Abstract
Objective
To establish the intracellular consequences of electrical stimulation to spiral ganglion neurons after deafferentation. Here we use a rat model to determine the effect of both low and high pulse rate acute electrical stimulation on activation of the proapoptotic transcription factor Jun in deafferented spiral ganglion neurons in vivo.
Study design
Experimental animal study.
Setting
Hearing research laboratories of the University of Iowa Departments of Biology and Otolaryngology
Methods
A single electrode was implanted through the round window of kanamycin-deafened rats at either postnatal day 32 (P32, n=24) or P60 (n=22) for four hours of stimulation (monopolar, biphasic pulses, amplitude twice eABR threshold) at either 100 or 5000 Hz. Jun phosphorylation was assayed by immunofluorescence to quantitatively assess the effect of electrical stimulation on proapoptotic signaling.
Results
Jun phosphorylation was reliably suppressed by 100 Hz stimuli in deafened cochleae of P32 but not P60 rats. This effect was not significant in the basal cochlear turns. Stimulation frequency may be consequential: 100 Hz was significantly more effective than was 5 kHz stimulation in suppressing phospho-Jun.
Conclusions
Suppression of Jun phosphorylation occurs in deafferented spiral ganglion neurons after only four hours of electrical stimulation. This finding is consistent with the hypothesis that electrical stimulation can decrease spiral ganglion neuron death after deafferentation.
Keywords: Spiral ganglion neuron, c-Jun, phosphorylation, deafness, cochlear implants, apoptosis
Introduction
Cochlear hair cells are the sole afferent input to spiral ganglion neurons (SGNs), and provide neurotrophic support. Hair cell death and dysfunction are the primary etiologies for human sensorineural hearing loss. After deafferentation, SGNs degenerate and die over a period of time ranging from months in rats1,2,3, to years in cats4, to decades in humans5,6. Cochlear implants (CIs) bypass the hair cell auditory transduction mechanism by providing direct depolarizing electrical stimulation (ES) to SGNs. Thus, the death of SGN cells and loss of dendrites are potentially limiting factors for the transmission of auditory information from CIs to the central nervous system.
Although positive correlation between speech performance and SGN survival has not been demonstrated in cochlear implantees7, electrophysiological and psychophysical parameters in cochlear implantees are correlated with SGN survival in animal studies8, human subjects9,10, and computational models11. Thus, prevention of SGN degeneration, apoptosis or loss of dendrites may enhance the benefits of CIs and improve auditory outcomes.
Although chronic depolarization is an effective trophic stimulus for SGNs in vitro12, studies differ regarding the ability of ES via an implanted electrode to prevent SGN death in vivo13,14,15,16,17. However, a survival-promoting effect of ES in vivo can be revealed when it is administered in combination with intracochlear infusion of neurotrophic factors18. To investigate the mechanism of potential anti-apoptotic effects of ES, we directly assessed apoptosis-related intracellular signaling by monitoring phosphorylation of the nuclear transcription factor Jun by the MAP kinase c-Jun N-terminal Kinase (JNK), an event known to occur in apoptotic neurons19 and to be necessary for neuronal apoptosis20. Our previous studies have established that the proapoptotic JNK-Jun signaling pathway is active in SGNs after deafferentation and is correlated with apoptosis21. Thus, we used immunofluorescent (IF) detection of phosphorylated Jun (pJun) in SGN nuclei as a reporter for apoptotic signaling in vivo. We asked whether ES suppresses Jun phosphorylation and, to the extent that it does so, what are the consequences of factors that may affect SGN survival after deafferentation: duration of deafness, proximity of the stimulating electrode to SGN somata, and pulse rate.
Methods
All protocols were approved by the University of Iowa Institutional Animal Use and Care Committee. Test rats were chemically deafened from postnatal day 8 (P8) to P16 using daily intraperitoneal injection of kanamycin (400 mg/kg, Apothecon)22. Profound hearing loss was tested by click auditory brainstem reponse (ABR) at P21 to exclude rats from any further study. Hair cell loss was definitively confirmed in the cochleae used for phosphoJun quantification by lack of calretinin IF (a marker of inner hair cells). Four nonconsecutive sections from each subject were labeled with anticalretinin in order to exclude subjects with any surviving inner hair cells.
