Abstract
Recent studies have shown that administration of peroxisome proliferator-activated receptor-β (PPARβ) agonists enhances fatty acid oxidation in rodent and human skeletal muscle and that muscle-restricted PPARβ overexpression affects muscle metabolic profile by increasing oxidative myofiber number, which raises the possibility that PPARβ agonists alter muscle morphology in adult animals. This possibility was examined in this study in which adult mice were treated with a PPARβ agonist, and the resulting changes in myofiber metabolic phenotype and angiogenesis were quantified in tibialis anterior muscles. The findings indicate a muscle remodeling that is completed within 2 days and is characterized by a 1.63-fold increase in oxidative fiber number and by a 1.55-fold increase in capillary number. These changes were associated with a quick and transient upregulation of myogenic and angiogenic markers. Both myogenic and angiogenic responses were dependent on the calcineurin pathway, as they were blunted by cyclosporine A administration. In conclusion, the data indicate that PPARβ activation is associated with a calcineurin-dependent effect on muscle morphology that enhances the oxidative phenotype.
Keywords: myogenesis, metabolism, Type 2 diabetes
skeletal muscle plays an important role in energy balance as a result of its mass, fuel needs, and ability to adapt to changes in substrate availability. Muscle energy expenditure, insulin sensitivity, and substrate preference are greatly affected by the muscle fiber composition, which determines muscle metabolic phenotype (14, 21). Myofibers with high mitochondrial content display high oxidative and low glycolytic capacity, whereas fibers with low mitochondrial content have the opposite metabolic phenotype. Importantly, myofiber composition and metabolic phenotype of a given muscle can be altered by physiological and pathological influences. Endurance exercise, which exerts beneficial metabolic actions, promotes a fiber type transition toward a more oxidative phenotype (3, 28), while physical inactivity and Type 2 diabetes lead to a reduction of the oxidative phenotype in various muscles (33, 47).
Thus increasing the oxidative capability of skeletal muscle would represent an effective therapeutic approach toward preventing or reversing the metabolic disturbances associated with Western lifestyle by enhancing energy expenditure. The peroxisome proliferator activated receptor-β (PPARβ) also called PPARδ is a promising pharmacological target, since it is the major PPAR isoform expressed in muscle and is involved in the regulation of muscle development and metabolism (24). In cultured myotubes, PPARβ overexpression and/or activation enhance fatty acid catabolism by upregulating genes that control fatty acid transport, β-oxidation, mitochondrial respiration, and energy uncoupling (16, 27, 46). Similar effects can be demonstrated in obese mouse models (46, 49) and healthy human subjects (43). Other evidence suggests that PPARβ is central to the adaptation of muscle to endurance exercise, which leads to the upregulation of muscle PPARβ in rodents (31) and humans (19, 50).
We and others (31, 49) have shown that muscle-specific PPARβ overexpression in mice promotes an increase in the number of oxidative fibers in muscle and augments resistance against diet-induced obesity. In contrast, muscle-selective PPARβ gene disruption reduces the percentage of oxidative fibers and increases susceptibility to diet-induced obesity (41).
Administration of PPARβ-specific agonists has been shown to improve the metabolic phenotype of obese and insulin-resistant animals by decreasing circulating and tissue lipids, by reducing insulinemia, and by increasing HDL-cholesterol (29, 36, 46, 49). Many of these beneficial actions have been attributed to changes of skeletal muscle metabolism. However, it is not known if the metabolic changes in muscle are associated with a remodeling of fiber composition and if this is case, then what is the length of time needed for this remodeling to occur. In this study, we examined the effects of a specific PPARβ agonist GW0742 administrated to adult mice on myofiber composition, as indicated by succinate dehydrogenase (SDH) histochemistry, and capillary density of the tibialis anterior. Our study indicates that PPARβ activation in mice leads to a very fast exercise-like muscle remodeling characterized by more SDH-positive fibers and increased capillary density.
As several studies have produced evidence for an important role for the calcineurin pathway in determining both oxidative phenotype of skeletal muscle and formation of new blood vessels, we explored the possible involvement of this regulatory pathway in PPARβ-controlled muscle remodeling. Calcineurin is a Ca2+-dependent protein phosphatase that acts as a Ca2+ sensor and couples prolonged changes in Ca2+ levels to reprogramming of gene expression in various cell types and organs, including endothelial cells, heart, and skeletal muscle. Activated calcineurin promotes dephosphorylation and nuclear translocation of nuclear factor of activated T cells (NFAT) transcription factors, which, in turn, activate the expression of several genes implicated in myofiber type switching toward oxidative phenotype (37, 42) and angiogenesis (4, 26). We demonstrated that inhibition of calcineurin activity by cyclosporine A (CsA) administration totally prevented both hyperplasic and angiogenic responses to PPARβ agonist treatment, suggesting the involvement of a calcineurin-dependent signaling pathway in PPARβ-promoted muscle remodeling.
