Abstract
Fibrosis is characterized by the excessive deposition of extracellular matrix components eventually resulting in organ dysfunction and failure. In dermatology, fibrosis is the hallmark component of many skin diseases, including systemic sclerosis, graft versus host disease, hypertrophic scars, keloids, nephrogenic systemic fibrosis, porphyria cutanea tarda, restrictive dermopathy and other conditions. Fibrotic skin disorders may be debilitating and impair quality of life. There are few FDA-approved anti-fibrotic drugs; thus, research in this area is crucial in addressing this deficiency. Recent investigations elucidating the pathogenesis of skin fibrosis have implicated endogenous reactive oxygen species produced by the multicomponent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) enzyme complex. In this review we discuss Nox enzymes and their role in skin fibrosis. An overview of the Nox enzyme family is presented and their role in the pathogenesis of skin fibrosis is discussed. The mechanisms that Nox enzymes influence specific skin fibrotic disorders are also reviewed. Finally, we describe the therapeutic approaches to ameliorate skin fibrosis by directly targeting Nox enzymes with the use of statins, p47phox subunit modulators, or GKT137831, a competitive inhibitor of Nox enzymes. Nox enzymes can also be targeted indirectly via scavenging ROS with antioxidants. We believe that Nox modulators are worthy of further investigation and have the potential to transform the management of skin fibrosis by dermatologists.
Keywords: skin fibrosis, NADPH oxidase, Nox, reactive oxygen species, ROS, transforming growth factor-beta
Introduction
Fibrosis, a frequent and progressive reaction to chronic injury or inflammation, is characterized by the excessive deposition of extracellular matrix (ECM) components eventually resulting in organ dysfunction and failure [83]. Fibrosis is a leading cause of organ dysfunction and it is hypothesized that approximately 45% of deaths in westernized countries are associated with fibrosis [101,110]. Fibrosis is evident in diseases such as hypertension, diabetes, chronic kidney disease, and idiopathic pulmonary fibrosis (IPF). In dermatology, fibrosis is the hallmark component of many skin diseases, including systemic sclerosis, graft versus host disease (GVHD), hypertrophic scars, keloids, nephrogenic systemic fibrosis, porphyria cutanea tarda, restrictive dermopathy and other conditions. Some of these fibrotic diseases have profound impact on duration and quality of life [16]. For instance, systemic sclerosis affects approximately 100,000 people in the United States with a median survival rate of 11 years [100]. Fibrotic skin disorders may be debilitating and impair quality of life, leading to low self-esteem, social isolation, prejudicial societal reactions, and job discrimination [1,16,21,31,33,32,126]. Subsequently, there is increased incidence of depression and other psychiatric comorbidities in these patients [73]. Despite this significant burden, there are few FDA-approved anti-fibrotic drugs [42]; thus, research in this area is crucial in addressing this deficiency.
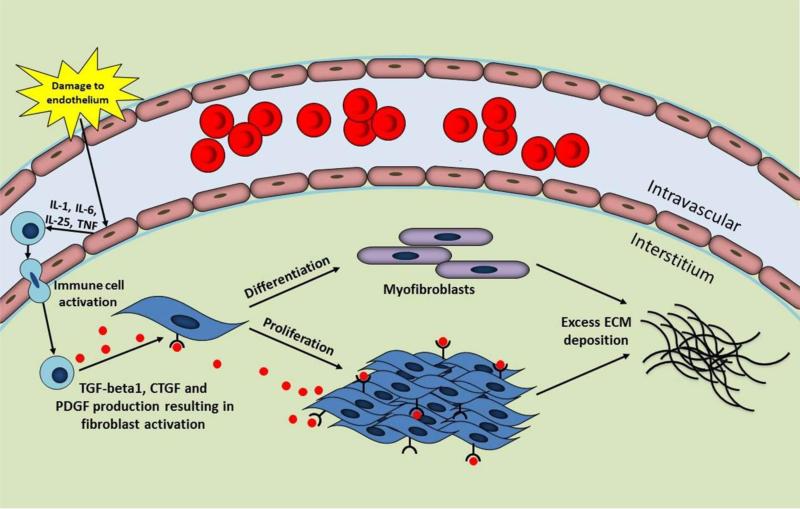
The complex process of fibrosis involves the interaction of various cell types, growth factors, and cytokines. A summary of the mechanisms of fibrosis is presented in Figure 1. Persistent injury, infection and inflammation are known triggers of fibrosis [112]. The trigger leads to disruption of endothelial cells in the vasculature, a feature associated with the onset of fibrotic disease [71]. Damaged endothelial cells secrete cytokines to recruit and activate immune cells such as neutrophils, macrophages, T-lymphocytes and B-lymphocytes [71,111,112]. These immune cells release key fibrotic growth factors, identified as transforming growth factor (TGF)-beta, connective tissue growth factor (CTGF) and platelet-derived growth factor (PDGF) [71]. TGF-beta1 is a widely recognized factor in organ fibrosis and upregulates the expressions of CTGF and PDGF. The actions of TGF-beta1, CTGF, and PDGF result in the activation and proliferation of fibroblasts and differentiation into myofibroblasts, cells with high proliferative capacity responsible for the excessive synthesis and accumulation of ECM that characterizes fibrosis. Furthermore, myofibroblasts express alpha-smooth muscle actin (alpha-SMA) and contract the matrix [71].
Fig. 1.
Overview of fibrosis. Fibrosis is triggered by an insult such as damage to the endothelial cells in the vasculature. These damaged endothelial cells secrete cytokines resulting in the recruitment of immune cells. Immune cells produce transforming growth factor (TGF)-beta1, connective tissue growth factor (CTGF), and platelet-derived growth factor (PDGF) leading to fibroblast activation, proliferation and differentiation into myofibroblasts. The downstream effect is excessive extracellular matrix (ECM) synthesis and deposition by fibroblasts and myofibroblasts, a hallmark feature of fibrosis.
IL, interleukin; TNF, tumor necrosis factor.
Recent investigations have implicated reactive oxygen species (ROS) in the pathogenesis of fibrosis [5,69,17,80]. The term ROS refers to the following compounds: hydrogen peroxide, superoxide anion and hydroxyl radicals [59]. The reaction of these compounds with other biomolecules can generate secondary reactive species: the reaction of hydrogen peroxide with chloride produces hypochlorous acid, and the reaction of superoxide with nitric oxide produces peroxynitrite [59]. ROS is primarily produced by phagocytic cells as a defense mechanism against pathogens. However, in the context of chronic injury, infection, or inflammation, ROS production becomes pathological and may promote key pro-fibrotic processes such as vascular damage, production of the profibrotic growth factors TGF-beta1, CTGF, and PDGF, promotion of fibroblast survival and differentiation into myofibroblasts, and excessive synthesis of ECM proteins.
The multicomponent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) enzyme complexes are the major producers of endogenous ROS [14]. Seven Nox enzymes (Nox1-5 and Duox1-2) have been identified in humans [14]. Apart from promoting fibrosis via ROS production, Nox enzymes are involved in various cellular processes, such as thyroid hormone synthesis, wound healing, release of cytokines, cell proliferation, cell mitosis, cell migration and angiogenesis [34,61,80,83]. Nox enzymes may provide a powerful clinical target in the management of skin fibrosis. Furthermore, characterization of the activity of Nox enzymes may provide a new vantage point for studying molecular mechanisms of skin fibrosis.
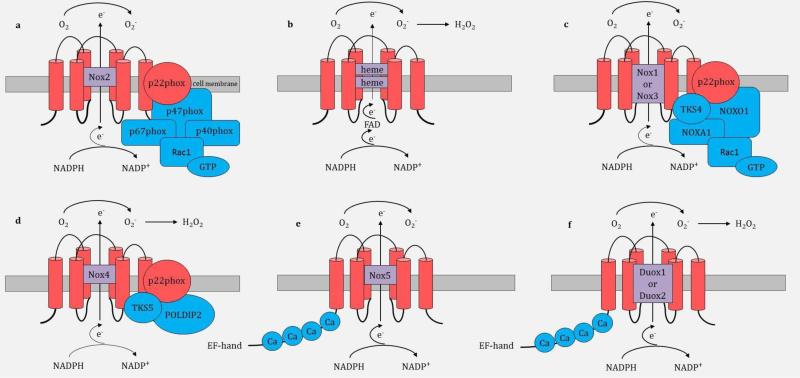
Nox enzymes generally consist of cell membrane and cytosolic components. Structural components of the Nox family are described in Figure 2. Nox2 was the first member of the Nox family to be described and is composed of cell membrane subunits (gp91phox and p22phox) and cytosolic regulatory subunits (p40phox, p67phox, p47phox, and Rac1-GTPase) (Figure 2a) [61]. Nox2, also referred to as gp91phox, is the catalytic subunit and has six amino-terminal transmembrane-spanning segments, conserved histidine residues that bind two hemes, and carboxy-terminal binding sites for flavin adenine dinucleotide (FAD) and NADPH [4,14]. The activation subunit, p67phox, primarily regulates Nox2 activity.
Fig. 2.
Structural components and molecular organization of the Nox family. Part a shows the molecular structure of Nox2. Rac1 is not membrane anchored and it binds to GTP. Part b shows the enzymatic reaction catalyzed by Nox enzymes. Nox1, Nox2 and Nox5 generate the superoxide anion (O2−); Nox4, Duox1 and Duox2 further dismutase O2− to hydrogen peroxide (H2O2) as their final product. Part c shows the molecular structure of Nox1; Nox3 has a similar structure to Nox1. Part d shows the molecular structure of Nox4. Parts e-f show the molecular structures of Nox5, Duox1 and Duox2 - these three enzymes have the similar structures with intracellular calcium (Ca) binding N-terminal cytoplasmic EF-hand domain regions e−, electron; FAD, flavin adenine dinucleotide; O2, molecular oxygen; NOXA1, Nox activator 1; NOXO1, Nox organizer 1; POLDIP2, DNA polymerase-delta-interacting protein; Ca, calcium.
Upon cell stimulation, Nox2 enzyme complex formation and activation are mediated by initial phosphorylation and conformational change of the p47phox subunit [14]. The p47phox subunit organizes the migration of p40phox and p67phox to the cell membrane and docks through the SRC homology domains to the proline-rich region of the p22phox subunit. Rac1 is recruited independently to the cell membrane and the Nox enzymatic complex is formed [14]. The complex mediates the transmembrane electron transfer from cytosolic NADPH to FAD, that in turn transfers electrons sequentially to the 2 hemes in the catalytic subunit and ultimately to molecular oxygen (O2) on the opposite side of the membrane to generate the superoxide anion (O2−) (Figure 2b) [35]; Nox1 and Nox5 also produce the superoxide anion [3]. Conversely, Nox4, Duox1 and Duox2 generate hydrogen peroxide (H2O2) from the dismutation of O2−; either spontaneously at an acidic pH or catalytically by superoxide dismutase (SOD)[4,14].
