Dear Editor,
Plant cell suspension cultures have been used as model systems to circumvent the problems associated with the analyses of a multi-factorial plant that is composed of multiple tissue and cell types exposed to diverse signals. A number of plant suspension cultures have proven to be valuable to study various topics including defense response, secondary metabolite formation, ion transport, gene regulation, and signal transduction (Roitsch and Sinha, 2002 and references therein). However, most cultures reported to date, including the cultures from model species such as Arabidopsis (Christie and Jenkins, 1996), require the presence of sugar in the medium and are characterized by no or negligible photosynthetic activity (Roitsch and Sinha, 2002). For only a very limited number of species, such as Chenopodium rubrum, have true photoautotrophic (PA) cultures been established (see references in Roitsch and Sinha, 2002). Such cultures combine the advantages of plant suspension cultures with carbon autotrophy and represent very powerful model systems for plant research. Unfortunately, for as-yet unknown reasons, it is very difficult to establish PA cultures (Widholm, 1992); hence, PA cultures from only a few crop species have been established. PA cultures have, however, been used to address various aspects of photosynthesis, herbicide effects, and secondary product formation from exclusively chloroplast localized pathways as well as in characterization of the metabolic changes occurring during the source–sink transition (Roitsch et al., 1995) and the coordinated regulation of primary metabolism and defense responses (Ehness et al., 1997). In parallel, a large number of mutant and transgenic Arabidopsis plants have been characterized with respect to their physiology, biochemistry, and molecular biology. That said, the establishment of a PA cell culture of Arabidopsis has, to date, proven elusive.
A photomixotrophic, chlorphyll containing cell suspension cultures of Arabidopsis thaliana, ecotype Columbia (Col-0) grown in continuous light with 2% supplementary sucrose, was used as starting material. Cultivation was initially carried out in two-tiered flasks with a carbonate buffer in the lower compartment that maintained approximately 2% CO2 in the atmosphere. This treatment was followed by a series of passages through medium containing stepwise 50% reductions of sucrose content during which growth rates initially dropped. When the growth rate had recovered, the next shift to a lower sucrose concentration was attempted. Finally, after a total period of about 2 years, stable growth in a sucrose-free medium was achieved.
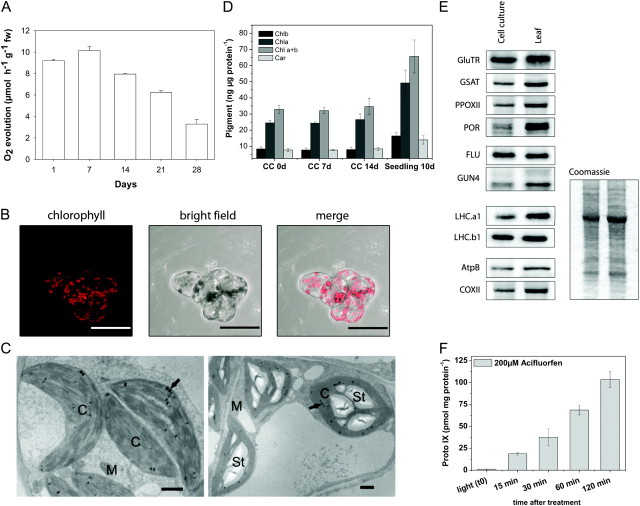
The generation of an Arabidopsis thaliana PA cell culture was ascertained by analyzing growth characteristics, photosynthetic, and metabolic parameters. When a half volume of the pre-culture was used as inoculum, maximal growth rates of the culture were approached 4 d after sub-culturing, but were reduced within the following days, resulting in a maximal doubling time of 10 d (Supplemental Figure 1; similar to that reported from several other PA cultures (Roitsch and Sinha, 2002)). The rate of oxygen evolution reached a maximum 7 d after sub-culturing, declined thereafter, and strongly declined after 21 d (Figure 1A). Thus, the time frame of optimal physiological activities of the green cells appears to be in the first 4–10 d after transfer.
Figure 1.
Characterization of the Photoautotrophic Cell Suspension Culture of A. thaliana.
(A) Measurement of O2 evolution during growth.
