Abstract
p27Kip1 (p27), a CDK inhibitor, migrates into the nucleus, where it controls cyclin–CDK complex activity for proper cell cycle progression. We report here that the classical bipartite-type basic amino-acid cluster and the two downstream amino acids of the C-terminal region of p27 function as a nuclear localization signal (NLS) for its full nuclear import activity. Importin α3 and α5, but not α1, transported p27 into the nucleus in conjunction with importin β, as evidenced by an in vitro transport assay. It is known that Akt phosphorylates Thr 157 of p27 and this reduces the nuclear import activity of p27. Using a pull-down experiment, 14-3-3 was identified as the Thr157-phosphorylated p27NLS-binding protein. Although importin α5 bound to Thr157-phosphorylated p27NLS, 14-3-3 competed with importin α5 for binding to it. Thus, 14-3-3 sequestered phosphorylated p27NLS from importin α binding, resulting in cytoplasmic localization of NLS-phosphorylated p27. These findings indicate that 14-3-3 suppresses importin α/β-dependent nuclear localization of Thr157-phosphorylated p27, suggesting implications for cell cycle disorder in Akt-activated cancer cells.
Keywords: importin, nuclear import, p27, phosphorylation, 14-3-3
Introduction
Cell cycle progression is controlled by a series of cyclins and cyclin-dependent kinases (CDKs), while p27, a CDK inhibitor, acts as an important cell cycle regulator (reviewed in Hengst and Reed, 1998; Sherr and Roberts, 1999). Cyclin–CDK complex activity is positively or negatively regulated by p27 to promote cell cycle progression (Cheng et al, 1999). p27 is stabilized post-translationally and accumulates in quiescent cells, and is localized in the nucleus where it regulates the cyclin–CDK complex (Reynisdottir et al, 1995). At G1–S transition, p27 is phosphorylated at Thr187 by cyclin E-CDK2 (Muller et al, 1997; Montagnoli et al, 1999). Skp2-containing E3 ubiquitin ligase, SCF, and its cofactor, Cdk subunit1 (Cks1), recognize Thr187-phosphorylated p27 and promote its degradation by proteasome (Carrano et al, 1999; Sutterluty et al, 1999). Recently, Ser10 was also found to be phosphorylated (Ishida et al, 2000), causing cytoplasmic localization of p27, leading to progression to the S/G2 phase (Rodier et al, 2001; Boehm et al, 2002). In addition, it has been reported that Jab1 promotes CRM1-mediated nuclear export of p27 (Tomoda et al, 1999). CRM1 directly binds to the nuclear export signal (NES) of p27 and exported it to the cytoplasm (Connor et al, 2003). Thus, p27 activity is controlled by its subcellular localization as well as by its concentration. A reduced p27 expression has been consistently observed in many human cancers and is correlated with tumor progression, while cytoplasmic localization of p27 in several cancers has also been implicated in promoting cell proliferation (reviewed in Slingerland and Pagano, 2000). Indeed, p27-deficient mice are prone to tumor development (Nakayama et al, 1996).
The phosphatidylinositol-3 kinase (PI3K)/Akt pathway regulates fundamental cellular functions such as cell proliferation, survival and motility (reviewed in Vanhaesebroeck and Alessi, 2000). Inappropriate activation of this kinase cascade has been implicated as a causative factor in the development of autoimmunity and cancer. In fact, the overexpression of Akt has been reported in several human cancers (reviewed in Vivanco and Sawyers, 2002). A recent study demonstrated that cytoplasmic localization of p21 in HER2/neu overexpressing cells was caused by phosphorylation of p21 by activated Akt (Zhou et al, 2001). Akt phosphorylates p27 at Ser10, Thr187 and Thr198, and phosphorylation of Thr198 promotes 14-3-3 binding and cytoplasmic localization of p27 (Fujita et al, 2002). Phosphorylation of Thr157 of p27 by Akt also induces cytoplasmic localization of p27 in breast cancer (Liang et al, 2002; Shin et al, 2002; Viglietto et al, 2002), although the mechanism for this is currently unclear.
Macromolecules traffic through nuclear pores that are located at the nuclear envelope. The nuclear pore complex (NPC) is composed of ∼30 different proteins called nucleoporins. Molecules with molecular weights greater than ∼40 kDa are selectively transported through the NPC by a mediated process. Most nuclear proteins contain nuclear localization signals (NLSs), which are largely divided into two types: classical-type and nonclassical-type NLS. Classical-type NLS is composed of basic amino-acid cluster(s) (Dingwall and Laskey, 1991), whereas nonclassical-type NLS has no typical similarity. Classical NLSs are recognized by importin α (karyopherin α), and form a complex with importin β (karyopherin β) (reviewed in Görlich and Mattaj, 1996; Kuersten et al, 2001). Cargo binding and release are regulated by a small GTPase Ran. Mammals possess at least six importin α isoforms, which can be largely classified into three subfamilies based on the similarities of their primary structures (Köhler et al, 1997; Tsuji et al, 1997). The existence of different importin α isoforms in mammals implies that they might recognize distinct substrates and transport them into the nucleus. Although it has been deduced that the bipartite-type sequence at the C-terminal region of p27 acts as its NLS (Zeng et al, 2000; Hirano et al, 2003), its precise NLS has not yet been determined. In addition, the issue of how p27 is imported into the nucleus has not been elucidated.
In this study, we report on the precise identification of the NLS of p27 located at the C-terminal region. It is composed of classical bipartite-type basic amino-acid clusters and extra two amino acids (152–168). Importin α3 and α5 transport it into the nucleus in conjunction with importin β. 14-3-3 was found to recognize specifically Thr157-phosphorylated p27NLS and prevented it from binding to importin α, resulting in the inhibition of nuclear import of p27. Thus, these results suggest that suppressing nuclear localization of p27 through Akt and 14-3-3 enhances cell proliferation.
Results
Nuclear import of p27 is mediated by its C-terminal region
In order to analyze the molecular mechanism involved in the nuclear import of p27, recombinant Flag-tagged p27 (Flag-p27) was microinjected into the HeLa cytoplasm. The Flag-p27 was translocated to the nucleus within 30 min at 37°C, whereas incubation at 4°C prevented its nuclear accumulation (Figure 1A). This indicated that p27 is actively transported into the nucleus, although Flag-p27 is small enough to pass through nuclear pores by diffusion.
Figure 1.
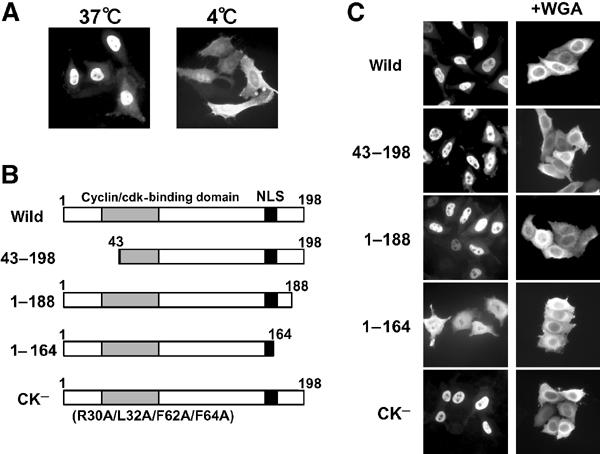
C-terminal region participates in the active nuclear import of p27. (A) Purified recombinant Flag-p27 was microinjected into the HeLa cytoplasm. After 30 min at 37 or 4°C, cells were fixed and the subcellular localization of Flag-p27 was detected by indirect immunofluorescence using anti-Flag antibody. (B) Flag-p27 mutants used in this experiment. (C) Nuclear import activity of Flag-p27 mutants. Purified Flag-p27 mutants were microinjected with or without WGA into the HeLa cytoplasm.
