Abstract
We compared the clinical outcomes of adults with acute leukemia that received single-unit umbilical cord blood transplantation (sUCBT) after conditioning with a busulfan/antithymocyte globulin (BU-ATG)-based regimen at University Hospital La Fe (n=102) or double-unit UCBT (dUCBT) after conditioning with a total body irradiation (TBI)-based regimen at the University of Minnesota (n=91). Non-relapse mortality, relapse and disease-free survival (DFS) were similar in the two groups. Multivariate analyses, showed more rapid neutrophil (HR 0.6; 95% CI 0.45 – 0.80; p = 0.0006) and platelet recovery (HR 0.59; 95% CI 0.43 – 0.83; p = 0.002) after the BU-ATG-based conditioning and sUCBT. While there was a lower risk of acute GVHD grade II–IV (HR 2.81; 95% CI 1.75 – 4.35; p < 0.001) after BU-ATG and sUCBT, the incidences of grade III–IV acute and chronic GVHD were similar between the two groups. Regarding disease-specific outcomes, DFS in both acute myeloid leukemia and acute lymphoblastic leukemia (ALL) patients were not significantly different; however, a significantly lower relapse rate was found in patients with ALL treated with TBI and dUCBT (HR 0.3; 95% CI 0.12 – 0.84; p = 0.02). In the context these specific treatment platforms our study demonstrates that sUCB and dUCBT offer similar outcomes.
Keywords: UCB transplant, acute leukemia
Introduction
Umbilical cord blood (UCB) has become a frequently used source of hematopoietic progenitor cells for allogeneic transplantation in patients with acute leukemia.1–4 Early studies in adults reported low rates of engraftment, which were mainly attributed to the low progenitor cell content of UCB grafts.5–7 For this reason, most centers have adopted a minimum cell dose threshold of 2.5 × 107 nucleated cells per kilogram actual body weight. Unfortunately such a threshold would exclude many adults from UCB transplantation. As a result, the double UCB transplant (dUCBT) strategy was established in order to overcome this limitation and make UCB an adequate source of hematopoietic stem cells (HSC) for nearly all adults8 and, many adults receive a dUCBT if an adequate single unit is not available.9
Recent improvements in treatment regimens and UCB unit selection algorithms, have led some centers to reevaluate the cell dose limit. In fact, previous reports have shown that high rates of engraftment can be achieved with lower cell dose contents using an optimized busulfan-antithymocyte globulin (BU-ATG)-based regimen conditioning regimen and CBU selection making sUCBT widely available.10 If the cell dose limit could be reduced, the added cost of the second unit might be avoided.
The aim of this study was to compare the clinical outcomes of adults with acute leukemia undergoing UCBT at two institutions using different transplant platforms regarding conditioning regimen, UCB unit selection, and graft-versus-host disease (GvHD) prophylaxis.
Patients and methods
Eligibility criteria
All consecutive adult patients over 15 years of age with acute leukemia undergoing first stem cell transplantation with UCBT from an unrelated donor using myeloablative conditioning regimen between January 2001 and December 2009. All patients at University Hospital La Fe (Valencia) were transplanted with a single unit. While about 25% of adults at the University of Minnesota (Minneapolis) receive a single unit containing >2.5 × 107 nucleated cells/kg, only recipients of a dUCBT were included in the study. The upper age limit for myeloablative UCBT was 45 and 55 years in Minneapolis and Valencia, respectively. Each center’s Institutional Review Boards approved treatment protocols and informed consent was obtained according to the principles of the Declaration of Helsinki.
The treatment plans including of unit selection, conditioning regimen, immune suppression and supportive care have been reported by University Hospital La Fe1–4,11 and University of Minnesota groups5–8,12 and are summarized below.
Umbilical Cord blood unit selection
Graft selection algorithm required that UCB units to be ≥ 4/6 HLA matched with the recipient (HLA class I antigens [A and B] considering the antigen level and class II antigen [DRB1] considering allele level resolution DNA typing).
