Abstract
The IMMEDIATE Trial of very early intravenous glucose-insulin-potassium (GIK) for acute coronary syndromes (ACS) in out-of-hospital emergency medical service (EMS) settings showed 80% reduction in infarct size at 30 days, suggesting potential longer-term benefit. Here we report 1-year outcomes. Pre-specified 1-year endpoints of this randomized, placebo-controlled, double-blind, effectiveness trial included all-cause mortality, and composites including cardiac arrest, mortality, or hospitalization for heart failure (HF). Among 871 participants randomized to GIK vs. placebo, respectively, death occurred within 1 year in 11.6% vs. 13.5% (unadjusted hazard ratio [HR] 0.83; 95% CI 0.57, 1.23, P=0.36). The composite of cardiac arrest or 1-year mortality was 12.8% vs. 17.0% (HR 0.71; 95% CI 0.50, 1.02, P=0.06). The composite of hospitalization for HF or mortality within 1 year was 17.2% vs. 17.2% (HR 0.98; 95% CI 0.70, 1.37, P=0.92). The composite of mortality, cardiac arrest, or HF hospitalization within 1 year was 18.1% vs. 20.4% (HR 0.85; 95% CI 0.62, 1.16, P=0.30). Among patients presenting with suspected ST elevation myocardial infarction (STEMI), hazard ratios for 1-year mortality and the 3 composites were, respectively, 0.65 (95% CI 0.33, 1.27, P=0.21); 0.52 (95% CI 0.30, 0.92, P=0.03); 0.63 (95% CI 0.35, 1.16, P=0.14); and 0.51 (95% CI 0.30, 0.87, P=0.01). Among patients with suspected ACS, serious endpoints generally were lower with GIK than placebo, but the differences were not statistically significant. However, among those with STEMI, the composites of cardiac arrest or 1-year mortality, and of cardiac arrest, mortality, or HF hospitalization within 1 year, were significantly reduced.
Keywords: acute myocardial infarction, acute coronary syndromes, emergency medical services, glucose-insulin-potassium, ACS, GIK
The IMMEDIATE (Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care) Trial was a randomized placebo-controlled, double-blind clinical effectiveness trial of the administration of intravenous glucose-insulin-potassium (GIK) very early in the evolution of acute coronary syndromes (ACS), by emergency medical service (EMS) paramedics, run in 13 US cities. (1–3) Prior to the IMMEDIATE Trial, experimental and clinical studies had shown GIK to protect myocardium from ischemic injury and lessen infarct size, and thereby to preserve left ventricular (LV) function, but clinical trials of the use of GIK for patients seen in hospitals for established acute myocardial infarction (AMI) had not generally shown these benefits. (4–13) In the IMMEDIATE Trial, rather than awaiting hospital diagnosis of AMI or ST elevation myocardial infarction (STEMI) to initiate GIK, as done in prior trials, GIK was delivered very early in the course of ACS, in the out-of-hospital setting. Paramedics, using electrocardiograph-based support by the acute cardiac ischemia time insensitive predictive instrument (ACI-TIPI) and the thrombolytic predictive instrument (TPI), initiated GIK as soon as possible in the community, and the infusion was continued in the receiving hospital for 12 hours total. (2,14,15) At 30 days, although the reduction in the primary endpoint of progression to AMI was not statistically significant, there were significant reductions in the composite endpoint cardiac arrest or mortality and an 80% reduction in infarct size, suggesting possible longer-term benefit. (1) One-year follow-up of trial participants for pre-specified study endpoints, including mortality, heart failure (HF), and the composite of cardiac arrest, mortality or HF, are reported here to address the degree to which such benefits might be sustained.
METHODS
This study was a double-blind, randomized, controlled clinical effectiveness trial of intravenous GIK; its design (3) and 30-day results, (1) including the primary study endpoint, have been previously published. Here we report 1-year results on the pre-specified clinical endpoints: all-cause mortality; the composite endpoint of mortality or hospitalization for HF within 1 year; and the composite endpoint of cardiac arrest, or mortality or HF hospitalization within 1 year.
Analyses were pre-specified to be examined for the cohort defined by intent-to-treat (ITT) for consenting participants with presumed ACS, and for a subset of the cohort presenting with suspected STEMI.
From December 2006 through July 2011, paramedics in 36 EMS systems in 13 cities across the US evaluated for study enrollment all patients age 30 or older for whom a 12-lead electrocardiogram (ECG) was obtained for chest pain or other possible ACS symptoms. Paramedics were aided by electrocardiograph-based software that printed the ACI-TIPI predicted patients’ probabilities of ACS and whether the TPI detected a likely STEMI. Paramedics were instructed to enroll patients who had an ACI-TIPI predicted probability of having ACS of ≥75%, had STEMI identified by the TPI, and/or who would have been identified as having STEMI by the community’s STEMI alert system. Excluded were patients with HF evidenced by more than basilar rales, those on dialysis for renal failure, or those unable to give assent. Assignment to study group was random by paramedic initiation of the blinded identical-appearing GIK or placebo study drug infusion packets.
As detailed elsewhere, (3) the trial used emergency exception from informed consent processes as in the Code of Federal Regulations 21CFR 50.24. (16) This process included community consultation and notification, institutional review board (IRB) approval, and having the paramedic read an information card to the patients prior to randomization and then asking for assent; full written consent was obtained once stabilized at the hospital. (3,17) Oversight was provided by a Data and Safety Monitoring Board (DSMB) appointed by the National Heart Lung and Blood Institute (NHLBI); there were no interim efficacy analyses. Funding was from NHLBI; insulin was donated by Eli Lilly and Company; ACI-TIPI/TPI defibrillator-electrocardiograph software was provided by the manufacturers of EMS systems’ equipment, Phillips Medical, Physio-Control, and Zoll Medical; no restrictions were imposed on study procedures or publication.
