Abstract
Maternal behaviour has profound, long-lasting implications for the health and well-being of developing offspring. In the monogamous California mouse (Peromyscus californicus), care by both parents is critical for offspring survival. We tested the hypothesis that similar to maternal care in rodents, paternal huddling and grooming (HG) behaviour can be transmitted to future generations via behavioural mechanisms. In California mice, testosterone maintains paternal HG behaviour. In the present study, we randomly assigned a group of male California mice to castration or sham-operated conditions and allowed them to raise their offspring normally. Adult sons of these males were paired with a female, and they were observed interacting with their own offspring. We found that like their fathers, the sons of castrated males huddled and groomed their young at lower levels than the sons of sham-operated fathers. The sons of castrates also retrieved pups more frequently. When both parents were present, the sons of castrates also showed a trend towards engaging in less exploratory behaviour. These data support the hypothesis that paternal behaviour, like maternal behaviour, can be transferred to future generations via epigenetic mechanisms and suggest that in a biparental species both parents contribute to offspring behavioural development.
Keywords: paternal, Peromyscus, epigenetic, biparental, monogamous, testosterone
1. Introduction
The concept that mammalian mothers influence the physical and behavioural development of their infants is intuitive; mother and infant are connected by the placenta during gestation and remain closely associated after birth, when the mother nurses and cares for her young. In species with altricial young, brain development continues through the postnatal period and reflects an additional period of sensitivity to cues from the environment [1]. The long-term effects of mother–infant interactions on the neuroendocrine system are well studied, particularly within the realm of stress physiology and the development of the hypothalamic–pituitary–adrenal (HPA) axis. Although mothers are typically the sole providers of infant care in mammals, paternal behaviour is observed in approximately 5 per cent of mammalian species [2]. The role of fathers in development is poorly understood, perhaps owing to fewer opportunities to develop studies that address these relationships.
The mammalian postnatal environment is largely characterized by interactions with the carer and siblings, so it follows that parental behaviour would be a means by which modifications to the developing neuroendocrine system of the infant could be triggered. In rats, the amount of licking, grooming and arched-back nursing (LG-ABN) that a mother performs has long-lasting behavioural and physiological effects on offspring [3]. Adult rats that experienced less of these nurturing behaviours during infancy show elevated HPA-axis activity, deficits in tests of spatial learning and memory and are behaviourally more fearful than the offspring of more nurturing mothers [4]. Moreover, the nurturing behaviour that a female pup experiences is strongly linked to the behaviour that she will eventually display towards her own young [5,6]. Similar relationships exist in other species as well; for example, the amount of contact a vervet monkey has with her mother in infancy predicts the amount of contact she will maintain with her own infant in its first six months of life [7]. Likewise, in rhesus macaques, high rates of infant rejection are transmitted from mother to daughter [8], and harsh and inconsistent maternal care in infancy is associated with heightened cortisol responses to stress in adulthood [9]. Furthermore, in zebra finches, reproductive success at hatching covaries with the brood size in which the mother was raised, indicating a long-lasting effect of stress during early development on future maternal behaviour [10]. Thus, variations in maternal behaviour during the postnatal environment can influence the development of both the endocrine system and future maternal behaviour.
When paternal care is normative or essential for a species both parents are expected to influence offspring growth and development. Paternal care may take the form of food provisioning as in many birds [2], and males may also be the sole providers of offspring care in certain non-mammalian species, such as in paternal mouth-brooding fish. Male care may enhance physical growth, as is the case with dung beetles, with male provisioning resulting in heavier brood masses than when females provide care alone [11]. The impact of male care may also be more complex; in the biparental rodent Octodon degus, pups raised without their fathers show reduced apical spine numbers and shorter apical dendrites in the orbitofrontal cortex [12], a brain area important for social interactions [13]. One study in the biparental California mouse (Peromyscus californicus) found that fathers required to wheel-run for food spent less time in contact with pups [14]. As a result, pups raised under this foraging demand showed both increased fearfulness in open-field tests, and decreased performance in novel object and place recognition tests. In studies examining the transmission of aggressive behaviour in the California mouse, adult males cross-fostered to the less aggressive white-footed mouse showed increased attack latencies in tests of resident–intruder aggression and decreased arginine vasopressin immunoreactivity (AVP-ir) in the bed nucleus of the stria terminalis (BNST) compared with California mice raised by fathers of their own species [15]; that is, cross-fostered males more closely resembled their foster parents’ behaviour. Mechanistically, paternal retrievals appear to be the postnatal environmental signal by which AVP-ir in the BNST and adult aggression are impacted [16,17]. Thus, fathers may influence offspring development when paternal involvement is typical for the species.