Several reports have indicated that NLS of p27 (p27NLS) is located at the C-terminal region (Zeng et al, 2000; Hirano et al, 2003), and that in the case of the Xenopus p27 homologue, Xic1, cyclin/CDK binding is required for its efficient nuclear import in addition to its own NLS (Chuang and Yew, 2001). Several deletion and point mutants of human p27 were prepared in an effort to identify an essential region for nuclear import (Figure 1B). Flag-p27 (CK−) has four point mutations (R30A/L32A/F62A/F64A) that inhibit cyclin D–CDK4 binding (Vlach et al, 1997). As expected, GST–Flag-p27 (CK-) did not precipitate cyclin D–CDK4 from HeLa whole-cell extract, whereas wild-type GST–Flag-p27 did (data not shown). When purified Flag-p27 mutants were microinjected into the HeLa cytoplasm, mutants lacking cyclin binding (43–198) or cyclin–CDK-binding activity (CK-) were translocated into the nucleus in a manner similar to the wild type (Figure 1C). In contrast, one of the C-terminal deletion mutants (1–164) was localized in the cytoplasm (Figure 1C). To exclude the possibility that deleted p27s translocated through the nuclear pore by diffusion, we performed microinjection of these mutant p27s with wheat germ agglutinin (WGA), which inhibits active nuclear-cytoplasmic protein transport. As shown in Figure 1C, p27 mutants did not translocate into the nucleus in the presence of WGA. To rule out the possibilities of protein degradation and facilitated protein nuclear export of these mutant p27s, the effects of both a proteasome inhibitor, MG132 (10 μM), and a nuclear export inhibitor, leptomycin B (10 ng/ml), were examined. They had no effects on the subcellular localizations of either Flag-p27 (wild) or 1–164 (data not shown). These results indicate that, in contrast to Xenopus Xic1, cyclin–CDK binding is not required for human p27 nuclear import and that its C-terminal region contains NLS activity.
Identification of p27NLS
In order to identify more precisely the p27NLS, we inserted candidate NLS-like sequences of p27 between GST and GFP of the GST–GFP fusion construct (Figure 2A), and microinjected purified recombinant fusion proteins into the HeLa cytoplasm. Consistent with the previous report (Tachibana et al, 1996), GST–GFP alone remained in the cytoplasm, whereas SV40 large T antigen NLS-inserted GST–GFP (SV40 NLS) was translocated into the nucleus (Figure 2Ba). Although the C-terminal region of p27 contains a typical bipartite-type NLS-like sequence (Figure 2A, NLS1) as reported previously (Zeng et al, 2000), this sequence showed only a weak nuclear import activity, compared with SV40 NLS (Figure 2Bb, NLS1). On the other hand, we found that two extra amino acids (Ala167 and Asn168), located downstream of the second basic amino-acid cluster, were required for full nuclear import activity (Figure 2Bb, NLS3). The protein containing a further amino acid, Arg169, also showed efficient nuclear import activity (data not shown). To exclude the possibility that these extra amino acids simply act as a spacer between p27NLS and GFP, three glycine residues were added to p27-NLS1 at its C-terminal (Figure 2A, NLS4). This sequence showed only a weak import activity similar to that of NLS1 (Figure 2Bb, NLS4), suggesting that the extra C-terminal two amino acids (Ala167 and Asn168) are essential for the full nuclear import activity of p27NLS. Deletion (NLS5) or amino-acid substitutions (NLS6) in the basic amino-acid clusters led to the complete loss of the nuclear import activity (Figure 2Bb), indicating that the basic amino-acid clusters in p27NLS are important, similar to other bipartite-type NLSs. Thus, the classical bipartite-type basic region plus the C-terminal extra two amino acids (152–168) function as the NLS of human p27.
Figure 2.
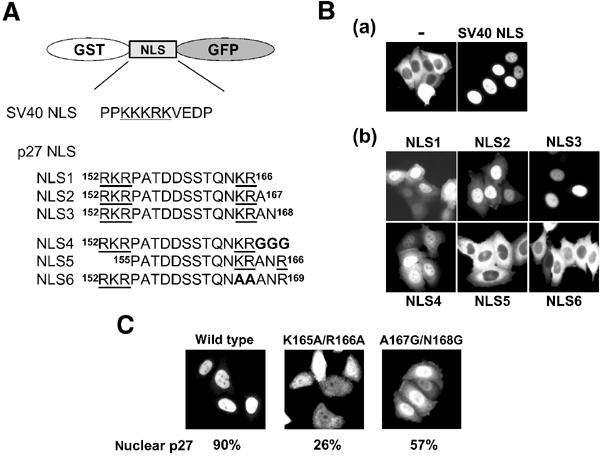
NLS of p27 is bipartite type. (A) Sequences inserted into GST–GFP fusion proteins are listed. The basic amino-acid clusters are underlined, and substituted or inserted amino acids are in bold letters. (B; a) GST–GFP alone (−) or NLS of SV40 (SV40 NLS)-inserted GST–GFP was microinjected into the HeLa cytoplasm. (b) Nuclear import activity of p27NLS-inserted GST–GFP fusion proteins. Proteins were microinjected into the HeLa cytoplasm. (C) Full-length Flag-p27 (wild type, K165A/R166A or A167G/N168G mutant) was microinjected into the HeLa cytoplasm. The relative nuclear to cytoplasmic ratio (average values for 20 injected cells) was represented as percentage.
To confirm the nuclear import activity of p27NLS in full-length protein, mutations were introduced into the NLS of full-length p27. Mutant Flag-p27 proteins (K165A/R166A and A167G/N168G) were purified and microinjected into HeLa cells. These mutations significantly affected the nuclear import activity of Flag-p27 (Figure 2C), indicating again that the classical bipartite-type sequence-containing region (152–168) plays an important role in the nuclear translocation of p27.
Importin α3 and α5 mediate the nuclear import of p27
As we found that p27 has a classical bipartite-type-like NLS, we hypothesized that importin α recognizes p27NLS. Three importin α's were used in this study: importin α1/Rch1, importin α3/Qip1 and importin α5/NPI-1. Purified Flag-p27 was immobilized to anti-Flag agarose, and GST or GST-importin α's were then added. Importin α3 and α5, but not α1, specifically bound to full-length p27 (Figure 3A, lanes 3–5). Similar results were obtained when GST-removed importin α's were used (data not shown). It is known that the affinity between importin α and NLS is dramatically increased in the presence of importin β (Catimel et al, 2001). We therefore performed the same experiments in the presence of HA-importin β. Although HA-importin β did not bind to p27 directly (Figure 3A, lane 6), importin β associated with p27 in conjunction with importin α3 or α5; however, importin α1 interacted with p27 only slightly even in the presence of importin β (Figure 3A, lanes 8–10). In contrast, p27 mutant (K165A/R166A), which is deficient in nuclear import activity (Figure 2C), did not associate with importin α (Figure 3A, lanes 13–15). These results indicate that p27 is recognized by specific importin α family members, importin α3 and α5, and forms a complex with importin β.