MATERIALS AND METHODS
Animals
Animals were maintained in a 12:12-h light-dark cycle and received food (UAR, Villemoisson sur Orge, France) and water ad libitum. All experimental procedures were conducted in accordance with the guidelines of the University of Nice-Sophia Antipolis and approved by the Campus Valrose Animal care and ethics committee.
PPARβ agonist administration.
Ten-week-old male C57BL6J (Janvier, France) were used in the various experiments. GW0742, a PPARβ-specific activator (23), was dissolved in cremophor (Sigma) and injected subcutaneously once a day (9 am) at 1 mg/kg. Control animals received the vehicle at 9 AM. Animals were killed after the indicated times by cervical dislocation, and tibialis anterior muscles were harvested immediately after death. Forty mice were used for histological analysis in a first series of experiments (time points for both treated and control mice: 5, 24, 48, and 96 h; 5 animals per time point and condition). Fifty mice were used for protein analysis in a second series of experiments (time points for both treated and control mice: 2, 5, 24, 48, and 96 h; 5 animals per time point and condition).
PPARβ agonist and CsA coadministration.
Twenty 10 wk-old male C57BL6J (five animals per group) were used. CsA (Sigma) treatments were performed twice a day (9 AM and 6 PM) by subcutaneous injections of the compound at 20 mg/kg in cremophor. GW0742 was dissolved in cremophor and injected subcutaneously once a day (2 pm) at 1 mg/kg. Injections of vehicle were performed at 9 AM, 2 PM, and 6 PM (control group); 9 AM and 6 PM (GW group); and 2 PM (CsA group). Animals were killed 48 h after the first injection of GW0742 by cervical dislocation, and tibialis anterior muscles were harvested immediately after death for histological and protein analyses.
Generation and maintenance of PPARβ human skeletal actin-Cre transgenic mice.
Animals overexpressing PPARβ specifically in skeletal muscle were generated as described previously (31). Briefly, B6D2 mice harboring a loxP-stop-loxP-PPARβ-hygromycine construction were crossed with B6D2 mice expressing Cre recombinase under human skeletal actin (HSA) promoter (34). All animals were maintained hemizygous for their transgene. The presence of the transgenes was verified by PCR analyses of tail DNA (REDExtract-N-Amp Tissue PCR kit, Sigma). Animals harboring the two transgenes were used as PPARβ-overexpressing mice, while animals harboring the HSA-Cre transgene were only used as controls.
Histological Analyses
Tibialis anterior muscles were harvested and frozen in tissue embedding medium (VWR International) immediately after the mouse was killed. Ten-micrometer cryosections were performed from the middle part of muscle, placed on poly-l-lysine coated slides (VWR International), and processed for histological analyses as described below.
SDH staining.
SDH activity was revealed by incubation of slides in 50 mM phosphate buffer pH 7.6, 50 mM sodium succinate, and 0.05% nitro blue tetrazolium for 30 min at room temperature. After a wash in sodium chloride 0.9%, the numbers of stained fibers, called SDH-positive myofibers, and unstained fibers, called SDH-negative myofibers, were determined in whole tibialis anterior sections by using Olympus DP-Soft software.
Capillary number determination.
Isolectin B4 was detected using an immunohistochemical method. Briefly, slides were incubated with a biotinylated antibody rose against isolectin B4 (Vector Laboratories) and signal was revealed using ABC and DAB kits (Vector Laboratories). Capillary numbers were determined in whole tibialis anterior sections by using Olympus DP-Soft software.
Protein Expression Analyses
Total proteins from tibialis anterior muscles were extracted in a buffer containing 50 mM Tris·HCl, pH 6.8, 10% glycerol, 10% SDS, 10 mM dithiothreitol, and Protease Inhibitor Complete Cocktail (Roche Molecular Biochemicals). Fifty micrograms of total protein were analyzed by SDS-PAGE and blotted on polyvinylidene fluoride membranes (Amersham Pharmacia Biotech). The antibodies used were as follows: Ms-351 (LabVision Neomarkers) for vascular endothelial growth factor-A (VEGF-A), sc-28188 (Santa Cruz Biotechnologies) for platelet endothelial cell adhesion molecule 1 (PECAM-1), and sc-302 (Santa Cruz biotechnologies) for Myf-5 and Ms-273 (LabVision Neomarkers) for MyoD1. Signals for the ubiquitously expressed TATA binding protein (TBP) were detected using sc-273 antibody (Santa Cruz Biotechnologies) and used for loading normalization. Signals were detected with horseradish perioxidase conjugated rabbit or mouse polyclonal antibody (Promega), using Uptilight chemiluminescence detection spray (Interchim) and quantified by digital imaging (Fuji LAS3000).
