Abstract
Optical coherence tomography (OCT) is a non-invasive imaging modality that is transforming clinical diagnosis in dermatology and other medical fields. OCT provides a cross-sectional evaluation of the epidermis and dermis and allows in vivo imaging of skin collagen. Upregulated collagen content is a key feature of fibrotic skin diseases. These diseases are often managed by the practitioner’s subjective assessment of disease severity and response to therapies. The purpose of this review is to provide an overview of the principles of OCT and present available evidence on the ability of OCT to image skin collagen in vivo for the diagnosis and management of diseases characterized by skin fibrosis. We review OCT studies that characterize the collagen content in normal skin and fibrotic skin diseases including systemic sclerosis and hypertrophic scars secondary to burn, trauma, and other injury. We also highlight several limitations of OCT and suggest enhancements to improve OCT imaging of skin fibrosis. We conclude that OCT imaging has the potential to serve as an objective, non-invasive measure of collagen’s status and disease progression for use in both research trials and clinical practice. The future use of OCT imagining as a quantitative imaging biomarker of fibrosis will help identify fibrosis and facilitate clinical examination in monitoring response to treatment longitudinally without relying on serial biopsies. The use of OCT technology for quantification of fibrosis is in the formative stages and we foresee tremendous growth potential, similar to the ultrasound development paradigm that evolved over the past 30 years.
Keywords: skin imaging, OCT, optical coherence tomography, collagen, skin fibrosis
Introduction
Optical coherence tomography (OCT) is a non-invasive imaging modality that is transforming clinical diagnosis in dermatology and other medical fields. OCT provides an in vivo cross-sectional image of tissues through the use of low-coherence interferometry [7,10,19,20,30,35]. OCT imaging was first used clinically by ophthalmologists to collect eye length measurements and has become the clinical standard for a number of eye diseases [8]. OCT has also expanded to other clinical and research uses in dermatology, cardiology, dentistry, gastroenterology, gynecology, rheumatology, surgery, and urology [4,5,33,8,10,16,32]. Since this technology expanded to dermatology in 1997, OCT has become increasingly used in the clinical assessment and research of skin diseases [10,36]. OCT can be used to image the epidermal and dermal layers of the skin, skin appendages, and blood vessels [10]. These OCT images can be used to non-invasively diagnose skin diseases and evaluate their progression longitudinally as an alternative to skin biopsies or surgical procedures.
One of OCT’s applications in dermatology is the imaging of skin collagen. Collagen proteins are the main extracellular matrix components of the skin that comprise approximately 80% of the dry weight of the dermis [14]. The orientation, organization, and reflective properties of skin collagen renders the tissue birefringent and detectable on OCT imaging [18]. Upregulated collagen content is a key feature of fibrotic skin disease. Fibrotic skin diseases, such as systemic sclerosis and hypertrophic scars, are most often managed by the practitioner’s subjective assessment of disease severity and response to therapy. More research is needed to definitively confirm OCT’s capabilities as a tool to assess normal skin collagen and skin fibrosis. Nonetheless, we envision that OCT imaging of skin collagen will eventually become universally accepted as the gold standard in the clinical management and research of fibrotic skin diseases.
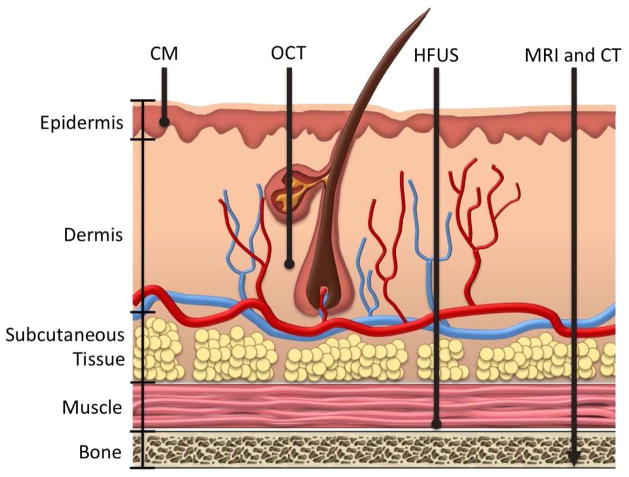
Several other non-invasive imaging modalities have been employed to assess skin tissue. With imaging techniques, there is generally an inverse relationship between penetration depth and resolution (Table 1) [30]. Figure 1 compares the penetration depths of different imaging modalities that have been applied to the skin. High-frequency ultrasound (US) is generally of lower resolution [6]. A typical 20 MHz high-frequency US system might have a penetration depth of approximately 15 mm but a resolution of approximately 300 μm that limits the ability to evaluate fine tissue variations [6,12]. Although possessing excellent penetration depth, computed tomography (CT) and magnetic resonance imaging (MRI) have restricted resolution (100 μm) and only enable assessment of architectural skin changes [12,7]. Confocal laser microscopy has high resolution (approximately 1 μm), but the limited penetration depth of 0.2 mm hinders the ability of this modality to study collagen alterations in skin [7,30]. In comparison, OCT’s penetration depth is approximately 2 mm, with resolution ranging from 4–10 μm. Therefore, OCT can image deeper structures than confocal microscopy while maintaining a resolution that exceeds that of US [21]. For the purpose of cutaneous fibrosis imaging, OCT provides an optimal balance between penetration depth and resolution.