Despite emerging evidence that paternal behaviour is important for development, it is not known whether nurturing behaviours, specifically huddling and grooming (HG) behaviours, are transmitted between generations in male mammals as they are in females. Here, we tested the hypothesis that paternal HG behaviours are non-genomically transferred between generations in the monogamous and biparental California mouse. In California mice, HG is influenced by testosterone; castration selectively reduces, and testosterone replacement restores, paternal HG behaviour [18,19]. To test our hypothesis, we surgically manipulated fathers to generate two groups of mice: (i) those that exhibited normative levels of HG towards their pups (sham-operated fathers) and (ii) those that showed reduced HG (castrated fathers). Castrated males still display some paternal behaviour and females only partially compensate for the reduction in male care [18]. Male offspring of manipulated fathers were raised to adulthood, at which time we paired each male with a mate and observed his subsequent behaviours with his own pups. By randomly assigning males to either sham or castrate groups, we limited the possibility that group differences in offspring paternal HG behaviours are heritable as opposed to being shaped by the early postnatal environment.
2. Material and methods
(a). Subjects
We used reproductively experienced California mice and their offspring, raised in a laboratory colony at the University of Wisconsin-Madison. Animals were housed in male–female breeding pairs in standard laboratory cages (48 × 27 × 16 cm) and provided with Purina mouse chow 5001 and water ad libitum. All offspring were weaned at 30-day and housed in same-condition, same-sex pairs. The colony was maintained on a 14 L : 10 D cycle with lights on at 2200 h. All behavioural testing was conducted at 1200 h under dim red light, with observations beginning 30 min to 2 h after lights out. No siblings were tested at any point during the study. For a timeline of the overall experimental design, please refer to figure 1.
Figure 1.
Breeding and behavioural testing timeline.
(b). Surgeries
Castration or sham surgeries were performed on adult males to generate mice that huddled and groomed their offspring at quantitatively different levels. Castration significantly reduces the mean HG scores to approximately 33 per cent of sham-operated fathers [13]. Breeding pairs were randomly assigned to sham or castration groups (n = 12 pairs per group) and checked daily for the birth of their first litter beginning at 31-day post-pairing. Female California mice experience a postpartum estrus that usually results in pregnancy within the first 1–3 days after a litter is born [14]. Thus, fathers had fully recovered and were continuously present during the postnatal development of the second litter. Second litter offspring were retained into adulthood for paternal behaviour observations with their own pups. Only one male offspring per litter was retained for testing in adulthood.
Male mice underwent bilateral castration or sham surgery as previously described [18,19]. Males recovered in isolation for 4 days and were given 5.0 mg kg−1 of i.p. ketoprofen for analgesia every 12 h for 2-day post-surgery. During recovery, the male's cage was placed next to the cage containing his mate and offspring to allow continuous olfactory and acoustic signals between mates. The male, female and young were reunited after the 4-day recovery period.
(c). Paternal behavioural testing of fathers with offspring
To reiterate, a pair's first litter served to aid in the timing of surgery. All behavioural measures were collected from second litters, beginning with observations of the manipulated fathers with their second litter offspring and continuing until the male offspring had pups of their own. On post-natal day one (PND1), the male, female and second litter were transferred to a Plexiglas observation cage with one small chamber (22 × 29 × 30 cm) and one large chamber (30 × 29 × 30 cm) divided by a Plexiglas insert with two holes to allow free passage between sides. The cage was equipped with food and water, a running wheel, and bedding. On PND3 we conducted a pup-displacement challenge (PDC) to measure paternal behaviour in the temporary absence of the female (as would occur naturally when females forage). The mother and pups were removed from the observation cage 90 s before the start of the trial, and the mother was placed in a standard housing cage. The pups were returned to the observation cage and placed in the location furthest from the nest. At the conclusion of the 10-min trial, the mother was returned to the family. On PND4, 1-h of undisturbed videotape was recorded after which families were returned to standard housing cages.