The study drug GIK solution had 30% glucose (300 g/L), 50 units of regular insulin per liter, and 80 mEq of KCl/L, which had been shown to improve myocardial perfusion. (18) The placebo was a 5% glucose solution in identical packaging. Per the study protocol, the study solution was to be administered at 1.5 ml/kg/hour (approximately 100 ml/hour for a 70 kg patient) for 12 hours intravenously via an infusion pump.
Collected were demographic (including race and ethnicity by self-report) and presenting data, including detailed information on EMS, emergency department (ED), and hospital care (including ECGs, cardiac biomarkers, cardiac catheterization, and other tests pertaining to ACS). For safety purposes, and reported elsewhere, (3) glucose and potassium blood tests were obtained upon ED arrival, at 6 hours after the start of the study drug, and once the study infusion was stopped. Also reported elsewhere (1) are data collected on biological mechanism cohort participants.
A Clinical Events Committee adjudicated final diagnoses and the causes of all hospitalization endpoints used for analyses. The Committee members assigned diagnoses of AMI (including by Killip Class), unstable angina pectoris (including by Canadian Class), non-ACS cardiac disease, and non-cardiac disease, based on out-of-hospital, ED, and 24-hour ECGs, biomarkers, and clinical data. In their review, they were blinded to study group, glucose and potassium tests, and whether the study infusion was stopped early. The analytic cohort of patients presenting with suspected STEMI was identified by having 3 cardiologists independently read the initial (out-of-hospital) ECG (blinded to study group) and determine whether the patient was sufficiently likely to be experiencing a STEMI to deserve immediate referral for cardiac catheterization and reperfusion.
All analyses were done on the ITT cohort with treatment assignment as randomized. In addition, primary analyses were conducted for the sub-cohort of those presenting with STEMI, as defined above. Analyses also were conducted on a modified-intent-to-treat (MITT) cohort, comprised of those in the ITT cohort whom ED physicians confirmed as having ACS and therefore continued on the study drug as GIK presumably would be used in clinical practice.
As described in the report of the 30-day outcomes, (1) the primary endpoint of the IMMEDIATE Trial was progression to MI based on biomarkers and ECGs. It was calculated that a sample size of 800 evaluable study participants would provide 90% power to detect a relative 20.5% reduction from 55.7% to 44.3% between the placebo and GIK groups for this endpoint. To accommodate attrition, 880 study participants were planned for randomization.
Time-to-event outcomes were analyzed using Cox proportional hazards regressions and the assumption of proportional hazards checked using a Kolmogorov-type supremum test. Robust variance estimators were used to account for potential clustering across multiple enrollments by individual participant’s time-to-event analyses. For composite events, the time to the first occurrence of any component of the composite was used. All statistical testing used 2-sided 0.05 level of significance, and were done using SAS software version 9.2.
RESULTS
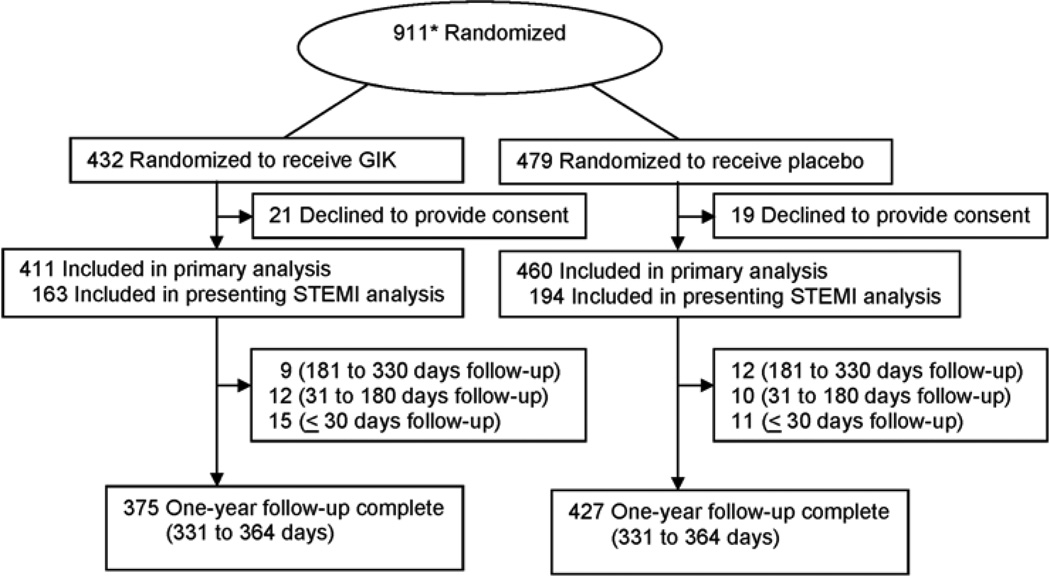
Of 911 patients randomized, there were 871 enrollments for 850 individuals, as shown in Figure 1. (Of those randomized, 40 initially agreed to have the study drug started in the ambulance but later declined written informed consent at the hospital and were excluded: 21 GIK group participants and 19 placebo group participants.) Enrollments were used as the unit of analysis. Demographic and clinical features are in Table 1. The average age was 63, 71% were men, and 86% presented with a chief complaint of chest pain. Randomization into the trial occurred at a median of 90 minutes after ischemic symptom onset. STEMI was suspected in 41%; 47% of participants received percutaneous coronary intervention (PCI). Characteristics of GIK and placebo participants were similar.
Figure 1.
Study participant flow chart (Includes 18 participants [8 GIK, 10 placebo] who did not meet eligibility criteria but were randomized. STEMI indicates ST elevation.)