Table 1.
Comparison of penetration depths and resolution of imaging techniques applied to skin imaging.
| Imaging Modality | Maximum Penetration Depth | Maximum Resolution |
|---|---|---|
| Confocal microscopy | 0.2 mm [7] | 0.5–1 μm [7] |
| High-definition optical coherence tomography | 0.57 mm [2,3] | <3 μm [2,3] |
| Optical coherence tomography | 2 mm [7] | 4–10 μm [7] |
| High-frequency ultrasound | 15 mm [6] | 300 μm [6] |
| Computed tomography | Total body penetration [7] | 100 μm [7] |
| Magnetic resonance imaging | Total body penetration [7] | 100 μm [7] |
Figure 1.
Diagram of the penetration depth of various imaging modalities. Computed tomography (CT), magnetic resonance imaging (MRI), and high-frequency ultrasound (HFUS) have high penetration depths but at the expense of reduced resolutions. Conversely, confocal microscopy (CM) has high resolution at a much lower penetration depth. Optical coherence tomography (OCT) can image deeper tissues than confocal microscopy while maintaining resolution exceeding those of CT, MRI, and HFUS. This makes OCT an excellent modality for imaging the increased dermal collagen that characterizes fibrotic skin disorders.
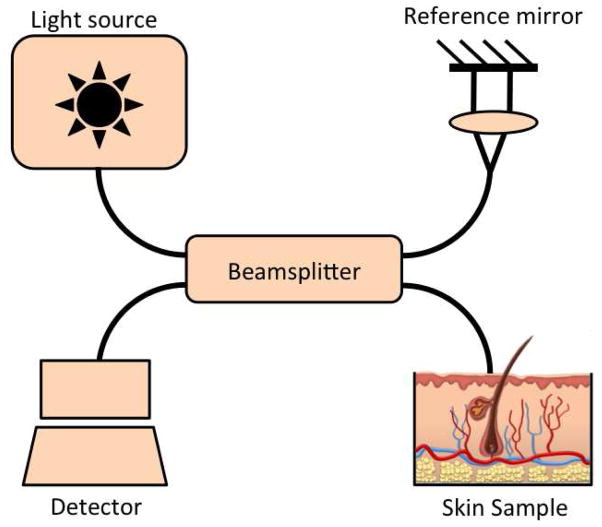
The principle of OCT imaging is analogous to US imaging. However, instead of sound, OCT uses laser light to generate images. The OCT system consists of an interferometer that is illuminated by light from an optical source. The light is split into two fractions by a beamsplitter, one of which is directed to a reference mirror, and the other to the patient skin (Figure 2) [27,21,11,31,37]. Lights reflected from both the reference mirror and patient skin are recombined at the beamsplitter and guided to a detection unit to form an image based on the interference signal [37,27]. OCT is able to generate 2-dimensional images and some specialized systems can also create composite 3-dimensional images. OCT provides vertical cross-sectional images of skin that is analogous to the tissue architecture observed in histology [37]. However, the histological information provided by conventional OCT is limited.
Figure 2.
Schematic of optical coherence tomography system. Light from an optical source is divided by a beamsplitter into 2 fractions. One fraction is guided towards the reference mirror and the other to the tissue. Light reflected from both the reference mirror and tissue are recombined at the beamsplitter and detected to form an interference signal.
The initial OCT systems employed the time-domain (TD) technique where the length of the reference arm could be adjusted to match desired depths within the tissue [30]. More recent OCT systems are based on the frequency-domain (FD) technique, where the entire depth of the tissue is analyzed simultaneously with respect to a static reference mirror [30]. The FD technique provides increased sensitivity and a higher image acquisition speed [30]. Swept-source (SS)-OCT is based on the FD technique and provides structural information about skin collagen. The ability to view collagen under SS-OCT is directly related to the density of collagen bundles and inversely related to the amount of extracellular fluids [24]. On SS-OCT images, areas with high collagen content appear white and areas with lower collagen content appear black. Figure 3 depicts an OCT image of an area of increased dense collagen. Another type of OCT, known as high-definition OCT (HD-OCT), permits morphologic analysis of skin structures in 3 dimensions and can provide images of individual cells [2,3].
Figure 3.