(B) Analysis of PA cell culture by fluorescence and bright field light microscopy and merge of the pictures.
(C) Effect of a source–sink transition induced by glucose on starch accumulation in chloroplasts. Ultrastructure of untreated 2-week-old control PA A. thaliana suspension culture cells (left) and after treatment with 50 mM glucose for 48 h (right). Arrows, chloroplasts with plastoglobuli; M, mitochondria; St, starch grains; Bar = 1 μm.
(D) Quantitative analysis of chlorophylla (Chla), Chlorophyllb (Chlb), sum of Chla and Chlb (Chla+b), total carotenoids (Car) from the Arabidopsis PA cell culture (CC), and from 10-day-old soil-grown seedlings.
(E) Protein levels of PA CC and Arabidopsis seedlings in light. Immunodetected protein bands for the following proteins are shown: glutamyl-tRNA reductase (GluTR), glutamate 1-semialdehyde aminotransferase (GSAT), protoporphyrinogenoxidase II (PPOXII), protochlorophyl lideoxidoreductase (POR), fluorescent in blue light (FLU), genome uncoupled 4 (GUN4), light harvesting chlorophyll binding proteins (LHCa1 and LHCb1), beta subunit of ATP-synthase (AtpB), and cytochrome c oxidase II (COXII). Coomassie blue staining of the protein extract preparation used for immunodetection.
(F) Manipulation of tetrapyrrole synthesis by acifluorfen. Arabidopsis PA culture cells were incubated with 200 μM acifluorfen in light at approximately 180 μmol photons m−2 s−1. Compared to the steady-state level (t 0), a linear accumulation of Proto IX was detected over a period of 2 h.
Light microscopic analyses revealed that, during cultivation, cells form small aggregates of a few cells that contain different amounts of chloroplasts. The difference in the number of chloroplasts in the individual cells was also demonstrated via chlorophyll fluorescence visualized by fluorescence microscopy (Figure 1B). Analysis of ultra-thin sections in a transmission electron microscope (TEM) reveals a good ultrastructural preservation of the cells including mitochondria and chloroplasts with grana stacks and plastoglobuli (Figure 1C).
To establish the PA cells as an additional model system, we compared different metabolic parameters and assessed differences in metabolite composition between Arabidopsis PA and heterotrophic cells and soil-grown Arabidopsis seedlings. Chlorophyll contents of the PA cell culture were constant within 14 d of cultivation in fresh media and were equivalent to 50% of the pigment levels documented for 10-day-old green Arabidopsis seedlings (Figure 1D). Under our growth conditions, the chlorophyll a/b ratio of the PA cell culture and seedlings were similar, indicating a similar relative composition of chlorophyll binding proteins of the antenna and core complexes of both photosystems.
Using GC–MS, the magnitude of relative metabolite levels observed for Arabidopsis PA cell suspension culture was quantitatively and significantly more similar to Arabidopsis seedlings than to Arabidopsis heterotrophic cell suspension cultures (Supplemental Table 1 and Supplemental Figure 2). The relative content of 45% of the metabolites was not significantly different between these two samples, while 6% of the metabolites were not significantly different between the Arabidopsis heterotrophic cell suspension culture and the soil-grown seedlings. These data suggest that the PA culture is suitable for addressing many, although not all, aspects of primary metabolism of photosynthetic organisms.
A specific advantage of PA cultures is the possibility to analyze source–sink transitions without the complication of the presence of multiple cell types (Roitsch, 1999). In order to check the response of photosynthetic source activities in response to the induction of sink metabolism by sugar, 2-week-old cultures were independently challenged with either 50 mM of sucrose or of its isomer palatinose (which can neither be taken up by plant cells nor metabolized by plant enzymes). The cell cultures were analyzed for maximum quantum yield (Fv/Fm) and effective quantum yield (ΦII) of photosystem II deduced from chlorophyll fluorescence measurements over different time periods. The Fv/Fm ratio remained unchanged up to 48 h, while ΦII declined only in the presence of sucrose (Supplemental Figure 3). High values for the Fv/Fm ratio indicate photosynthetic efficiency of the cell culture. Further, the fact that a decrease in the ΦII value was only prompted by sucrose and not palatinose is in accordance with previously characterized PA cell cultures (Sinha et al., 2002).