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants by Treatment Group (N=871).*
| Characteristics | GIK (n=411) | Placebo (n=460) |
|---|---|---|
| Age, mean ± SD, (years) | 63.9±13.9 | 63.3±14.1 |
| Women | 113 (27.5%) | 140 (30.4%) |
| Men | 298 (72.5%) | 320(69.6%) |
| White | 332/403 (82.4%) | 392/451 (86.9%) |
| Black | 52/403 (12.9%) | 42/451 (9.3%) |
| Asian | 6/403 (1.5%) | 3/451 (0.7%) |
| American Indian or Alaskan Native | 8/403 (2.0%) | 8/451 (1.8%) |
| Native Hawaiian or other Pacific Islander | 2/403 (0.5%) | 2/451 (0.4%) |
| Other | 4/403 (1.0%) | 4/451 (0.9%) |
| Hispanic ethnic group | 44/402 (10.9%) | 58/445 (13.0%) |
| Chief complaint upon presentation | ||
| Chest pain | 358/411 (87.1%) | 391/460 (85.0%) |
| Dyspnea | 15/411 (3.6%) | 19/460 (4.1%) |
| Initial out-of-hospital blood pressure – (mm Hg) | ||
| Systolic | 143.3±32.0 | 143.4±34.9 |
| Diastolic | 84.4±23.6 | 85.0±25.1 |
| Initial out-of-hospital heart rate –(beats/min) | 86.8±24.7 | 86.6±25.6 |
| Initial out-of-hospital respiratory rate – (breaths/min) | 19.3±4.2 | 19.5±4.4 |
| Time from symptom onset to study drug (≤ 24 hours) | ||
| Median | 90.0 | 90.0 |
| Interquartile range | 50.0–159.3 | 52.0–159.3 |
| Time from symptom onset to study drug | ||
| 0 to 30 minutes | 24/401 (6.0%) | 20/457 (4.4%) |
| 31 to 60 minutes | 101/401 (25.2%) | 121/457 (26.5%) |
| 61 to 90 minutes | 60/401 (15.0%) | 74/457 (16.2%) |
| 91 to 180 minutes | 66/401 (16.5%) | 82/457 (17.9%) |
| 181 to 360 minutes | 46/401 (11.5%) | 55/457 (12.0%) |
| 361 minutes to 24 hours | 37/401 (9.2%) | 36/457 (7.9%) |
| Within 24 hours - unspecified | 31/401 (7.7%) | 34/457 (7.4%) |
| > 24 hours | 36/401 (9.0%) | 35/457 (7.7%) |
| STEMI on presenting out-of-hospital ECG (%) | 163/411 (39.7%) | 194/460 (42.2%) |
| ACI-TIPI score ‡ | 74.6±22.6 | 76.9±20.6 |
| TPI triggered – No./Total (%) | 84/411 (20.4%) | 116/460 (25.2%) |
| Diabetes mellitus | 121/411 (29.4%) | 121/460 (26.3%) |
| Heart failure | 68/411 (16.5%) | 77/460 (16.7%) |
| Myocardial infarction | 152/411 (37.0%) | 159/460 (34.6%) |
| Hospital acute reperfusion treatment | ||
| Thrombolytic therapy | 3/411 (0.7%) | 8/460 (1.7%) |
| Percutaneous coronary intervention | 198/411 (48.2%) | 208/460 (45.2%) |
| Coronary artery bypass graft | 12/411 (2.9%) | 13/460 (2.8%) |