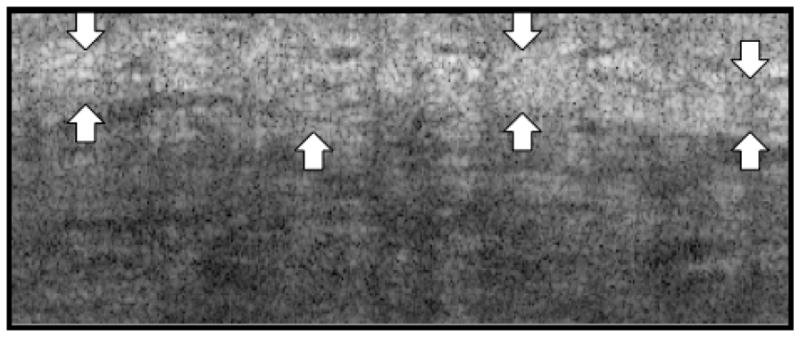
Swept-source (SS)-OCT image of dense collagen. The arrows outline a dense deposit of collagen. (Image courtesy of Michelson Diagnostics)
In addition to structural information, OCT can obtain functional and quantitative information on collagen status [29]. Polarization sensitive OCT (PS-OCT) images identify birefringent skin tissue regions by measuring and displaying changes in the polarization state of reflected light. The rate at which the polarization state of light changes is directly related to the collagen content of the skin tissue, and is depicted by the rate the phase retardation angle changes with tissue depth [25]. Tissues with high collagen content, such as skin fibrosis, rapidly change the polarization state of light and have high phase retardation rates; tissues with decreased or normal collagen content slowly change the polarization state of light and have low phase retardation rates [25]. Other extracellular matrix components of the skin also contribute to skin anisotropy and further research is needed to elucidate all the factors that contribute to skin anisotropy. Nonetheless, since collagen is the major component and primary driver of skin anisotropy, PS-OCT collagen imaging may prove valuable in the clinical assessment of fibrotic skin diseases.
OCT offers several advantages in clinical practice and research. In contrast to biopsy, OCT has the advantage of time-efficiency as it can be used to examine skin sites in less than 10 seconds [1]. This provides valuable information to physicians and patients in real-time. OCT benefits patient care, as it is non-invasive, rapid, comfortable, and painless. OCT also demonstrates excellent intra-observer and inter-observer reliabilities that are useful in standardizing clinical diagnosis [1]. Furthermore, OCT requires minimal operator training and allows users to easily save, store, or share image files [1]. We believe that OCT holds promise to significantly change the way we manage and research cutaneous fibrotic diseases. The qualitative and quantitative nature of OCT images will facilitate digital recording and evaluation of blinded data analysis in clinical trials, monitoring patient collagen status, measuring response to therapy longitudinally, and will facilitate evaluation and management of patients remotely via teledermatology aided by OCT.
The purpose of this review is to present the available evidence on the ability of OCT to image normal and pathological skin collagen in vivo for the diagnosis and management of diseases that feature skin fibrosis.
Methods
We employed the following search strategy to identify the clinical evidence reported in the biomedical literature: in July 2013, we searched Medline, PubMed, EMBASE, Web of Science, Google Scholar, and Cochrane databases (January 1990-present) using the following search terms: “optical coherence tomography,” “OCT,” “skin,” “collagen,” and “skin fibrosis.” The relevant articles that met the following criteria were selected for inclusion: research studies evaluating OCT imaging of collagen in normal skin and skin fibrosis. Papers published in languages other than English were excluded.
Results
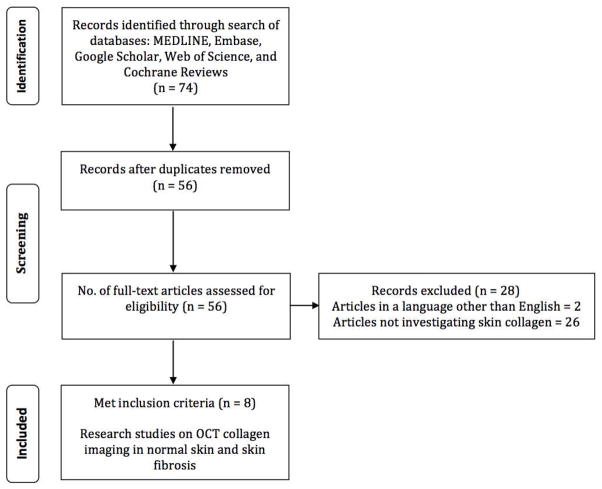
Our search resulted in 74 articles (Figure 4). After duplicates were removed, a total of 56 unique articles were considered and screened. 2 articles were in languages other than English. Of the remaining 54 articles, 8 articles investigating OCT imaging of collagen in normal skin and skin fibrosis met inclusion criteria and are included in this review. A detailed list of included studies is presented in Table 2.
Figure 4.