University Hospital La Fe
A total nucleated cell dose (TNC) ≥ 1.5 × 107/kg recipient’s body weight was required until 2005. From 2006 TNC ≥ 2 × 107/kg and CD34+ cell dose ≥ 1 × 105/kg recipient’s body weight were required. Cell dose was considered the most important criteria for unit selection. All patients for which donor search was initiated had a suitable UCB unit available according to the above-mentioned criteria.
University of Minnesota
All patients from the University of Minnesota included in this analysis received dUCB grafts selected according to the institutional algorithm. The combined minimum TNC dose was ≥ 2.5 × 107/kg of recipient’s body weight with one unit have a cell dose ≥ 1.5 × 107/kg. The 2 UCB units were infused randomly within 30 minutes of each other.
Conditioning regimen and GvHD prophylaxis
University Hospital La Fe
All patients received thiotepa, busulfan, cyclophospamide or fludarabine, and ATG.4,8 Until March 2005, 30 patients received thiotepa (10 mg/kg), busulfan (9.6 mg/kg IV), cyclophosphamide (120 mg/kg) and ATG (Thymoglobulin®, Genzyme Transplant, Cambridge, MA; 8 mg/kg). From March 2005, the remaining 72 patients received the same preparative regimen but replacing cyclophosphamide by fludarabine (150 mg/m2).
For GvHD prophylaxis, all patients received cyclosporine combined with either long course prednisone in the first 62 patients (0.5 mg/kg/d on days +7 to +14, 1 mg/kg/d on days +14 to +28, with slow tapering until discontinuation on day +180), mycophenlate-mofetil (MMF) (15 mg/kg/12h until day +28) in the following 35 patients, or a short course of prednisone in the last 5 patients (1 mg/kg/d on days +14 to +28).
University of Minnesota
All patients received myeloablative conditioning consisting of cyclophosphamide 120 mg/kg IV divided in daily 2 doses, fludarabine 75 mg/m2 IV divided in 3 daily doses and total body irradiation (1,320 cGy) delivered in 8 fractions over 4 days. The GvHD prophylaxis with cyclosporine A, starting IV on day −3 with a target through level of 200–400 mcg/l, that in case of no GVHD was tapered over 10 weeks starting at day +100. Patients also received MMF starting IV at day −3, before 2006 at a dose of 2 g/day and from 2006 3 g/day, that was discontinued at day +30 in case of no acute GVHD.8,9
Definitions
Myeloid recovery was defined as the first day of an absolute neutrophil count of 0.5 × 109/L lasting for 3 or more consecutive days. Platelet recovery was defined as the first day of a platelet count of 20 × 109/L or higher, without transfusion support for 7 consecutive days. Patients who survived more than 28 days after transplantation and who failed to achieve myeloid engraftment were considered as primary graft failure. Acute and chronic GVHD were defined and graded according to standard criteria.10,13–15 Disease stage at the time of transplantation was classified as follows: i) early stage: first complete remission (CR1); ii) intermediate stage: second or further CR; and iii) advanced stage: not in remission. Donor and recipient HLA match for dUCBT was considered taking into account the CBU with the highest HLA disparity. Non-relapse mortality (NRM) was defined as death from any cause without evidence of relapse. Leukemia-free survival was defined as survival from the time of transplantation without evidence of leukemia relapse.
Statistical analysis
Patient and transplant characteristics from different series were compared using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. The probabilities of engraftment, NRM, GVHD, and relapse were estimated by the cumulative incidence method (marginal probability).16,17 For cumulative incidence analyses of engraftment, GVHD, and relapse, death in CR was considered as a competing cause of failure, whereas relapse was the competing event for non-relapse mortality (NRM). Unadjusted time-to-event analyses were performed using the Kaplan–Meier estimate,18 and, for comparisons, the log-rank tests.19 Disease-free survival (DFS) was calculated from the date of UCBT. In the analysis of LFS, relapse or death in CR, whichever occurred first, was considered an uncensored event. The follow-up of the patients was updated on October 1, 2012. A Cox proportional hazards model20 or the Fine and Gray method for competing events21 were used for multivariable analysis. Variables included in the models were: treatment platform, age, gender, recipient body weight, transplant period, recipient CMV serostatus, diagnosis (AML vs ALL), disease stage at transplantation and HLA compatibility. Statistical analysis were conducted using R version 2.12.2 (The CRAN project) with packages, survival v2.36-10, Design 2.3-0, prodlim v1.2.1 and cmprsk v2.2-2.22
Results
Patient, umbilical Cord blood unit and transplant characteristics
Table 1 summarizes the demographic characteristics of the 102 and 91 patients at the University Hospital La Fe and the University of Minnesota, respectively. Patient and disease characteristics were similar in both groups, except for a higher proportion of patients that were CMV seropositive (77% vs. 54%; p = 0.0009), and with relapsed or refractory disease at time of transplantation (27% vs. 8%; p = 0.003) in the Valencia group. The median follow-up for surviving patients was 76 months (range, 39–130) and 61 months (range, 23–121), respectively. The median date of transplantation was December 2006 in the Valencia cohort and June 2006 in the Minneapolis group. Table 2 summarizes the characteristics of the cord blood units.