Abbreviations: STEMI, ST elevation myocardial infarction; ACI-TIPI, acute cardiac ischemia time-insensitive predictive instrument; TPI, thrombolytic predictive instrument.
Plus-minus values are means±SD.
Race was self-reported.
ACI-TIPI score ≥ 75% was part of the inclusion criteria.
19 patients had 21 repeat visits.
No significant differences were noted between GIK and placebo.
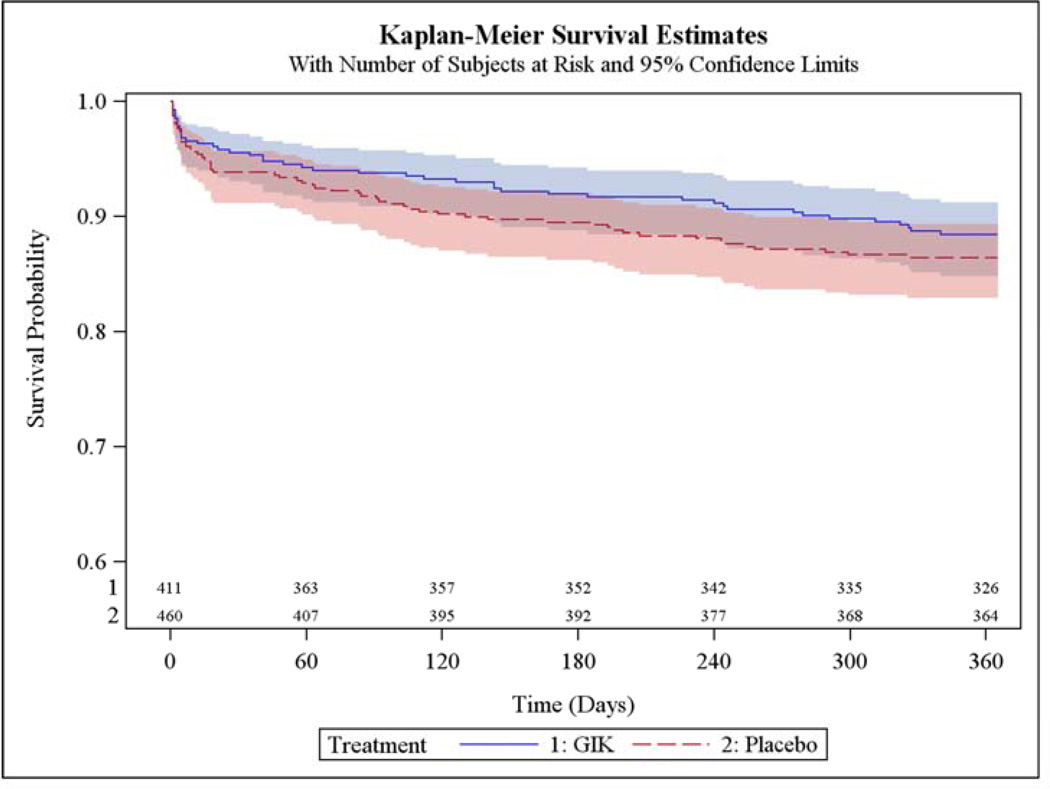
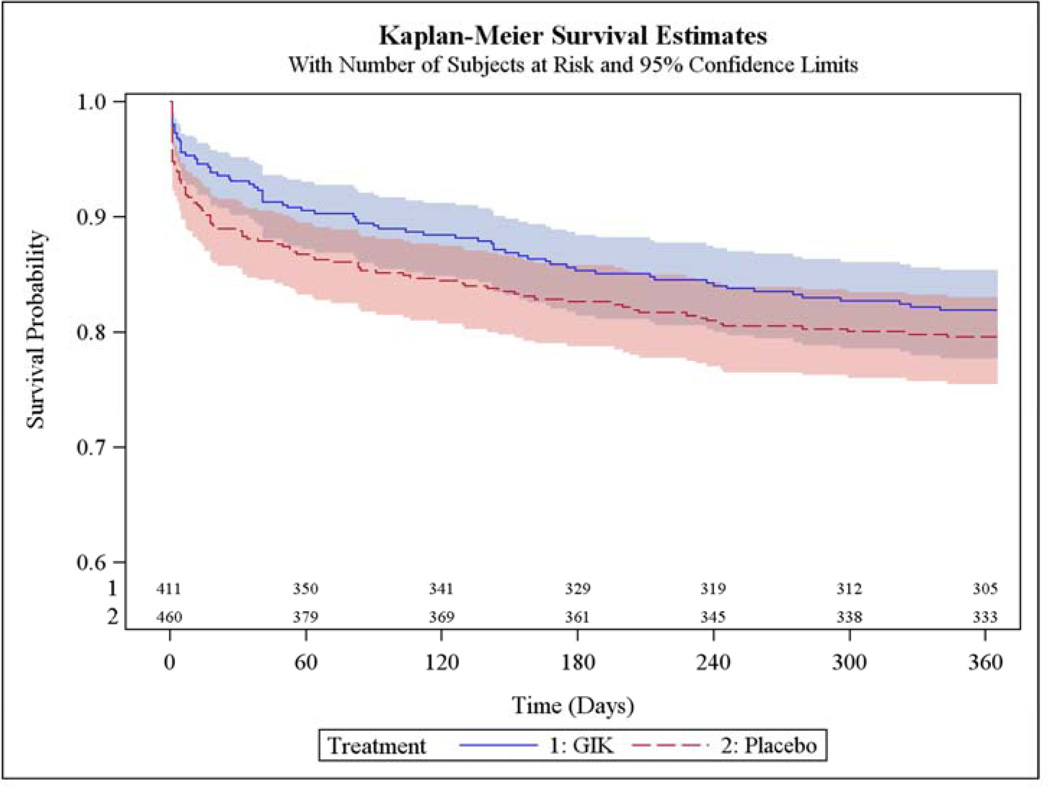
The main results are in Table 2. Among all participants, the 1-year mortality rates estimated from unadjusted Kaplan-Meier (K-M) curves were 11.6% with GIK vs. 13.5% with placebo (unadjusted hazard ratio [HR] 0.83; 95% CI 0.57, 1.23, P=0.36); the K-M rates of the composite of cardiac arrest or 1-year mortality were 12.8% for GIK and 17.0% for placebo (HR 0.71; 95% CI 0.50, 1.02, P=0.06); the K-M rates for the composite of hospitalization for HF or mortality within 1 year were 17.2% for both GIK and placebo (HR 0.98; 95% CI 0.70, 1.37, P=0.92); and the K-M rates for the composite of cardiac arrest, 1-year mortality, or HF hospitalization within 1 year were 18.1% with GIK vs. 20.4% with placebo (HR 0.85; 95% CI 0.62, 1.16, P=0.30). The causes of death are in Table 3.
Table 2.
IMMEDIATE TRIAL INCIDENCE OF IN-HOSPITAL CARDIAC ARREST/1-YEAR MORTALITY OVERALL AMONG ALL PATIENTS IN THE INTENT-TO-TREAT COHORT
| Outcome | Treatment | Unadjusted Rates |
Unadjusted Kaplan- Meier Rate |
Unadjusted Hazard Ratio (GIK vs. Placebo) |
95% Confidence Interval of Unadjusted Hazard Ratio |
Unadjusted Cox Regression P-Value |
|---|---|---|---|---|---|---|
| 1-Year Mortality | GIK | 45/411 (10.9%) | 11.6% | 0.83 | 0.57, 1.23 | 0.355 |
| Placebo | 60/460 (13.0%) | 13.5% | ||||
| Cardiac Arrest or Mortality | GIK | 50/411 (12.2%) | 12.8% | 0.71 | 0.50, 1.02 | 0.062 |
| Placebo | 76/460 (16.5%) | 17.0% | ||||
| In-hospital Cardiac Arrest | GIK | 15/411 (3.6%) | 0.56 | 0.30, 1.02 | 0.060 | |
| Placebo | 29/460 (6.3%) | |||||
| 1-Year HF or Mortality | GIK | 67/411 (16.3%) | 17.2% | 0.98 | 0.70, 1.37 | 0.915 |
| Placebo | 76/460 (16.5%) | 17.2% | ||||
| 1-Year HF | GIK | 35/411 (8.5%) | 9.6% | 1.22 | 0.74, 2.02 | 0.439 |
| Placebo | 32/460 (7.0%) | 7.8% | ||||
| Cardiac Arrest, HF, or Mortality | GIK | 71/411 (17.3%) | 18.1% | 0.85 | 0.62, 1.16 | 0.299 |
| Placebo | 91/460 (19.8%) | 20.4% |
Table 3.