Statistical Analyses
All values are presented as means ± SD. Two-way ANOVA tests were performed for comparisons between groups and duration of treatment. When significant changes were observed in ANOVA tests, Fisher's paired least significant difference post hoc test was applied to locate the source of significant differences. Analyses were performed with Stat View Abacus Concept version 5. No significant differences were observed for any variable among control groups at the different time points.
RESULTS
PPARβ Pharmacological Activation Increases Oxidative Myofiber Number in Tibialis Anterior Muscles
To characterize the effects of PPARβ activation on myofiber composition of tibialis anterior muscles, adult C57Bl6J male mice received a daily subcutaneous injection of a specific PPARβ agonist, GW0742 (1 mg/kg). Cross-sections around the midportion of muscles were prepared at various times and treated for in situ staining of SDH activity, a marker of mitochondrial complex II content. This method allows for the distinction between oxidative fibers, i.e., rich in mitochondria and appearing in dark, and glycolytic fibers, i.e., poor in mitochondria and remaining unstained. In control animals, the tibialis anterior muscle contains almost equal amounts of SDH-negative and SDH-positive myofibers (Fig. 1A). The number of both oxidative and glycolytic fibers remained unchanged in mice treated up to 24 h with the PPARβ agonist. By contrast, after 48 h of treatment, the total myofiber number was significantly increased (+600 fibers; P < 0.05; n = 5). This increment in myofiber number is predominantly related to an increased oxidative fiber number (+500; P < 0.05; n = 5), whereas the SDH-negative fiber number was not significantly changed (Fig. 1, B and C).
Fig. 1.
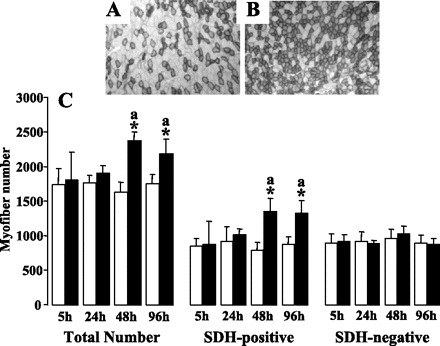
GW0742 administration to adult mice promotes hyperplasic response in tibialis anterior muscles. A and B: typical pictures of succinate dehydrogenase (SDH) detection in tibialis anterior muscles from adult C57BL6J untreated (A) or treated with GW0742 (B) for 48 h. C: quantification of total myofiber number, SDH-positive myofiber number, and SDH-negative myofiber number in tibialis anterior from C57BL6J mice untreated (open bars) and treated for increasing times with GW0742 (filled bars). Myofiber numbers were determined using Olympus DP Soft software. Values are means ± SD from 5 animals of each group. *P < 0.01 vs. controls; aP < 0.05 vs. 5- and 24-h GW-treated groups.
PPARβ activation also promoted an important and time-dependent reduction of the diameter of both SDH-positive (Fig. 2A) and SDH-negative fibers (Fig. 2C). Fiber diameter was unchanged up to 24 h and reduced by ∼25% after 48 h of treatment in both fiber types. Fiber size remained unchanged for longer treatment. Interestingly, the analysis of fiber size distribution after 48 h of treatment revealed that the reduction of mean diameter affects the totality of the SDH-positive (Fig. 2B) and SDH-negative (Fig. 2D) fibers.
Fig. 2.
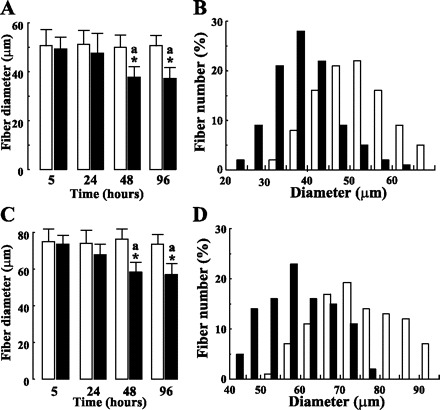
GW0742 administration promotes a fiber diameter reduction in tibialis anterior muscles of adult mice. Diameters of at least 300 fibers of SDH-positive (A and B) and SDH-negative (C and D) fibers were determined. Values presented in A and C are means ± SD from groups of 5 animals receiving for indicated times either vehicle (open bars) or GW0742 (filled bars). *P < 0.01 vs. controls; aP < 0.05 vs. 5- and 24-h GW-treated groups. B and D: fiber diameter distribution for animals receiving for 48-h vehicle alone (open bars) or GW0742 (filled bars).