Similarly to Nox2, activation of Nox1 and Nox3 requires association with p22phox, activator subunit NOXA1 (homologous to p67phox), organizer subunit NOXO1 (homologous to p47phox) and Rac1-GTPase (Figure 2c) [35,61]. NOXA1 regulates the activities of Nox1 and Nox3. Nox1 and Nox3 also each have their own unique six transmembrane spanning alpha helix catalytic subunit, similar to gp91phox in Nox2 enzyme [4,35]. In addition, Nox1 and Nox3 interact with tyrosine kinase substrate 4 (TKS4) [14]. Nox4 (Figure 2d) is distinct from Nox1, 2 and 3 as it only requires the six transmembrane catalytic subunit and p22phox but appears to be constitutively active without the need for activation of other cofactors [61]. In some instances, Nox4 assembles with proteins that enhance its activity, identified as tyrosine kinase substrate 5 (TKS5) and DNA-polymerase-delta-interacting protein 2 (POLDIP2). Nox5, Duox1, and Duox2 (Figure 2e and 2f) differ from other Nox isoforms and contain just the catalytic subunit and additional intracellular Ca2+ binding N-terminal cytoplasmic EF-hand domain regions, and are primarily regulated by Ca2+ signaling, without the need for assembly with p22phox or cytosolic cofactors [61].
In this review we discuss Nox enzymes and their role in skin fibrosis. To the best of our knowledge, there are no published reviews that focus on the role of Nox enzymes in skin fibrosis. We discuss the mechanisms via Nox enzymes promote skin fibrosis highlighting specific fibrotic skin disorders. Finally, we describe the therapeutic approaches to ameliorate skin fibrosis by directly targeting Nox enzymes with the use of statins, p47phox subunit modulators, or GKT137831, a competitive inhibitor of Nox enzymes. Nox enzymes can also be targeted indirectly via scavenging ROS with antioxidants.
Methods
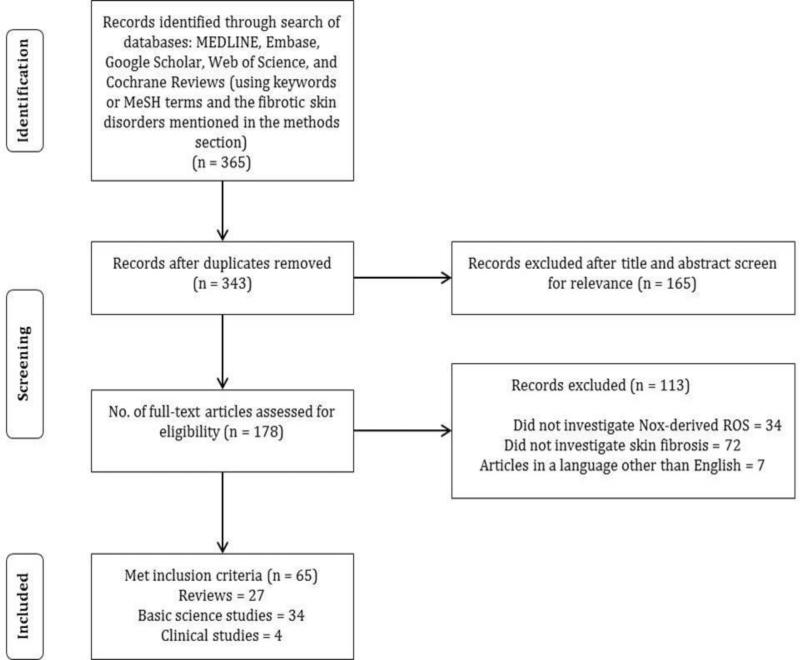
A review of published literature from January 1, 1980 to present on the role of Nox in skin fibrosis was performed in February, 2013 (Figure 3). A search of the Medline database using PubMed, EMBASE, Web of Science, Google Scholar and Cochrane databases was conducted utilizing specific keywords or MeSH terms. “Skin fibrosis” was combined with: “NADPH oxidase,” “Nox,” “Nox1,” “Nox2,” “Nox3,” “Nox4,” “Nox5,” “ROS,” and “oxidative stress.” Additional search terms on therapeutics include: “Nox inhibitors skin,” “NADPH oxidase inhibitors skin,” and “antioxidants skin fibrosis.”
Fig. 3.
Flowchart illustrating the literature search strategy and results.
Fibrotic skin disorders were identified by reviewing the textbook Dermatology [18]. The following fibrotic skin conditions were identified: acral fibrokeratoma, amyloidosis, atypical fibroxanthoma, bleomycin-induced skin fibrosis, cutaneous angiofibroma, dermatofibroma, dermatofibroma protuberans, eosinophilia-myalgia syndrome, eosinophilic fasciitis, epithelioid cell histiocytoma, epithelioid sarcoma, fibroblastic rheumatism, fibroma of the tendon sheath, fibrosarcoma, fibrous hamartoma, graft versus host disease, hypertrophic scars, infantile digital fibroma, infantile myofibromatosis, keloids, lipodermatosclerosis, mixed connective tissue disease, multinucleate cell angiohistiocytoma, nephrogenic systemic fibrosis, nodular fasciitis, porphyria cutanea tarda, restrictive dermopathy, scleredema, scleredema diabeticorum, scleroderma, scleromyxedema, sclerotic fibroma of the skin, stiff skin syndrome, superficial fascial fibromatosis, taxane-induced skin fibrosis, toxic oil syndrome, and Winchester syndrome. Each of these disorders was combined with “NADPH oxidase,” “Nox1,” “Nox2,” “Nox3,” “Nox4” and “Nox5” and researched in all the databases mentioned above.
The relevant articles that met the following criteria were selected for inclusion: reviews, guidelines, and research support studies of Nox and oxidative stress in skin fibrosis. Papers published in a language other than English were excluded. Additional articles were identified from a review of the bibliography of articles meeting the search criteria.
Results
Our search resulted in 312 articles from the Medline, EMBASE, and Cochrane databases. Google Scholar and Web of Science yielded additional 53 articles for a total of 365 articles (Figure 3). After duplicates were removed, a total of 343 articles were considered and screened. 165 articles were excluded after title and abstract screen. Of the remaining 178 articles, 113 were excluded: 34 articles did not investigate Nox-derived ROS, 72 articles did not investigate skin fibrosis and 7 articles in a language other than English. This resulted in 65 articles included in this review: 27 review articles, 34 basic science studies, and 4 clinical studies.
The Role of Nox in Skin Fibrosis
Nox-derived ROS are involved at various levels of skin fibrosis and interfere with redox-sensitive intracellular signaling pathways, including inhibition of protein tyrosine phosphates, activation of certain transcription factors, and modulation of enzymes[4]. Cysteine residues of proteins are particular targets of ROS [85]. Nox1, Nox2, and Nox4 have been primarily linked to skin fibrosis and interact with profibrotic cytokines: Nox1 and Nox2 are induced by PDGF in fibrotic skin disorders and Nox4 is induced by TGF-beta to mediate fibrotic effects [83,87]. Nox enzymes regulate ECM synthesis, ECM degradation and the survival of fibroblasts.
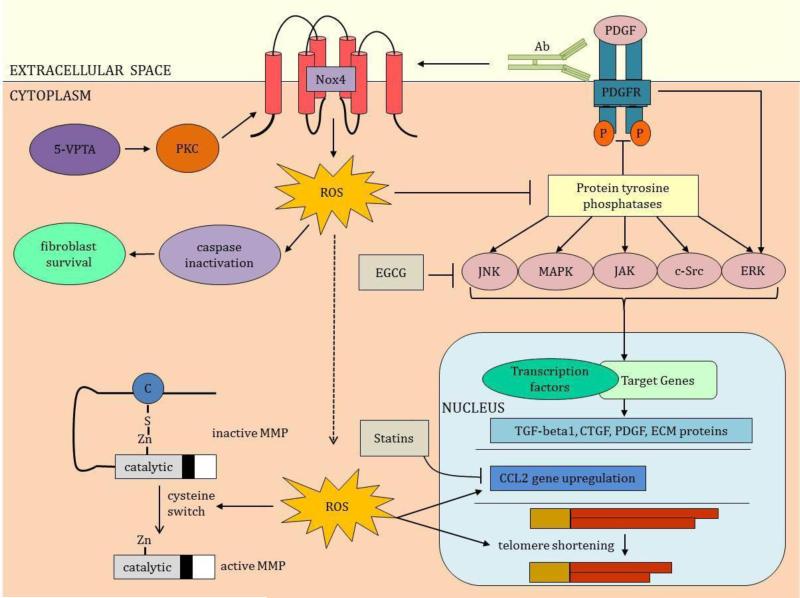
Nox enzymes play a key role in excessive ECM protein synthesis via indirect activation of key protein tyrosine kinases [85]. ROS reversibly inactivates cysteine-dependent serine/threonine protein tyrosine phosphatases resulting in increased activity of various kinases (Figure 4) [85]. These kinases include c-jun amino-terminal kinase (JNK), mitogen-activated protein kinases (MAPK), Janus kinase (JAK), c-Src, and extracellular signal-regulated kinases (ERK). As a result, signaling cascade phosphorylation is promoted, transcription factors are induced, and the downstream effect is increased expression of fibrotic genes encoding TGF-beta1, CTGF, and PDGF [83,85]. Phenotypic modifications expressed as myofibroblast induction and subsequent excessive ECM deposition are associated with skin fibrosis [83].
Fig. 4.
Mechanistic Involvement of Nox in skin fibrosis. Nox enzymes influence several pathways that result in skin fibrosis. In toxic oil syndrome, 5-VPTA stimulates PKC to activate Nox. Nox enzymes produce ROS that inactivate the proapoptic caspases promoting survival of fibroblasts, the key cell involved in matrix deposition. ROS also inactivates protein tyrosine phosphatases to promote the activities of the tyrosine kinases, JNK, MAPK, JAK, c-Src, and ERK (EGCG modulates the tyrosine kinases). Inhibition of the protein tyrosine phosphatase-1B by ROS increases the activity of the tyrosine kinase, PDGFR. PDGFR's interaction with PDGF further upregulates ERK. Autoantibodies (Ab), identified in scleroderma, stimulate the PDGFR and its downstream pathway, ERK. PDGFR induces Nox1 and Nox2 and autoantibodies enhance this induction. All these tyrosine kinases enhance the transcription of genes involved in skin fibrosis, including TGF-beta, CTGF, PDGF and ECM proteins. ROS promotes the activation of MMPs in the process termed cysteine switch. ROS also upregulates expression of the chemokine ligand-2 (CCL-2) implicated in scleroderma and GVHD; statins antagonize this upregulation. ROS promotes telomere shortening, a process implicated in keloids and restrictive dermopathy. 5-VPTA, N-(5-vinyl-1,3-thiazolidin-2-ylidene) phenylamine; PKC, protein kinase C; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; EGCG, epigallocatechin-3-gallate; Zn, zinc; C, cysteine.
Nox enzymes may interfere with ECM degradation (Figure 4). ECM degradation occurs predominantly from the actions of matrix metalloproteinases (MMPs) [51,78]. The majority of MMPs is secreted as pro-enzymes and become activated upon proteolysis of their cysteine-zinc active site in a process termed “cysteine-switch [34].” ROS modify the cysteine-switch with subsequent MMP activation [34].
Nox enzymes also promote survival of fibroblasts by transiently inhibiting caspases (Figure 4). Caspase proteins are involved in the intrinsic apoptotic cell death pathway. ROS may inhibit caspases transiently through reversible inactivation of their cysteine active-site [34]. If this process occurs in fibroblasts, they become resistant to apoptosis resulting in persistent matrix deposition, a key feature of skin fibrosis [34].