As shown in Table 1, 24 deafened rats underwent unilateral cochlear implantation at either P32 or P60, of which three at either age received no ES. Controls included the nonoperated contralateral cochleae; age-matched, unoperated deafened rats; and age-matched hearing rats both operative and nonanesthetized unoperated. These numbers, summarized in Table 1, are comparable to those used in a previous study (Alam et al.21) showing Jun phosphorylation in deafened rats.
Table 1.
Subject groups for pJun IF quantitation.
| P32 (n=24) | N | P60 (n=23) | N | |
|---|---|---|---|---|
| DEAF (n=30) | ES 5 kHz | 5 | ES 5 kHz | 4 |
| ES 100 Hz | 5 | ES 100 Hz | 4 | |
| surgery, no ES | 3 | surgery, no ES | 3 | |
|
| ||||
| nonanesthetized, unoperated | 3 | nonanesthetized, unoperated | 3 | |
|
| ||||
| HEARING (n=16) | surgery, no ES | 5 | surgery, no ES | 6 |
|
| ||||
| nonanesthetized, unoperated | 3 | nonanesthetized, unoperated | 3 | |
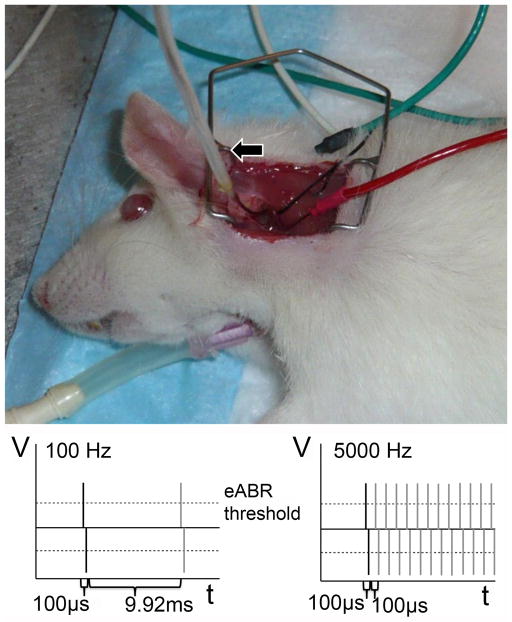
Rats were anesthetized using intramuscular Ketamine (90 mg/kg)/Xylazine (10 mg/kg) injection. Ketamine (45 mg/kg) was subsequently injected as needed to maintain surgical plane per pedal and blink reflexes. Ventral tracheotomy was performed and a Harvard Rodent Ventilator was used throughout the 4 hour stimulation period. A dorsal approach was used to open the bulla and access the round window as previously described23,24 (Fig. 1, upper panel).
Figure 1.
Upper panel: tracheotomy; ABR electrodes are vertex (green), test (red) and ground (white). Lower panels: ES protocol schematic. Left: low rate (100 Hz); Right: high rate (5 kHz).
The electrically evoked auditory brainstem response (eABR) was recorded to verify neurological responses. Subdermal needle electrodes were placed at the vertex (common positive), contralateral nuchal skin (ground), and sternocleidomastoid muscle adjacent to the test ear (Fig. 1, upper panel).
The electrode – Teflon-insulated platinum/iridium wire with 1 mm of its tip stripped of insulation – was inserted, via the cochleostomy, 1–3 mm into scala tympani. The return electrode for monopolar stimulation was placed in the left forepaw25. EABRs were recorded directly after electrode implantation to determine electrically-evoked response thresholds and again after completion of ES. Fig. 1 lower panels diagram the electrical stimulation current patterns for the ES protocols: low rate (100 Hz) ES was 100 μs biphasic pulses with an interpulse interval of 9.92 ms; high rate (5 kHz) ES was 100 μs biphasic pulses with an interpulse interval of 100 μs. The stimulating current amplitude was twice eABR threshold (typically 0.5–1.2 mA) with threshold defined as the current amplitude that evoked a 1 V or greater replicable waveform. Control hearing and deafened animals underwent surgery, electrode insertion, and maintenance of 4 hours in surgical plane anesthesia without ES.