(d). Pairing procedure for male offspring
Pups were weaned at 30 days and housed in same-sex, same-condition groups of two to four animals. When males were 6- to 12-month old, one male pup from each litter was paired with a female and courtship and paternal behavioural data were collected. Twenty-four hours prior to pairing, fur was shaved from either the flanks or lower back of the animals for visual identification on videotape. Animals were weighed, and each male was placed in a 91 × 46 × 43 cm clear polycarbonate home cage equipped with a water bottle, food, nestlet, aspen bedding and red transparent tube 15 × 4.8 cm in diameter. Males were placed in testing cages 24-h before females to allow the male time to establish a territory [18,20], mimicking the female-biased dispersal pattern of this species [21]. Pairs remained undisturbed, except for weekly cage changes, until the birth of their first litter.
(e). Paternal behaviour testing of male offspring
To assess paternal behaviour in the adult sons of castrated and sham fathers, we used two measures of paternal behaviour; the PDC on PND3 and 1-h of unmanipulated behavioural observations with both parents present on PND4. Behaviours were scored from videotape by a single observer blind to condition. Latencies to approach the young and time spent huddled over and/or grooming the young (HG) were recorded. HG was defined as sitting crouched on or over the young as well as licking and grooming young. Pup retrievals were defined as grasping the pup by the skin of the neck or back and lifting it off the ground. In some cases, the pup was carried to another location in the testing arena but often the pup was placed back in its original location. We scored departures from the pups (leaving pups unattended), rears (number of times the animal stands up on back paws to sniff the air) and exploratory behaviour (time spent sniffing bedding, slowly walking around the cage, rearing). For observations with both parents present, the above behaviours were scored for each parent. To assess pair affiliation we recorded time huddled together in the nest and proximity (time spent together within one longitudinal cage quadrant).
(f). Statistical analysis
Data were analysed using SPSS for Macintosh (v. 16.0.1, SPSS Inc., Chicago, IL, USA). We performed a manipulation check on paternal behaviour during the PDC in surgically manipulated fathers to confirm that the castration indeed reduced HD behaviour in fathers using t-tests for independent samples. Group differences in the behaviour of adult sons were assessed using one-way ANOVA or ANCOVA. Several data points were dropped owing to equipment failure, resulting in a final sample size of 19 (10 castrates and nine sham) for the PDC manipulation check on fathers, and 18 males for PDC observations of sons, representing nine adult male offspring per experimental group. In the 1-h observations, analyses of 11 sons of castrates and nine sons of sham-operated animals are presented. Data are archived in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.46tv4).
3. Results
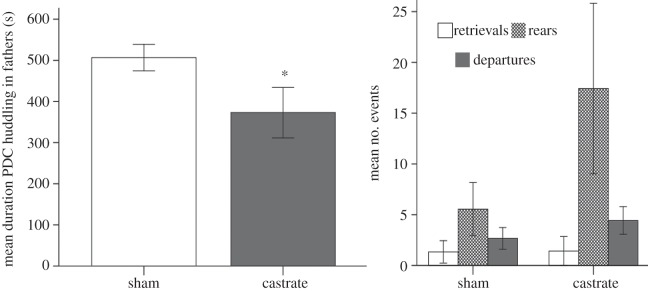
We replicated the reduction in HG behaviour by castration as previously reported [13,14]. Sham-operated fathers (M = 506.55 s, s.e.m. = 32.31) spent significantly more time performing HG behaviour during the PDC than castrated fathers (M = 359.40 s, s.e.m. = 56.58) (t17 = 2.13, p = 0.048, n = 19; figure 2). Groups did not differ in the number of pup retrievals, rears, or departures (all p-values > 0.22). At the beginning of the 10-min PDC trial, sham-operated fathers also approached and began to care for their pups more quickly (M = 17.44 s, s.e.m. = 7.23) than castrated fathers (M = 82.50 s, s.e.m. = 32.76) (t17 = 2.56, p = 0.020).
Figure 2.
Pup-displacement challenge (PDC) behaviours of castrated and sham-operated fathers. Bars represent ± 1 s.e.m. Asterisk (*) represents p < 0.05.