We next examined the effects of PPARβ activation on the expression levels of regulatory transcription factors implicated in myogenesis, MyoD1 and Myf5 (20). Western blot analyses revealed that PPARβ activation led to a transient accumulation of both myogenic markers in tibialis anterior muscles (Fig. 3). The effect on Myf5 was already detectable after 2 h, maximal after 5 h (1.7-fold), returned to the control level at 24 h, while longer treatments led to important reduction of the protein content (2-fold decrease after 48 h of treatment). PPARβ activation led to a more marked (∼3-fold) and delayed effect on MyoD1 protein accumulation. Upregulation of MyoD1 protein was detectable at 5 h, persisted up to 48 h, and returned to control value after 96 h of chronic treatment.
Fig. 3.
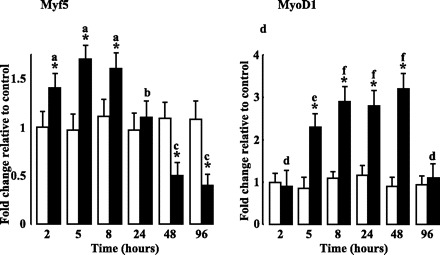
GW0742 administration induces expression of myogenic markers. Densitometric analyses of tibialis anterior lysates from animals treated or not with GW0742 for the indicated times. Expression levels for Myf-5 and MyoD1 were determined as described in materials and methods. TATA binding protein (TBP) was used as internal standard. For each time and condition, values are plotted as fold change vs. animals receiving vehicle alone for 2 h and are means ± SD from 5 animals of each group. *P < 0.05 vs. controls; aP < 0.05 vs. 24, 48, and 96 h of GW treatment; bP < 0.05 vs. 2, 5, 8, 48, and 96 h of GW treatment; cP < 0.05 vs. 2, 5, 8, and 24 h of GW treatment; dP < 0.05 vs. 5, 8, 24, and 48 h of GW treatment; eP < 0.05 vs. 8, 24, and 48 h of GW treatment; fP < 0.05 vs. 2, 5, and 96 h of GW treatment.
PPARβ Pharmacological Activation Promotes Muscle Angiogenesis
Endurance exercise training that leads to myofiber transition toward a more oxidative phenotype is also characterized by increased muscle vascularization. To determine the effects of PPARβ activation on capillary density, we performed in situ staining of isolectin B4, a glycoprotein expressed in endothelial cell membranes, in cross-sections around the midportion of tibialis anterior muscles from adult mice receiving or not daily subcutaneous injections of GW0742 at 1 mg/kg. Typical pictures of isolectin B4 detection are shown for tibialis anterior from untreated (Fig. 4A) and 48 h GW0742-treated (Fig. 4B) animals. Quantification of the capillary number in whole muscle sections revealed a significant increase in the capillary number after 24 h in GW0742-treated animals. After 48 h, the capillary number per muscle section was increased by 1.5-fold and did not significantly change for longer periods of treatment (Fig. 4C).
Fig. 4.
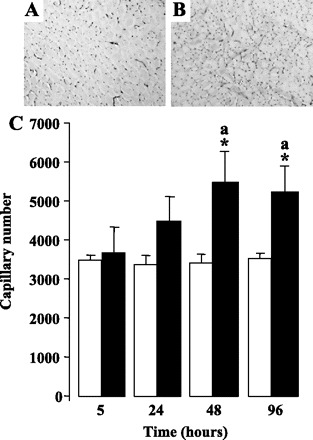
PPARβ pharmacological activation promotes muscle angiogenic response. A and B: typical pictures of isolectin B4 detection in the middle part of tibialis anterior muscles from adult C57BL6J untreated (A) or treated with GW0742 (B) for 48 h. C: capillary number in tibialis anterior from C57BL6J mice untreated (open bars) and treated for the indicated times (filled bars) with GW0742. Quantifications were determined using Olympus DP Soft software. Values are means ± SD from 5 animals of each group. *P < 0.01 vs. controls; aP < 0.05 vs. 5- and 24-h GW-treated groups.
To further investigate this angiogenic response to PPARβ activation, we quantified by Western blot the expression levels of VEGF-A, a potent angiogenic peptide that elicits mitogenic action on endothelial cells (18), and PECAM-1, a typical endothelial marker, in tibialis anterior muscles from animals treated for various times with the PPARβ agonist. As shown in Fig. 5, these experiments revealed that the effects of PPARβ activation on VEGF-A protein amounts were detectable after 2 h, peaked at 5 h (8-fold induction), and remained valuable up to 24 h. The induction was reduced for longer periods of treatment, and after 96 h, the VEGF-A signal was significantly reduced (3-fold decrease) compared with untreated animals. PPARβ activation also exerted a potent and fast action on PECAM-1 protein content (∼8-fold induction after 8 h). PECAM-1 amounts were reduced for longer periods of treatment but remained significantly higher than in control animals up to 96 h of treatment.
Fig. 5.