| Adjudicated Cause of Death | ||
|---|---|---|
| Subgroup | # Deaths by Treatment |
|
| GIK | Placebo | |
| ALL: total # deaths (1 year analysis) | 45 | 60 |
| Death Cardiac - Arrythmic | 3 | 2 |
| Death Cardiac - Heart failure | 6 | 10 |
| Death Cardiac - Ischemia | 13 | 22 |
| Death Cardiac - Not otherwise specified | 12 | 9 |
| Death Non-Cardiac - specify | 11 | 17 |
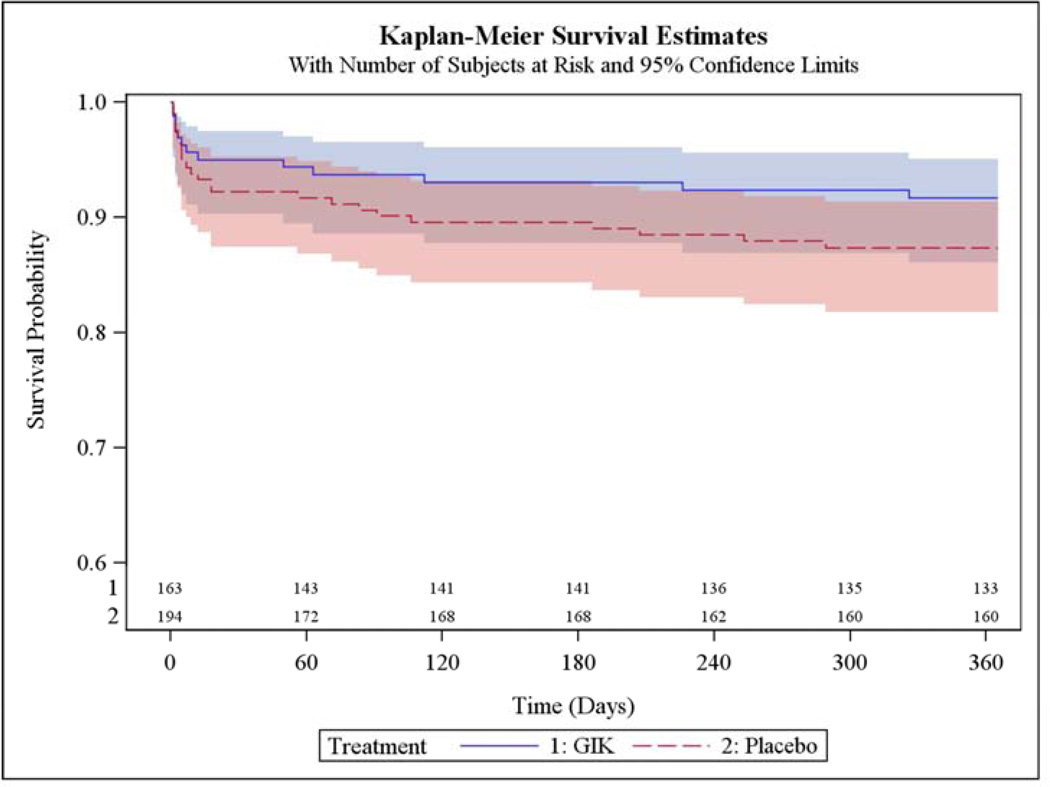
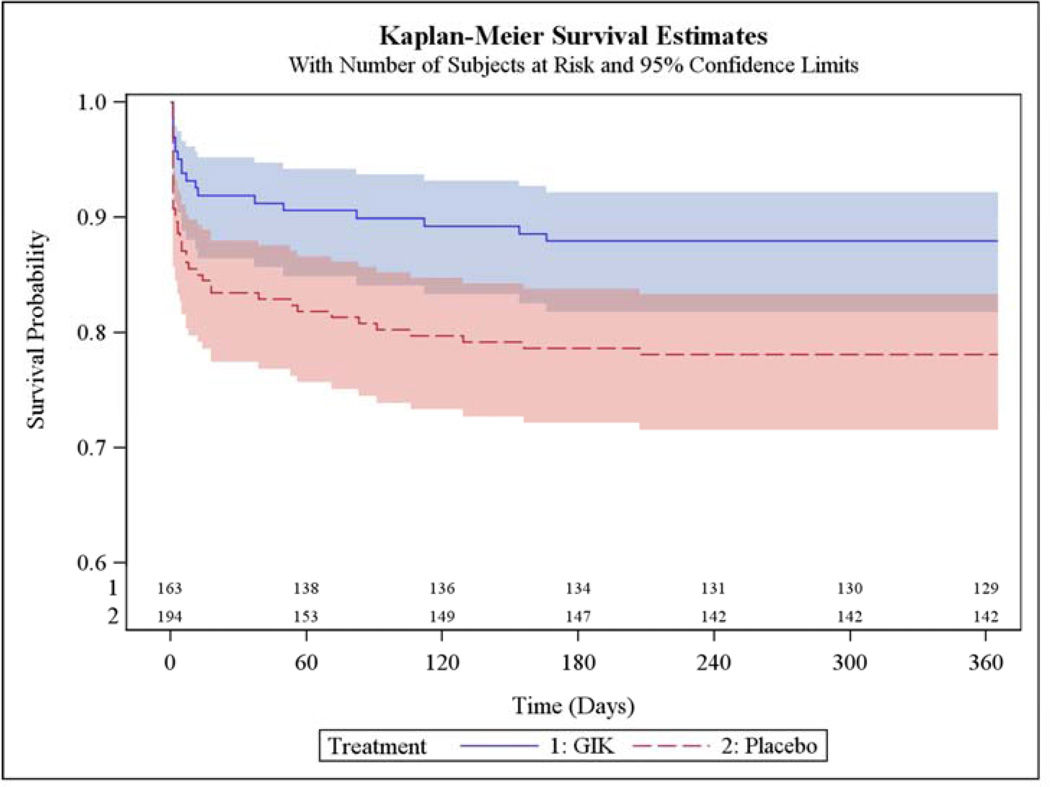
In Table 4 are results among participants who presented with suspected STEMI, including 163 who received GIK and 194 who received placebo. One-year mortality rates estimated from unadjusted K-M curves were 8.3% with GIK vs. 12.6% with placebo (HR 0.65; 95% CI 0.33, 1.27, P=0.21); the K-M rates of the composite of cardiac arrest or mortality within 1 year were 10.8% with GIK vs. 19.3% with placebo (HR 0.52; 95% CI 0.30, 0.92, P=0.03); the composite of hospitalization for HF or mortality within 1 year was 10.2% with GIK vs. 15.7% with placebo (HR 0.63; 95% CI 0.35, 1.16, P=0.14); and the composite outcome of cardiac arrest, 1-year mortality, or HF hospitalization within 1 year was 12.0% with GIK vs. 21.9% with placebo (HR 0.51; 95% CI 0.30, 0.87, P=0.01).
Table 4.
IMMEDIATE TRIAL INCIDENCE OF IN-HOSPITAL CARDIAC ARREST/1-YEAR MORTALITY OVERALL AMONG PATIENTS PRESENTING WITH ECG ST ELEVATION
| Outcome | Treatment | Unadjusted Rates |
Unadjusted Kaplan- Meier Rate |
Unadjusted Hazard Ratio (GIK vs. Placebo) |
95% Confidence Interval of 95% Unadjusted Hazard Ratio |
Unadjusted Cox Regression P-Value |
|---|---|---|---|---|---|---|
| 1-Year Mortality | GIK | 13/163 (8.0%) | 8.3% | 0.65 | 0.33, 1.27 | 0.208 |
| Placebo | 24/194 (12.4%) | 12.6% | ||||
| Cardiac Arrest or Mortality | GIK | 17/163 (10.4%) | 10.8% | 0.52 | 0.30, 0.92 | 0.025 |
| Placebo | 37/194 (19.1%) | 19.3% | ||||
| In-hospital Cardiac Arrest | GIK | 9/163 (5.5%) | 0.49 | 0.23, 1.03 | 0.061 | |
| Placebo | 21/194 (10.8%) | |||||
| 1-Year HF or Mortality | GIK | 16/163 (9.8%) | 10.2% | 0.63 | 0.35, 1.16 | 0.139 |
| Placebo | 30/194 (15.5%) | 15.7% | ||||
| 1-Year HF | GIK | 6/163 (3.7%) | 4.2% | 0.65 | 0.24, 1.74 | 0.389 |