Table 1.
Characteristics of patients*
| Valencia cohort | Minneapolis cohort | P | |
|---|---|---|---|
| No. of patients | 102 | 91 | |
| Age, yr | 0.1 | ||
| Median | 30 | 28 | |
| Range | 16–52 | 15–45 | |
| Male Sex, no. (%) | 65 (64) | 49 (54) | 0.2 |
| Median Weight, kg | 70 | 73 | 0.4 |
| Range | 37–112 | 43–149 | |
| Diagnosis, no. (%) | 0.4 | ||
| AML | 49 (48) | 50 (55) | |
| ALL | 53 (52) | 41 (45) | |
| Disease stage at transplant, no. (%) | 0.0 | ||
| First complete remission | 54 (53) | 49 (54) | |
| Second or beyond complete remission | 20 (20) | 35 (38) | |
| Relapsed or refractory | 28 (27) | 7 (8) | |
| CMV seropositive recipient, no. (%) | 79 (77) | 49 (54) | 0.0 |
| Median Time from diagnosis to transplantation for patients in CR1, months | 182 | 127 | 0,4 |
| Range | 27–331 | 67–343 |
Percentages may not sum to 100 because of rounding.
Poor-risk cytogenetics included: complex karyotype, del(7q)/-7, t(6;9), EVI1, t(9;22), MLL rearrangement. Poor-risk molecular markers included FLT3-ITD mutated.
Table 2.
Graft- and transplantation-related characteristics*
| Valencia platform | Minneapolis platform | P value | |
|---|---|---|---|
| HLA compatibility, no. (%)1 | 0.24 | ||
| 6 of 6 | 7 (7) | 6 (7) | |
| 5 of 6 | 21 (21) | 28 (31) | |
| 4 of 6 | 74 (73) | 57 (63) | |
| ABO blood group mismatch, no. (%) | < 0.0001 | ||
| Major | 24 (24) | 43 (47) | |
| Minor | 34 (33) | 35 (38) | |
| None | 44 (43) | 13 (14) | |
| No. of nucleated cells infused × 107/kg, median (range) | 2.5 (1–5.8) | 3.6 (2–6.3)2 | < 0.0001 |
Percentages may not sum to 100 because of rounding
For dUCT the unit with the highest HLA disparity was considered
After adding the cell dose of 2 cord blood units infused
Hematopoietic Engraftment
Myeloid engraftment
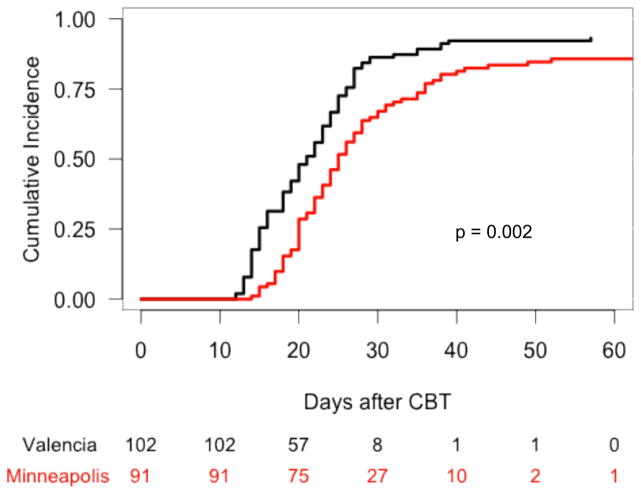
In the Valencia cohort, 2 patients died on days 12 and 19 after UCB infusion without evidence of myeloid engraftment, 3 patients experienced primary graft failure, 2 patients with initial neutrophil recovery subsequently lost the graft, and the remaining 95 patients achieved stable myeloid engraftment at a median time of 20 days (range, 12–57). In the Minneapolis cohort, 12 patients experienced primary graft failure, 1 patient with initial neutrophil recovery subsequently lost the graft, and the remaining 78 patients achieved stable myeloid recovery at a median time of 24 days (range, 14–52). The cumulative incidence of sustained myeloid recovery at 60 days in the Valencia and Minneapolis cohorts was 93% and 86%, respectively (p = 0.002) (Figure 1). In multivariable analysis, Valencia platform was associated with a better neutrophil recovery (p = 0.0006) (Table 3).