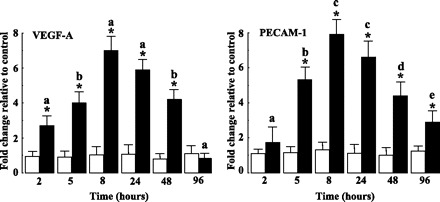
PPARβ activation induces expression of angiogenic markers. Densitometric analyses of tibialis anterior lysates from GW0742-treated adult mice. Expression levels of vascular endothelial growth factor-A (VEGF-A) and platelet endothelial cell adhesion molecule 1 (PECAM-1) were determined as described in materials and methods. TBP was used as internal standard. For each time and condition, values are plotted as fold change vs. animals receiving vehicle only for 2 h and are means ± SD from 5 animals of each group. *P < 0.05 vs. controls; aP < 0.05 vs. other time points of GW treatment; bP < 0.05 vs. 2, 8, 24, and 96 h of GW treatment; cP < 0.05 vs. 2, 5, 8, and 96 h; dP < 0.05 vs. 2, 8, and 24 h of GW treatment; eP < 0.05 vs. 2, 5, 8, and 24 h of GW treatment.
Collectively, these results clearly indicate that pharmacological activation of PPARβ promotes a fast and impressive enhancement of the oxidative phenotype of myofibers that involves upregulation of myogenic markers and an angiogenic response. In that respect, treatment of wild-type animals with the PPARβ agonist leads to a muscle remodeling that is reminiscent of that induced by regular physical training.
Calcineurin Signaling is Implicated in PPARβ-Promoted Muscle Remodeling
Given the fact that activation of calcineurin pathway has been implicated in the transition process toward a more oxidative phenotype in skeletal muscle (2, 10, 32), we examined the effects of alteration of the calcineurin signaling on muscle remodeling promoted by PPARβ pharmacological activation. To that purpose, adult animals were treated with GW0742 in the presence or the absence of CsA at a concentration known to block the calcineurin signaling pathway (9). The numbers of SDH positive and negative myofibers and the capillary number were determined after 48 h of coadministration of the compounds (Fig. 6). These analyses established that CsA had no significant effect on both the SDH-positive and SDH-negative myofiber numbers and on capillary number in tibialis anterior muscles from GW0742-untreated mice. By contrast, CsA administration totally prevented the muscle remodeling induced by GW0742 treatment, i.e., the increases in SDH-positive fibers, in total myofiber number (Fig. 6A) and in capillary density (Fig. 6B).
Fig. 6.
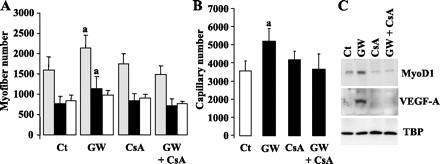
GW0742-promoted muscle remodeling is dependent upon the calcineurin pathway; 10 wk-old C57BL6J mice were treated for 48 h with GW0742 (GW), cyclosporine A (CsA), or a combination of both compounds (GW + CsA). Control animals (Ct) received vehicle alone. A: tibialis anterior sections from the various animals were reacted for SDH activity. Total myofiber number (gray bars), SDH-positive myofiber number (filled bars) and SDH-negative myofiber number (hatched bars) were determined in whole muscle sections. B: quantification of capillary number in tibialis anterior sections from the various animals was performed by isolectin B4 immunodetection. In A and B quantifications were determined using Olympus DP Soft software. Values are means ± SD from 5 animals of each group. aP < 0.05 vs. the other conditions. C: Western blot analyses of tibialis anterior proteins from control animals (Ct), GW0742-treated animals, cyclosporine A-treated mice, and animals that received a combination of both molecules. Detection of MyoD1 and VEGF-A was performed as described in materials and methods. TBP was used as internal standard.
We next investigated the effects of CsA administration on the expression levels of MyoD1 and VEGF-A proteins in tibialis anterior muscles from mice treated or not with GW0742 for 48 h. As shown in Fig. 6C, CsA administration totally abolished the PPARβ-promoted accumulation of myogenic and angiogenic markers in muscles.
These observations indicated that the calcineurin pathway is necessary for PPARβ-promoted muscle remodeling.
Muscle-Specific PPARβ Overexpression Promotes Angiogenesis
We have previously shown that muscle-targeted PPARβ overexpression promoted an increase in the number of oxidative myofibers in various mouse muscles (31). To compare the muscle remodeling induced by PPARβ pharmacological activation or muscle-specific PPARβ overexpression, we determined the capillary number and total myofiber number in tibialis anterior muscles from 10-wk-old double transgenic mice (harboring both HSA-Cre and Stop-PPARβ transgenes) and their control littermates (animals harboring the HSA-Cre transgene only). These data are reported in Table 1 together with the values obtained for adult C57Bl6J wild-type mice treated for 2 days with GW0742. In accordance with our previous observations (31), PPARβ overexpression in skeletal muscle promoted an increase of 37% of total myofiber number. Despite a significantly reduced myofiber number in untreated C57Bl6J mice compared with control B6D2 mice, PPARβ pharmacological activation led after 48 h to a similar 37% increase in total myofiber number. By contrast, PPARβ overexpression clearly appeared less effective than PPARβ activation (25 vs. 55% increase in capillary number, respectively) in promoting angiogenesis.