(a). Paternal behaviour of adult sons during the pup-displacement challenge
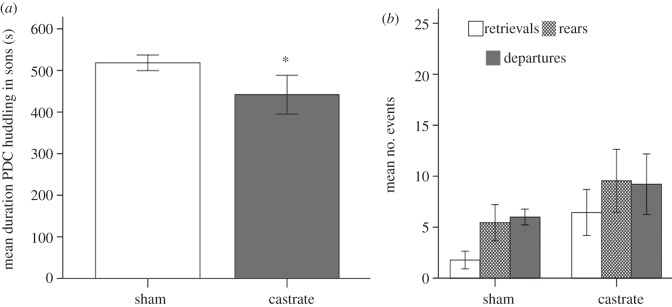
In the adult sons of manipulated fathers, paternal age was correlated with duration of HD (r = 0.51, p = 0.001), number of rears (r = −0.44, p = 0.070) and time spent investigating the cage (r = −0.49, p = 0.039), and for this reason we used paternal age as a covariate in analyses of these behaviours. We used latency to approach pups during the PDC as a covariate for total time huddling over pups, pup retrievals and departures, because these scores are by definition influenced by pup approach latency. During the PDC, sons of castrated fathers spent significantly less time in HG behaviour than sons of sham-operated fathers (see table 1 for raw and adjusted means; F1,14 = 5.21, p = 0.039), and performed more retrievals than sons of sham-operated fathers (F1,15 = 4.94, p = 0.042; figure 3). There were no statistically significant differences between treatment groups in the number of departures (F1,15 = 1.16, p = 0.29) or rears (F1,15 = 2.06, p = 0.17) or time spent investigating the cage (F1,15 = 0.29, p = 0.59).
Table 1.
Behaviour of sons during PDC, raw and adjusted means.
| measure | group | mean | s.e.m. | adjusted mean |
|---|---|---|---|---|
| huddling over pups (s) | castrate | 441.78 | 46.57 | 450.40 |
| sham | 518.37 | 18.69 | 509.80 | |
| number of retrievals | castrate | 6.44 | 2.25 | 6.74 |
| sham | 1.78 | 0.86 | 1.48 | |
| number of departures | castrate | 9.22 | 2.97 | 9.34 |
| sham | 6.00 | 0.78 | 5.89 | |
| number of rears | castrate | 9.56 | 3.08 | 9.82 |
| sham | 5.44 | 1.76 | 5.18 | |
| investigating (s) | castrate | 45.02 | 22.59 | 47.1 |
| sham | 35.82 | 15.95 | 33.7 |
Figure 3.
Pup-displacement challenge (PDC) behaviours of adult sons of castrated and sham-operated males. In (a), mean duration of huddling and grooming (HG), and mean number of PDC events on (b). Bars represent ± 1 s.e.m. Asterisk (*) represents p < 0.05.
(b). Paternal behaviour of adult sons during 1-h observation
In the PND4 1-h observation with both the male and female present, there were no differences in male HG duration, female HG duration, time spent huddling together over the pups or the time mates spent in close proximity (all p-values > 0.25). Sons of sham-operated males spent more time exploring the cage than sons of castrates though the difference was a trend (corrected t9.89 = −2.19, p = 0.054). Retrievals were infrequently observed and thus excluded from analysis.
4. Discussion
Here, we demonstrate that decreased paternal HG during development influences paternal behaviour in the adult male offspring. When the mother is absent from the nest, the sons of castrated fathers perform less HG than the sons of sham-operated fathers and also increase their pup retrieval frequencies. Because we were able to randomly assign males to either low or high HG conditions by surgical manipulation, we can infer that the observed similarity between the paternal behaviour of fathers and sons is not solely attributable to shared genetic material but that the postnatal behavioural environment experienced by offspring also shapes the systems that support paternal behaviour in adulthood.
The transmission of HG behaviour between fathers and sons was evident when we temporarily removed the mother from the cage. Under field conditions, males need to care for pups without assistance to allow the mother to forage [18]. The importance of using the PDC observation is consistent with our previous findings in which testosterone response to courtship predicts paternal behaviour specifically in the PDC [22]. HD behaviour during the PDC could reflect several underlying factors; first, higher levels of HG may simply be better-quality paternal care that is highlighted by the challenge of the paradigm. Second, paternal care during the PDC may reflect the male's response to a stressful situation or to compensate for a mate's absence during foraging. Still, mothers are held in a cage within a few feet of the father and young during the PDC and can communicate using ultrasonic vocalizations [23]. Regardless, continued pup care in the absence of the female is likely advantageous because it conveys continued protection and thermoregulation to the young in the absence of care from the mother.