Figure 1.
Cumulative incidence of neutrophil recovery after UCB transplantation with either Valencia or Minneapolis platforms.
Table 3.
Multivariable analysis of short and long-term outcomes for all patients according to UCBT platform
| Outcome | Relative Risk (95% CI) | P | |
|---|---|---|---|
|
| |||
| Valencia platform | Minneapolis platform | ||
| Myeloid engraftment | 1 | 0.6 (0.449 – 0.802) | 0.0006 |
| Platelet engraftment | 1 | 0.59 (0.43 – 0.83) | 0.002 |
| Acute GvHD, grade II–IV | 1 | 2.81 (1.75 – 4.35) | < 0.0001 |
| Chronic GvHD | 1 | 0.7 (0.31 – 1.60) | 0.4 |
| Transplant-related mortality | 1 | 1.06 (0.66 – 1.71) | 0.8 |
| Relapse | 1 | 0.52 (0.25 – 1.08) | 0.08 |
| Leukemia-free survival | 1 | 1,27 (0.86 – 1.88) | 0.23 |
Time to neutrophil recovery and engraftment correlated with CD34+ cell dose in the Valencia (HR 1.19; CI 95% 1.06 – 1.34; P = 0.003) and in the Minneapolis (HR 1.15; CI 95% 1.07 – 1.24; P = 0.0003) cohorts.
Platelet engraftment
Of the 95 patients with myeloid engraftment in the Valencia cohort, 16 patients died between 22 and 250 days after transplantation without platelet recovery. The remaining 79 patients had platelet engraftment at a median time of 53 days (range, 23–142). Of the 78 patients with myeloid engraftment in the Minneapolis cohort, 17 patients died between 44 and 143 days after transplantation without platelet recovery. The remaining 61 patients had platelet engraftment at a median time of 97 days (range, 35–450). The cumulative incidence of sustained platelet engraftment at 100 days in the Valencia and Minneapolis cohorts was 77% and 62%, respectively (p = 0.001). In multivariable analysis, Valencia platform was associated with a better platelet recovery (p = 0.002) (Table 3).
GVHD
Acute GVHD
In the Valencia cohort, 51 of the 95 evaluable patients with stable engraftment developed aGVHD. Acute GVHD grade I was observed in 25 patients, grade II in 10 patients, grade III in 12 patients, and grade IV in 4 patients. The median time to the development of acute GVHD grade II to IV was 16 days (range, 8–91). In the Minneapolis cohort, 59 of the 78 evaluable patients with stable engraftment developed aGVHD. Acute GVHD grade I was observed in 5 patients, grade II in 34 patients, grade III in 16 patients, and grade IV in 4 patients. The median time to the development of acute GVHD grade II to IV was 23 days (range, 13–69).
The cumulative incidence of aGVHD at 100 days in the Valencia and Minneapolis cohorts was 28% and 69% for grade II–IV, respectively (p < 0.0001), whereas for grade III–IV it was 17% and 26%, respectively (p = 0.2). In multivariable analysis, Minneapolis platform was associated with an increased risk of aGvHD grades II–IV (p < 0.0001) (Table 3). No factor was associated with the risk of grade III–IV aGVHD.