Table 1.
PPARβ overexpression or pharmacological activation increases myofiber and capillary numbers
| Control | Transgenic | %Increase | Nontreated | 48-h Treated | %Increase | |
|---|---|---|---|---|---|---|
| Myofiber number | 2,085±256 | 2,855±220† | 37 | 1,730±208 | 2,375±125‡ | 37 |
| SDH positive, % | 45 | 62 | 47.7 | 56.9 | ||
| SDH negative, % | 55 | 38 | 52.3 | 43.1 | ||
| Capillary number | 4,393±321 | 5,464±214* | 24.4 | 3,507±92 | 5,492±538‡ | 54.6 |
Values are means ± SD; n = 5 mice of each genotype. PPARβ, peroxisome proliferator-activated receptor-β; SDH, succinate dehydrogenase.
P < 0.01,
P < 0.005 vs. control animals.
P < 0.001 vs. nontreated animals.
DISCUSSION
In this study, we demonstrate that treatment of adult mice by a specific PPARβ agonist leads to a very fast remodeling of tibilalis anterior muscles. Our data indicate that administration of GW0742 to adult mice induces a 1.37-fold increment of total myofiber number largely accounted for by an increase in SDH-positive myofibers (Fig. 1; Table 1) associated with a 1.55-fold increase in capillary density (Fig. 4; Table 1). Interestingly, this muscle remodeling took place very rapidly, was complete after 2 days of treatment and did not change thereafter despite continued PPARβ agonist administration (Figs. 1 and 4). A time-course study of the molecular events in response to PPARβ activation confirmed the histological observations and showed rapid but transient upregulation of both myogenic (Myf5 and MyoD1) and angiogenic markers (VEGF-A and PECAM-1) in the tibialis anterior muscle (Figs. 3 and 5).
These observations indicate that PPARβ agonist treatment promotes histological and biochemical changes of skeletal muscle that are reminiscent of those taking place during exercise-induced adaptive remodeling. Muscle remodeling in response to muscular activity differs according to the type of activity involved. Three weeks of chronic slow frequency electrical stimulation of the motor nerve of rabbit tibialis anterior muscles led to a twofold increase in capillary number and oxidative fiber switching (7). Similar increases in capillary density and oxidative fiber number were also reported after several weeks of long-term endurance training and voluntary running in rodent models (1, 7, 12, 25). Clearly, the muscle remodeling induced by PPARβ agonist administration is very fast compared with the period of time required for remodeling in response to muscular activity.
Additionally, the effects of PPARβ agonist on myogenic and angiogenic markers also recapitulated those observed during muscle adaptation to physical exercise. Several studies revealed that, in trained animals, upregulation of myogenic and angiogenic markers occurred within hours or days, returned to control values rapidly, and preceded muscle remodeling (30, 48, 51).
An intriguing observation is the GW0742-promoted reduction in cross-section area of both SDH-positive and SDH-negative fibers in the tibialis anterior. This phenotype was not observed during endurance exercise-induced muscle remodeling (1) and is more suggestive of the initiation of an atrophy program. Interestingly, it has been recently reported (11) that GW0742 administration upregulated the expression of two muscle specific E3 ligases, atrogin-1/MAFbx and MuRF-1, that play important roles in ubiquitin-proteasome-dependent muscle proteolysis. Further investigations are needed to evaluate the implications of such an effect of PPARβ activation. However, it should be noted that fasting induces muscle atrophy and also promotes PPARβ upregulation in mouse skeletal muscle (27), suggesting a potential role of the nuclear receptor in this physiological process.
PPARβ is expressed in several cell types present in adult skeletal muscle, including myofibers, myoblasts (27), and endothelial cells (39, 44), and it is likely that these cell types are contributing to the muscle remodeling promoted by PPARβ agonist treatment. Two hypotheses can be proposed to explain the formation of new fibers, which requires myoblast recruitment. PPARβ activation in myoblasts could initiate terminal differentiation by promoting a transient upregulation of myogenic genes and formation of the new fibers. Alternatively or concomitantly, PPARβ activation in myofibers could promote the production of signals that, in turn, trigger myoblast terminal differentiation. The existence of signals produced by myofibers to activate differentiation and/or fusion of myoblasts, such as specific interleukins or growth factors, has been documented (15, 45). The fiber hyperplasia produced by administration of the PPARβ agonist was very similar to that observed with HSA-Cre-mediated overexpression of PPARβ (Table 1). This could argue in favor of a prominent role of the functional fibers in myoblast recruitment, since in the transgenic model, PPARβ overexpression occurred specifically in functional myofibers and not in myoblasts (31, 34).