How might differing levels of HG give rise to long-term changes in offspring behaviour. In rats, the LG-ABN experienced by offspring is positively associated with expression of estrogen receptor alpha (ERα) in the medial preoptic area (MPOA) of the brain, rendering animals with higher receptor levels more sensitive to estrogen [24]. Differences in ERα receptor expression between offspring of low- and high-LG-ABN mothers are attributable to methylation of the ERα promoter region, and are detectable within the first week postpartum [25]. These epigenetic modifications to gene expression are persistent, predicting how a female will behave towards her future offspring. In the California mouse, it is possible that a similar mechanism is involved because testosterone (T) promotes HG behaviour via conversion to oestradiol (E2) [19]. California mouse fathers have significantly more aromatase activity in the MPOA compared with mated non-fathers, indicating that with fatherhood comes a regional increase in conversion of T to E2. Thus, the actions of E2 in the MPOA are also important for parental behaviour [26]. Future studies will need to examine whether epigenetic modifications to ER expression underlie transmission of HG behaviour across generations, as is the case for maternal behaviour.
We did not quantify stress reactivity as a consequence of the paternal environment, but there are several reasons to suspect group differences in HPA axis activity. Adult rats who experienced less LG-ABN as pups show physiological changes consistent with higher anxiety, including higher corticosterone responses to acute stress, decreased glucocorticoid feedback sensitivity, and increased behavioural fearfulness in response to novelty [3,4]. Animals who are more stress reactive also show decreased exploratory behaviour in an open-field maze [4], similar to our finding that the sons of castrated animals show a trend for spending less time exploring than sons of sham-operated fathers. In another study, adult male offspring of castrated California mice had increased plasma progesterone and a trend for increased corticosterone compared with males raised by intact fathers; these corticosterone levels were significantly negatively correlated with the paternal grooming the animal experienced during postnatal development [17]. In the same study, AVP-ir in the paraventricular nucleus of the hypothalamus (PVN) was increased in the sons of castrated fathers. AVP activity in the PVN can be an important trigger for HPA-axis activity [27], pointing to the impact of reduced HG in the development of the stress response. This concept is further supported by the finding that separation from the mother leads to hypomethylation of AVP in the PVN in mice [28].
While castration itself does not alter paternal retrievals or aggression [17], we found that the sons of castrates perform more retrievals than their fathers. The emergence of this change in behaviour indicates that the changes in the behavioural environment during development influences additional behaviours in the offspring and could relate to adrenal activity. In male prairie voles, high plasma corticosterone is associated with increased frequency of pup retrievals [29]. It has been proposed that retrievals reflect a restrictive, or even ‘rough’ or abusive parenting style because fathers retrieve pups in the absence of any threat perceptible to experimenters [30]. Likewise, some models of maternal abuse in rodents show that stressed mothers tend to drag, grab, or roughly handle pups more than non-stressed mothers, suggesting that the behaviour of a stressed parent may resemble that of an abusive one [31]. In the California mouse, retrievals appear to be a behavioural signal that programs male offspring to be more aggressive as adults [17,32]. Interestingly, when male California mouse pups are retrieved by their fathers, they experience a spike in T 45 min later that could be the neuroendocrine signal linking retrieval behaviour with aggression in adulthood [32]. It would be interesting to know if a reduction in HG would ultimately increase male aggression several generations later. The association and dissociation between HD and retrieval behaviours in various studies of California mice appears to be complex. It was previously thought that HD were dissociated [18,16]. By contrast, this study suggests at least a cross-generational interaction between these groups of behaviours.
A critical question is whether these transgenerational effects are due to decreases in overall care or an effect that is specific to decreased care by the father. The amount of HG pups receive is higher in sham-operated pairs even though females paired with castrates increase their HG behaviour in an attempt to compensate for the male [18]. The distinct chemosensory profiles of male and female mice [33] could permit sex-specific effects, beyond the different tactile cues that pups receive during nursing. Similarly, it is possible that changes to the male pheromonal profile as a result of castration impact offspring development independent of the care itself. Future work should investigate whether fathers still influence offspring development when their behavioural role has been rescued by another means, and to what extent olfactory cues from the father impact behavioural development of offspring.
In sum, we have shown that a reduction in paternal care influences the development of offspring paternal behaviour. Our work suggests that in species characterized by paternal care, these behaviours shape the offspring in ways that will determine the developmental climate of subsequent generations. As with maternal behaviour, paternal behaviour may be an important indicator of environmental conditions that by virtue of being transmitted to the next generation serves an important adaptive value. In the present social atmosphere that increasingly encourages human paternal involvement, understanding how mammalian fathers influence their offspring, whether positively or negatively, will continue to be an important direction for the study.