Chronic GVHD
Thirty-nine of 78 patients at-risk in the Valencia cohort developed chronic GVHD (cGvHD) at a median time of 140 days (range, 70–415). Chronic GVHD was limited in 16 patients and extensive in 23 patients. In the Minneapolis cohort, 24 of 67 patients at-risk developed cGVHD at a median time of 153 days (range, 92–558). Chronic GVHD was limited in 5 patients and extensive in the remaining 19 patients.
The 2-year cumulative incidence of any cGVHD and extensive cGVHD in the Valencia and Minneapolis cohorts was 50% and 36% (p = 0.06) and 29% and 28% (p = 0.8), respectively. No factor was associated with the risk of cGVHD in multivariable analysis.
NRM
Thirty-six patients in the Valencia cohort died without prior relapse at a median time of 125 days after transplantation (range, 12–2535), whereas in the Minneapolis cohort, 32 patients died at a median time of 62 days after transplantation (range, 24–765). The cumulative incidence of NRM was similar in both groups (p = 0.8). For patients in the Valencia cohort, the incidence of NRM at 100 days, 180 days, and 5 years was 13%, 20%, and 34%, respectively. For patients in the Minneapolis cohort, the incidence of NRM at 100 days, 180 days, and 5 years was 24%, 30%, and 35%, respectively. No factor was associated with the risk of NRM in multivariable analysis.
Relapse
Overall, 32 patients in the Valencia cohort relapsed at a median time of 4.5 months (range, 1–50) and 12 patients in the Minneapolis cohort relapsed at a median time of 6.8 months (range, 1–22). All relapsed patients died at a median time of 64 days (range, 2–1129). Relapse incidence was different depending on diagnosis, with a 5-year cumulative incidence of relapse of 40% and 24% for patients with ALL and AML, respectively (p = 0.04). The 5-year cumulative incidence of relapse was 42% for Valencia cohort and 19% for Minneapolis cohort (p = 0.004). The 5-year cumulative incidence of relapse was 70% vs 66% (p = 0.9) for patients in advanced stage, and 31% vs 14% (p = 0.04) for patients in CR for the Valencia and Minneapolis cohorts, respectively. However, in multivariable analysis, only advanced disease status at time of transplantation was associated with increased risk of relapse (p < 0.0001).
When analysis was restricted to patients with AML, the 5-year cumulative incidence of relapse was 23% and 13% for Valencia and Minneapolis cohorts, respectively (p = 0.3). In multivariable analysis, advanced disease status at time of transplantation (p < 0.0001) and better HLA match (6/6 vs. 5/6 vs. 4/6) (p < 0.001) were the only independent factors independently associated with an increased risk of relapse (Table 4). In patients with ALL, the 5-year cumulative incidence of relapse was 40% and 12% for Valencia and Minneapolis cohorts, respectively (p = 0.003). In multivariable analysis, advanced disease status at time of transplantation (p = 0.0006) and Valencia platform (p = 0.02) was associated with an increased risk of relapse (Table 4).
Table 4.