Figure 3.
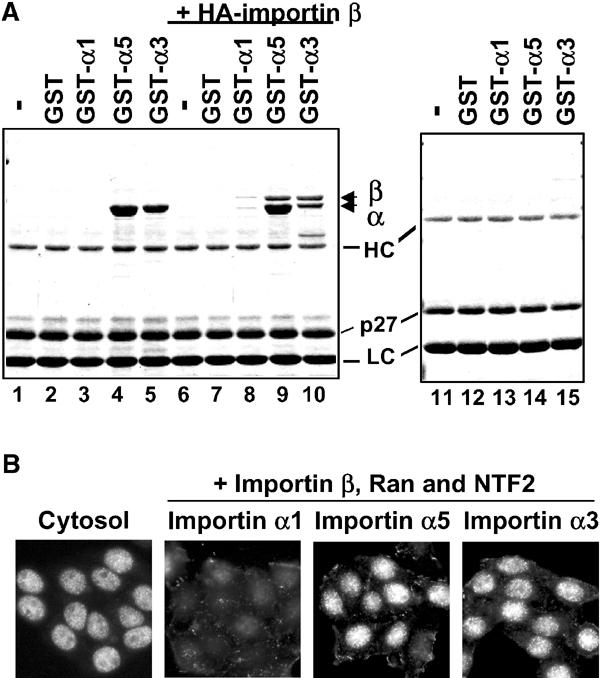
Importin α3- and α5-dependent nuclear import of p27. (A) Solution-binding assay. Flag-p27 was incubated with GST or GST-importin α's with (lanes 1–5) or without (lanes 6–10) HA-importin β in the presence of anti-Flag agarose. The same experiment was carried out using Flag-p27 (K165A/R166A) without importin β (lanes 11–15). The bound proteins were eluted and analyzed by SDS–PAGE. GST-importin α1, α3 and α5 are indicated as GST-α1, -α3 and -α5. HC: heavy chain and LC: light chain. (B) In vitro transport assay. Flag-p27 was applied to the in vitro reconstituted transport assay system supplemented with Ehrlich cytosol, or importin α/β with Ran and NTF2.
In order to confirm whether specific importin α's participate in p27 nuclear import, we attempted an in vitro reconstituted transport assay using recombinant importin α/β in digitonin-permeabilized cells. When Ehrlich total cytosol was used, Flag-p27 was effectively transported into the nucleus. Consistent with the binding assay, importin α3 and α5, but not importin α1, transported Flag-p27 into the nucleus (Figure 3B). From these findings, we concluded that importin α3 and α5 specifically recognize and transport p27 into the nucleus in conjunction with importin β.
Phosphorylation of Thr157 reduces nuclear import of p27
Recent studies indicate that Akt phosphorylates Thr157 of p27, causing the cytoplasmic localization of p27 in breast cancer cells (Liang et al, 2002; Shin et al, 2002; Viglietto et al, 2002). Flag-p27 co-transfected with constitutively active (CA) Akt to HeLa cells distributed throughout the cells, although co-transfection with dominant-negative (DN) Akt did not affect its nuclear accumulation (Figure 4A). In contrast, a mutant p27 (T157A), in which Thr157 was substituted to Ala, clearly accumulated in the nucleus irrespective of active Akt (Figure 4A), indicating that the phosphorylation of Thr157 by Akt reduces the nuclear import activity of p27. As mentioned above, we identified p27NLS as 152–168. Thr157 is located within this sequence, suggesting that phosphorylation of Thr157 may alter NLS activity. To address whether phosphorylation of Thr157 affects importin α binding, we performed immunoprecipitation assay. Co-expression of active Akt (CA) strongly inhibited the binding of importin α5 to p27 (Figure 4B, lane 2), suggesting that cytoplasmic localization of Thr157-phoshorylated p27 is caused by reduced importin α binding.
Figure 4.
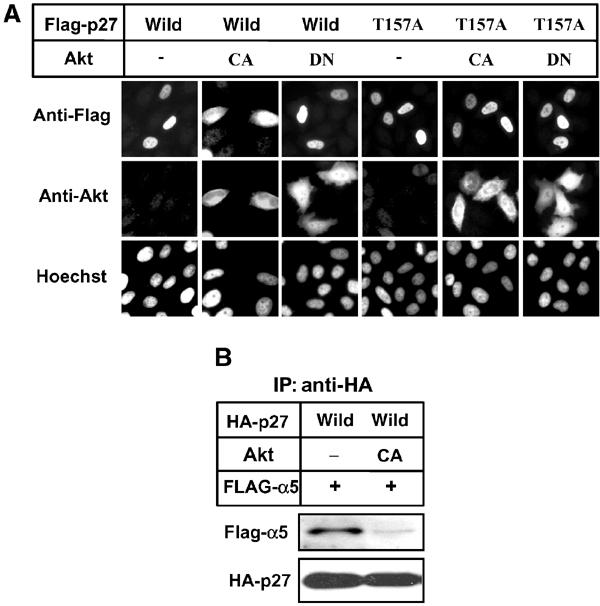
Phosphorylation by Akt increased cytoplasmic p27. (A) HeLa cells were transfected with pcDNA-Flag-p27 (wild or T157A) together with empty vector (−) or pcDNA-Akt (CA or DN) at 1:9 ratio for 24 h. DNA was stained by Hoechst dye. CA, constitutively active form. DN, dominant-negative form. (B) Phosphorylation of p27 by Akt reduced importin α5 binding. 293T cells were transfected with pcDNA-HA-p27 (wild) and pcDNA-Flag-importin α5 (Flag-α5) together with empty vector (−) or pcDNA-Akt (CA). HA-p27 was immunoprecipitated (IP) and co-precipitated proteins were analyzed by Western blotting using anti-Flag or anti-HA antibody.
To investigate more precisely as to whether the phosphorylation of Thr157 reduces p27 nuclear import activity, we substituted Thr157 of p27 to Asp (T157D) to mimic phosphorylated state. Unexpectedly, Flag-p27 (T157D) showed nuclear accumulation and importin α-binding activity like wild type (Supplementary Figure S1). We then synthesized peptides corresponding to p27NLS with or without phospho-Thr157, and conjugated them to biotin-labeled BSA (Figure 5A). When microinjected into the HeLa cytoplasm, the nonphosphorylated conjugate (Figure 5B, NLS) was efficiently accumulated in the nucleus. In contrast, the major portion of the phosphorylated conjugate remained in the cytoplasm (Figure 5B, pNLS). These results suggest that the nuclear import activity of p27NLS is affected by Thr157 phosphorylation in vivo.
Figure 5.