Literature search. Flowchart illustrating the literature search strategy and results employed in this article.
Table 2.
Summary of Studies Investigating the use of OCT to Image Skin Fibrosis
| Authors | Study Aim | Population Characteristics | Findings | Limitations |
|---|---|---|---|---|
| Normal Skin | ||||
| Mogensen et al [19] | To describe normal skin collagen morphology using PS-OCT imaging | Healthy volunteers aged 0.5–59 years; (n = 20); examined forehead, ear lobe, nose, cheek, chin, back of the neck, chest, hands, arms and calf | PS-OCT images showed characteristic structures due to the birefringence differences between epidermis, papillary, and reticular dermis | Small proportion of children relative to adults in the study |
| Pierce et al [25] | To measure anatomic variations in birefringence using PS-OCT | Healthy volunteers aged 24–35 years; (n = 5); examined lower back, temple, and hand | Mean phase retardation highest for skin of the lower back and lowest for skin of the temple | Study limited to male patients; small sample size |
| Pircher et al [27] | To use phase resolved PSOCT to investigate polarization properties of different regions of human skin in vivo | Healthy volunteers; (n not specified); examined fingertip and hand | 3-D PS-OCT has potential to increase contrast and quantify retardation and orientation of birefringent structures in skin | Selected skin regions examined |
| Yasuno et al [37] | To investigate normal skin birefringence using PS-OCT | Healthy volunteers | PS-OCT successfully revealed the birefringent nature of human skin tissue | Limited information on volunteers |
| Fibrosis | ||||
| Abignano et al [1] | To determine if SS-OCT could offer a potential sensitive imaging biomarker to assess and quantify skin fibrosis | Systemic sclerosis patients with mean age of 54 years; (n = 21); examined fingers, hands, and forearms | Systemic sclerosis affected skin displayed consistent decrease of optical density in the papillary dermis, this directly correlated with mRSS score | Selected skin regions scanned; Only two patients underwent skin biopsies and from sites with MRSS of 3 |
| Kunzi-Rapp et al [14] | Use OCT to evaluate new collagen synthesis after scar treatment with the Er:YAG laser | Post-traumatic and acne scar patients aged 12–39 years; (n = 12); examined face and extremities | OCT demonstrated was the production of new collagen bundles after scar treatment with the Er:YAG laser | N/A – OCT was not the main goal of study but rather used in assessment |
| Liew et al [16] | To investigate the utility of OCT to quantify vascularity in hypertrophic scars | Hypertrophic scar patients with mean age of 32 years; (n = 8); examined burn scar sites | Increase in mean density of vasculature in hypertrophic scar tissues (38%) when compared with normal, unscarred skin (22%); proliferation of larger vessels | Pilot study – small sample size; scans acquired in the severely affected areas of each scar |
| Pierce MC, Strasswimmer J et al [26] | To demonstrate the capability of OCT in detecting features of skin fibrosis | Fibrotic scar patient; (n = 1); examined hand | PS-OCT imaging quantitatively demonstrated polarization differences between normal skin and the fibrotic site | Fibrosis examination restricted to the hand; small sample size |
Discussion
OCT Imaging of Normal Skin Collagen
Collagen is present in all skin. OCT measurement of skin collagen varies by anatomical location due to differences in local skin characteristics such as collagen content and epidermal thickness [26]. To establish a range of normal skin characteristics, researchers have used OCT to measure normal skin and characterize the variations in collagen content seen at different anatomic sites [28,20,38]. One study collected PS-OCT images from the back, temple, and dorsum of the hand of healthy patients and measured skin birefringence from these images [26]. In these images, the mean measured phase retardation rates of skin varied by anatomical site; the lower back had the highest values, the hand had intermediate values, and the temple had the lowest values [26]. These observations support what we expect based on the relative collagen distribution at these anatomic sites. Although this study was limited to only three anatomic sites, it illustrates that OCT is sensitive enough to distinguish differences in baseline collagen status of different anatomic sites. These OCT images of normal skin collagen establish baseline values for purposes of comparison that enable clinicians to accurately identify, understand, and characterize various diseases that feature altered collagen content.
Another study used OCT to examine normal skin of the forehead, ear lobe, nose, cheek, chin, neck, chest, hands, arms, and calf in healthy volunteers aged 6 months to 59 years [20]. They found that PS-OCT was able to identify the boundaries that differentiate the epidermis, papillary dermis, and reticular dermis [20]. These disruptions in the epidermal boundaries are characteristic of many fibrotic disorders [13]. Therefore, these results suggest that OCT could be used to identify the precise location of excess collagen deposition in the dermis and evaluate the skin to highlight pathologic disruptions in their boundaries.