In contrast, the amplitude of capillarization was significantly higher in GW0742-treated animals than in the muscles of PPARβ overexpressing mice (Table 1). This suggests that activation of the PPARβ pathway specifically in functional fibers induced a limited angiogenic response, while the angiogenic response in agonist-treated mice could involve other cell types, such as endothelial cells. Involvement of both myofibers and endothelial cells in the production of VEGF-A and in the remodeling of skeletal muscle capillary network has been shown. Several studies have demonstrated that VEGF-A mRNA and protein are upregulated in myofibers during training-induced muscle remodeling (5, 6, 8) and that endothelial cells from skeletal muscle are also able to produce VEGF-A (13).
Noteworthy, a proangiogenic action of various PPARβ agonists implicating upregulation of VEGF-A and VEGF receptor was recently described in murine aortic and human umbilical endothelial cells (39, 44). The mechanisms of PPARβ-mediated activation of VEGF-A gene expression remain unclear. The presence of a PPAR-responsive element in the VEGF-A gene promoter has been reported and implicated in the repression of the gene by PPARγ agonists in adenocarcinoma cells (38). However, Fauconnet et al. (17) showed that the PPARβ agonist-mediated activation of VEGF-A gene expression in bladder cancer cells involves an indirect mechanism requiring the synthesis of an intermediary regulatory protein through the MAPK pathway.
Our data demonstrating a complete blunting of PPARβ agonist-mediated muscle remodeling by coadministration of CsA (Fig. 6) strongly suggest an indirect action through the calcineurin pathway rather than a direct transactivation of myogenic and angiogenic markers. It has been established that calcineurin, a Ca2+/calmodulin-regulated phosphatase, plays an important function in myofiber type specification and angiogenesis by dephosphorylation and nuclear translocation of the transcription factors of the NFAT family. Activation of the calmodulin/calcineurin/NFAT signaling pathway leads to increased transcription of genes expressed in oxidative fibers and results in enhanced mitochondrial biogenesis (2, 10, 32). Other findings (22, 35, 40) have implicated the calcineurin/NFAT signaling pathway in endothelial cell proliferation and angiogenesis.
In summary, our results confirmed the role of PPARβ in adaptive responses of skeletal muscle and demonstrate for the first time that pharmacological activation of the nuclear receptor results in a very fast enhancement of oxidative capability of the tissue by increasing both oxidative fiber number and capillary density. As already proposed for exercise-induced muscle remodeling, these actions of PPARβ on muscle morphology implicate an activation of the calcineurin pathway. How PPARβ pharmacological activation is affecting the calcineurin signaling remains to be investigated.
GRANTS
This work was funded by the Institut National de la Santé et de la Recherche Médicale, the Programme National de Recherche sur le Diabète (n°A04074AS) from the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche and the Programme Cardiovasculaire, Obésité et Diabète from the Agence Nationale de la Recherche (ANR-05-PCOD-012). C. Gaudel was supported by a doctoral fellowship from the Fondation Recherche Médicale.
Acknowledgments
We thank Dr. T. M. Willson (GlaxoSmithKline) for the generous gift of GW0742 and Dr. A. S. Rousseau (Institut National de la Santé et de la Recherche Médicale U907, Nice, France) for statistical analyses of the data.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90: 1900–1908, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem 276: 43524–43533, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand 99: 123–125, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Armesilla AL, Lorenzo E, Gomez del Arco P, Martinez-Martinez S, Alfranca A, Redondo JM. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol Cell Biol 19: 2032–2043, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birot OJ, Koulmann N, Peinnequin A, Bigard XA. Exercise-induced expression of vascular endothelial growth factor mRNA in rat skeletal muscle is dependent on fibre type. J Physiol 552: 213–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Brown MD, Cotter MA, Hudlicka O, Vrbova G. The effects of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflügers Arch 361: 241–250, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Brutsaert TD, Gavin TP, Fu Z, Breen EC, Tang K, Mathieu-Costello O, Wagner PD. Regional differences in expression of VEGF mRNA in rat gastrocnemius following 1 hr exercise or electrical stimulation. BMC Physiol 2: 8–18, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakkalakal JV, Stocksley MA, Harrison MA, Angus LM, Deschenes-Furry J, St-Pierre S, Megeney LA, Chin ER, Michel RN, Jasmin BJ. Expression of utrophin A mRNA correlates with the oxidative capacity of skeletal muscle fiber types and is regulated by calcineurin/NFAT signaling. Proc Natl Acad Sci USA 100: 7791–7796, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12: 2499–2509, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantin D, Constantin-Teodosiu D, Layfield R, Tsintzas K, Bennett AJ, Greenhaff PL. PPARdelta agonism induces a change in fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function in rat skeletal muscle. J Physiol 583: 381–390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper J, Hudlicka O. Effect on muscle fatigue of the changes in the capillary bed induced by long-term stimulation [proceedings]. J Physiol 263: 155P–156P, 1976 [PubMed] [Google Scholar]