Multivariable analysis of disease-specific outcomes: relapse risk and leukemia-free survival
| Acute myeloid leukemia | Acute lymphoblastic leukemia | ||||
|---|---|---|---|---|---|
|
| |||||
| Outcome | Variable | Relative risk (95% CI) | P | Relative risk (95% CI) | P |
| Relapse | |||||
| UCBT platform | 0.3 | 0.02 | |||
| Valencia | 1 | 1 | |||
| Minneapolis | 0.6 (0.24 – 1.57) | 0.3 (0.12 – 0.84) | |||
| Disease status | < 0.0001 | 0.0006 | |||
| Complete remission | 1 | 1 | |||
| Relapse/refractory | 10.1 (3.50 – 29.1) | 4.32 (1.86 – 9.74) | |||
| HLA match | |||||
| 6/6 | 1 | ||||
| 5/6 | 0.12 (0.02 – 0.60) | 0.01 | - | ||
| 4/6 | 0.09 (0.03 – 0.31) | < 0.0001 | - | ||
| Leukemia-free survival | |||||
| UCBT platform | 0.4 | 0.15 | |||
| Valencia | 1 | 1 | |||
| Minneapolis | 1.26 (0.73 – 2.19) | 1.48 (0.87 – 2.54) | |||
| Disease status | 0.007 | ||||
| Complete remission | - | 1 | |||
| Relapse/refractory | - | 0.44 (0.24 – 0.80) | |||
LFS
Thirty-four patients in the Valencia cohort and 47 patients in the Minneapolis cohort are alive and leukemia free after UCBT at last follow-up. The overall LFS at 5 years was 34% and 52% for the Valencia and Minneapolis cohorts, respectively (p = 0.05). The 5-year LFS was 21% vs 29% (p = 0.9) for patients in advanced stage, and 40% vs 53% (p = 0.2) for patients in CR for the Valencia and Minneapolis cohorts, respectively. The 5-year LFS was 49% for patients with AML and 35% for patients with ALL (p = 0.04). In multivariable analysis, advanced disease status at time of transplantation was the only factor associated with higher risk of treatment failure (p < 0.0001).
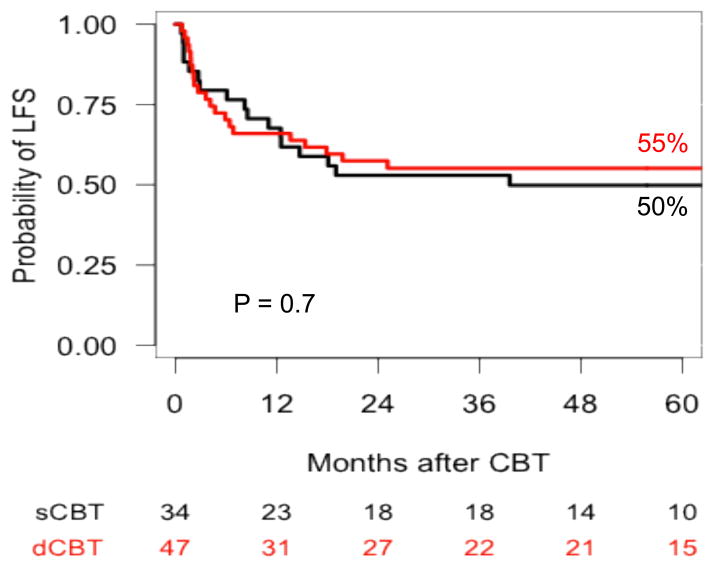
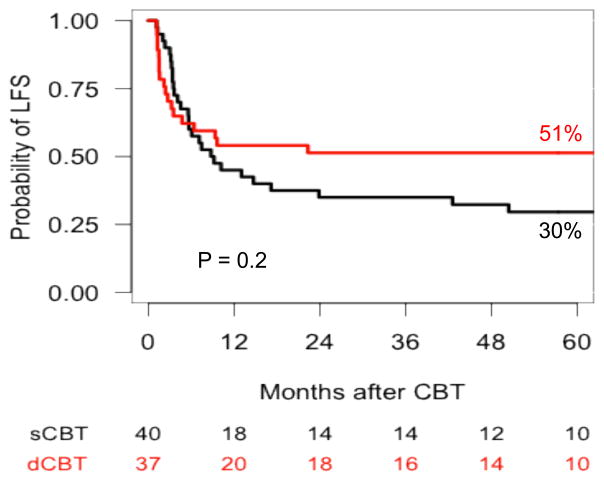
The 5-year LFS for patients with AML was 45% for patients in the Valencia cohort and 54% in the Minneapolis cohort (p = 0.4), and 50% vs 55% (p = 0.7) for patients in CR, respectively (Figure 2). No factor was significantly associated with LFS in patients with AML in multivariable analysis. In patients with ALL, the 5-year LFS was 24% for patients in the Valencia cohort and 49% for those in the Minneapolis cohort (p = 0.08), and 30% vs 51% (p = 0.2) for patients in CR, respectively (Figure 3). In multivariable analysis, advanced disease status at time of transplantation was the only factor associated with higher risk of treatment failure (p = 0.007) (Table 4).