| Placebo | 11/194 (5.7%) | 6.1% | ||||
| Cardiac arrest, HF, or Mortality | GIK | 19/163 (11.7%) | 12.0% | 0.51 | 0.30, 0.87 | 0.014 |
| Placebo | 42/194 (21.6%) | 21.9% |
Figure 2 shows the K-M plot for the endpoint of all-cause mortality for all participants; and Figure 3 for the composite outcome of in-hospital cardiac arrest or HF or mortality for all participants. Figure 4 and Figure 5 present the K-M plots for participants presenting with ST-segment elevation for the outcomes of all-cause mortality and the composite outcome of in-hospital cardiac arrest or HF or mortality respectively.
Figure 2.
Cumulative Incidence of Freedom from Death through 1 Year (ITT Participants)
Figure 3.
Cumulative Incidence of Freedom from In-Hospital Cardiac Arrest or HF or Death through 1 Year (ITT Participants)
Figure 4.
Cumulative Incidence of Freedom from Death through 1 Year (Participants Presenting with ST-Segment Elevation)
Figure 5.
Cumulative Incidence of Freedom from In-Hospital Cardiac Arrest or HF or Death through 1 Year (Participants Presenting with ST-Segment Elevation)
DISCUSSION
This placebo-controlled, double-blind, randomized, clinical effectiveness trial of community-based EMS use of GIK for ACS was intended to translate the effects seen in experimental laboratory research into an approach that could work in widespread clinical practice. In previous trials, GIK was administered in hospitals once a diagnosis of AMI was established. In the IMMEDIATE trial, to better duplicate the experimental benefits, GIK was administered immediately following evaluation by paramedics in the community based on their clinical impression of ACS, supplemented by ACI-TIPI and TPI predictions printed on the out-of-hospital ECGs. (2,3) This approach allowed initiation of GIK at a median of 90 minutes after the onset of ischemic symptoms, rather than the typical 6 hours seen in previous GIK trials. (2) Thereby, benefits from GIK that the IMMEDIATE Trial sought to detect were reductions in myocardial damage, acute cardiac arrest, and acute mortality. The degree to which this 1-time 8-hour GIK treatment might be reflected a year later has not been previously reported.