We next conducted an ultrastructural analysis using a TEM in order to visualize starch accumulation and distribution in chloroplasts. The PA culture of A. thaliana was treated with glucose for 12, 24, and 48 h, samples were fixed, and ultra-thin sections analyzed by TEM in comparison to untreated control samples. Chloroplasts of control samples were predominantly free of starch grains (Figure 1C). In contrast, the majority of the volume of chloroplasts from 50 mM glucose-treated samples was occupied by starch granules of variable size after 48 h (Figure 1C). These results are in agreement with the consequences of feedback inhibition of sugars on photosynthesis and confirm similar metabolic responses to those from green seedlings (Avelange et al., 1990).
Tetrapyrrole biosynthesis is one of the essential pathways for PA growth. PA cultures have the attraction to assay changes in the expression and activity of the pathway almost instantaneously after supply of any inhibitory or stimulatory agent. As a measure for rapid changes in the metabolic flow of chlorophyll biosynthesis, steady-state levels of tetrapyrrole intermediates, transcript and protein levels, and enzyme activities of the pathway can be easily determined (Figure 1E). Protein contents involved in chlorophyll synthesis and photosynthesis were compared between the PA cell cultures and the leaves of young seedlings by Western blot analysis. Related to total protein, all analyzed proteins were detectable in similar amounts with the exception of protochlorophyllide oxidoreductase (Figure 1E), arguing for similar quantitative metabolic activities of cell cultures and green seedlings and suggesting that the cell cultures are an adequate alternative system for the exploration of rapid regulatory processes in the metabolic pathway of tetrapyrrole biosynthesis.
To confirm the applicability of the PA culture for measurements of rapid metabolic responses, we performed an initial inactivation experiment in which the peroxidizing herbicide acifluorfen was applied, which inhibits the central enzyme of the pathway, protoporphyrinogen oxidase. Within 15 min of acifluorfen application, protoporphyrin(ogen) IX accumulated more than 25 times compared with the start of the inhibition experiment (Figure 1F), indicating the immediate uptake of the agent followed by instantaneous inhibition of tetrapyrrole biosynthesis.
When taken together, the data presented here demonstrate that the described PA culture of A. thaliana will likely be useful for addressing fast responses such as those involved in signaling cascades as well as in studying photosynthesis per se, tetrapyrrole metabolism, and many questions in primary metabolism such as source–sink regulation and metabolic control by sugars.
SUPPLEMENTARY DATA
Supplementary Data are available at Molecular Plant Online.
Acknowledgments
The skillful technical assistance of Peter Krbez and Tanja Mayer is greatly acknowledged. No conflict of interest declared.
References
- Avelange M-H, Sarrey F, Rébillé F. Effects of glucose feeding on respiration and photoautotrophic Dianthus caryophyllus cells: mass spectrometric determination of gas exchange. Plant Physiol. 1990;94:1157–1162. doi: 10.1104/pp.94.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Jenkins GI. Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell. 1996;8:1555–1567. doi: 10.1105/tpc.8.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt D, Roitsch T. Glucose and stress independently regulate source/sink relations and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell. 1997;9:1825–1841. doi: 10.1105/tpc.9.10.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T. Source sink regulation by sugar and stress. Curr. Opin. Plant Biol. 1999;2:198–206. doi: 10.1016/S1369-5266(99)80036-3. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Sinha AK. Application of photoautotrophic suspension cultures in plant science. Photosynthetica. 2002;40:481–492. [Google Scholar]
- Roitsch T, Bittner M, Godt DE. Induction of apoplastic invertase of Chenopodium rubrum by D-glucose and a glucose analogue and tissue specific expression suggest a role in sink source regulation. Plant Physiol. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK, Hofmann MG, Römer U, Köckenberger W, Elling L, Roitsch T. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002;128:1480–1489. doi: 10.1104/pp.010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JM. Properties and uses of photoautotrophic plant cell cultures. Int. Rev. Cytol. 1992;132:109–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.