In addition to conventional 2-dimensional OCT, 3-dimensional OCT imaging systems have been designed and utilized in structural imaging of normal skin [1,7,23]. We anticipate future use of 3-dimensional OCT imaging will allow clinicians to evaluate collagen fiber orientation and its impact on local anatomic structure and will lead to a better understanding of the anatomic impact of fibrotic skin disorders. We believe that OCT will become widely accepted as an objective measure of skin fibrosis. Further characterization of the range of OCT measurements in normal skin is critical to enabling clinicians to diagnose and treat fibrotic diseases earlier by measuring variations in baseline collagen content before they show clinical signs.
OCT Imaging of Skin Fibrosis as a Feature of Systemic Sclerosis
Systemic sclerosis is a disease characterized by fibrosis that arises due to excess collagen deposition in the dermis of the skin and in other organ systems [9,13,34]. Skin fibrosis is a major prognostic factor in systemic sclerosis and is often the primary outcome in clinical studies, however, there are currently no validated imaging biomarkers to quantify skin fibrosis [1]. Most studies utilize the modified Rodnan skin score (MRSS), a clinical assessment scoring system for skin fibrosis, as their primary outcome measure [9]. The MRSS is calculated by palpating and scoring skin thickness at 17 anatomic sites; therefore, MRSS is subject to limitations that include inter-observer bias and requires a high level of skill for effective utilization [1,9]. OCT skin imaging addresses the limitations of the MRSS by providing a reproducible and time-efficient approach to quantifying and monitoring skin fibrosis.
OCT has the potential to become the first quantitative imaging biomarker for rapid clinical assessment of systemic sclerosis [1]. A study investigated the use of OCT on 21 systemic sclerosis patients [1]. In this study, SS-OCT images of the hands and forearms of patients were obtained and correlated with histology and the patient’s MRSS [1]. As systemic sclerosis progressed on histologic evaluation, MRSS increased and the dermal-epidermal junction became increasingly difficult to visualize on OCT images [1]. This correlation between OCT images, histology, and the MRSS highlights the potential usefulness of OCT for monitoring systemic sclerosis progression. Furthermore, the authors were unable to distinguish between different epidermal layers using OCT generated images of the epidermis from patients with systemic sclerosis [1]. They concluded that OCT could show a decrease in the distinction between the papillary and reticular dermis in systemic sclerosis images compared with images of normal skin [1].
Randomized clinical trials are needed to characterize the ability of OCT to classify the severity of scleroderma and other forms of fibrosis. Earlier diagnosis will likely be achieved by measuring preclinical collagen changes in the dermis of patients. We hypothesize that OCT will become a valuable tool in clinical trials and other studies to evaluate fibrotic disease diagnosis, progression, and response to therapy. In future studies, stratification of patient fibrosis severity and further evaluations of the diagnostic sensitivity and specificity of this method are needed.
OCT Imaging of Fibrotic Scars
OCT has been used to diagnose and evaluate hypertrophic scars, a fibrotic disorder that results from a cutaneous injury such as a surgical wound or burn wound. When a hypertrophic scar occurs, these cutaneous injuries lead to the production of excess collagen synthesis and local vascular proliferation [22,17]. Three studies used OCT to evaluate changes in skin fibrosis due to burn scars, hypertrophic scars, and various scar types pre- and post-laser treatment.
A descriptive case report used PS-OCT to investigate the collagen content of a hypertrophic scar on the hand of a patient [27]. The PS-OCT image revealed increased birefringence in the fibrotic scar site compared with normal skin site and showed that OCT can be used to differentiate scar tissue from normal tissue [27]. A similar scar imaged by OCT is depicted in Figure 5.
Figure 5.
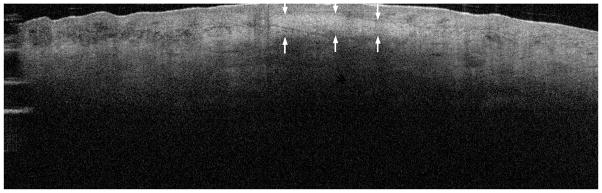
Swept-source (SS)-OCT image of a scar. The arrows depict the scar site that appears white due to increased collagen deposition. (Image courtesy of Michelson Diagnostics)
In another study, OCT was used to evaluate response to therapy after treatment with 2940- nm Erbium:YAG laser treatment for facial rejuvenation, post-traumatic scars, and acne scars [15]. The authors utilized OCT to monitor post-treatment dermal remodeling, inflammation, and newly synthesized collagen [15]. These results suggest that OCT can monitor the response to laser treatments for scars.