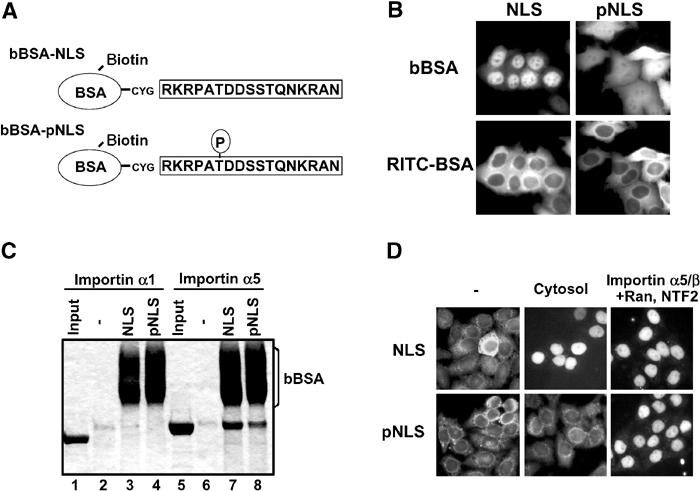
Phosphorylation at Thr157 reduces the nuclear import activity of p27NLS. (A) Conjugates used in this study. BSA was labeled with biotin, and then conjugated with nonphosphorylated or Thr157-phosphorylated p27NLS peptides. (B) Nuclear import activity of conjugates. Conjugates were microinjected into the HeLa cytoplasm with RITC-BSA. (C) Solution-binding assay. Conjugates were incubated with Flag-importin α1 (lanes 2–4) or α5 (lanes 6–8) in the presence of avidin agarose. The bound proteins were eluted and analyzed by SDS–PAGE. Minus (−) indicates incubation of importin α's with avidin agarose only. (D) In vitro transport assay. Conjugates were applied to the in vitro reconstituted transport assay system using Ehrlich cytosol, or importin α5/β with Ran and NTF2.
To explore whether the phosphorylation of NLS causes insufficient binding to nuclear import factor, we examined the direct binding activity of Thr157-phosphorylated p27NLS to importin α5. Solution-binding assay revealed that the phosphorylated conjugate (pNLS) showed only a slightly reduced but still considerable binding affinity to importin α5, compared with the nonphosphorylated peptide conjugate (NLS; Figure 5C, lanes 7 and 8). These findings indicate that the direct association between p27NLS and importin α5 was not greatly influenced by Thr157 phosphorylation. We therefore considered the possibility that cytosolic factor(s) might play an important role in cytoplasmic localization of phosphorylated p27NLS in vivo. To determine whether a cytosolic factor is involved in the cytoplasmic localization of p27, an in vitro transport assay was carried out. The nonphosphorylated conjugate (NLS) was transported into the nucleus by both Ehrlich cytosol and importin α5/β system (Figure 5D upper), whereas the phosphorylated conjugate (pNLS) was transported into the nucleus by importin α5/β, but not Ehrlich cytosol (Figure 5D lower). These results support the view that cytosolic factor(s) might bind to the Thr157-phosphorylated p27NLS, thus inhibiting the binding of p27NLS to importin α.
14-3-3 associates with Thr157-phosphorylated p27NLS
To identify the Thr157-phosphorylated p27NLS-binding protein, we performed a pull-down experiment. HeLa cytosol was incubated with bBSA-NLS or bBSA-pNLS immobilized to avidin agarose. After extensive washing, bound proteins were eluted and analyzed by SDS–PAGE. Two major bBSA-pNLS-specific bands around 30 kDa were detected (Figure 6A, arrows). Based on their size (∼30 kDa) and phosphothreonine binding character, we deduced these proteins as 14-3-3 family proteins. Therefore, we subjected the same samples to Western blotting to determine whether 14-3-3 proteins were included in bBSA-pNLS binding proteins. As shown in Figure 6A (right panel), 14-3-3 was detected in Thr157-phosphorylated p27NLS-bound proteins but not in nonphosphorylated p27NLS-bound proteins, indicating that 14-3-3 associates with Thr157-phosphorylated p27NLS.
Figure 6.
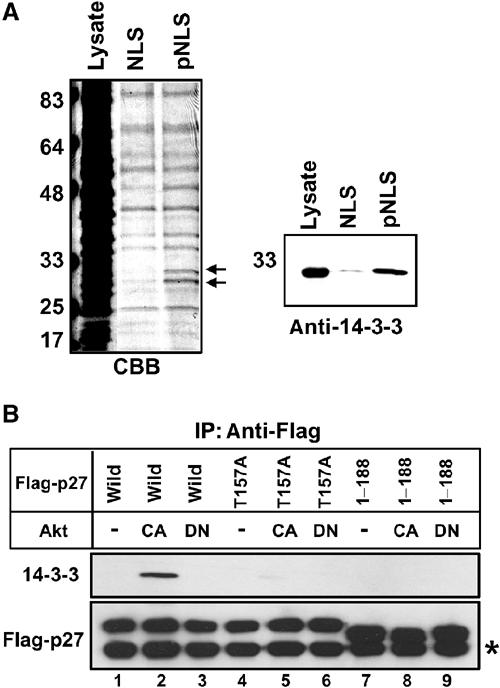
14-3-3 associates with Thr157-phosphorylated p27NLS. (A) Identification of Thr157-phosphorylated p27NLS-binding protein. HeLa cytosol was incubated with bBSA-NLS or bBSA-pNLS immobilized to avidin agarose. Bound proteins were separated by SDS–PAGE. Arrows indicate pNLS-specific binding proteins (left panel). The same samples were subjected to Western blotting and bound protein was detected by anti-14-3-3 antibody (right panel). (B) 14-3-3 associates with Thr157-phosphorylated p27 in vivo. 293T cells were transfected with pcDNA-Flag-p27 (wild, T157A or 1–188) together with empty vector (−; lanes 1,4 and 7) or pcDNA-Akt (CA or DN; lanes 2, 3, 5, 6, 8 and 9) at 1:9 ratio. Flag-p27 was immunoprecipitated (IP), and co-precipitated proteins were analyzed by Western blotting using anti-Flag or anti-14-3-3 antibody. Asterisk (*) indicates the light chain of IgG.
To confirm the in vivo binding of 14-3-3 to Thr157-phosphorylated p27, an immunoprecipitation assay was carried out. Flag-p27 (wild) expression vector was transfected with CA or DN Akt expression vectors, and Flag-p27 was immunoprecipitated with anti-Flag antibody. 14-3-3 was co-precipitated from active Akt-expressing cells, indicating that the phosphorylation of p27 by Akt induced 14-3-3 binding in vivo (Figure 6B, lane 2). We then used the Flag-p27 mutant (T157A) to examine whether the interaction between 14-3-3 and p27 was dependent on Thr157 phosphorylation. Co-expression of Flag-p27 mutant (T157A) with active or inactive Akt failed to promote the binding of 14-3-3 to Flag-p27 (Figure 6B, lanes 5 and 6). These findings clearly indicate that Thr157 phosphorylation of p27 by Akt creates the binding site for 14-3-3 in p27NLS. On the other hand, a recent study indicated that Akt also phosphorylated Thr198 and 14-3-3 recognized Thr198-phosphorylated p27 (Fujita et al, 2002). Consistently, Flag-p27 mutant (1–188) that lacks Thr198 did not associate with 14-3-3, even in the presence of active Akt (Figure 6B, lane 8). These results suggest that phosphorylation of Thr157 by Akt is necessary but not sufficient for in vivo binding to 14-3-3 (see Discussion).