Nox enzymes play a central role in several fibrotic skin disorders. Our understanding of their role in skin disorders continues to expand as scientific findings demonstrate their involvement in skin fibrosis. Specific mechanisms of Nox involvement in various fibrotic skin disorders are discussed in the remainder of the section and are summarized in Table 1.
| DISEASE | FEATURES | NOX INVOLVEMENT |
|---|---|---|
| Bleomycin-induced skin fibrosis | Fibrotic disorder developing from exposure to the chemotherapeutic agent, bleomycin; pulmonary fibrosis can also occur | Nox promotes type I collagen, alpha-smooth muscle actin, TGF-beta and CTGF expression and fibroblast differentiation [64,119,120] |
| Chronic graft versus host disease | Multisystemic disorder arising as a complication of allogeneic hematopoietic stem cell transplantation | PDGFR autoantibodies stimulate ROS in fibroblasts and promote type I collagen expression [93] |
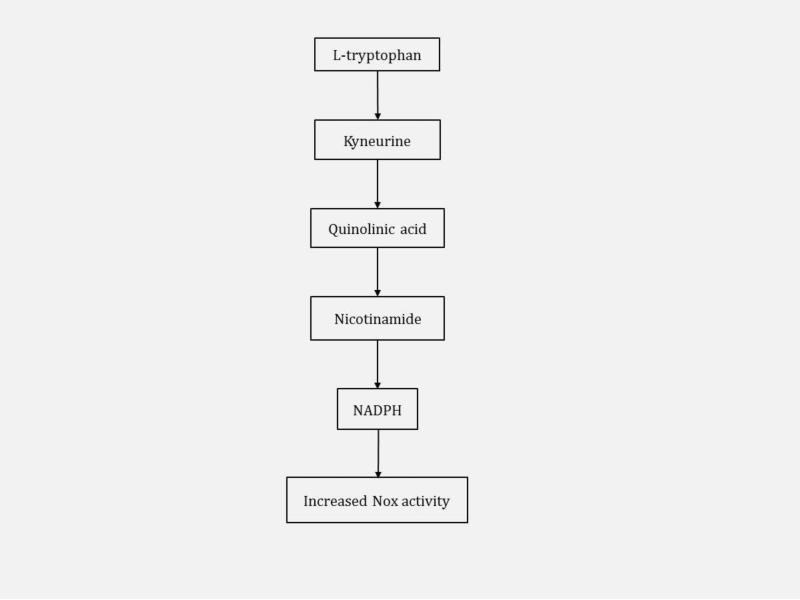
| Eosinophilia-myalgia syndrome | Fibrosing skin disorder with associated eosinophilic infiltration | L-tryptophan ingestion increases NADPH concentration and Nox activity [74] |
| Hypertrophic scars | Fibrotic skin disorder resulting from injuries to the deep dermis | Nox upregulates pyridinoline cross-link formation in collagen [104,105] |
| Keloids | Fibroproliferative disorder resulting from abnormal wound healing | Nox stimulates telomere shortening [27] |
| Nephrogenic systemic fibrosis | Systemic disorder with associated skin fibrosis associated with exposure to MRI contrast agents | Contrast exposure enhances Nox4 activity [103] |
| Porphyria Cutanea Tarda | Manifested by skin fragility, erosions, bullae, milia and scars on sun exposed skin | Sun exposure enhances ROS production by Nox [17] |
| Restrictive dermopathy | Genetic disorder linked to mutations in the lamin A gene | Nox4 stimulates telomere shortening [88] |
| Scleroderma | Complex connective tissue disorder involving multiple organ systems, including the skin | Nox promotes fibroblast proliferation and differentiation, collagen-gene expression, TGF-beta1 upregulation, alpha-smooth muscle actin upregulation, chemokine ligand-2 upregulation, and PDGFR upregulation [29,48,68,96,115,116,120] |
| Taxane-induced skin fibrosis | Fibrotic disorder developing from exposure to the chemotherapeutic agents, paclitaxel or docetaxel | Taxanes stimulate translocation of Rac1 to the cell membrane and enhance Nox activation [3,22] |
| Toxic oil syndrome | Systemic disorder with associated skin fibrosis extending into the subcutaneous fat; develops after ingestion of adulterated rapeseed oil | Protein kinase C phosphorylates and activates Nox [39] |
Systemic sclerosis (scleroderma)
Nox modulation is a potential target for the treatment of scleroderma. Scleroderma is a complex connective tissue disorder characterized by the excessive deposition of ECM proteins in the skin and internal organs [19]. The pathogenesis of this skin disorder is not fully elucidated but inflammation, autoantibodies, and vasculopathy have been implicated [34,47,122]. TGF-beta and PDGF, two key factors upregulated in scleroderma, are interfered by Nox enzymes to promote ECM deposition (Figure 4) [120].
Oxidative stress, a state of excessive ROS production, may contribute to the pathogenesis of scleroderma [29,34,117,118]. The source of oxidative stress in scleroderma is attributed to ROS production by fibroblasts and activated leukocytes via Nox enzymes [29]. One study found that ROS are important for skin fibroblast proliferation and the expression of type I collagen in scleroderma [84]. Animal models suggest that ROS induce EC damage, a process linked to the onset of systemic sclerosis, and upregulate TGF-beta1, collagen, and the myofibroblast marker, alpha-SMA [10,48,114,120].
Nox enzymes are also involved in the recruitment of inflammatory cells in scleroderma via induction of chemokine ligand (CCL)-2, a chemoattractant for monocytes and T lymphocytes [116]. Furthermore, in vitro studies reveal that CCL-2 upregulates the expression of type I collagen messenger RNA (mRNA) and enhances the expression of MMP-1, MMP-2, and tissue inhibitor of metalloproteinase (TIMP)-1 in cultured skin fibroblasts [116]. CCL-2 has been associated with other human fibrotic conditions and considering the multiple roles of CCL-2 in skin fibrosis, it presents an attractive therapeutic target in skin fibrosis.
The profibrotic PDGF and its receptor, PDGFR, are other substrates of Nox enzymes [68,96]. PDGF's binding to PDGFR triggers phosphorylation and activation of the receptor. Excessive phosphorylation is mitigated by the phosphatase, protein tyrosine phosphatase (PTP)-1B [96]. However, Nox-derived ROS inactivates PTP1B, leading to unregulated activation of PDGFR, induction of the ERK1/2 pathway, and downstream elevation of type I collagen [96]. Therefore restoring PTP-1B activity via Nox inhibition may be useful in sustaining homeostasis in the PDGF pathway associated with skin fibrosis.
These observations highlight the importance of Nox enzymes in systemic sclerosis and may potentially lead to the development of Nox-based anti-fibrotic therapies. This field of study is crucial as there are currently limited agents to treat the cutaneous fibrosis associated with scleroderma [42]. Furthermore, by investigating the role of Nox enzymes in systemic sclerosis, new clues on the pathogenesis of the disease may be elucidated.
Chronic graft versus host disease
Nox-derived ROS contribute to skin fibrosis in graft versus host disease (GVHD), a similar disease to systemic sclerosis [50,127]. GVHD arises as a complication of allogeneic hematopoietic stem cell transplantation (HSCT) and occurs in a significant percentage (20% to 70%) of subjects surviving more than 100 days [93,114]. GVHD represents the major cause of late death in this group of patients and is often associated with severe impairment of quality of life in survivors [93]. Cutaneous scleroderma-like changes represent a devastating morbidity for patients [55]. Many of the other manifestations of GVHD share clinical characteristics with scleroderma including fibrosis of visceral organs [50]; and much like systemic sclerosis, there are limited treatment options for GVHD.
It is recognized that Nox enzymes play a role in GVHD. The sources of Nox-derived ROS in the disease have been linked to activated CD4+ T lymphocytes and plasmacytoid dendritic cells [50]. Like scleroderma, ROS upregulate the expression of CCL-2 in the dermal fibroblasts of GVHD patients (Figure 4). It is suggested that autoantibodies exist in GVHD and stimulate ROS production [93]. These autoantibodies (Figure 4) recognize PDGFR and induce tyrosine phosphorylation of the receptor [93]. Furthermore, the autoantibodies induce ROS in fibroblasts and stimulate the expression of type I collagen gene through the Ha-Ras-ERK1/2-ROS signaling pathway [93]. The biologic activity of these autoantibodies and their interaction with ROS suggests a role in the development of skin fibrosis [93]. The presence of these receptor autoantibodies in scleroderma is not fully established as one study demonstrated the existence of these autoantibodies in the disease, however, another study failed to replicate this finding [7,24]. In addition, a recent study on the presence of PDGFR antibodies in patients with chronic GVHD failed to show an association between autoantibody levels and disease severity [90].
Nox modulators may potentially aid the management of GVHD and improve patient survival. Further investigations on therapies directed toward CCL-2 and autoantibodies may lead to therapeutic breakthrough. It may be worthwhile to elucidate the involvement of Nox enzymes in GVHD immune response, particularly B cell activation and production of antibodies. Furthermore, clarifying the role and mechanisms of autoantibodies in systemic sclerosis and GVHD may be useful in establishing differences between the two diseases and may reveal how these differences can be harnessed therapeutically in the management of the diseases.
Hypertrophic scars
Hypertrophic scars are common, ensuing from injuries involving the deep dermis [53]. As a general feature of fibrosis, there is excessive accumulation of collagen in hypertrophic scars. One of the final steps in the synthesis of collagen is cross-linking which can occur via one of two routes: allysine or hydroxyallysine [99]. Cross-linking in the skin normally occurs via the allysine route [99]. However, in skin fibrosis, there is a switch to the hydroxyallysine route resulting in the formation of pyridinoline cross-links [99]. These pyridinoline cross-links are resistant to degradation leading to the persistence and accumulation of collagen that characterizes fibrosis [99]. Hence, pyridinoline cross-link formation is often viewed as a marker of fibrosis irreversibility and is associated with various skin fibrotic diseases (hypertrophic scars, keloids, systemic sclerosis, and lipodermatosclerosis), [6,20,43,82,72,98] as well as pulmonary, hepatic, and renal fibrotic diseases [60,81,99]. Given their widespread nature, pyridinoline cross-links are believed to be a general fibrotic phenomenon [82,99].
There is evidence that Nox enzymes are responsible for the formation of pyridinoline cross-links in hypertrophic scars as pyridinoline cross-links were formed in normal human skin tissue ex vivo (from post mortem or plastic surgery waste specimens) after exposure to Nox-derived ROS [104]. The involvement of ROS is further supported by the finding that catalase, an antioxidant, was effective at scavenging hydroxyl radicals in dermal fibroblasts and significantly reduced pyridinoline cross-link formation [105]. It is likely that Nox enzymes also perpetuate the formation of pyridinoline cross-links in other fibrotic disease states. Considering that pyridinoline cross-links impact various fibrotic diseases, investigating how inhibition of Nox-induced ROS may prevent the formation of these cross-links may transform the management of skin fibrosis and fibrosis in general.
Keloids
Keloid scars are another fibrotic disorder where Nox modulation may be therapeutic. Keloid scars are skin fibroproliferative disorders resulting from abnormal wound healing response to an injury [49]. The etiology of keloids is not fully understood and keloids can be challenging to manage [49]. Evidence suggests ROS may be involved in the pathogenesis of keloids as studies reveal a significant increase in ROS generation in keloid fibroblasts [27]. These ROS are hypothesized to contribute to the inflammatory status of the disease [27].