In the previously-reported 30-day results, the reduction of progression to biomarker-confirmed AMI was not significant, but the degree of LV protection, as evidenced by the mitigation of infarct size in the biological mechanism cohort, was significant at 30 days. These participants had SPECT cardiac imaging at 30 days: among those presenting with ACS, infarct size was reduced from 10% of LV mass to 2% of LV mass with GIK (P=0.01), and for those presenting with STEMI, infarct size in the placebo patients was 12% of LV mass compared to only 3% with GIK (P=0.05). If sustained, this preservation of myocardium would be expected to lead to longer-term benefits of better survival and avoidance of HF. Indeed, some such effect could have been inferred from the 30-day results (1) that included a 40% reduction in the composite incidence of cardiac arrest, 30-day mortality or HF hospitalization within 30 days (P=0.03), and a 54% reduction for this composite among those presenting with STEMI (P=0.02).
The 1-year outcomes (Table 2) generally demonstrate point estimates favoring GIK for individual events and for composites, but confidence intervals overlap 1.0 in the majority of analyses. However, this study was not specifically powered to detect a significant difference in these outcome measures. Nonetheless, selected biologically plausible outcomes did reach significance in favor of GIK, consistent with the 30-day results. For those treated with GIK, the composite of cardiac arrest and 1-year mortality was lower among all those with suspected ACS, 12.8% for GIK vs. 17.0% for placebo (HR 0.71; 95% CI 0.50, 1.02, P=0.06) and among those presenting with ST elevation, 10.8% with GIK vs. 19.3% with placebo (HR 0.52; 95% CI 0.30, 0.92, P=0.03). This also was the case for the 3-way composite of cardiac arrest, 1-year mortality, or HF hospitalization within 1 year among those presenting with STEMI (HR 0.51; 95% CI 0.30, 0.87, P=0.01). These results are supportive of the potential impact of GIK and that a larger trial should be designed to have power to detect such effects – both at 30 days and at 1 year.
In suggesting the need for another larger GIK trial, it should be noted that, although the results presented here and in the 30-day IMMEDIATE Trial results (1) are consistent with experimental results, (5) they are distinct in some ways from prior GIK clinical trials, most dramatically, the over 20,000-patient CREATE-ECLA-OASIS Trials. (11,19) However, the IMMEDIATE Trial study design and execution is very different from other GIK clinical trials, which likely explains the disparate outcomes. The other trials were not double-blind placebo-controlled randomized trials, as was IMMEDIATE. Most importantly, IMMEDIATE tested GIK administered to patients with ACS, as early as possible, emulating prior successful animal experiments. All previous clinical trials gave GIK only after the patient had arrived at the hospital and had an AMI documented, typically 6 hours after symptom onset, compared with a median of 90 minutes in IMMEDIATE. This delayed administration is not consistent with the concept of GIK providing metabolic support during ischemia prior to re-establishment of coronary perfusion, and therefore would not be expected to show significant results. In CREATE-ECLA, 17.4% did not get reperfusion, and 68.3% were given GIK after, not before, coronary reperfusion – so for 85.7% there was no opportunity at all for the GIK to provide metabolic support that might have prolonged the window of benefit from reperfusion. (6,20) On the other hand, in IMMEDIATE, the results of the biological mechanism cohort in which infarct size was 80% smaller with GIK is consistent with such metabolic support.
In summary, the 30-day results of IMMEDIATE previously reported, and the 1-year results presented here demonstrate trends toward, and in some cases statistically significant, improved clinical outcomes. Such scientifically plausible findings warrant additional clinical trials to further evaluate this simple, inexpensive and potentially very impactful treatment for ACS and particularly STEMI.
Acknowledgements
We thank the research coordinators who completed the 1-year follow-up and thank Catherine Griffin and Muriel Powers for expert manuscript preparation.
Funding/Support: Funded by National Institutes of Health (NIH) Cooperative Agreement from National Heart, Lung and Blood Institute (NHLBI) grants: U01HL077821, U01HL077826, U01HL077823. Role of the Sponsor: Patrice Desvigne-Nickens, MD, Yves Rosenberg, MD, and Xin Tian, PhD from NHLBI, respectively, were involved as part of the NIH Cooperative Agreement process, in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or the NIH. The National Center for Research Resources (UL1RR025752) provided support for specimen processing and testing. Insulin was donated by Eli Lilly and Company, Indianapolis, IN. Electrocardiograph-based decision support software was donated by Physio-Control, Redmond, WA; and Zoll Medical, Chelmsford, MA. The National Center for Research Resources, Eli Lilly and Company, Physio-Control and Zoll Medical did not participate in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D’Agostino RB, Ruthazer R, Atkins JA, Sayah AJ, Levy MK, Richards ME, Aufderheide TP, Braude DA, Pirrallo RG, Doyle DD, Frascone RJ, Kosiak DJ, Leaming JM, Van Gelder CM, Walter G-P, Wayne MA, Woolard RH, Opie LH, Rackley CE, Apstein CS, Udelson JE. Effect of Out-of-Hospital Administration of Intravenous Glucose, Insulin, and Potassium (GIK) in Patients with suspected Acute Coronary Syndromes: The IMMEDIATE Randomized Controlled Trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selker HP, Beshansky JR, Ruthazer R, Sheehan PR, Sayah AJ, Atkins JM, Aufderheide TP, Pirrallo RG, D’Agostino RB, Massaro JM, Griffith JL. Emergency medical service predictive instrument aided diagnosis and treatment of acute coronary syndromes and ST elevation myocardial infarction in the IMMEDIATE Trial. Prehospital Emergency Care. 2011;15:139–148. doi: 10.3109/10903127.2010.545478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selker HP, Behansky JR, Griffith JL, D’Agostino RB, Massaro JM, Udelson JE, Rashba EJ, Ruthazer R, Sheehan PR, Desvigne-Nickens P, Rosenberg YD, Atkins JM, Sayah AJ, Aufderheide TP, Rackley CE, Opie LH, Lambrew CT, Cobb LA, MacLeod BA, Ingwall JS, Zalenski RJ, Apstein CS. Study design for the IMMEDIATE (Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care) Trial: a double-blind randomized controlled trial of intravenous glucose, insulin, and potassium (GIK) for acute coronary syndromes in emergency medical services. Am Heart J. 2012;163:315–322. doi: 10.1016/j.ahj.