After four hours of continuous ES, eABR threshold and growth function recordings were repeated. Still in surgical plane, the animals were then euthanized by transcardial perfusion with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (PFA). Cochleae were further fixed by intrascalar perfusion with 0.5 ml PBS/4% PFA gently injected into the round window. Cochleae were than placed in in PBS/4% PFA for 24 hr at 4°C with rotation. After rinsing for 24 hr with PBS, the cochleae were decalcified in 0.12 M EDTA for 7 days at 4°C. In preparation for sectioning, cochleae underwent infiltration with a graded series of sucrose solutions to 30%, and then infiltration under gentle vacuum with degassed and heated OCT11826. After 24 hours, the cochleae were embedded in OCT and frozen for cryosectioning. Serial 6 μm sections parallel to the midmodiolar plane were cut on a Leica freezing microtome and placed on polylysine-coated Fisher Superfrost slides.
Immunohistochemistry was performed as described previously21. Rabbit anti-pJun (1:400, Cell Signaling) was used to label nuclei with phosphorylated Jun and combined mouse antibodies to 200 kDa neurofilament (NF200, 1:400, Sigma), β3 tubulin (TuJ1, 1:400, Abcam), and 150 kDa neurofilament 2H3 (1:400, Developmental Studies Hybridoma Bank) to label neurons and their axons. Sections used for pJun labeling were also labeled with all three anti-neuronal antibodies. Adjacent sections were labeled with rabbit anti-calretinin (1:400, Millipore, formerly Chemicon) to label any surviving inner hair cells. Fluorescently-labeled anti-rabbit (for calretinin, pJun) and anti-mouse (for neurons) secondary antibodies (Alexa dyes, Molecular Probes) were then applied. After a wash with PBS, the sections were stained with Hoechst 33342 to label nuclei and coverslipped. Tissue sections were viewed on a Zeiss Axiovert 200 microscope. Images of control and experimental sections were captured in the same session using identical settings to allow quantitative comparison. Each image was assigned a random number irrelevant to the experimental condition; thus, subsequent analyses were performed by a person blind to the experimental conditions. NIH ImageJ software was used to quantify pJun IF and to correct each nucleus individually for local cytoplasmic or background fluorescence.
Nuclear pJun quantitative immunofluorescence (IF) at each cochlear location was compared among subjects for all spiral ganglion neurons scored. Cochlear location was defined by ½-turn increments proceeding from base to apex, starting at the basal-most ½-turn (adjacent to the round window and inserted electrode) - indicated by Rosenthal Canal 1 (RC1) and proceeding apically to RC4 (Fig. 2). For any given turn (RC), three to four perimodiolar sections were used to quantify pJun IF level. All neurons within each section were sampled and averaged for each RC. pJun IF averages for all cells of a given cochlea included all cells from RC1, RC2 and RC3. SGNs in RC4 were excluded as some subjects had surviving calretinin-positive hair cells in or adjacent to the helicotrema. Values for IF are in arbitrary optical density units consistent among all subjects and replicates. N is defined as the number of cochleae in each subgroup. This number is given in each column in Figures 4–7 in cases where n differs among the columns. All figures show mean ± standard error of the mean (SEM).
Figure 2.