To better understand the etiology of keloids, one study investigated the effect of ROS on telomeres in keloids [27]. It was discovered that the activity of telomerase, an enzyme that normally counteracts telomere shortening, was absent in keloid tissues and was linked to increased ROS generation. The role of telomere shortening in skin fibrosis has not been established, but in the lungs, telomere shortening is hypothesized to promote fibrosis by causing the death of stem cells and alveolar type 2 cells [62,79,112]. It is possible that telomere shortening also promotes injury that initiates the process of fibrosis in keloids. Further investigations are needed to fully delineate how Nox-derived ROS may be involved in keloids and how ROS-induced telomerase impairment may be targeted to effectively manage this treatment-resistant disease.
Nephrogenic systemic fibrosis
Nephrogenic systemic fibrosis (NSF) is a multisystemic disorder with associated skin fibrosis. NSF develops after exposure to the magnetic resonance imaging contrast agents in the setting of impaired glomerular filtration rate. This disease has been linked to Nox activity. In a study using a rodent model of NSF, there was increased superoxide anion production in the skin and this was correlated with an increase in Nox4 [103]. Nox4 is believed to be dominant isoform in NSF as the activities of Nox1 and Nox2 were negligible [103]. Furthermore, preincubation with the antioxidant, SOD, abrogated the increased ROS production [103]. Although the study was conducted on animals, it further supports the involvement of Nox in skin fibrosis and suggests that Nox inhibitors and antioxidants may be important treatments in the management of fibrotic skin diseases.
Porphyria cutanea tarda
Porphyria cutanea tarda (PCT) is a metabolic disorder of heme biosynthesis caused by decreased activity of uroporphyrinogen decarboxylase and concomitant increase in circulating porphyrins [13]. PCT is manifested by skin fragility, erosions, bullae, milia and scars on sun exposed skin [13]. Nox enzymes may be involved in the pathogenesis of PCT as it was discovered that the ROS, O2− and H2O2, are produced in PCT [17]. The ROS mediate the skin fibrosis in PCT and various antioxidants (vitamins C and E, lycopene, and beta-carotene) successfully inhibited porphyrin-induced cellular phototoxicity [17,77]. More research is needed to fully elucidate the involvement of Nox enzymes in the pathogenesis of PCT. It was noted that 2-3% of PCT patients exhibit scleroderma-like lesions [13].
Restrictive dermopathy
Restrictive dermopathy (RD) is a rare lethal genetic disorder that is characterized by congenital tautness of the skin with associated fibrosis of the dermis [107,123]. This skin disorder has been linked to mutations in the lamin A gene and recent data suggest a relationship between lamin mutations and ROS accumulation via Nox4 [88]. Elevated ROS levels in RD are linked to fibroblast telomere shortening (Figure 4) however the impact of this finding on the cutaneous fibrotic feature of the disease has not been demonstrated [88].
Taxane-induced skin fibrosis
Taxane is a chemotherapeutic agent and has been linked to skin fibrosis, especially with prolonged exposure at higher doses [44,65]. This may be explained by the fact that taxane induces and enhances Nox activation by stimulating the translocation of Rac1 to the cell membrane [3,22]. It is hypothesized that the disturbance of microtubule polymerization by taxane affects the migration of Rac1 to the cell membrane and enhances its stability in the active Nox complex [3]. Interestingly, low dose paclitaxel has been found to lessen skin fibrosis in mice by suppressing Smad2 and Smad3 phosphorylation and collagen deposition [65]. We hypothesize that this anti-fibrotic phenomenon occurs secondary to the stimulation of endogenous antioxidants in response to the oxidative stress created by the low dose exposure to taxanes.
Bleomycin-induced skin fibrosis
Bleomycin is another chemotherapeutic agent known to trigger skin fibrosis. Bleomycin is commonly used to induce skin fibrosis in animal models of fibrosis [116]. Bleomycin stimulates Nox-derived ROS generation which upregulates type I collagen, alpha-SMA, myofibroblast differentiation and TGF-beta1 resulting in the induction of scleroderma-like skin fibrosis [34,67,116,119,120]. Investigations suggest CTGF is required for bleomycin-induced skin fibrosis in mice [64]. Therefore, blocking CTGF directly, or inhibiting ROS upregulation of CTGF, may prove beneficial in treating bleomycin-induced skin fibrosis.
Toxic oil syndrome & eosinophilia-myalgia syndrome
Toxic oil syndrome (TOS) was associated with the consumption of adulterated rapeseed oil distributed in Spain in the early 1980s [40,63]. Many years after exposure, patients developed cutaneous manifestations characterized by indurated plaques of the pretibial areas, and occasionally, the forearms and abdomen, with marked fibrosis extending into the subcutaneous fat [63]. N-(5-vinyl-1,3-thiazolidin-2-ylidene)phenylamine (5-VPTA) has been suggested as one of the etiologic agents of TOS [27]. 5-VPTA stimulates protein kinase C activity that phosphorylates and activates Nox (Figure 4) [39].
Potential pathogenic links between TOS and eosinophilia-myalgia syndrome (EMS) have been suggested. EMS, like TOS, is a fibrosing skin disorder with associated eosinophilic infiltration [63]. EMS has been associated with the ingestion of large amounts of L-tryptophan [63]. Increased consumption of L-tryptophan results in increased levels of its metabolite, NADPH, and may increase the activity of Nox enzyme approximately 1.5 fold [74]. The involvement of NADPH and Nox activity in the metabolism of L-tryptophan is featured in Figure 5. While these two diseases are less common, the role of Nox enzymes in the pathogenesis highlights the importance of Nox enzymes in skin fibrosis.
Fig. 5.
Metabolism of L-tryptophan to NADPH in eosinophilia-myalgia syndrome (EMS). Increased ingestion of L-tryptophan in EMS results in increased NADPH concentration with an associated increase in the activity of Nox enzymes.
The Role of Nox in the Fibrosis of Other Organ Systems
Nox enzymes play a role in the fibrotic processes of several other organ systems. Similarly to the skin, Nox1, Nox2, and Nox4 are the major contributors to fibrosis. Studies found that mice with deletions of p47phox were protected against bleomycin-induced lung and liver fibrosis suggesting Nox mediated fibrotic injury in the tissues [54]. It was also shown that ROS stimulate myofibroblasts differentiation and death of epithelial cells in the lungs [59]. Furthermore, fibroblasts isolated from the lungs of patients with IPF generated increased H2O2 in response to TGF-beta1 and induced death of the respiratory epithelial cells [59,102]. ROS are the predominant insult to the kidney contributing to glomerulosclerosis and tubulointerstitial fibrosis [36,54]. There is also evidence of Nox involvement in heart: TGF-beta1 stimulates Nox4 generation of ROS leading to increased expression of ECM proteins (collagen and fibronectin), CTGF and differentiation of fibroblasts into myofibroblasts [26].
There is significant crosstalk between the renin-angiotensin-aldosterone system (RAAS), Nox and TGF-beta1 in organ system fibrosis mediated by the activities of Nox1, Nox2 and Nox4 [2,14,37,91,94,97,106,108]. Angiotensin II of the RAAS is believed to elicit fibrotic effects by inducing Nox activity, stimulating TGF-beta1 production and promoting the proliferation of fibroblasts and differentiation into myofibroblasts [37]. In the liver, angiotensin II induces hepatic fibrosis via TGF-beta1 stimulation, Nox activation, and collagen upregulation in hepatic stellate cells, the primary cells involved in hepatic fibrosis [8,9]. There is also crosstalk between angiotensin II and TGF-beta1 and Nox enzymes in cardiac and renal fibrosis [37,91,94,106,108]. Therapies targeting the RAAS system are currently being evaluated. Nox modulators represent potential therapeutic modalities to treat fibrosis in other organ systems.
Nox-Associated Therapeutic Agents in Skin Fibrosis
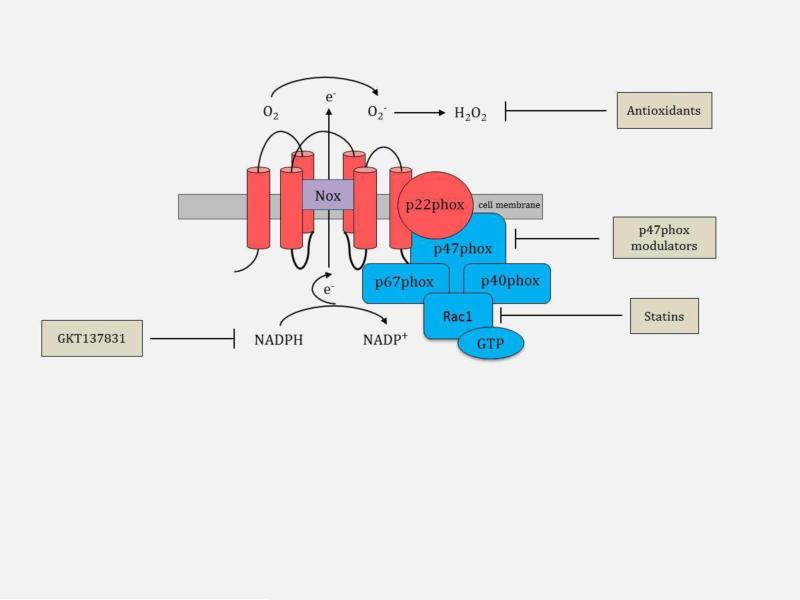
The therapeutic potential of Nox enzymes’ inhibition to modulate skin fibrosis has gained the attention of the pharmaceutical industry. The complex activation mechanism provides several opportunities to target these enzymes (Figure 6). Inhibiting the migration of it cytosolic subunits to the cell membrane can attenuate the activation of Nox enzymes. We hypothesize that an alternate strategy may encompass preventing the interaction of p47phox with other subunits. Another possibility is the competitive inhibition of Nox enzymes with molecules mimicking NADPH. Several current pharmaceuticals (statins), small molecule inhibitors, peptides, and antioxidants have been identified and are being investigated for their potential use in organ fibrosis (Table 2).
Fig. 6.
Nox-associated therapeutic agents in skin fibrosis. Therapeutic agents can be directed at modulating the p47phox subunit, inhibiting the migration of Rac1 to the cell membrane (statins), competitively inhibiting Nox with molecules mimicking NADPH (GKT137831), or scavenging reactive oxygen species, O2− and H2O2 (antioxidants).
Table 2.
