubMed] [Google Scholar]
- 75.Levi B, Longaker MT. 2011. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 29, 576–582 10.1002/stem.612 (doi:10.1002/stem.612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith DM, Cooper GM, Afifi AM, Mooney MP, Cray J, Rubin JP, Marra KG, Losee JE. 2011. Regenerative surgery in cranioplasty revisited: the role of adipose-derived stem cells and BMP-2. Plast. Reconstr. Surg. 128, 1053–1060 10.1097/PRS.0b013e31822b65e4 (doi:10.1097/PRS.0b013e31822b65e4) [DOI] [PubMed] [Google Scholar]
- 77.Cowan CM, et al. 2004. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 22, 560–567 10.1038/nbt958 (doi:10.1038/nbt958) [DOI] [PubMed] [Google Scholar]
- 78.Grimaud E, Heymann D, Redini F. 2002. Recent advances in TGF-β effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 13, 241–257 10.1016/S1359-6101(02)00004-7 (doi:10.1016/S1359-6101(02)00004-7) [DOI] [PubMed] [Google Scholar]
- 79.Olivos-Meza A, Fitzsimmons JS, Casper ME, Chen Q, An KN, Ruesink TJ, O'Driscoll SW, Reinholz GG. 2010. Pretreatment of periosteum with TGF-β1 in situ enhances the quality of osteochondral tissue regenerated from transplanted periosteal grafts in adult rabbits. Osteoarthritis Cartilage 18, 1183–1191 10.1016/j.joca.2010.06.003 (doi:10.1016/j.joca.2010.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, Firestein GS. 2002. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J. Immunol. 168, 1405–1412 [DOI] [PubMed] [Google Scholar]
- 81.Roelen BA, Dijke P. 2003. Controlling mesenchymal stem cell differentiation by TGFβ family members. J. Orthop. Sci. 8, 740–748 10.1007/s00776-003-0702-2 (doi:10.1007/s00776-003-0702-2) [DOI] [PubMed] [Google Scholar]
- 82.Ali AM, Sharawy M. 1995. An immunohistochemical study of the effects of surgical induction of anterior disc displacement in the rabbit craniomandibular joint on type I and type II collagens. Arch. Oral Biol. 40, 473–480 10.1016/0003-9969(95)00005-A (doi:10.1016/0003-9969(95)00005-A) [DOI] [PubMed] [Google Scholar]
- 83.DeLise AM, Fischer L, Tuan RS. 2000. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309–334 10.1053/joca.1999.0306 (doi:10.1053/joca.1999.0306) [DOI] [PubMed] [Google Scholar]
- 84.Revell CM, Reynolds CE, Athanasiou KA. 2008. Effects of initial cell seeding in self assembly of articular cartilage. Ann. Biomed. Eng. 36, 1441–1448 10.1007/s10439-008-9524-x (doi:10.1007/s10439-008-9524-x) [DOI] [PMC free article] [PubMed] [Google Scholar]