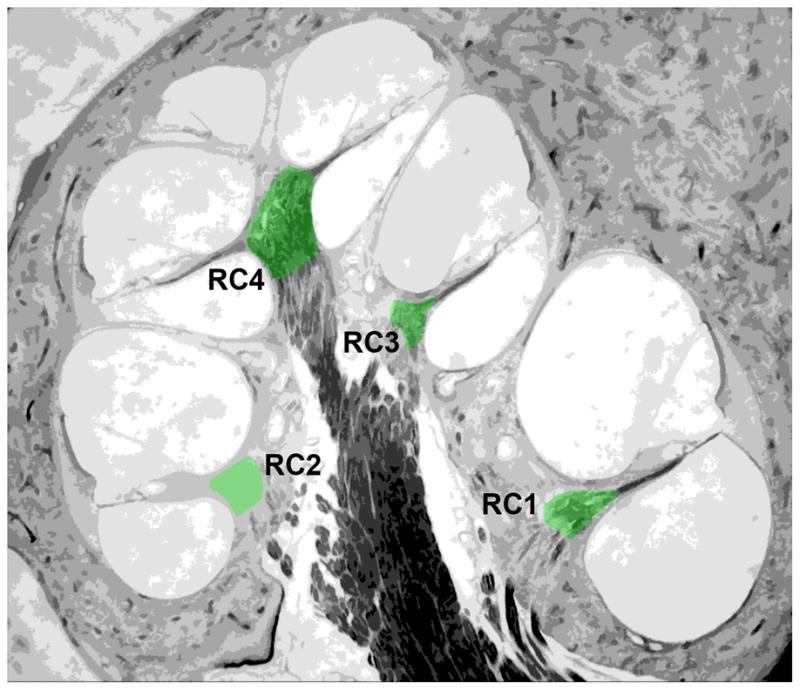
The spiral ganglion (green) in a perimodiolar cross-section of rat cochlea is shown with the designations for location within the ganglion, RC1-4, base to apex.
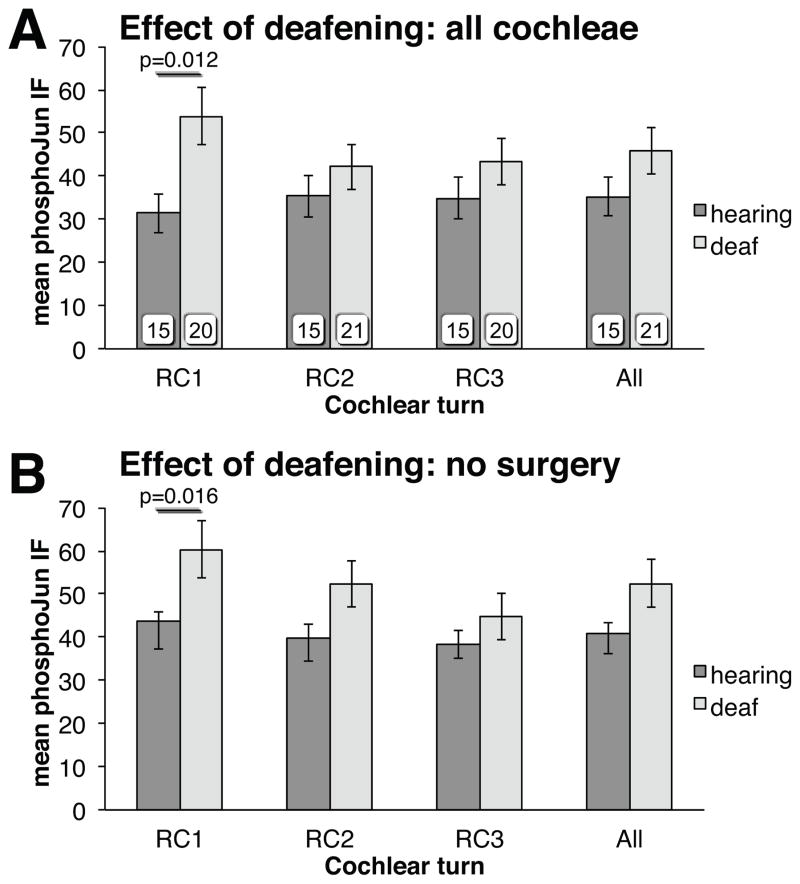
Figure 4.
Effect of deafening on pJun qIF. (A) all deafened unstimulated cochleae; (B) only deafened nonoperated controls, n=11.