Nox Inhibitors and Antioxidants in Skin Fibrosis.
| AGENT | ACTION | TARGETS | COMMENTS |
|---|---|---|---|
| Nox Inhibitors | |||
| Statins | Prevents Rac1 migration to the cell membrane | Nox1 and 2, CCL-2, collagen, skin inflammation, dermal thickening | Non-specific Nox inhibitor |
| p47phox modulators | |||
| AEBSF | Small molecule Nox inhibitor - inhibits p47phox subunit | General Nox enzyme inhibitor | Non-specific, general serine protease inhibitor |
| Apocynin | Small molecule Nox inhibitor - inhibits p47phox subunit | General Nox enzyme inhibitor | Non-specific inhibitor, antioxidative effects |
| Diphenyl iodonium (DPI) | Small molecule Nox inhibitor - inhibits p47phox subunit | General Nox enzyme inhibitor | Non-specific, general flavoprotein inhibitor, toxic |
| Gp91ds-tat | Peptide Nox inhibitor - binds p47phox and prevents interaction with other subunits | Nox1 and Nox2 | May be limited to parenteral administration given its peptide origin |
| PR-39 | Peptide Nox inhibitor - binds SH3 domain of p47phox limiting Nox activity | Nox1 and Nox2 | Non-specific - may interact with any protein with SH3 domain, may be limited to parenteral administration given its peptide origin |
| S17834 | Nox inhibitor - prevents the binding of p47phox to the membrane complex | Nox1 and Nox2 | Further investigations needed |
| Competitive inhibitors of Nox | |||
| GKT137831 | Small molecule Nox inhibitor - likely competitive substrate inhibitor since it structurally mimics NADPH | Nox1 and Nox4 selective | Currently under clinical investigation in phase I clinical trial |
| Nox inhibitors with unspecified mechanism | |||
| Fulvene-5 | Small molecule Nox inhibitor | Nox2 and Nox4 | Specificity and activity towards other Nox isoforms is unknown |
| M090 | Small molecule Nox inhibitor | Nox1-selective | |
| ML171 | Small molecule Nox inhibitor | Nox1-selective | Potential antipsychotic effects due to phenothiazine structure |
| VAS2870 | Small molecule Nox inhibitor - likely inhibits assembly of Nox once translocation to the membrane has occurred. | General Nox enzyme inhibitor | Investigations needed for off-target effects and toxicity |
| Antioxidants | |||
| Naturallv-ocurring antioxidants | |||
| Beta-carotene | Antioxidant | Reactive oxvgen species | Naturally present in many fruits, grains, oils, and vegetables |
| Caffeine | Antioxidant | Reactive oxvgen species | Caffeine in combination with EGCG reduced ROS in human skin fibroblasts |
| EGCG | Antioxidant - free radical scavenger, attenuates Nox expression | Signaling pathwavs, PDGF, TGF-beta, type I collagen, alpha- SMA, fibronectin, MMP-1, TIMP-1, CTGF, fibroblast proliferation and differentiation | Natural antioxidant in green tea |
| Resveratrol | Antioxidant - free radical scavenger | Reactive oxygen species | Natural antioxidant in red wine, skin of grapes, other fruits, and plants |
| Selenium | Antioxidant - free radical scavenger | Reactive oxygen species | Dietary selenium is derived from meat, mushroom, fish, eggs, cereals, and nuts |
| Vitamin C | Antioxidant - free radical scavenger | Reactive oxygen species | Clinical study concluded that oral vitamin C ingestion at a dose of 2g resulted in no benefit in endothelial function in 11 scleroderma patients (skin fibrosis not examined) |
| Vitamins E | Antioxidant - free radical scavenger | Reactive oxygen species | Clinical study concluded that 3-week oral vitamin E treatment at doses 500 to 1000 mg/day has limited benefit in scleroderma patients |
| Vitamin E and pentoxyphylline | Mechanism of action of pentoxyphylline not fully elucidated | Reactive oxygen species | Small clinical investigation involving 12 scleroderma patients exploring the combined treatment (800 UI of vitamin E and 800 mg of pentoxyphylline daily) revealed improvement in MRSS score (mean reduction from 25.7 to 18.7 at 16th week); patients reported no serious side effects |
| Synthetic antioxidants | |||
| Ebselen | Antioxidant | leukocyte infiltration and activation, collagen contraction, TGF-beta1 activation | Further investigations needed to examine potential as a novel therapeutic in skin fibrosis |
| Edaravone | Antioxidant - free radical scavenger | inflammatory cell migration to the skin, TGF-beta1 production | Further investigations needed to examine potential as a novel therapeutic in skin fibrosis |
Statins
The translocation of the cytosolic subunit, Rac1, to the cell membrane is an important process in Nox enzyme activation and ROS generation with statins executing their anti-fibrotic effect by impeding Rac1 translocation (Figure 6) [57]. It is hypothesized that some of the beneficial effects of statins in lowering cholesterol may be the due to the inhibition of vascular Nox [59]. To elucidate the effect of statins in skin fibrosis, pravastatin was studied in an in vivo BALB/c mouse model of chronic GVHD induced by allogeneic HSCT [117,121]. Mice treated with pravastatin following transplantation exhibited significantly reduced CCL-2 expression (Figure 4), dermal thickening, dermal collagen deposition, and skin inflammation in comparison to untreated mice [121]. Although this is a murine model, the data suggests that statins may demonstrate anti-fibrotic properties in skin. In addition to GVHD, statins may also be useful in patients with scleroderma where upregulated CCL-2 expression has been identified. Since statins are an established therapeutic option for cardiovascular disease with known pharmacologic safety profile, statins may represent an easily translatable treatment for skin fibrosis. Optimal method of statin delivery to skin (topical versus oral), statin class and dosing also need to be determined.
The p47phox subunit modulators
The inhibition of the organizer subunit, p47phox, represents a strategy in modulating skin fibrosis and is a target of several small molecules and peptides (Figure 6). Apocynin, DPI, AEBSF, and S17834 are small molecules with activities against p47phox and block its interaction with other enzymatic subunits [86]. Apocynin, DPI, and AEBSF are widely used in studies to inhibit Nox activity as evidence of the involvement of Nox enzymes in various physiological and pathological conditions. However, the potential use of these small compounds as treatments for fibrotic diseases is limited as they lack specificity and influence the activation of several other enzymes[4,109]. Furthermore, it has been shown that apocynin functions as an antioxidant rather than a Nox inhibitor in non-phagocytic cells [41], DPI possesses undesirable hypoglycemic properties and AEBSF has very weak potency making them unattractive therapeutic options [15,59]. S17834 was found to inhibit Nox enzymes and superoxide anion production in endothelial cells in vitro [59]. One study found S17834 to be beneficial in hyperlipidemia and atherosclerosis in a diabetes mouse model although this finding was not directly linked to Nox inhibition [124]. There is insufficient evidence to assess the efficacy of S17834 as a Nox inhibitor [59].
Considering the non-specificity of these small molecule inhibitors, attention has shifted to peptides. Peptides have the advantage of generally being more specific than small molecule inhibitors. The peptide-based inhibitors, gp91ds-tat and PR39, targeting p47phox have been identified. However, they are also unlikely to translate into clinically useful drugs as they lack oral bioavailability and have low stability [86]. Furthermore, PR39 is not completely specific for Nox enzymes and may influence other proteins possessing the SRC homology domain. The use of these peptides may be limited to parenteral administration [86].
Competitive inhibitors of Nox enzymes
Competitively inhibiting molecules involved in the Nox enzymatic process is another therapeutic strategy. A competitive inhibitor, GKT137831, has been identified [38]. This small molecule structurally mimics NADPH and is selective for Nox1 and Nox4 (Figure 6) [38,87]. GKT137831 is the first inhibitor fulfilling the key requirements needed in a Nox inhibitor, including high oral bioavailability, suitable toxicity profile, specificity for Nox enzymes, selectivity for Nox isoforms, and lack of antioxidant activity [58]. GKT137831 established a model for the discovery of other competitive inhibitors of Nox enzyme class of drugs and is currently under investigation in a phase I clinical trial for the treatment of diabetic nephropathy [38,52,57,87]. GKT137831 has been found effective in several preclinical disease models, including diabetic nephropathy, atherosclerosis, idiopathic pulmonary fibrosis, liver fibrosis, and angiogenesis. Selectivity of GKT137831 for the fibrotic Nox isoforms, Nox1 and Nox4, makes GKT137831 a promising therapeutic option for fibrotic skin diseases.
Nox inhibitors with unspecified mechanisms of action
Other Nox inhibitors have been identified although their mechanisms of action have not been fully elucidated. These inhibitors include VAS2870 and fulvene-5 [57,86]. VAS2870 is hypothesized to inhibit the assembly of Nox enzymes following translocation of the cytosolic subunits to the cell membrane[4]. VAS2870 prevented ROS generation in endothelial cells in response to oxidized low-density lipoprotein [92]. While VAS2870 does not have high potency, it is proposed that modification of the chemical structure may strengthen potency and permit specificity in targeting Nox isoforms [59]. Fulvene-5 is another inhibitor that successfully inhibited endothelial cell ROS production by Nox2 and Nox4 in an in vitro model of hemangioma [12]. Data on the efficacy of fulvene-5 is limited as there is only in vitro support for its use. Characterizing the mechanism of action may be an important step in elucidating the potential of fulvene-5 in disease management.
Antioxidants
Restoring cellular redox homeostasis with the application of antioxidants represents a therapeutic strategy against the oxidative stress created by Nox enzymes in skin fibrosis (Figure 6) [85,89]. Vitamins C and E are widely recognized as antioxidants although they appear to have less promising roles in the modulation of skin fibrosis. Clinical investigations of oral vitamins C and E supplementation in scleroderma patients yielded limited benefits [25,69]. Nonetheless there may be a potential for these vitamins to be efficacious when combined with other anti-fibrotic agents. One study evaluated the combined use of vitamin E and pentoxyphylline, a theobromine methylxanthine analog with an unknown pharmacological effect that has been shown to inhibit the proliferation of human-derived fibroblasts in keloids and scleroderma [11,28]. In this small clinical investigation, there was significant reduction in skin thickness of patients and minimal associated side effects [28]. In the future, studies may evaluate the combined use of vitamins and Nox inhibitors for possible synergistic effects. There are also various topical antioxidants used for anti-aging and anti-cancer purposes that may possess anti-Nox functionality. Research may be directed at eliciting these potential underlying Nox inhibitory activities. Perhaps in the future, topical anti-Nox pharmaceuticals may be of substantive use in combating fibrotic skin disorders.
A common problem that often arises with vitamins is the potential for adverse effects when ingested at high levels. The botanically-derived antioxidants resveratrol, epigallocatechin-3-gallate (EGCG) and caffeine do not have this risk and may present a therapeutic avenue in the management of skin fibrosis. Resveratrol is found in red wine and in many plants, including skin of grapes and mulberries and EGCG is found in green tea extracts [29,66]. In vitro data revealed that resveratrol, EGCG alone, and in combination with caffeine, modulate ROS in human skin fibroblasts [45,46]. Resveratrol modulates telomere length and may be useful in the management of keloids where telomere shortening has been identified [113]. EGCG also regulates several organ fibrotic processes such as ECM protein synthesis, ECM degradation by MMPs, the profibrotic growth factor CTGF and fibroblast differentiation into myofibroblasts [23,30,56,70,76,95,125]. Furthermore, EGCG can counteract TGF-beta-induced ROS and suppress TGF-beta signaling in human dermal fibroblasts [30,56]. Expanding these findings to clinical trials may help in clarifying the role of resveratrol and EGCG in skin fibrosis.
Another approach in treating skin fibrosis is to target synthetic antioxidants. Edaravone and ebselen are two synthetic antioxidants demonstrating promise in this respect. In a murine model of scleroderma, edaravone significantly reduced dermal thickening and fibrosis, infiltration of inflammatory cells into the skin, and production of H2O2 and TGF-beta1 [122]. Another murine study demonstrated that ebselen inhibits the activation of Nox enzymes and blocks the infiltration of leukocytes into the skin leading to the attenuation of H2O2 level [75]. Future studies will need to carefully elucidate the potential adverse effects that may be associated with these synthetic antioxidants.