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opie LH, Bruyneel K, Owen P. Effects of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation. 1975;52:49–57. doi: 10.1161/01.cir.52.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Grossman AN, Opie LH, Beshansky JR, Ingwall JS, Rackley CE, Selker HP. Glucose-insulin-potassium revived: current status in acute coronary syndromes and the energy-depleted heart. Circulation. 2013;127:1040–1048. doi: 10.1161/CIRCULATIONAHA.112.130625. [DOI] [PubMed] [Google Scholar]
- 6.Apstein CS. Glucose-insulin-potassium for acute myocardial infarction: remarkable results from a new, prospective randomized trial. Circulation. 1998;98:2223–2226. doi: 10.1161/01.cir.98.21.2223. [DOI] [PubMed] [Google Scholar]
- 7.Apstein CS, Opie LH. Glucose-insulin-potassium (GIK) for acute myocardial infarction: A negative study with a positive value. Cardiovasc Drugs Ther. 1999;13:185–189. doi: 10.1023/a:1007757407246. [DOI] [PubMed] [Google Scholar]
- 8.Diaz R, Paolasso EA, Piegas LS, Tajer CD, Moreno MG, Corvalan R, Isea JE, Romero G. Metabolic modulation of acute myocardial infarction: the ECLA (Estudios Cardiologicos Latinoamerica) Collaborative Group. Circulation. 1998;98:2227–2234. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- 9.Van der Horst I, Zijlstra F, van’t Hof AWJ, Doggen CJM, de Boer MJ, Suryapranata H, Hoorntje JCA, Dambrink JHE, Gans ROB, Gilo HJG on behalf of the Zwolle Infarct Study Group. Glucose-insulin-potassium infusion in patients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin-potassium study: a randomized trial. J Am Coll Cardiol. 2003;42:784–791. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 10.Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, Wedel H, Welin L. Randomized trial of insulin-glucose- infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at one year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 11.The CREATE-ECLA Trial Group Investigators. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction, The CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 12.Apstein CS. Glucose-insulin-potassium infusion and mortality in the CREATE-ECLA trial. JAMA. 2005;293:2596–2597. doi: 10.1001/jama.293.21.2596. [DOI] [PubMed] [Google Scholar]
- 13.Timmer JR, Svilaas T, Ottervanger JP, Henriques JP, Dambrink J-H, Van den Broek SAJ, van der Horst ICC, Zjilstra F. Glucose-insulin-potassium infusion in patients with acute myocardial infarction without signs of heart failure: the Glucose-Insulin-Potassium Study (GIPS)-II. J Am Coll Cardiol. 2006;47:1730–1731. doi: 10.1016/j.jacc.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Selker HP, Beshansky JR, Griffith JL, Aufderheide TP, Ballin DS, Bernard SA, Crespo SG, Feldman JA, Fish SS, Gibler WB, Kiez DA, McNutt RA, Moulton AW, Ornator JP, Podrid PJ, Pope JH, Salem DN, Sayre MR, Woolard RH. Use of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) to assist emergency department triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia: a multicenter controlled clinical trial. Ann Intern Med. 1998;129:845–855. doi: 10.7326/0003-4819-129-11_part_1-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Selker HP, Beshansky JR, Griffith JL for the TPI Trial Investigators. Use of the electrocardiograph-based thrombolytic predictive instrument to assist thrombolytic and reperfusion therapy for acute myocardial infarction. Ann Intern Med. 2002;137:87–95. doi: 10.7326/0003-4819-137-2-200207160-00006. [DOI] [PubMed] [Google Scholar]
- 16.Exception from informed consent requirements for emergency research. [Accessed November, 2011];Code of Federal Regulations. Title 21, Section 50.24. 2011 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.24.
- 17.Beshansky JR, Sheehan PR, Hadar N, Klima K, Selker H. National implementation of exception from informed consent requirements for emergency research (21CFR50.24) process and 1-year experience in the IMMEDIATE trial. Clinical Trials. 2010;7:428. [Google Scholar]
- 18.Stanley AW, Jr, Moraski RE, Russell RO, Rogers WJ, Mantle JA, Kreisberg RA, McDaniel HG, Rackley CE. Effects of glucose-insulin-potassium on myocardial substrate availability and utilization in stable coronary artery disease: studies on myocardial carbohydrate, lipid and oxygen arterial coronary sinus differences in patients with coronary artery disease. Am J Cardiol. 1975;36:929–937. doi: 10.1016/0002-9149(75)90085-5. [DOI] [PubMed] [Google Scholar]
- 19.Diaz R, Goyal A, Mehta SR, Afzal R, Xavier D, Pais P, Chrolavicius S, Zhu J, Kazmi K, Liu L, Budaj A, Zubaid M, Avezum A, Ruda M, Yusuf S. Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA. 2007;298:2399–2405. doi: 10.1001/jama.298.20.2399. [DOI] [PubMed] [Google Scholar]
- 20.Cobb LA, Killip T, Lambrew CT, MacLeod BA, Rackley CE, Selker HP, Zalenski RJ. Glucose-insulin-potassium infusion and mortality in the CREATE-ECLA trial. JAMA. 2005;293:2597. doi: 10.1001/jama.293.21.2597-a. [DOI] [PubMed] [Google Scholar]