14-3-3 competes with importin α5 for binding to Thr157-phosphorylated p27NLS
It is well known that 14-3-3 proteins are phosphopeptide-binding proteins that regulate cellular events. It is most likely that 14-3-3 binds to Thr157-phosphorylated p27NLS and downregulates its nuclear import activity. Purified recombinant HA-14-3-3 isoforms (β, ɛ, γ, σ, θ and ζ) were incubated with bBSA-NLS or bBSA-pNLS to detect direct binding in vitro. As shown in Figure 7A (lanes 1–6), HA-14-3-3β, ɛ and γ were efficiently associated with Thr157-phosphorylated p27NLS, but not with nonphosphorylated p27NLS. These results indicate that some of the 14-3-3 isoforms directly bind to Thr157-phosphorylated p27NLS with different binding affinities. As HA-14-3-3γ showed the strongest binding activity among 14-3-3 isoforms tested, we used HA-14-3-3γ in the experiments described below.
Figure 7.
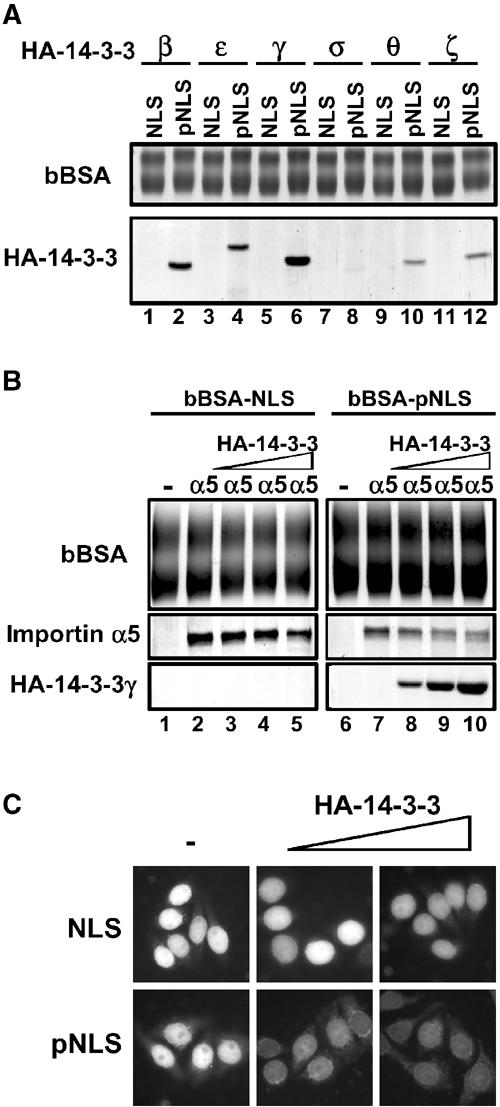
14-3-3 masks phosphorylated-p27NLS from importin α binding. (A) Specific 14-3-3 isoforms bind to phosphorylated p27NLS. Recombinant HA-14-3-3 isoforms (β, ɛ, γ, σ, θ and ζ) were incubated with bBSA-NLS (lanes 1, 3, 5, 7, 9 and 11) or bBSA-pNLS (lanes 2, 4, 6, 8, 10 and 12). The bound proteins were eluted and analyzed by SDS–PAGE. (B) 14-3-3 competes with importin α for binding to Thr157-phosphorylated p27NLS. Importin α5 was incubated with bBSA-NLS (lanes 2–5) or bBSA-pNLS (lanes 7–10) in the presence of increasing amounts of HA-14-3-3γ (lanes 3–5 and 8–10). The amounts of added HA-14-3-3 are 2 (lanes 3 and 8), 6 (lanes 4 and 9) and 16 (lanes 5 and 10) molar excess of importin α5. (C) 14-3-3 inhibits the nuclear import of Thr157-phosphorylated p27NLS in vitro. Conjugates were subjected to the in vitro reconstituted transport assay using importin α5/β with Ran and NTF2 in the presence of increasing amounts of HA-14-3-3γ. The amounts of added HA-14-3-3 are 8 and 24 molar excess of importin α5.
To clarify whether 14-3-3 competes with importin α5 for binding to Thr157-phosphorylated p27NLS, we performed a competitive binding assay. bBSA-NLS or bBSA-pNLS was incubated with importin α5 in the presence of HA-14-3-3γ. As shown in Figure 7B (lanes 1–5), the association between p27NLS and importin α5 was not impaired by 14-3-3γ, because 14-3-3γ did not bind to nonphosphorylated p27NLS at all. In contrast, HA-14-3-3γ inhibited the binding of importin α5 to phosphorylated p27NLS in a dose-dependent manner (Figure 7B, lanes 7–10), indicating that 14-3-3 competes with importin α5 for binding to Thr157-phosphorylated p27NLS.
To explore whether 14-3-3 suppresses the nuclear localization of phosphorylated p27NLS, we examined the effects of 14-3-3γ on the in vitro reconstituted transport of Thr157-phosphorylated p27NLS. Addition of the recombinant HA-14-3-3γ to the assay solution led to the inhibition of nuclear import of Thr157-phosphorylated p27NLS dose-dependently (Figure 7C lower). On the other hand, the nuclear import of nonphosphorylated p27NLS was not affected by the addition of 14-3-3γ, indicating that 14-3-3 recognizes Thr157-phosphorylated p27NLS and prevents it from binding to importin α by masking the NLS. Based on the above findings, we conclude that 14-3-3 binding causes the cytoplasmic localization of Thr157-phosphorylated p27.
Discussion
Nuclear localization of p27 in proper cell cycle phase is critical for its function; however, the precise mechanism of nuclear import of human p27 has not been determined. We report here that importin α/β recognizes the bipartite-type-like NLS located in the C-terminal region of p27 and transports it into the nucleus (Figure 1B and C). The p27NLS (152RKRPATDDSSTQNKRAN168) sequence identified in this study contains a typical bipartite-type NLS motif (152–166), but two extra amino acids (Ala167 and Asn168) located at its C-terminal are required for its full nuclear import activity (Figure 2B). Basic residues and the two downstream amino acids of p27NLS are well conserved in human, rodents and other mammalian p27 homologues. Substitution of Ala167 and Asn168 to glycine residues caused insufficient nuclear accumulation, suggesting that both amino acids are required for proper recognition by importin α (Figure 2B). Three importin α subfamily members showed different binding affinities to p27, and importin α3 and α5 were found to bind strongly to it (Figure 3A). Importin α3 and α5, but not importin α1, transported p27 into the nucleus in conjunction with importin β, as revealed by the in vitro transport system (Figure 3B). These results indicate that classical importin α/β transport system functions for p27 nuclear localization, suggesting that p27 may be effectively transported into the nucleus throughout the cell cycle except for the M phase, in which nuclear envelope breaks down. Indeed, exogenously injected p27 was translocated into the nucleus within 30 min at G1/S, S and G2 phases (Supplementary Figure S2). Thus, the nuclear import machinery for p27 (importin α/β system) functions throughout the cell cycle. On the other hand, it was previously shown that p27 protein levels are increased at G0/G1 and rapidly decreased in the S phase (Reynisdottir et al, 1995). These findings indicate that the cell cycle-dependent nuclear localization of p27 is primarily regulated by the protein level of p27, which is controlled by its protein degradation following nuclear export.