There are several barriers to the development of Nox modulators. Considering that Duox1 and Duox2 are involved in thyroid hormone synthesis, hypothyroidism is a potential side effect which can be treated with hormone replacement therapy. Furthermore, inhibition of the phagocyte innate immune response from Nox2 inhibition may increase susceptibility to infections [59]. In the development of Nox-modulators, selectivity will be needed to avoid unintentional effects on Nox2, Duox1 and Duox2. Selectivity is a significant obstacle to the development of Nox-based drugs. While peptides are generally more selective than small molecules, delivery of peptides remains a significant challenge. A potential strategy is to screen libraries of currently available drugs and antioxidants for Nox modulation, as this may be a more effective way of identifying Nox inhibitors rather than developing new drugs.
Conclusion
Nox enzymes are expressed in the skin and generate ROS not only in physiologic processes but also contribute to pathologic processes. Nox enzymes are important in dermatology because they are key modulators of signaling pathways involved in skin fibrosis. Nox enzymes play a role in a variety of fibrotic skin diseases, including scleroderma, graft versus host disease, keloids, hypertrophic scars, nephrogenic systemic fibrosis and other fibrotic skin disorders. Modulation of Nox enzyme activity and restoration of redox homeostasis with anti-Nox pharmacotherapy has the potential to re-establish the normal wound healing process and ameliorate fibrosis. We believe that Nox modulators are worthy of further investigation and have the potential to transform the management of skin fibrosis by dermatologists.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award TL1 TR000133.
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award KL2 TR000134.
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R33AI080604.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CCL
Chemokine ligand
- CTGF
Connective tissue growth factor
- ECM
Extracellular matrix
- EGCG
Epigallocatechin-3-gallate
- EMS
Eosinophilia-myalgia syndrome
- ERK
Extracellular signal-regulated kinase
- FAD
Flavin adenine dinucleotide
- GVHD
Graft versus host disease
- HSCT
Hematopoietic stem cell transplantation
- IPF
Idiopathic pulmonary fibrosis
- JAK
Janus kinase
- JNK
c-jun amino-terminal kinase
- MAPK
Mitogen activated protein kinase
- MMP
Matrix metalloproteinase
- mRNA
Messenger RNA
- NADPH
Nicotinamide adenine dinucleotide phosphate
- Nox
Nicotinamide adenine dinucleotide phosphate oxidase
- NSF
Nephrogenic systemic fibrosis
- PCT
Porphyria cutanea tarda
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- PKC
Protein kinase C
- POLDIP
DNA-polymerase-delta-interacting protein
- PTP
Protein tyrosine phosphatase
- RAAS
Renin-angiotensin-aldosterone system
- RD
Restrictive dermopathy
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TGF
Transforming growth factor
- TIMP
Tissue inhibitor of metalloproteinase
- TKS
Tyrosine kinase substrate
- TOS
Toxic oil syndrome
- 5-VPTA
N-(5-vinyl-1,3-thiazolidin-2-ylidene)phenylamine
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Abdullah A, Blakeney P, Hunt R, Broemeling L, Phillips L, Herndon DN, Robson MC. Visible scars and self-esteem in pediatric patients with burns. The Journal of burn care & rehabilitation. 1994;15(2):164–168. doi: 10.1097/00004630-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertension research : official journal of the Japanese Society of Hypertension. 2006;29(10):813–820. doi: 10.1291/hypres.29.813. doi:10.1291/hypres.29.813. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer research. 2007;67(8):3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. doi:10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 4.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cellular and molecular life sciences : CMLS. 2012;69(14):2327–2343. doi: 10.1007/s00018-012-1010-9. doi:10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56(6):2316–2327. doi: 10.1002/hep.25938. doi:10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey AJ, Bazin S, Sims TJ, Le Lous M, Nicoletis C, Delaunay A. Characterization of the collagen of human hypertrophic and normal scars. Biochimica et biophysica acta. 1975;405(2):412–421. doi: 10.1016/0005-2795(75)90106-3. [DOI] [PubMed] [Google Scholar]
- 7.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, Fraticelli P, Sambo P, Funaro A, Kazlauskas A, Avvedimento EV, Gabrielli A. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. The New England journal of medicine. 2006;354(25):2667–2676. doi: 10.1056/NEJMoa052955. doi:10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 8.Bataller R, Sancho-Bru P, Gines P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxidants & redox signaling. 2005;7(9-10):1346–1355. doi: 10.1089/ars.2005.7.1346. doi:10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]
- 9.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. The Journal of clinical investigation. 2003;112(9):1383–1394. doi: 10.1172/JCI18212. doi:10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batteux F, Kavian N, Servettaz A. New insights on chemically induced animal models of systemic sclerosis. Current opinion in rheumatology. 2011;23(6):511–518. doi: 10.1097/BOR.0b013e32834b1606. doi:10.1097/BOR.0b013e32834b1606. [DOI] [PubMed] [Google Scholar]
- 11.Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. The British journal of dermatology. 1990;123(3):339–346. doi: 10.1111/j.1365-2133.1990.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. The Journal of clinical investigation. 2009;119(8):2359–2365. doi: 10.1172/JCI33877. doi:10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleasel NR, Varigos GA. Porphyria cutanea tarda. The Australasian journal of dermatology. 2000;41(4):197–206. doi: 10.1046/j.1440-0960.2000.00437.x. quiz 207-198. [DOI] [PubMed] [Google Scholar]
- 14.Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nature reviews Cancer. 2012;12(9):627–637. doi: 10.1038/nrc3339. doi:10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloxham DP. The relationship of diphenyleneiodonium-induced hypoglycaemia to the specific covalent modification of NADH-ubiquinone oxidoreductase. Biochemical Society transactions. 1979;7(1):103–106. doi: 10.1042/bst0070103. [DOI] [PubMed] [Google Scholar]
- 16.Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Archives of dermatological research. 2006;297(10):433–438. doi: 10.1007/s00403-006-0651-7. doi:10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 17.Bohm F, Edge R, Foley S, Lange L, Truscott TG. Antioxidant inhibition of porphyrin- induced cellular phototoxicity. Journal of photochemistry and photobiology B, Biology. 2001;65(2-3):177–183. doi: 10.1016/s1011-1344(01)00259-7. [DOI] [PubMed] [Google Scholar]
- 18.Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology 3e. Mosby; St. Louis: 2012. [Google Scholar]
- 19.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochimica et biophysica acta. 2013;1832(7):1049–1060. doi: 10.1016/j.bbadis.2012.09.014. doi:10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinckmann J, Notbohm H, Tronnier M, Acil Y, Fietzek PP, Schmeller W, Muller PK, Batge B. Overhydroxylation of lysyl residues is the initial step for altered collagen cross-links and fibril architecture in fibrotic skin. The Journal of investigative dermatology. 1999;113(4):617–621. doi: 10.1046/j.1523-1747.1999.00735.x. doi:10.1046/j.1523-1747.1999.00735.x. [DOI] [PubMed] [Google Scholar]
- 21.Brych SB, Engrav LH, Rivara FP, Ptacek JT, Lezotte DC, Esselman PC, Kowalske KJ, Gibran NS. Time off work and return to work rates after burns: systematic review of the literature and a large two-center series. The Journal of burn care & rehabilitation. 2001;22(6):401–405. doi: 10.1097/00004630-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Cao DX, Qiao B, Ge ZQ, Yuan YJ. Comparison of burst of reactive oxygen species and activation of caspase-3 in apoptosis of K562 and HL-60 cells induced by docetaxel. Cancer letters. 2004;214(1):103–113. doi: 10.1016/j.canlet.2004.03.047. doi:10.1016/j.canlet.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Chen A, Zhang L, Xu J, Tang J. The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. The Biochemical journal. 2002;368(Pt 3):695–704. doi: 10.1042/BJ20020894. doi:10.1042/BJ20020894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Classen JF, Henrohn D, Rorsman F, Lennartsson J, Lauwerys BR, Wikstrom G, Rorsman C, Lenglez S, Franck-Larsson K, Tomasi JP, Kampe O, Vanthuyne M, Houssiau FA, Demoulin JB. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis and rheumatism. 2009;60(4):1137–1144. doi: 10.1002/art.24381. doi:10.1002/art.24381. [DOI] [PubMed] [Google Scholar]
- 25.Cracowski JL, Girolet S, Imbert B, Seinturier C, Stanke-Labesque F, Bessard J, Boignard A, Bessard G, Carpentier PH. Effects of short-term treatment with vitamin E in systemic sclerosis: a double blind, randomized, controlled clinical trial of efficacy based on urinary isoprostane measurement. Free radical biology & medicine. 2005;38(1):98–103. doi: 10.1016/j.freeradbiomed.2004.09.032. doi:10.1016/j.freeradbiomed.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circulation research. 2005;97(9):900–907. doi: 10.1161/01.RES.0000187457.24338.3D. doi:10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 27.De Felice B, Wilson RR, Nacca M. Telomere shortening may be associated with human keloids. BMC medical genetics. 2009;10:110. doi: 10.1186/1471-2350-10-110. doi:10.1186/1471-2350-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza RB, Macedo AR, Kuruma KA, Macedo PA, Borges CT. Pentoxyphylline in association with vitamin E reduces cutaneous fibrosis in systemic sclerosis. Clinical rheumatology. 2009;28(10):1207–1212. doi: 10.1007/s10067-009-1202-3. doi:10.1007/s10067-009-1202-3. [DOI] [PubMed] [Google Scholar]
- 29.Dooley A, Bruckdorfer KR, Abraham DJ. Modulation of fibrosis in systemic sclerosis by nitric oxide and antioxidants. Cardiology research and practice. 2012;2012:521958. doi: 10.1155/2012/521958. doi:10.1155/2012/521958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooley A, Shi-Wen X, Aden N, Tranah T, Desai N, Denton CP, Abraham DJ, Bruckdorfer R. Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatology. 2010;49(11):2024–2036. doi: 10.1093/rheumatology/keq208. doi:10.1093/rheumatology/keq208. [DOI] [PubMed] [Google Scholar]
- 31.Engrav LH, Heimbach DM, Reus JL, Harnar TJ, Marvin JA. Early excision and grafting vs. nonoperative treatment of burns of indeterminant depth: a randomized prospective study. The Journal of trauma. 1983;23(11):1001–1004. doi: 10.1097/00005373-198311000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Fauerbach JA, Heinberg LJ, Lawrence JW, Bryant AG, Richter L, Spence RJ. Coping with body image changes following a disfiguring burn injury. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2002;21(2):115–121. [PubMed] [Google Scholar]