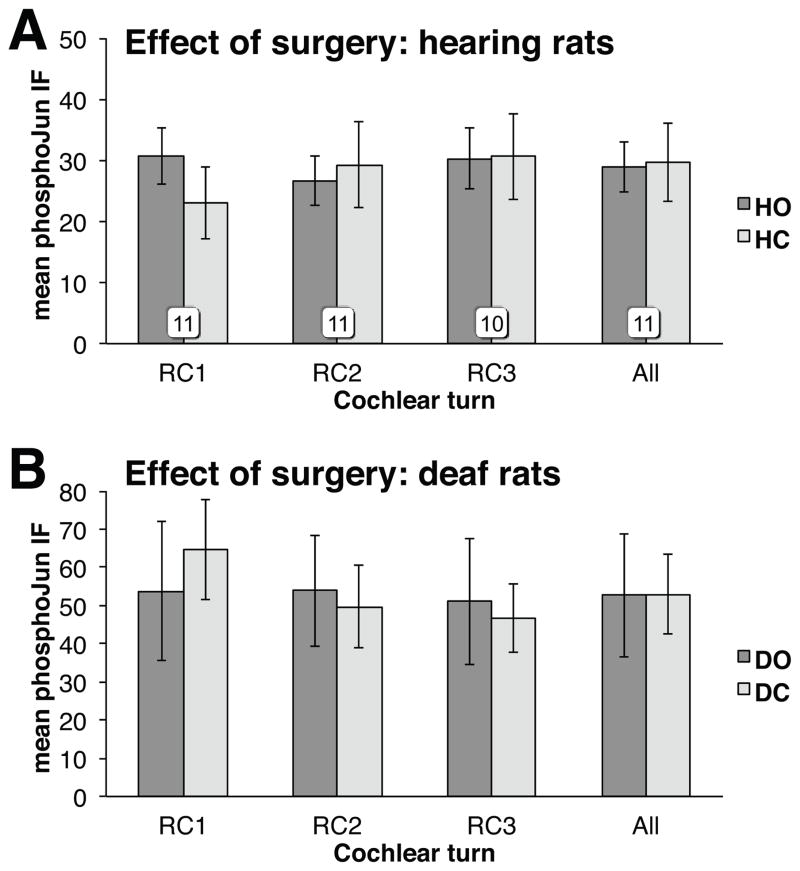
Figure 7.
Effect of the operation on pJun qIF. HO: Hearing Operated; HC: Hearing Contralateral control; DO: Deaf Operated; DC: Deaf Contralateral control. In 7B, n=6.
Non-parametric tests were used to compare groups. In most cases, two-sample Student’s T-tests with a Satterthwaite adjustment for unequal variances agreed with the nonparametric tests. We also report the effect size value η2 from the t-test which measures the percentage of variance in the outcome variable which is explained by the difference between the two groups being compared.
Results
Rats at P32 and at P60 were implanted with electrodes for 4 hr of ES at 100 or 5000 Hz and then euthanized. These two ages were chosen based on our previous data (Alam et al.21) showing that P32 is the earliest time point at which a significant reduction in SGN death can be observed and P60 is the time point at which about half of the SGNs have died.. Thus, we are assessing the effect of ES on proapoptotic signaling in ears at an early stage post-deafening when most SGNs are still alive (P32) and at a later stage when neuronal degeneration is well advanced (P60).