Acknowledgements
Animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the University of Wisconsin-Madison IACUC.
We thank E. Herget H. Loomans K. Schultz M. Fuxjager J. Pultorak, and C. Snowdon. Support was provided by NSF Grant IOS-0620042 and the Wisconsin Alumni Research Foundation and NSF DDIG 1010799.
References
- 1.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. 2006. Fetal programming of the hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J. Physiol. 571, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clutton-Brock TH, Vincent ACJ. 1991. Sexual selection and the potential reproductive rates of males and females. Nature 351, 58–60. 10.1038/351058a0 (doi:10.1038/351058a0) [DOI] [PubMed] [Google Scholar]
- 3.Liu D, et al. 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277, 1659–1662. 10.1126/science.277.5332.1659 (doi:10.1126/science.277.5332.1659) [DOI] [PubMed] [Google Scholar]
- 4.Caldji C, Tannenbaum B, Sharma S, Francis DD, Plotsky PM, Meaney MJ. 1998. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl Acad. Sci. USA 95, 5335–5340. 10.1073/pnas.95.9.5335 (doi:10.1073/pnas.95.9.5335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis DD, Diorio J, Liu D, Meaney MJ. 1999. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286, 1155–1158. 10.1126/science.286.5442.1155 (doi:10.1126/science.286.5442.1155) [DOI] [PubMed] [Google Scholar]
- 6.Champagne FA, Francis DD, Mar A, Meaney MJ. 2003. Naturally-occurring variations in maternal care in the rat as a mediating influence for the effects of environment on the development of individual differences in stress reactivity. Physiol. Behav. 79, 359–371. 10.1016/S0031-9384(03)00149-5 (doi:10.1016/S0031-9384(03)00149-5) [DOI] [PubMed] [Google Scholar]
- 7.Fairbanks LA. 1989. Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Dev. Psychobiol. 22, 669–681. 10.1002/dev.420220703 (doi:10.1002/dev.420220703) [DOI] [PubMed] [Google Scholar]
- 8.Maestripieri D, Lindell SG, Higley JD. 2007. Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Dev. Psychobiol. 49, 165–171. 10.1002/dev.20200 (doi:10.1002/dev.20200) [DOI] [PubMed] [Google Scholar]
- 9.Maestripieri D. 2005. Effects of early experience on female behavioural and reproductive development in rhesus macaques. Proc. R. Soc. B 272, 1243–1248. 10.1098/rspb.2005.3059 (doi:10.1098/rspb.2005.3059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naguib M, Nemitz A, Gil D. 2006. Maternal developmental stress reduces reproductive success of female offspring in zebra finches. Proc. R. Soc. B 273, 1901–1905. 10.1098/rspb.2006.3526 (doi:10.1098/rspb.2006.3526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt J, Simmons LW. 2000. Maternal and paternal effects on offspring phenotype in the dung beetle Onthophagus taurus. Evolution 54, 936–941. [DOI] [PubMed] [Google Scholar]
- 12.Helmeke C, Seidel K, Poeggel G, Bredy TW, Abraham A, Braun K. 2009. Paternal deprivation during infancy results in dendrite- and time-specific changes of dentritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience 163, 790–798. 10.1016/j.neuroscience.2009.07.008 (doi:10.1016/j.neuroscience.2009.07.008) [DOI] [PubMed] [Google Scholar]
- 13.Kolb B, Pellis S, Robinston TE. 2004. Plasticity and function of the orbitofrontal cortex. Brain Cogn. 55, 104–115. 10.1016/S0278-2626(03)00278-1 (doi:10.1016/S0278-2626(03)00278-1) [DOI] [PubMed] [Google Scholar]
- 14.Bredy T, Brown RE, Meaney MJ. 2007. Effect of resource availability on biparental care, and offspring neural and behavioral development in the California mouse (Peromyscus californicus). Eur. J. Neurosci. 25, 567–575. 10.1111/j.1460-9568.2006.05266.x (doi:10.1111/j.1460-9568.2006.05266.x) [DOI] [PubMed] [Google Scholar]
- 15.Bester-Meredith JK, Marler CA. 2001. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus). Horm. Behav. 40, 51–64. 10.1006/hbeh.2001.1666 (doi:10.1006/hbeh.2001.1666) [DOI] [PubMed] [Google Scholar]
- 16.Bester-Meredith JK, Marler CA. 2003. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci. 117, 455–463. 10.1037/0735-7044.117.3.455 (doi:10.1037/0735-7044.117.3.455) [DOI] [PubMed] [Google Scholar]