We believe that OCT can be extended to evaluate the treatment response of other lasers. OCT may be useful for measurement of immediate ablative endpoints, depth of penetration of ablative lasers, and long-term non-ablative endpoints such as collagen remodeling post non-ablative treatment. OCT may provide an objective evaluation of treatment settings and help guide clinical laser therapy to improve patient outcomes. In addition to collagen imaging, vasculature has been studied in fibrotic skin diseases. A clinical study used OCT to quantify the vascularity of hypertrophic scars in 8 patients and found an increased mean vascular density in hypertrophic scar tissue (38%) when compared with normal, unscarred skin (22%) [17]. The study revealed there was a proliferation of larger vessels (diameter ≥ 100 μm) in hypertrophic scars that was absent from normal scars and normal skin [17]. This study demonstrates OCT’s assessment of vascularity may aid in diagnosis and monitoring of response to therapy in hypertrophic scars. The role of vascularity in other fibrotic skin diseases is worthy of further investigation and we believe that OCT imaging is an optimal diagnostic tool for this purpose.
Despite the lack of OCT studies directly evaluating keloids or other scar types, we hypothesize that OCT has utility in identifying disease and monitoring and quantifying scar response to treatment without biopsy. The future use of OCT imagining as a quantitative imaging biomarker of fibrosis will help identify fibrosis and facilitate clinical examination in monitoring response to treatment longitudinally without relying on serial biopsies that may worsen scars. In turn, this will lead to earlier and more effective treatment implementation and improved patient outcomes.
Current Limitations and Future Direction of OCT
Although there are currently no randomized clinical trials (RCTs) evaluating the efficacy of OCT in dermatology, there are apparent differences between normal and fibrotic skin images. We would like to see OCT evaluated as a quantitative imaging biomarker for other similar fibrotic skin diseases such as morphea, chronic graft-versus-host disease, and nephrogenic fibrosing dermopathy. In addition, researchers need to evaluate if OCT can be used to measure and distinguish between different types of collagen deposition in skin. Future study of OCT technology will allow additional clinicopathologic comparisons between skin biopsy– the current gold standard for diagnosis, physical exam findings, and OCT data.
To further advance OCT, some technical limitations need to be addressed. Penetration depth is a significant limitation that may be overcome by combining OCT with other compatible acousto-optics imaging systems. Increasing wavelength may increase depth of penetration, however, the resulting decreased scatter and increased absorption may result in limited gains.
We believe OCT is useful in the evaluation of skin fibrosis. Using OCT, clinicians can obtain a rapid qualitative visual assessment of OCT images, however, clinicians cannot yet make a quantitative visual assessment of collagen content. Currently, third party applications and algorithms are employed in collagen data analysis and provide the quantitative data to clinicians. More advanced computer software and algorithms that allow clinicians to make rapid quantitative measurements, in the clinic or remotely, would enhance OCT’s clinical utility.
OCT can add significant value to the teledermatologic evaluation of patients with fibrotic skin diseases. These remote evaluations of skin fibrosis would be enhanced by using OCT to provide the user with an unbiased evaluation of the patient’s initial skin state and response to therapy. OCT’s ability to remotely provide visual and quantitative analyses of skin fibrosis may also assist in overcoming teledermatology’s tactile limitations when examining patients with skin fibrosis remotely.
Currently, the cost and portability of OCT are significant obstacles that hinder widespread adoption. Unfortunately, while there is an element of limited competition at the present, raw technology costs are currently high. Innovations in the telecoms sector will feed down to OCT and the resulting reduction in cost and size of key components will enable radical innovation and cost reduction. This will have cost benefits for the end consumer. As costs decline, adoption will increase among both clinicians and researchers. Despite these limitations, the use of OCT technology for quantification of fibrosis is in the formative stages and we foresee tremendous growth potential, similar to the ultrasound development paradigm that evolved over the past 30 years.
Conclusion
OCT is able to characterize normal and pathologic collagen variations by providing high-resolution skin images similar to the tissue architecture observed in histology. OCT imaging provides a non-traumatic and time-efficient approach to the diagnosis and management of a number of fibrotic skin conditions while ensuring patient comfort. OCT images may help in elucidating correlations between histopathology for integrated evaluation in research. This integrated approach may transform dermatologic tissue imaging and provide new insights into the physiology, pathology, and management of cutaneous diseases. Advances in OCT technology and further research on clinical applications have the potential to contribute to the trend toward non-surgical evaluation.
Acknowledgments
Funding Source:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award TL1 TR000133.
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award KL2 TR000134.
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R33AI080604.