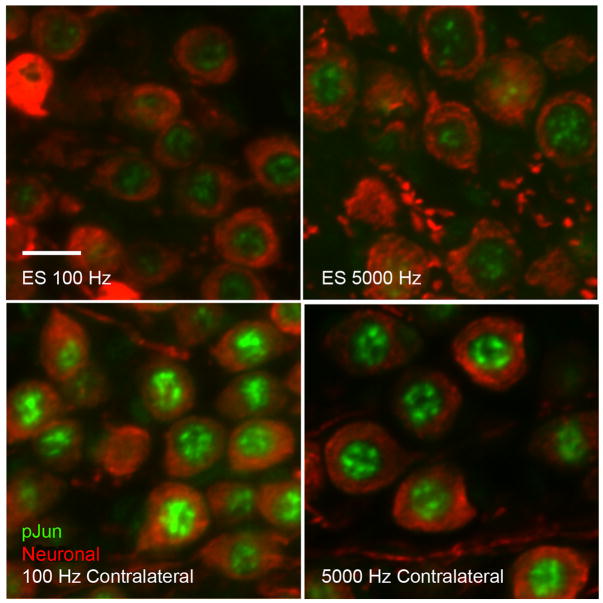
Consistent with the previous results of Alam et al.21 we observed increased pJun immunofluorescence (IF) in the basal turn of deafened rats at P32; moreover, the pJun IF was reduced by ES. Figure 3 shows examples of RC1 pJun IF in electrically stimulated cochleae, at 100 and 5 kHz (upper panels), and the contralateral unstimulated cochlea from the same rat (lower panels). PhosphoJun (nuclear) is labeled green with anti-rabbit IgG and the combined mouse antibodies – NF200, TuJ1, and 2H3 – labeled red with anti-mouse IgG. A striking feature of pJun IF was the high degree of heterogeneity among SGNs within each subject, evident from the high SEM. For example, the cochlear positions in subject R21 had pJun quantitative immunofluorescence (qIF) values: RC1, 16.7±9.9 (SEM); RC2, 13.5±7.8; RC3, 13.1±7.6; all: 14.3±8.5. This is consistent with previous observations after deafening1,21 in that, at any one time, only some rat SGNs in a section exhibit elevated pJun. There was also variability among rats, so change in pJun qIF due to ES in each rat was determined relative to the contralateral unstimulated cochlea.
Figure 3.
Examples of nuclear pJun IF (green) in RC1 SGNs (red). Upper panels: 100 Hz and 5 kHz ES. Lower panels: contralateral unstimulated control. Scale bar = 10 μm.
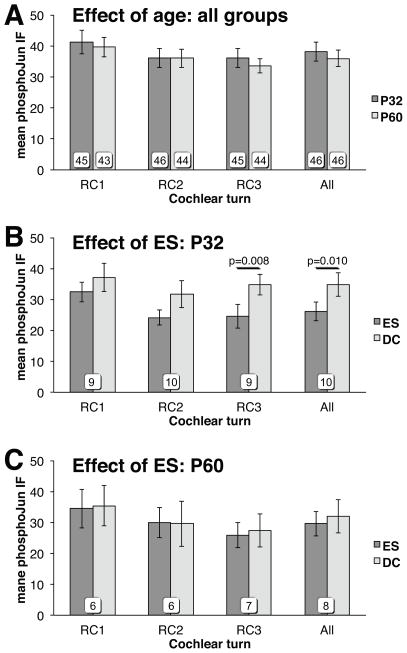
Excluding the electrically stimulated rats, we observed an increase in Jun phosphorylation in all deafened ears relative to all age-matched hearing controls. That is, SGNS in RC1 in cochleae from all deafened unstimulated rats have significantly higher pJun qIF than do RC1 SGNs in control hearing cochleae (Fig. 4A, Mann-Whitney U test, p=0.012, η2=0.17). The increase was significant only in the basal-most region (RC1) of the spiral ganglion. The increase in pJun qIF in RC1 SGNs was also significant when the comparison was limited just to the subset of nonoperative controls (Fig. 4B, Mann-Whitney U test, p=0.016, η2=0.23). These data confirm the results of Alam et al.21 and are consistent with studies showing the cochlea base to be more susceptible to aminoglycoside ototoxicity27.
We next asked whether ES suppresses Jun phosphorylation in SGNs and whether this effect is dependent on deafness duration. Combining data from all unstimulated deafened cochleae, age alone had no significant effect on pJun qIF (Fig. 5A). That is, pJun qIF levels in deafened unstimulated cochleae are not significantly different between P32 and P60 rats (Mann-Whitney U test). ES (high and low rate combined) suppressed Jun phosphorylation in SGNs in P32 cochleae, but not in P60 cochleae (Figs. 5B,C). In SGNs in deafened rat cochleae at P32, pJun qIF was reduced relative to unstimulated contralateral cochlea. The effect was significant over SGNs in all turns (Wilcoxon matched-pairs signed-ranks, p=0.010) and in RC3 (Wilcoxon matched-pairs signed-ranks, p=0.008) but not evident in the basal region. These data imply that an anti-apoptotic effect of ES diminishes rapidly with time after hair cell loss and is less evident in the base where SGNs die sooner. We note that apoptotic signaling, manifested as Jun phosphorylation, is highest in the base after deafening, and that increased Jun phosphorylation is harder to reverse by ES in basal SGNs that have higher levels of Jun phosphorylation prior to ES.