- 33.Fauerbach JA, Heinberg LJ, Lawrence JW, Munster AM, Palombo DA, Richter D, Spence RJ, Stevens SS, Ware L, Muehlberger T. Effect of early body image dissatisfaction on subsequent psychological and physical adjustment after disfiguring injury. Psychosomatic medicine. 2000;62(4):576–582. doi: 10.1097/00006842-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Gabrielli A, Svegliati S, Moroncini G, Amico D. New insights into the role of oxidative stress in scleroderma fibrosis. The open rheumatology journal. 2012;6:87–95. doi: 10.2174/1874312901206010087. doi:10.2174/1874312901206010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS chemical biology. 2010;5(10):981–993. doi: 10.1021/cb100219n. doi:10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxidants & redox signaling. 2006;8(9-10):1597–1607. doi: 10.1089/ars.2006.8.1597. doi:10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 37.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxidants & redox signaling. 2009;11(10):2505–2516. doi: 10.1089/ars.2009.2599. doi:10.1089/ARS.2009.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hecker L, Cheng J, Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cellular and molecular life sciences : CMLS. 2012;69(14):2365–2371. doi: 10.1007/s00018-012-1012-7. doi:10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiskanen KM, Naarala J, Savolainen KM. The effects of N-(5-vinyl-1,3-thiazolidin-2- ylidene)phenylamine (5-VTPA) on the changes in free intracellular calcium and the production of reactive oxygen metabolites in human leukocytes. Toxicology. 1995;100(1-3):195–202. doi: 10.1016/0300-483x(95)03090-3. [DOI] [PubMed] [Google Scholar]
- 40.Heiskanen KM, Savolainen KM. Effects of 3-phenylamino-1,2-propanediol and its mono-and dioleylesters on the production of reactive oxygen metabolites in human polymorphonuclear leukocytes. Toxicology letters. 1997;91(1):39–45. doi: 10.1016/s0378-4274(96)03862-3. [DOI] [PubMed] [Google Scholar]
- 41.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51(2):211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. doi:10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 42.Hummers LK, Wigley FM. Current Rheumatology Diagnosis & Treatment. 2nd edn McGraw-Hill; New York: 2007. Scleroderma. [Google Scholar]
- 43.Istok R, Bely M, Stancikova M, Rovensky J. Evidence for increased pyridinoline concentration in fibrotic tissues in diffuse systemic sclerosis. Clinical and experimental dermatology. 2001;26(6):545–547. doi: 10.1046/j.1365-2230.2001.00886.x. [DOI] [PubMed] [Google Scholar]
- 44.Itoh M, Yanaba K, Kobayashi T, Nakagawa H. Taxane-induced scleroderma. The British journal of dermatology. 2007;156(2):363–367. doi: 10.1111/j.1365-2133.2006.07597.x. doi:10.1111/j.1365-2133.2006.07597.x. [DOI] [PubMed] [Google Scholar]
- 45.Jagdeo J, Adams L, Lev-Tov H, Sieminska J, Michl J, Brody N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. Journal of drugs in dermatology : JDD. 2010;9(12):1523–1526. [PubMed] [Google Scholar]
- 46.Jagdeo J, Brody N. Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. Journal of drugs in dermatology : JDD. 2011;10(7):753–761. [PubMed] [Google Scholar]
- 47.Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. The Journal of dermatology. 2010;37(1):11–25. doi: 10.1111/j.1346-8138.2009.00738.x. doi:10.1111/j.1346-8138.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 48.Jinnin M, Ihn H, Yamane K, Tamaki K. Interleukin-13 stimulates the transcription of the human alpha2(I) collagen gene in human dermal fibroblasts. The Journal of biological chemistry. 2004;279(40):41783–41791. doi: 10.1074/jbc.M406951200. doi:10.1074/jbc.M406951200. [DOI] [PubMed] [Google Scholar]
- 49.Kashiyama K, Mitsutake N, Matsuse M, Ogi T, Saenko VA, Ujifuku K, Utani A, Hirano A, Yamashita S. miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. The Journal of investigative dermatology. 2012;132(6):1597–1604. doi: 10.1038/jid.2012.22. doi:10.1038/jid.2012.22. [DOI] [PubMed] [Google Scholar]
- 50.Kavian N, Marut W, Servettaz A, Laude H, Nicco C, Chereau C, Weill B, Batteux F. Arsenic trioxide prevents murine sclerodermatous graft-versus-host disease. Journal of immunology. 2012;188(10):5142–5149. doi: 10.4049/jimmunol.1103538. doi:10.4049/jimmunol.1103538. [DOI] [PubMed] [Google Scholar]
- 51.Keeley FW, Murray RK. The Extracellular Matrix. In: Bender DA, Botham KM, Weil PA, Kennelly PJ, Murray RK, Rodwell VW, editors. Harper's Illustrated Biochemistry. 29th edn McGraw-Hill; New York: 2011. [Google Scholar]
- 52.Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P. NADPH oxidase inhibitors: a patent review. Expert opinion on therapeutic patents. 2011;21(8):1147–1158. doi: 10.1517/13543776.2011.584870. doi:10.1517/13543776.2011.584870. [DOI] [PubMed] [Google Scholar]
- 53.Kim JS, Choi IG, Lee BC, Park JB, Kim JH, Jeong JH, Jeong JH, Seo CH. Neuregulin induces CTGF expression in hypertrophic scarring fibroblasts. Molecular and cellular biochemistry. 2012;365(1-2):181–189. doi: 10.1007/s11010-012-1258-2. doi:10.1007/s11010-012-1258-2. [DOI] [PubMed] [Google Scholar]
- 54.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Experimental biology and medicine. 2008;233(2):109–122. doi: 10.3181/0707-MR-190. doi:10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 55.Kitko CL, White ES, Baird K. Fibrotic and sclerotic manifestations of chronic graft- versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(1 Suppl):S46–52. doi: 10.1016/j.bbmt.2011.10.021. doi:10.1016/j.bbmt.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klass BR, Branford OA, Grobbelaar AO, Rolfe KJ. The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-beta1-stimulated wound contraction. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2010;18(1):80–88. doi: 10.1111/j.1524-475X.2009.00552.x. doi:10.1111/j.1524-475X.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 57.Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhofer S, Radermacher KA, Janssen B, Gorlach A, Schmidt HH. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. Journal of molecular medicine. 2012;90(12):1391–1406. doi: 10.1007/s00109-012-0963-3. doi:10.1007/s00109-012-0963-3. [DOI] [PubMed] [Google Scholar]
- 58.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. Journal of medicinal chemistry. 2010;53(21):7715–7730. doi: 10.1021/jm100773e. doi:10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 59.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Seminars in immunopathology. 2008;30(3):339–363. doi: 10.1007/s00281-008-0123-6. doi:10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 60.Last JA, King TE, Jr., Nerlich AG, Reiser KM. Collagen cross-linking in adult patients with acute and chronic fibrotic lung disease. Molecular markers for fibrotic collagen. The American review of respiratory disease. 1990;141(2):307–313. doi: 10.1164/ajrccm/141.2.307. doi:10.1164/ajrccm/141.2.307. [DOI] [PubMed] [Google Scholar]
- 61.Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochemical pharmacology. 2012;84(5):581–590. doi: 10.1016/j.bcp.2012.05.005. doi:10.1016/j.bcp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, Driscoll B. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. American journal of physiology Lung cellular and molecular physiology. 2009;296(1):L57–70. doi: 10.1152/ajplung.90411.2008. doi:10.1152/ajplung.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leiferman K, Peters M. Eosinophils in Cutaneous Diseases. In: Fitzpatrick's Dermatology in General Medicine. (8th edn.) 2012 [Google Scholar]
- 64.Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis and rheumatism. 2011;63(1):239–246. doi: 10.1002/art.30074. doi:10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Zhu S, Wang T, Hummers L, Wigley FM, Goldschmidt-Clermont PJ, Dong C. Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS medicine. 2005;2(12):e354. doi: 10.1371/journal.pmed.0020354. doi:10.1371/journal.pmed.0020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. Journal of cellular physiology. 1999;179(3):297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. doi:10.1002/(SICI)1097-46521(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 67.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respiratory research. 2005;6:11. doi: 10.1186/1465-9921-6-11. doi:10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis and rheumatism. 2010;62(6):1733–1743. doi: 10.1002/art.27443. doi:10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 69.Mavrikakis ME, Lekakis JP, Papamichael CM, Stamatelopoulos KS, Kostopoulos Ch C, Stamatelopoulos SF. Ascorbic acid does not improve endothelium-dependent flow-mediated dilatation of the brachial artery in patients with Raynaud's phenomenon secondary to systemic sclerosis. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2003;73(1):3–7. doi: 10.1024/0300-9831.73.1.3. [DOI] [PubMed] [Google Scholar]
- 70.Meng M, Li YQ, Yan MX, Kou Y, Ren HB. Effects of epigallocatechin gallate on diethyldithiocarbamate-induced pancreatic fibrosis in rats. Biological & pharmaceutical bulletin. 2007;30(6):1091–1096. doi: 10.1248/bpb.30.1091. [DOI] [PubMed] [Google Scholar]
- 71.Moinzadeh P, Denton C, Krieg T, Black C. Scleroderma. Fitzpatrick's Dermatology in General Medicine. (8th edn.) 2012 [Google Scholar]
- 72.Moriguchi T, Fujimoto D. Crosslink of collagen in hypertrophic scar. The Journal of investigative dermatology. 1979;72(3):143–145. doi: 10.1111/1523-1747.ep12530609. [DOI] [PubMed] [Google Scholar]
- 73.Mura G, Bhat KM, Pisano A, Licci G, Carta M. Psychiatric symptoms and quality of life in systemic sclerosis. Clinical practice and epidemiology in mental health : CP & EMH. 2012;8:30–35. doi: 10.2174/1745017901208010030. doi:10.2174/1745017901208010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murrell DF. A radical proposal for the pathogenesis of scleroderma. Journal of the American Academy of Dermatology. 1993;28(1):78–85. doi: 10.1016/0190-9622(93)70014-k. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura Y, Feng Q, Kumagai T, Torikai K, Ohigashi H, Osawa T, Noguchi N, Niki E, Uchida K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. The Journal of biological chemistry. 2002;277(4):2687–2694. doi: 10.1074/jbc.M109641200. doi:10.1074/jbc.M109641200. [DOI] [PubMed] [Google Scholar]
- 76.Park G, Yoon BS, Moon JH, Kim B, Jun EK, Oh S, Kim H, Song HJ, Noh JY, Oh C, You S. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. The Journal of investigative dermatology. 2008;128(10):2429–2441. doi: 10.1038/jid.2008.103. doi:10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- 77.Pinelli A, Trivulzio S, Tomasoni L, Bertolini B, Pinelli G. High-dose vitamin E lowers urine porphyrin levels in patients affected by porphyria cutanea tarda. Pharmacological research : the official journal of the Italian Pharmacological Society. 2002;45(4):355–359. doi: 10.1006/phrs.2002.0956. doi:10.1006/phrs.2002.0956. [DOI] [PubMed] [Google Scholar]
- 78.Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft- versus-host disease and scleroderma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9(7):417–425. doi: 10.1016/s1083-8791(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 79.Proctor CJ, Kirkwood TB. Modelling telomere shortening and the role of oxidative stress. Mechanisms of ageing and development. 2002;123(4):351–363. doi: 10.1016/s0047-6374(01)00380-3. [DOI] [PubMed] [Google Scholar]
- 80.Rezvani HR, Rossignol R, Ali N, Benard G, Tang X, Yang HS, Jouary T, de Verneuil H, Taieb A, Kim AL, Mazurier F. XPC silencing in normal human keratinocytes triggers metabolic alterations through NOX-1 activation-mediated reactive oxygen species. Biochimica et biophysica acta. 2011;1807(6):609–619. doi: 10.1016/j.bbabio.2010.12.006. doi:10.1016/j.bbabio.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ricard-Blum S, Bresson-Hadni S, Vuitton DA, Ville G, Grimaud JA. Hydroxypyridinium collagen cross-links in human liver fibrosis: study of alveolar echinococcosis. Hepatology. 1992;15(4):599–602. doi: 10.1002/hep.1840150408. [DOI] [PubMed] [Google Scholar]