How does importin α recognize p27NLS? Several reports have shown that importin α subfamily has different substrate-binding features (Miyamoto et al, 1997; Köhler et al, 1999). Based on the crystallographic analysis, the classical NLS-binding region of importin α was found to be located in armadillo (arm) repeats (Conti et al, 1998; Fontes et al, 2000). However, several transport substrates bind to the C-terminal region of specific importin α (Sekimoto et al, 1997; Henderson et al, 2002). Similarly, p27NLS showed a high binding affinity to the specific importin α family, importin α3 and α5 (Figure 3A). However, association between p27 and importin α3 or α5 was dose-dependently inhibited by the addition of GST–SV40NLS–GFP fusion protein, and the addition of GST–SV40NLS–GFP also prevented nuclear import of p27 in the in vitro transport system (data not shown). These results suggest that the binding site of p27 in importin α may be close to that of SV40NLS, although the possibility that the p27-binding site of importin α is sterically hindered by SV40NLS cannot be excluded. The role of the additional two amino acids of p27NLS in specific importin α binding needs to be examined further. Similarly, it has been shown that non-basic amino acids were important for nuclear import activity of c-Myc NLS in addition to basic amino acids (Makkerh et al, 1996).
Subcellular localization of numerous proteins is regulated by phosphorylation and dephosphorylation. Phosphorylation of p27 is a key event in controlling subcellular localization. It has been shown that phosphorylation of Thr187 by CDK2, and of Ser10 by human kinase interacting stathmin (hKIS) induced nuclear export (Muller et al, 1997; Montagnoli et al, 1999; Boehm et al, 2002), whereas phosphorylation of Thr198 induced cytoplasmic localization of p27 (Fujita et al, 2002, 2003). In breast cancer cells, phosphorylation of Thr157 by Akt also caused cytoplasmic localization of p27 (Liang et al, 2002; Shin et al, 2002; Viglietto et al, 2002). Cytoplasmic localization of p27 by phosphorylation of Ser10 or Thr187 has been demonstrated to be due to facilitated CRM1-mediated nuclear export. As Thr157 was found to be located in the p27NLS, we expected that cytoplasmic localization of Thr157-phosphorylated p27 would be caused by an impaired nuclear localization, but not by promoted nuclear export. The peptide corresponding to p27NLS with phosphorylated Thr157 was conjugated to biotin-labeled BSA, and this conjugate showed cytoplasmic localization when microinjected into the HeLa cytoplasm (Figure 5B). This result clearly indicates that the phosphorylation of Thr157 prevented the nuclear import of p27NLS. It is noteworthy that Thr157 is not conserved in rodents, raising the possibility that phosphorylation at different sites may inhibit the nuclear import of p27 in different species. Indeed, several Akt phosphorylation sites of p27 have been reported (Ishida et al, 2000).
We identified 14-3-3 proteins as Thr157-phosphorylated p27NLS binding factors (Figure 5A). 14-3-3 proteins were first discovered as phospho-serine-binding proteins and are thought to play a pivotal role in signal transduction, cell cycle checkpoint control and apoptosis. In particular, it has been shown that 14-3-3 is able to act as a subcellular localization modifier for several phosphorylated proteins. For example, 14-3-3 promotes cytoplasmic localization of CDC25 (Kumagai and Dunphy, 1999; Lopez-Girona et al, 1999) and FKHRL1 (Brunet et al, 1999), while it promotes nuclear localization of TERT (Seimiya et al, 2000) and TLX-2 (Tang et al, 1998). Two 14-3-3-binding motifs have been identified thus far: RSXpS/TXP and RXXXpS/TXP (Yaffe et al, 1997; Rittinger et al, 1999). The Akt-phosphorylation site in p27NLS, 152RKRPAT157, is similar to the 14-3-3-binding motif. In fact, five 14-3-3 isoforms associated directly with Thr157-phosphorylated p27NLS (Figure 7A). In addition, we found that 14-3-3 competes with importin α5 for interacting with phosphorylated p27NLS (Figure 7B), resulting in the cytoplasmic localization of p27NLS (Figure 7C). Therefore, 14-3-3 proteins bind to Thr157-phosphorylated p27 to modulate its subcellular localization. On the other hand, recent studies have shown that phosphorylation of Thr198 of p27 by Akt and RSK promoted 14-3-3 binding, which caused cytoplasmic localization of Thr198-phosphorylated p27 (Fujita et al, 2002, 2003), although the issue of whether cytoplasmic localization of Thr198-phosphorylated p27 is caused by preventing nuclear import or by facilitated nuclear export should be further examined. In this study, we found that p27-lacking Thr198 (1–188) failed to associate with 14-3-3 in the presence of active Akt (Figure 6B) and also that p27 (1–188) showed nuclear localization even in the presence of active Akt as similar to p27 (T157A) when it was co-transfected with active Akt to HeLa cells (data not shown). From these findings, it is likely that phosphorylation of both Thr157 and Thr198 is required for efficient 14-3-3 binding in vivo, although 14-3-3 can bind to Thr157-phosphoryalted p27NLS in vitro.
p21, another CDK inhibitor, is also required for proper cell cycle progression, and the cellular localization of p21 is critical for its function (Sherr and Roberts, 1995; Asada et al, 1999). The cytoplasmic localization of p21 was observed in HER-2/neu overexpressing cells that were resistant to apoptosis and were promoted to proliferate (Zhou et al, 2000, 2001). In these cells, Thr145, which is located in the NLS of p21, was phosphorylated by activated Akt, and the phosphorylation of this residue caused cytoplasmic localization of p21 (Zhou et al, 2001). Thus, the subcellular localization of these two CDK inhibitors is regulated by Akt. The phosphorylation site of p21, 140RKRRQT145, is similar to the Akt-phosphorylation site of p27, suggesting that 14-3-3 may also prevent the nuclear import of phosphorylated p21 in Akt-activating cells. On the other hand, two groups have shown that Akt inactivated p21 but had no effect on its nuclear localization (Rossig et al, 2001; Li et al, 2002). Thus, unlike p27, cytoplasmic localization of p21 in human cancers is not frequently observed, suggesting that the mislocalization of p21 alone may contribute only to the development of some particular cancer.
Proper subcellular localization of proteins is important for their functions. Cytoplasmic localization of p27 frequently occurs in various human cancers. Although cyclin D3 overexpression caused cytoplasmic retention of p27 in thyroid tumor cells (Baldassarre et al, 1999), the mechanism of altered subcellular localization of phosphorylated p27 has not been elucidated. In this study, we revealed that 14-3-3 suppresses nuclear import of Thr157-phosphorylated p27. Our findings link the modification of nuclear-cytoplasmic protein transport with the development of human breast cancer. In conclusion, 14-3-3 prevents the binding of importin α to Thr157-phosphorylated p27, and consequently p27 is localized in the cytoplasm and cyclin–CDK complex activity is out of control, leading to cell cycle disorder.
Materials and methods
Cell culture
HeLa and 293T cells were maintained in Dulbecco's modified Eagle's Medium (Sigma) supplemented with 10% fetal bovine serum and antibiotics. For microinjection, HeLa cells were seeded on the day before injection at 2 × 105 on cover slips in 35 mm dishes. To prepare cells for the in vitro transport assay, HeLa cells were seeded at 1 × 106 on eight-well slide glass (ICN) 24–48 h before assay.