- 17.Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. 2006. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm. Behav. 50, 699–707. 10.1016/j.yhbeh.2006.06.035 (doi:10.1016/j.yhbeh.2006.06.035) [DOI] [PubMed] [Google Scholar]
- 18.Trainor BC, Marler CA. 2001. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus). Horm. Behav. 40, 32–42. 10.1006/hbeh.2001.1652 (doi:10.1006/hbeh.2001.1652) [DOI] [PubMed] [Google Scholar]
- 19.Trainor BC, Marler CA. 2002. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. R. Soc. Lond. B 269, 823–829. 10.1098/rspb.2001.1954 (doi:10.1098/rspb.2001.1954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bester-Meredith JK, Young LJ, Marler CA. 1999. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm. Behav. 36, 25–38. 10.1006/hbeh.1999.1522 (doi:10.1006/hbeh.1999.1522) [DOI] [PubMed] [Google Scholar]
- 21.Ribble DO. 1991. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprint. Behav. Ecol. Sociobiol. 29, 161–166. 10.1007/BF00166397 (doi:10.1007/BF00166397) [DOI] [Google Scholar]
- 22.Gleason ED, Marler CA. 2010. Testosterone response to courtship predicts future paternal behavior in the California mouse, Peromyscus californicus. Horm. Behav. 57, 147–154. 10.1016/j.yhbeh.2009.10.006 (doi:10.1016/j.yhbeh.2009.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalcounis-Rueppell MC, Petric R, Briggs JR, Carney C, Marshall MM, Willse JT, Rueppell O, Ribble DO, Crossland JP. 2010. Differences in ultrasonic vocalizations between wild and laboratory California mice (Peromyscus californicus). PLoS ONE 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ. 2003. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology 144, 4720–4724. 10.1210/en.2003-0564 (doi:10.1210/en.2003-0564) [DOI] [PubMed] [Google Scholar]
- 25.Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. 2006. Maternal care associated with methylation of the estrogen receptor alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147, 2909–2915. 10.1210/en.2005-1119 (doi:10.1210/en.2005-1119) [DOI] [PubMed] [Google Scholar]
- 26.Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. 2003. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology 78, 36–44. 10.1159/000071704 (doi:10.1159/000071704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Kawasaki M, Ohnishi H, Nakamura T, Ueta Y. 2009. Regulatory mechanism of the arginine vasopressin-enhanced green fluorescent protein fusion gene expression in acute and chronic stress. Peptides 30, 1763–1770. 10.1016/j.peptides.2009.05.025 (doi:10.1016/j.peptides.2009.05.025) [DOI] [PubMed] [Google Scholar]
- 28.Murgatroyd C, et al. 2009. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 12, 1559–1566. 10.1038/nn.2436 (doi:10.1038/nn.2436) [DOI] [PubMed] [Google Scholar]
- 29.Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. 2006. Effects of stress on paternal care are sexually dimorphic in prairie voles. Physiol. Behav. 87, 424–429. 10.1016/j.physbeh.2005.11.002 (doi:10.1016/j.physbeh.2005.11.002) [DOI] [PubMed] [Google Scholar]
- 30.Marler CA, Trainor BC, Davis ES. 2005. Paternal behavior and offspring aggression. Curr. Dir. Psychol. Sci. 14, 163–166. 10.1111/j.0963-7214.2005.00351.x (doi:10.1111/j.0963-7214.2005.00351.x) [DOI] [Google Scholar]
- 31.Roth TL, Lubin FD, Funk AJ, Sweatt JD. 2009. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 65, 760–769. 10.1016/j.biopsych.2008.11.028 (doi:10.1016/j.biopsych.2008.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker EA, Moore BM, Auger CJ, Marler CA. 2010. Paternal behavior increases testosterone levels in offspring of the California mouse. Horm. Behav. 58, 385–389. 10.1016/j.yhbeh.2010.03.019 (doi:10.1016/j.yhbeh.2010.03.019) [DOI] [PubMed] [Google Scholar]
- 33.Brennan PA, Kendrick KM. 2006. Mammalian social odours: attraction and individual recognition. Proc. R. Soc. B 361, 2061–2078. 10.1098/rstb.2006.1931 (doi:10.1098/rstb.2006.1931) [DOI] [PMC free article] [PubMed] [Google Scholar]