Figure 2.
Probability of leukemia-free survival in patients with acute myeloid leukemia in remission after UCB transplantation with either Valencia or Minneapolis platforms.
Figure 3.
Probability of leukemia-free survival in patients with acute lymphoblastic leukemia in remission after UCB transplantation with either Valencia or Minneapolis platforms.
Discussion
This study shows the relative efficacy of two UCB transplantation platforms in adults with acute leukemia, which were carried out at two institutions using either a BU-ATG-based conditioning with sUCBT (Valencia platform) and TBI-based conditioning with dUCBT (Minneapolis platform). The main observation of this study is that sUCBT even can be a suitable graft source. While numerous reports have previously established the minimum cell dose threshold of 2.5 × 107 nucleated cells/kg, it is possible that this needs to be reconsidered especially in the context of specific conditioning regimens. Whether the same results would have been observed with a sUCB unit after cyclophosphamide, fludarabine and TBI as used in Minneapolis is not known. This might be addressed in future studies.
In the present study, we have had the opportunity to compare the outcome of two relatively large single center series of adult patients with acute leukemia undergoing UCBT in two institutions experienced in UCBT. High rates of myeloid and platelet engraftment were achieved in the Valencia cohort and compare favorably with the Minneapolis cohort and with those reported in registry-based studies.23,24 The addition of thiotepa to a BU-ATG-based conditioning regimen as used in the Valencia cohort appears to permit the routine use of lower dosed single units. Furthermore, it is possible that MMF may delay or reduce engraftment in some patients, as myelosuppression is a known complication and has been previously reported.25–27 Why a faster engraftment and a lower graft failure rate in the Valencia cohort did not translate into a lower NRM remains unexplained and is probably multifactorial.
As previously described,28 grade II acute GvHD with involvement principally of the skin was more frequently observed in recipients of dUCBT as compared sUCBT without a clear impact on NRM. The reason for the relatively low GvHD rate in the Valencia cohort is unproven but we speculate that the use of rabbit ATG in the conditioning regimen may have contributed to this observation. While it is possible that a lower CD3 cell dose inherent in the use of a single unit may have a played a role, prior studies have not observed an association between CD3 cell dose and acute GVHD. Incidences of acute grade III–IV and chronic GvHD were comparable in both cohorts. While the addition of ATG could hampered immune reconstitution in the Valencia cohort, a detailed analysis is unavailable. However there was no significant difference in NRM in the present study and the incidence of cytomegalovirus reactivation previously reported from both groups was also similar.29,30
Regarding relapse, it has been suggested a lower rate of relapse for patients receiving dUCBT indicating a potentially higher graft-versus-leukemia (GvL) effect.12,31,32 However, this is, to date, an unexplained finding that has not been uniformly confirmed.33 In the present study, the only variable with independent prognostic value for relapse was disease stage at time of transplantation, and after adjusting for this variable and various known risk factors, relapse was not associated with cohort. Interestingly, relapse risk may be more associated with degree of HLA mismatch rather than the use of a dUCBT as has been previously suggested by other studies.12 In fact, for both groups we observed an impressive reduction in the risk of relapse, in patients with AML, suggesting an enhanced GVL effect, for patients transplanted with UCB units with a higher HLA disparity. In this regard, previous registry-based studies from Eurocord34 and the Japanese Society for Hematopoietic Cell Transplantation group35 have also found that UCBTs with a higher HLA disparity had a lower probability of relapse. In contrast to pediatric patients, this effect does not seem to be counterbalanced with an increased NRM as suggested by large registry-based studies.35–37 While there was a lower risk of relapse in Minneapolis patients with ALL, it is unclear whether this was due to the conditioning that included TBI, the use of two units or absence of in vivo T cell depletion with ATG.
In conclusion, this retrospective study demonstrated that in the context of specific treatment platforms employed in Valencia and Minneapolis, sUCB and dUCBT offer similar outcomes. Future studies are needed to determine whether this finding is true for all or only specific scenarios. In addition, the finding that HLA mismatch does not compromise LFS and may indeed enhance the GVL effect also needs further investigation. Together, these two findings could have a significant impact on the practice of transplant medicine.