Plasmids and proteins
cDNA of full-length human p27 and 14-3-3 isoforms were cloned from HeLa total RNA by RT–PCR and verified by sequencing. N-terminal Flag-tagged p27 and HA-tagged 14-3-3s were inserted into the pGEX-6P-3 and pGEX-4T-3 (Pharmacia), respectively, and introduced into Escherichia coli BL21(DE3) strain. IPTG (0.1 mM) was added at 20°C for 16 h to induce protein expression, and GST-fusion proteins were purified by glutathione sepharose (Pharmacia) according to the manufacturer's instruction. The GST moiety was cleaved with PreScission protease (Pharmacia) for Flag-p27 and with thrombin (Pharmacia) for 14-3-3s. Site-directed mutagenesis was performed using a QuikChange mutagenesis kit (Stratagene) with appropriate oligonucleotides. Deleted p27 was generated by PCR amplification. To prepare GST–p27NLS–GFP fusion proteins, appropriate double-strand oligonucleotides were inserted into pGEX-GFP (Tachibana et al, 1996). HA-importin β, Ran, NTF2, GST-importin α1/Rch1, α3/Qip1 and α5/NPI-1 were expressed and purified as described previously (Imamoto et al, 1995a; Sekimoto et al, 1996; Miyamoto et al, 1997).
Synthetic peptides, CYGRKRPATDDSSTQNKRAN (NLS) or CYGRKRPApTDDSSTQNKRAN (pNLS; synthesized by Sigma Genosys) were conjugated to biotinylated bovine serum albumin (bBSA) as described previously (Imamoto et al, 1995b). Both conjugates contained 7–10 peptides per bBSA, as judged by SDS–PAGE. RITC-labeled BSA (RITC-BSA) was prepared as essentially described previously (Tachibana et al, 1996).
Indirect immunofluorescence
Cells were fixed by treatment with 3.7% formaldehyde, permeabilized with 0.5% Triton X-100 and blocked with 3% skim milk. The subcellular localization of Flag-p27 was then detected using anti-Flag M2 antibody (Sigma), followed by Alexa Fluor 488-labeled goat anti-mouse IgG (Molecular Probes). For double staining, polyclonal anti-Flag antibody (Sigma) and monoclonal anti-Akt antibody (BD Transduction Lab) were applied to the cells, followed by Alexa Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes) and Alexa Fluor 546-labeled goat anti-mouse IgG (Molecular Probes). For staining bBSA, cells were fixed and permeabilized as described above. After blocking with 3% BSA, the cells were stained with Alexa Fluor 488-labeled streptavidin (Molecular Probes).
Microinjection
Purified Flag-p27 (1 mg/ml) was microinjected into the cytoplasm of HeLa cells grown on cover slips and incubated at 37°C for 30 min unless otherwise stated. WGA was co-injected at 1 mg/ml. For microinjection of bBSA, 2 mg/ml bBSA-NLS or bBSA-pNLS was injected with RITC-BSA into the HeLa cytoplasm, and the cells were incubated at 37°C for 30 min.
For quantitation, the fluorescence intensities of cytoplasm and nuclei were calculated by integrating the pixel intensities of the fluorescence images using Adobe Photoshop.
In vitro transport assay
An in vitro cell free transport assay was performed as described previously (Nagoshi et al, 1999). Import substrates were Flag-p27, bBSA-NLS or bBSA-pNLS (10 pmol). After incubation at 28°C for 30 min, the cells were fixed and protein localization was detected by indirect immunofluorescence.
Solution-binding assay
Purified Flag-p27 (5 μg) and GST (5 μg) or GST-importin αs (18 μg) were mixed in 100 μl of transport buffer containing 0.1% Triton X-100 in the presence of anti-Flag M2 agarose (Sigma) at 4°C for 3 h. After extensive washing, bound proteins were eluted by SDS-sample buffer, applied on 12.5% SDS–PAGE and stained with Coomassie Brilliant Blue (CBB). For binding to bBSA, bBSA-NLS or bBSA-pNLS (20 μg) immobilized to ImmunoPure immobilized avidin (avidin agarose; Pierce) was incubated with Flag-importin α's (10 μg) or HA-14-3-3 isoforms (10 μg) in a transport buffer containing 0.1% Triton X-100 at 4°C for 3 h. Bound proteins were eluted and analyzed as described above. In a competition assay, HA-14-3-3γ was added to the above bBSA-NLS- or bBSA-pNLS-containing assay solutions in the presence of importin α5. The amounts of added recombinant proteins are specified in each figure legend.
Transfection, immunoprecipitation and Western blot analysis
HeLa or 293T cells were transfected with the combination of appropriate plasmids using Effecten transfection reagent (Qiagen) according to the manufacturer's instruction. Plasmids used for transfection were pcDNA-Flag-p27 (wild, T157A or 1-188), pcDNA-HA-p27 (wild), pcDNA-Flag-importin α5, pcDNA-Akt1 (myristylated ΔPH; constitutively active form referred as CA) and pcDNA-Akt1 (K179A/T308A/S473A; dominant-negative form referred as DN).
For immunoprecipitation, cells were lysed in transport buffer supplemented with 0.5% NP-40, 1 mM Na3VO4, 5 mM NaF, 1 mM PMSF, 10 μg/ml each aprotinin, leupeptin and pepstatin at 4°C for 30 min. After clarification, cell extracts were incubated with anti-HA agarose or anti-Flag agarose at 4°C for 2 h. The bound proteins were subjected to SDS–PAGE and transferred to a PVDF membrane. The membrane was incubated with anti-Flag M2 antibody, anti-HA antibody (Sigma) or polyclonal anti-14-3-3 antibody (#06–511, Upstate), following by HRP-conjugated goat secondary antibody. Signals were detected by an enhanced chemiluminescence system (ECL, Amersham).
Pull-down experiment
HeLa cells were lysed in hypotonic buffer (10 mM HEPES (pH 7.9), 10 mM KCl, 1.5 mM MgCl2 with protease and phosphatase inhibitors) using a Dounce homogenizer and dialyzed in transport buffer. HeLa cytosol was incubated with bBSA-NLS or bBSA-pNLS in the presence of avidin agarose at 4°C for 3 h in a transport buffer containing 0.1% Triton X-100. After extensive washing, the bound proteins were eluted by 0.1 M glycine–HCl (pH 3.0). The eluted proteins were precipitated with cold acetone and applied to SDS–PAGE.
Supplementary Material
Supplementary Materials
Materials and Methods
Acknowledgments
We thank Dr Yukiko Gotoh (The University of Tokyo) for the gift of pcDNA-Akt1 expression vectors. This work was supported by Grant-in-Aid for Scientific Research on Priority Areas (B; no. 11237202), Grant-in-Aid for Scientific Research (B; no. 12CE2007) from the Japanese Ministry of Education, Science, Sports and Culture, and the Human Frontier Science Program.