Abbreviations
- CT
Computed tomography
- FD
Frequency domain
- MRI
Magnetic resonance imaging
- MRSS
Modified Rodnan skin score
- OCT
Optical coherence tomography
- PS-OCT
Polarization-sensitive optical coherence tomography
- SS-OCT
Swept-source optical coherence tomography
- TD
Time domain
- US
Ultrasound
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Abignano G, Aydin SZ, Castillo-Gallego C, Liakouli V, Woods D, Meekings A, Wakefield RJ, McGonagle DG, Emery P, Del Galdo F. Virtual skin biopsy by optical coherence tomography: the first quantitative imaging biomarker for scleroderma. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2012-202682. [DOI] [PubMed] [Google Scholar]
- 2.Boone M, Norrenberg S, Jemec G, Del Marmol V. High-definition optical coherence tomography: adapted algorithmic method for pattern analysis of inflammatory skin diseases: a pilot study. Archives of dermatological research. 2013;305 (4):283–297. doi: 10.1007/s00403-012-1311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boone MA, Norrenberg S, Jemec GB, Del Marmol V. High-definition optical coherence tomography imaging of melanocytic lesions: a pilot study. Archives of dermatological research. 2013 doi: 10.1007/s00403-013-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill RA, Mortensen NJ. Intraoperative augmented reality for laparoscopic colorectal surgery by intraoperative near-infrared fluorescence imaging and optical coherence tomography. Minerva chirurgica. 2010;65 (4):451–462. [PubMed] [Google Scholar]
- 5.Chu CR, Izzo NJ, Irrgang JJ, Ferretti M, Studer RK. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. Journal of biomedical optics. 2007;12 (5):051703. doi: 10.1117/1.2789674. [DOI] [PubMed] [Google Scholar]
- 6.Crisan M, Crisan D, Sannino G, Lupsor M, Badea R, Amzica F. Ultrasonographic staging of cutaneous malignant tumors: an ultrasonographic depth index. Archives of dermatological research. 2013;305 (4):305–313. doi: 10.1007/s00403-013-1321-1. [DOI] [PubMed] [Google Scholar]
- 7.Dalimier E, Salomon D. Full-field optical coherence tomography: a new technology for 3D high-resolution skin imaging. Dermatology. 2012;224 (1):84–92. doi: 10.1159/000337423. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature biotechnology. 2003;21 (11):1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 9.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. The New England journal of medicine. 2009;360 (19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 10.Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Archives of dermatological research. 2011;303 (7):457–473. doi: 10.1007/s00403-011-1152-x. [DOI] [PubMed] [Google Scholar]
- 11.Gladkova ND, Petrova GA, Nikulin NK, Radenska-Lopovok SG, Snopova LB, Chumakov YP, Nasonova VA, Gelikonov VM, Gelikonov GV, Kuranov RV, Sergeev AM, Feldchtein FI. In vivo optical coherence tomography imaging of human skin: norm and pathology. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2000;6 (1):6–16. doi: 10.1034/j.1600-0846.2000.006001006.x. [DOI] [PubMed] [Google Scholar]
- 12.Han JH, Kang JU, Song CG. Polarization sensitive subcutaneous and muscular imaging based on common path optical coherence tomography using near infrared source. Journal of medical systems. 2011;35 (4):521–526. doi: 10.1007/s10916-009-9388-0. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Annals of internal medicine. 2004;140 (1):37–50. [PubMed] [Google Scholar]
- 14.Krieg T, Aumailley M, Koch M, Chu M, Uitto J. Fitzpatrick’s Dermatology in General Medicine. 8. McGraw-Hill; New York: 2012. Collagens, Elastic Fibers, and Other Extracellular Matrix Proteins of the Dermis. [Google Scholar]
- 15.Kunzi-Rapp K, Dierickx CC, Cambier B, Drosner M. Minimally invasive skin rejuvenation with Erbium: YAG laser used in thermal mode. Lasers in surgery and medicine. 2006;38 (10):899–907. doi: 10.1002/lsm.20380. [DOI] [PubMed] [Google Scholar]
- 16.Lamirel C, Newman N, Biousse V. The use of optical coherence tomography in neurology. Reviews in neurological diseases. 2009;6 (4):E105–120. [PubMed] [Google Scholar]
- 17.Liew YM, McLaughlin RA, Gong P, Wood FM, Sampson DD. In vivo assessment of human burn scars through automated quantification of vascularity using optical coherence tomography. Journal of biomedical optics. 2013;18 (6):061213. doi: 10.1117/1.JBO.18.6.061213. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Vercollone C, Brezinski ME. Towards improved collagen assessment: polarization-sensitive optical coherence tomography with tailored reference arm polarization. International journal of biomedical imaging. 2012;2012:892680. doi: 10.1155/2012/892680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matcher SJ. Practical aspects of OCT imaging in tissue engineering. Methods in molecular biology. 2011;695:261–280. doi: 10.1007/978-1-60761-984-0_17. [DOI] [PubMed] [Google Scholar]