Footnotes
Auhorship: JS, JW, GS and CB conceived the study and interpreted the data; JS, JW, and CB wrote the paper; JS and TD performed the statistical analyses; MS, PM, VB, IL and EW reviewed the manuscript and contributed to the final draft.
The authors have no conflict of interest to declare.
References
- 1.Eapen M, Rubinstein P, Zhang M-J, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. The Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 2.Eapen M, Rocha V, Sanz GF, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanz J, Sanz MA, Saavedra S, et al. Cord Blood Transplantation from Unrelated Donors in Adults with High-Risk Acute Myeloid Leukemia. Biology of Blood and Marrow Transplantation. 2010;16(1):86–94. doi: 10.1016/j.bbmt.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 Recipients of Placental-Blood Transplants from Unrelated Donors. N Engl J Med. 1998;339(22):1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 6.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 7.Cornetta K, Laughlin MJ, Carter SL, et al. Umbilical cord blood transplantation in adults: Results of the prospective cord blood transplantation (COBLT) Biology of Blood and Marrow Transplantation. 2005;11(2):149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Crotta A, Ruggeri A, et al. Double cord blood transplantation: extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23(2):223–229. doi: 10.1016/j.beha.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Sanz J, Boluda JCH, Martín C, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47(10):1287–1293. doi: 10.1038/bmt.2012.13. [DOI] [PubMed] [Google Scholar]
- 11.Sanz GF, Saavedra S, Planelles D, et al. Standardized, unrelated donor cord blood transplantation in adults with hematologic malignancies. Blood. 2001;98(8):2332–2338. doi: 10.1182/blood.v98.8.2332. [DOI] [PubMed] [Google Scholar]
- 12.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 15.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 16.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. 2012 [Google Scholar]
- 23.Rocha V, Labopin M, Sanz GF, et al. Transplants of Umbilical-Cord Blood or Bone Marrow from Unrelated Donors in Adults with Acute Leukemia. N Engl J Med. 2004;351(22):2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after Transplantation of Cord Blood or Bone Marrow from Unrelated Donors in Adults with Leukemia. N Engl J Med. 2004;351(22):2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 25.Pidala J, Kim J, Perkins J, et al. Mycophenolate mofetil for the management of steroid-refractory acute graft vs host disease. Bone Marrow Transplant. 2010;45(5):919–924. doi: 10.1038/bmt.2009.252. [DOI] [PubMed] [Google Scholar]
- 26.Okamura A, Shimoyama M, Ishii S, et al. Delayed neutrophil engraftment in cord blood transplantation with intensive administration of mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant. 2011;46(1):148–149. doi: 10.1038/bmt.2010.59. [DOI] [PubMed] [Google Scholar]
- 27.Sanz J, Picardi A, Hernández Boluda JC, et al. Impact of Graft-versus-Host Disease Prophylaxis on Outcomes after Myeloablative Single-Unit Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2013;19(9):1387–1392. doi: 10.1016/j.bbmt.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 28.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker CM, van Burik J-AH, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13(9):1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Montesinos P, Sanz J, Cantero S, et al. Incidence, risk factors, and outcome of cytomegalovirus infection and disease in patients receiving prophylaxis with oral valganciclovir or intravenous ganciclovir after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2009;15(6):730–740. doi: 10.1016/j.bbmt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues CA, Sanz GF, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(2):256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 32.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gluckman E, Rocha V, Arcese W, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32(4):397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Atsuta Y, Kanda J, Takanashi M, et al. Different effects of HLA disparity on transplant outcomes after single-unit cord blood transplantation between pediatric and adult patients with leukemia. Haematologica. 2013 doi: 10.3324/haematol.2012.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen YC, Scaradavou A, Stevens CE, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: a pooled analysis of three international registries. Bone Marrow Transplant. 2011;46(1):70–76. doi: 10.1038/bmt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arcese W, Rocha V, Labopin M, et al. Unrelated cord blood transplants in adults with hematologic malignancies. Haematologica. 2006;91(2):223–230. [PubMed] [Google Scholar]