- 13.Da Silva-Azevedo L, Baum O, Zakrzewicz A, Pries AR. Vascular endothelial growth factor is expressed in endothelial cells isolated from skeletal muscles of nitric oxide synthase knockout mice during prazosin-induced angiogenesis. Biochem Biophys Res Commun 297: 1270–1276, 2002 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15: 666–673, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17: 2477–2493, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fauconnet S, Lascombe I, Chabannes E, Adessi GL, Desvergne B, Wahli W, Bittard H. Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. J Biol Chem 277: 23534–23543, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280: C1358–C1366, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fritz T, Kramer DK, Karlsson HK, Galuska D, Engfeldt P, Zierath JR, Krook A. Low-intensity exercise increases skeletal muscle protein expression of PPARdelta and UCP3 in type 2 diabetic patients. Diabetes Metab Res Rev 22: 492–498, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Funk WD, Ouellette M, Wright WE. Molecular biology of myogenic regulatory factors. Mol Biol Med 8: 185–195, 1991 [PubMed] [Google Scholar]
- 21.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49: 235–261, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105: 863–875, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(−/−) mice. Atherosclerosis 181: 29–37, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Grimaldi PA. Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie 87: 5–8, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol 81: 619–626, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med 193: 607–620, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holst D, Luquet S, Nogueira V, Kristiansen K, Leverve X, Grimaldi PA. Nutritional regulation and role of peroxisome proliferator-activated receptor delta in fatty acid catabolism in skeletal muscle. Biochim Biophys Acta 1633: 43–50, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Jansson E, Kaijser L. Muscle adaptation to extreme endurance training in man. Acta Physiol Scand 100: 315–324, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Leibowitz MD, Fievet C, Hennuyer N, Peinado-Onsurbe J, Duez H, Bergera J, Cullinan CA, Sparrow CP, Baffic J, Berger GD, Santini C, Marquis RW, Tolman RL, Smith RG, Moller DE, Auwerx J. Activation of PPARdelta alters lipid metabolism in db/db mice. FEBS Lett 473: 333–336, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol 284: H1668–H1678, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J 17: 2299–2301, 2003 [DOI] [PubMed] [Google Scholar]
- 32.McCullagh KJ, Calabria E, Pallafacchina G, Ciciliot S, Serrano AL, Argentini C, Kalhovde JM, Lomo T, Schiaffino S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA 101: 10590–10595, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercier J, Perez-Martin A, Bigard X, Ventura R. Muscle plasticity and metabolism: effects of exercise and chronic diseases. Mol Aspects Med 20: 319–373, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res 27: e27, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namgaladze D, Shcherbyna I, Kienhofer J, Hofer HW, Ullrich V. Superoxide targets calcineurin signaling in vascular endothelium. Biochem Biophys Res Commun 334: 1061–1067, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Oliver WR Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA 98: 5306–5311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson EN, Williams RS. Calcineurin signaling and muscle remodeling. Cell 101: 689–692, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN. PPARgamma represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis 8: 373–379, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, Tanabe T, Warner TD, Bishop-Bailey D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol 27: 63–69, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Rafiee P, Heidemann J, Ogawa H, Johnson NA, Fisher PJ, Li MS, Otterson MF, Johnson CP, Binion DG. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun Signal 2: 3, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4: 407–414, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol 266: 1–16, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Sprecher DL, Massien C, Pearce G, Billin AN, Perlstein I, Willson TM, Hassall DG, Ancellin N, Patterson SD, Lobe DC, Johnson TG. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol 27: 359–365, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res 64: 3162–3170, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Stewart CE, Rittweger J. Adaptive processes in skeletal muscle: molecular regulators and genetic influences. J Musculoskelet Neuronal Interact 6: 73–86, 2006 [PubMed] [Google Scholar]
- 46.Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 100: 15924–15929, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise? FASEB J 19: 94–96, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2: e294, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watt MJ, Southgate RJ, Holmes AG, Febbraio MA. Suppression of plasma free fatty acids upregulates peroxisome proliferator-activated receptor (PPAR) alpha and delta and PPAR coactivator 1alpha in human skeletal muscle, but not lipid regulatory genes. J Mol Endocrinol 33: 533–544, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98: 1745–1752, 2005 [DOI] [PubMed] [Google Scholar]