- 20.Mogensen M, Morsy HA, Thrane L, Jemec GB. Morphology and epidermal thickness of normal skin imaged by optical coherence tomography. Dermatology. 2008;217 (1):14–20. doi: 10.1159/000118508. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen M, Thrane L, Joergensen TM, Andersen PE, Jemec GB. Optical coherence tomography for imaging of skin and skin diseases. Seminars in cutaneous medicine and surgery. 2009;28 (3):196–202. doi: 10.1016/j.sder.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira GV, Chinkes D, Mitchell C, Oliveras G, Hawkins HK, Herndon DN. Objective assessment of burn scar vascularity, erythema, pliability, thickness, and planimetry. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2005;31 (1):48–58. doi: 10.1111/j.1524-4725.2005.31004. [DOI] [PubMed] [Google Scholar]
- 23.Pan Y, Farkas DL. Noninvasive imaging of living human skin with dual-wavelength optical coherence tomography in two and three dimensions. Journal of biomedical optics. 1998;3 (4):446–455. doi: 10.1117/1.429897. [DOI] [PubMed] [Google Scholar]
- 24.Phillips KG, Wang Y, Levitz D, Choudhury N, Swanzey E, Lagowski J, Kulesz-Martin M, Jacques SL. Dermal reflectivity determined by optical coherence tomography is an indicator of epidermal hyperplasia and dermal edema within inflamed skin. Journal of biomedical optics. 2011;16 (4):040503. doi: 10.1117/1.3567082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce MC, Sheridan RL, Hyle Park B, Cense B, de Boer JF. Collagen denaturation can be quantified in burned human skin using polarization-sensitive optical coherence tomography. Burns : journal of the International Society for Burn Injuries. 2004;30 (6):511–517. doi: 10.1016/j.burns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Pierce MC, Strasswimmer J, Hyle Park B, Cense B, De Boer JF. Birefringence measurements in human skin using polarization-sensitive optical coherence tomography. Journal of biomedical optics. 2004;9 (2):287–291. doi: 10.1117/1.1645797. [DOI] [PubMed] [Google Scholar]
- 27.Pierce MC, Strasswimmer J, Park BH, Cense B, de Boer JF. Advances in optical coherence tomography imaging for dermatology. The Journal of investigative dermatology. 2004;123 (3):458–463. doi: 10.1111/j.0022-202X.2004.23404.x. [DOI] [PubMed] [Google Scholar]
- 28.Pircher M, Goetzinger E, Leitgeb R, Hitzenberger C. Three dimensional polarization sensitive OCT of human skin in vivo. Optics express. 2004;12 (14):3236–3244. doi: 10.1364/opex.12.003236. [DOI] [PubMed] [Google Scholar]
- 29.Sakai S, Yamanari M, Lim Y, Nakagawa N, Yasuno Y. In vivo evaluation of human skin anisotropy by polarization-sensitive optical coherence tomography. Biomedical optics express. 2011;2 (9):2623–2631. doi: 10.1364/BOE.2.002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattler E, Kastle R, Welzel J. Optical coherence tomography in dermatology. Journal of biomedical optics. 2013;18 (6):061224. doi: 10.1117/1.JBO.18.6.061224. [DOI] [PubMed] [Google Scholar]
- 31.Tadrous PJ. Methods for imaging the structure and function of living tissues and cells: 3. Confocal microscopy and micro-radiology. The Journal of pathology. 2000;191 (4):345–354. doi: 10.1002/1096-9896(200008)191:4<345::AID-PATH696>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 32.Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, Fujimoto JG. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276 (5321):2037–2039. doi: 10.1126/science.276.5321.2037. [DOI] [PubMed] [Google Scholar]
- 33.Unterhuber A, Povazay B, Bizheva K, Hermann B, Sattmann H, Stingl A, Le T, Seefeld M, Menzel R, Preusser M, Budka H, Schubert C, Reitsamer H, Ahnelt PK, Morgan JE, Cowey A, Drexler W. Advances in broad bandwidth light sources for ultrahigh resolution optical coherence tomography. Physics in medicine and biology. 2004;49 (7):1235–1246. doi: 10.1088/0031-9155/49/7/011. [DOI] [PubMed] [Google Scholar]
- 34.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. The Journal of clinical investigation. 2007;117 (3):557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welzel J. Optical coherence tomography in dermatology: a review. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2001;7 (1):1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 36.Welzel J, Lankenau E, Birngruber R, Engelhardt R. Optical coherence tomography of the human skin. Journal of the American Academy of Dermatology. 1997;37 (6):958–963. doi: 10.1016/s0190-9622(97)70072-0. [DOI] [PubMed] [Google Scholar]
- 37.Wessels R, De Bruin DM, Faber DJ, Van Leeuwen TG, Van Beurden M, Ruers TJ. Optical biopsy of epithelial cancers by optical coherence tomography (OCT) Lasers in medical science. 2013 doi: 10.1007/s10103-013-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuno Y, Makita S, Sutoh Y, Itoh M, Yatagai T. Birefringence imaging of human skin by polarization-sensitive spectral interferometric optical coherence tomography. Optics letters. 2002;27 (20):1803–1805. doi: 10.1364/ol.27.001803. [DOI] [PubMed] [Google Scholar]