References
- Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S (1999) Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J 18: 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre G, Belletti B, Bruni P, Boccia A, Trapasso F, Pentimalli F, Barone MV, Chiappetta G, Vento MT, Spiezia S, Fusco A, Viglietto G (1999) Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J Clin Invest 104: 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG (2002) A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J 21: 3390–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1: 193–199 [DOI] [PubMed] [Google Scholar]
- Catimel B, Teh T, Fontes MR, Jennings IG, Jans DA, Howlett GJ, Nice EC, Kobe B (2001) Biophysical characterization of interactions involving importin-alpha during nuclear import. J Biol Chem 276: 34189–34198 [DOI] [PubMed] [Google Scholar]
- Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ (1999) The p21(Cip1) and p27(Kip1) CDK ‘inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 18: 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LC, Yew PR (2001) Regulation of nuclear transport and degradation of the Xenopus cyclin-dependent kinase inhibitor, p27Xic1. J Biol Chem 276: 1610–1617 [DOI] [PubMed] [Google Scholar]
- Connor MK, Kotchetkov R, Cariou S, Resch A, Lupetti R, Beniston RG, Melchior F, Hengst L, Slingerland JM (2003) CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell 14: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Uy M, Leighton L, Blobel G, Kuriyan J (1998) Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell 94: 193–204 [DOI] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA (1991) Nuclear targeting sequences—a consensus? Trends Biochem Sci 16: 478–481 [DOI] [PubMed] [Google Scholar]
- Fontes MR, Teh T, Kobe B (2000) Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-alpha. J Mol Biol 297: 1183–1194 [DOI] [PubMed] [Google Scholar]
- Fujita N, Sato S, Katayama K, Tsuruo T (2002) Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem 277: 28706–28713 [DOI] [PubMed] [Google Scholar]
- Fujita N, Sato S, Tsuruo T (2003) Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem 278: 49254–49260 [DOI] [PubMed] [Google Scholar]
- Görlich D, Mattaj IW (1996) Nucleocytoplasmic transport. Science 271: 1513–1518 [DOI] [PubMed] [Google Scholar]
- Henderson MJ, Russell AJ, Hird S, Munoz M, Clancy JL, Lehrbach GM, Calanni ST, Jans DA, Sutherland RL, Watts CK (2002) EDD, the human hyperplastic discs protein, has a role in progesterone receptor coactivation and potential involvement in DNA damage response. J Biol Chem 277: 26468–26478 [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI (1998) Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol 227: 25–41 [DOI] [PubMed] [Google Scholar]
- Hirano K, Zeng Y, Hirano M, Nishimura J, Kanaide H (2003) Sequence requirement for nuclear localization and growth inhibition of p27Kip1R, a degradation-resistant isoform of p27Kip1. J Cell Biochem 89: 191–202 [DOI] [PubMed] [Google Scholar]
- Imamoto N, Shimamoto T, Kose S, Takao T, Tachibana T, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y (1995a) The nuclear pore-targeting complex binds to nuclear pores after association with a karyophile. FEBS Lett 368: 415–419 [DOI] [PubMed] [Google Scholar]
- Imamoto N, Tachibana T, Matsubae M, Yoneda Y (1995b) A karyophilic protein forms a stable complex with cytoplasmic components prior to nuclear pore binding. J Biol Chem 270: 8559–8565 [DOI] [PubMed] [Google Scholar]
- Ishida N, Kitagawa M, Hatakeyama S, Nakayama K (2000) Phosphorylation at serine 10, a major phosphorylation site of p27(Kip1), increases its protein stability. J Biol Chem 275: 25146–25154 [DOI] [PubMed] [Google Scholar]
- Köhler M, Ansieau S, Prehn S, Leutz A, Haller H, Hartmann E (1997) Cloning of two novel human importin-alpha subunits and analysis of the expression pattern of the importin-alpha protein family. FEBS Lett 417: 104–108 [DOI] [PubMed] [Google Scholar]
- Köhler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Görlich D, Hartmann E (1999) Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol Cell Biol 19: 7782–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuersten S, Ohno M, Mattaj IW (2001) Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol 11: 497–503 [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG (1999) Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev 13: 1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dowbenko D, Lasky LA (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277: 11352–11361 [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM (2002) PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160 [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A, Furnari B, Mondesert O, Russell P (1999) Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397: 172–175 [DOI] [PubMed] [Google Scholar]
- Makkerh JP, Dingwall C, Laskey RA (1996) Comparative mutagenesis of nuclear localization signals reveals the importance of neutral and acidic amino acids. Curr Biol 6: 1025–1027 [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Imamoto N, Sekimoto T, Tachibana T, Seki T, Tada S, Enomoto T, Yoneda Y (1997) Differential modes of nuclear localization signal (NLS) recognition by three distinct classes of NLS receptors. J Biol Chem 272: 26375–26381 [DOI] [PubMed] [Google Scholar]
- Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M (1999) Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev 13: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, Ansorge W, Huttner W, Eilers M (1997) Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene 15: 2561–2576 [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Imamoto N, Sato R, Yoneda Y (1999) Nuclear import of sterol regulatory element-binding protein-2, a basic helix–loop–helix-leucine zipper (bHLH-Zip)-containing transcription factor, occurs through the direct interaction of importin beta with HLH-Zip. Mol Biol Cell 10: 2221–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY (1996) Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85: 707–720 [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Polyak K, Iavarone A, Massague J (1995) Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 9: 1831–1845 [DOI] [PubMed] [Google Scholar]
- Rittinger K, Budman J, Xu J, Volinia S, Cantley LC, Smerdon SJ, Gamblin SJ, Yaffe MB (1999) Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol Cell 4: 153–166 [DOI] [PubMed] [Google Scholar]
- Rodier G, Montagnoli A, Di Marcotullio L, Coulombe P, Draetta GF, Pagano M, Meloche S (2001) p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J 20: 6672–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S (2001) Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol 21: 5644–5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimiya H, Sawada H, Muramatsu Y, Shimizu M, Ohko K, Yamane K, Tsuruo T (2000) Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J 19: 2652–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y (1997) Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J 16: 7067–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y (1996) Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem 271: 31017–31020 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9: 1149–1163 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512 [DOI] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL (2002) PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8: 1145–1152 [DOI] [PubMed] [Google Scholar]
- Slingerland J, Pagano M (2000) Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 183: 10–17 [DOI] [PubMed] [Google Scholar]
- Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W (1999) p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol 1: 207–214 [DOI] [PubMed] [Google Scholar]
- Tachibana T, Hieda M, Sekimoto T, Yoneda Y (1996) Exogenously injected nuclear import factor p10/NTF2 inhibits signal-mediated nuclear import and export of proteins in living cells. FEBS Lett 397: 177–182 [DOI] [PubMed] [Google Scholar]
- Tang SJ, Suen TC, McInnes RR, Buchwald M (1998) Association of the TLX-2 homeodomain and 14-3-3eta signaling proteins. J Biol Chem 273: 25356–25363 [DOI] [PubMed] [Google Scholar]
- Tomoda K, Kubota Y, Kato J (1999) Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398: 160–165 [DOI] [PubMed] [Google Scholar]
- Tsuji L, Takumi T, Imamoto N, Yoneda Y (1997) Identification of novel homologues of mouse importin alpha, the alpha subunit of the nuclear pore-targeting complex, and their tissue-specific expression. FEBS Lett 416: 30–34 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR (2000) The PI3K–PDK1 connection: more than just a road to PKB. Biochem J 346 (Part 3): 561–576 [PMC free article] [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, Fusco A, Santoro M (2002) Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 8: 1136–1144 [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2: 489–501 [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Amati B (1997) Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J 16: 5334–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91: 961–971 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Hirano K, Hirano M, Nishimura J, Kanaide H (2000) Minimal requirements for the nuclear localization of p27(Kip1), a cyclin-dependent kinase inhibitor. Biochem Biophys Res Commun 274: 37–42 [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC (2000) HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem 275: 8027–8031 [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3: 245–252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials
Materials and Methods