- 82.Ricard-Blum S, Esterre P, Grimaud JA. Collagen cross-linking by pyridinoline occurs in non-reversible skin fibrosis. Cellular and molecular biology. 1993;39(7):723–727. [PubMed] [Google Scholar]
- 83.Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cellular signalling. 2013;25(1):264–268. doi: 10.1016/j.cellsig.2012.10.003. doi:10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sambo P, Baroni SS, Luchetti M, Paroncini P, Dusi S, Orlandini G, Gabrielli A. Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis and rheumatism. 2001;44(11):2653–2664. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 85.Sampson N, Berger P, Zenzmaier C. Therapeutic targeting of redox signaling in myofibroblast differentiation and age-related fibrotic disease. Oxidative medicine and cellular longevity. 2012;2012:458276. doi: 10.1155/2012/458276. doi:10.1155/2012/458276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascular pharmacology. 2012;56(5-6):216–231. doi: 10.1016/j.vph.2012.02.012. doi:10.1016/j.vph.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, Touyz RM. Oxidative stress, Nox isoforms and complications of diabetes--potential targets for novel therapies. Journal of cardiovascular translational research. 2012;5(4):509–518. doi: 10.1007/s12265-012-9387-2. doi:10.1007/s12265-012-9387-2. [DOI] [PubMed] [Google Scholar]
- 88.Sieprath T, Darwiche R, De Vos WH. Lamins as mediators of oxidative stress. Biochemical and biophysical research communications. 2012;421(4):635–639. doi: 10.1016/j.bbrc.2012.04.058. doi:10.1016/j.bbrc.2012.04.058. [DOI] [PubMed] [Google Scholar]
- 89.Simonini G, Pignone A, Generini S, Falcini F, Cerinic MM. Emerging potentials for an antioxidant therapy as a new approach to the treatment of systemic sclerosis. Toxicology. 2000;155(1-3):1–15. doi: 10.1016/s0300-483x(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 90.Spies-Weisshart B, Schilling K, Bohmer F, Hochhaus A, Sayer HG, Scholl S. Lack of association of platelet-derived growth factor (PDGF) receptor autoantibodies and severity of chronic graft-versus-host disease (GvHD). Journal of cancer research and clinical oncology. 2013;139(8):1397–1404. doi: 10.1007/s00432-013-1451-z. doi:10.1007/s00432-013-1451-z. [DOI] [PubMed] [Google Scholar]
- 91.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148(8):3773–3780. doi: 10.1210/en.2006-1691. doi:10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 92.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochemical and biophysical research communications. 2006;344(1):200–205. doi: 10.1016/j.bbrc.2006.03.114. doi:10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 93.Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, Moroncini G, Bacigalupo A, Leoni P, Avvedimento EV, Gabrielli A. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110(1):237–241. doi: 10.1182/blood-2007-01-071043. doi:10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 94.Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, Nakata T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin aldosterone system in diabetic rats. European journal of pharmacology. 2008;589(1-3):264–271. doi: 10.1016/j.ejphar.2008.06.019. doi:10.1016/j.ejphar.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 95.Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273(1-3):45–52. doi: 10.1016/j.tox.2010.04.014. doi:10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 96.Tsou PS, Talia NN, Pinney AJ, Kendzicky A, Piera-Velazquez S, Jimenez SA, Seibold JR, Phillips K, Koch AE. Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts. Arthritis and rheumatism. 2012;64(6):1978–1989. doi: 10.1002/art.34336. doi:10.1002/art.34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Current pharmaceutical design. 2007;13(12):1247–1256. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- 98.Uzawa K, Marshall MK, Katz EP, Tanzawa H, Yeowell HN, Yamauchi M. Altered posttranslational modifications of collagen in keloid. Biochemical and biophysical research communications. 1998;249(3):652–655. doi: 10.1006/bbrc.1998.8955. doi:10.1006/bbrc.1998.8955. [DOI] [PubMed] [Google Scholar]
- 99.van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MM, Middelkoop E, Boers W, Karel Ronday H, DeGroot J, Huizinga TW, Bank RA. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix biology : journal of the International Society for Matrix Biology. 2004;23(4):251–257. doi: 10.1016/j.matbio.2004.06.001. doi:10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Varga J. Systemic Sclerosis (Scleroderma) and Related Disorders. 2012.
- 101.Vettori S, Gay S, Distler O. Role of MicroRNAs in Fibrosis. The open rheumatology journal. 2012;6:130–139. doi: 10.2174/1874312901206010130. doi:10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(7):854–856. doi: 10.1096/fj.04-2882fje. doi:10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 103.Wagner B, Tan C, Barnes JL, Ahuja S, Davis TL, Gorin Y, Jimenez F. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. The American journal of pathology. 2012;181(6):1941–1952. doi: 10.1016/j.ajpath.2012.08.026. doi:10.1016/j.ajpath.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 104.Wan KC, Evans JH. Free radical involvement in hypertrophic scar formation. Free radical biology & medicine. 1999;26(5-6):603–608. doi: 10.1016/s0891-5849(98)00245-7. [DOI] [PubMed] [Google Scholar]
- 105.Wan KC, Wu HT, Chan HP, Hung LK. Effects of antioxidants on pyridinoline cross-link formation in culture supernatants of fibroblasts from normal skin and hypertrophic scars. Clinical and experimental dermatology. 2002;27(6):507–512. doi: 10.1046/j.1365-2230.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 106.Wang P, Tang F, Li R, Zhang H, Chen S, Liu P, Huang H. Contribution of different Nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: effect of valsartan. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55(5):408–417. doi: 10.1016/j.phrs.2007.01.016. doi:10.1016/j.phrs.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 107.Wesche WA, Cutlan RT, Khare V, Chesney T, Shanklin D. Restrictive dermopathy: report of a case and review of the literature. Journal of cutaneous pathology. 2001;28(4):211–218. doi: 10.1034/j.1600-0560.2001.028004211.x. [DOI] [PubMed] [Google Scholar]
- 108.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension. 2008;51(2):474–480. doi: 10.1161/HYPERTENSIONAHA.107.102467. doi:10.1161/HYPERTENSIONAHA.107.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. British journal of pharmacology. 2011;164(3):866–883. doi: 10.1111/j.1476-5381.2011.01249.x. doi:10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature reviews Immunology. 2004;4(8):583–594. doi: 10.1038/nri1412. doi:10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wynn TA. Cellular and molecular mechanisms of fibrosis. The Journal of pathology. 2008;214(2):199–210. doi: 10.1002/path.2277. doi:10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature medicine. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. doi:10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xia L, Wang XX, Hu XS, Guo XG, Shang YP, Chen HJ, Zeng CL, Zhang FR, Chen JZ. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. British journal of pharmacology. 2008;155(3):387–394. doi: 10.1038/bjp.2008.272. doi:10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamamoto A, Ashihara E, Nakagawa Y, Obayashi H, Ohta M, Hara H, Adachi T, Seno T, Kadoya M, Hamaguchi M, Ishino H, Kohno M, Maekawa T, Kawahito Y. Allograft inflammatory factor-1 is overexpressed and induces fibroblast chemotaxis in the skin of sclerodermatous GVHD in a murine model. Immunology letters. 2011;135(1-2):144–150. doi: 10.1016/j.imlet.2010.10.015. doi:10.1016/j.imlet.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 115.Yamamoto T. Experimental mouse model of scleroderma:induction by bleomycin. In: Lawrence SC (ed) Animal models of human inflammatory skin diseases. CRC Press; Boca Raton, FL: 2004. pp. 535–47. doi:10.1684/ejd.2008.0570. [Google Scholar]
- 116.Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Archives of dermatological research. 2006;297(8):333–344. doi: 10.1007/s00403-005-0635-z. doi:10.1007/s00403-005-0635-z. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto T. Scleroderma--pathophysiology. European journal of dermatology : EJD. 2009;19(1):14–24. doi: 10.1684/ejd.2008.0570. doi:10.1684/ejd.2008.0570. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto T. Autoimmune mechanisms of scleroderma and a role of oxidative stress. Self/nonself. 2011;2(1):4–10. doi: 10.4161/self.2.1.14058. doi:10.4161/self.2.1.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamamoto T, Nishioka K. Animal model of sclerotic skin. V: Increased expression of alpha-smooth muscle actin in fibroblastic cells in bleomycin-induced scleroderma. Clinical immunology. 2002;102(1):77–83. doi: 10.1006/clim.2001.5138. doi:10.1006/clim.2001.5138. [DOI] [PubMed] [Google Scholar]
- 120.Yamamoto T, Takagawa S, Katayama I, Mizushima Y, Nishioka K. Effect of superoxide dismutase on bleomycin-induced dermal sclerosis: implications for the treatment of systemic sclerosis. The Journal of investigative dermatology. 1999;113(5):843–847. doi: 10.1046/j.1523-1747.1999.00758.x. doi:10.1046/j.1523-1747.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 121.Yoon HK, Lim JY, Kim TJ, Cho CS, Min CK. Effects of pravastatin on murine chronic graft-versus-host disease. Transplantation. 2010;90(8):853–860. doi: 10.1097/TP.0b013e3181f2c92b. doi:10.1097/TP.0b013e3181f2c92b. [DOI] [PubMed] [Google Scholar]
- 122.Yoshizaki A, Yanaba K, Ogawa A, Iwata Y, Ogawa F, Takenaka M, Shimizu K, Asano Y, Kadono T, Sato S. The specific free radical scavenger edaravone suppresses fibrosis in the bleomycin-induced and tight skin mouse models of systemic sclerosis. Arthritis and rheumatism. 2011;63(10):3086–3097. doi: 10.1002/art.30470. doi:10.1002/art.30470. [DOI] [PubMed] [Google Scholar]
- 123.Youn GJ, Uzunyan M, Vachon L, Johnson J, Winder TL, Yano S. Autosomal recessive LMNA mutation causing restrictive dermopathy. Clinical genetics. 2010;78(2):199–200. doi: 10.1111/j.1399-0004.2010.01385.x. doi:10.1111/j.1399-0004.2010.01385.x. [DOI] [PubMed] [Google Scholar]
- 124.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55(8):2180–2191. doi: 10.2337/db05-1188. doi:10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, Messadi DV, Le AD. Green tea extract and (−)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. The Journal of investigative dermatology. 2006;126(12):2607–2613. doi: 10.1038/sj.jid.5700472. doi:10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]
- 126.Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15(Suppl 1):S32–39. doi: 10.1111/j.1524-475X.2007.00223.x. doi:10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ziegler TR, Panoskaltsus-Mortari A, Gu LH, Jonas CR, Farrell CL, Lacey DL, Jones DP, Blazar BR. Regulation of glutathione redox status in lung and liver by conditioning regimens and keratinocyte growth factor in murine allogeneic bone marrow transplantation. Transplantation. 2001;72(8):1354–1362. doi: 10.1097/00007890-200110270-00004. [DOI] [PubMed] [Google Scholar]