Figure 5.
Effect of deafness duration and of electrical stimulation on pJun qIF. (A) deafened cochleae. (B,C) deafened cochleae with ES and paired contralateral unstimulated deafened control (DC) cochleae.
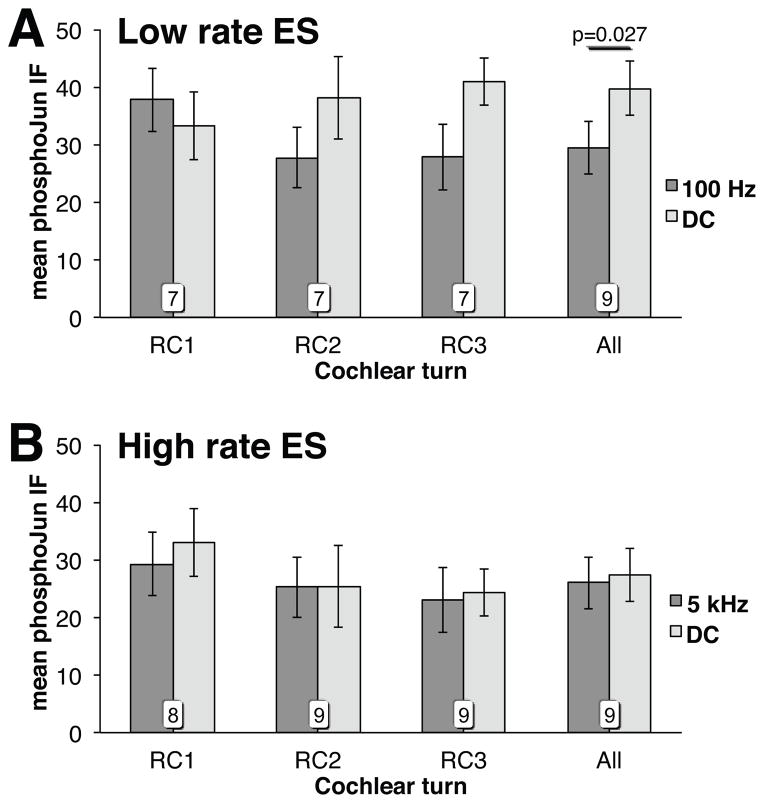
Because some cochlear implant ES strategies involve rates as high as 5 kHz, we compared the ability of 5 kHz ES to that of 100 Hz ES to reduce Jun phosphorylation. As above, pairwise comparisons were used between the stimulated and contralateral unstimulated cochlea in each deafened rat. As shown in Fig. 6A, 100 Hz ES significantly (Wilcoxon matched-pairs signed-ranks, p=0.027, η2=0.24) suppressed Jun phosphorylation in SGNs relative to contralateral unstimulated cochlea when all cochlear locations were averaged (All). In contrast, there was no significant change in pJun qIF with 5 kHz ES (Fig. 6B.)
Figure 6.
Effect of ES rate on pJun qIF in deafened cochleae with ES and paired contralateral unstimulated deafened control (DC) cochleae: 100 Hz (A) or 5 kHz (B).
To test the possibility that round window cochleostomy affects Jun phosphorylation, we assessed pJun qIF in hearing and deafened rats (P32 and P60) subjected to a surgical protocol identical to the one used to insert an electrode into stimulated subjects. As shown in Fig. 7, the procedure did not result in increased pJun qIF in the cochleae of either hearing (Fig. 7A) or deafened (Fig. 7B) rats.
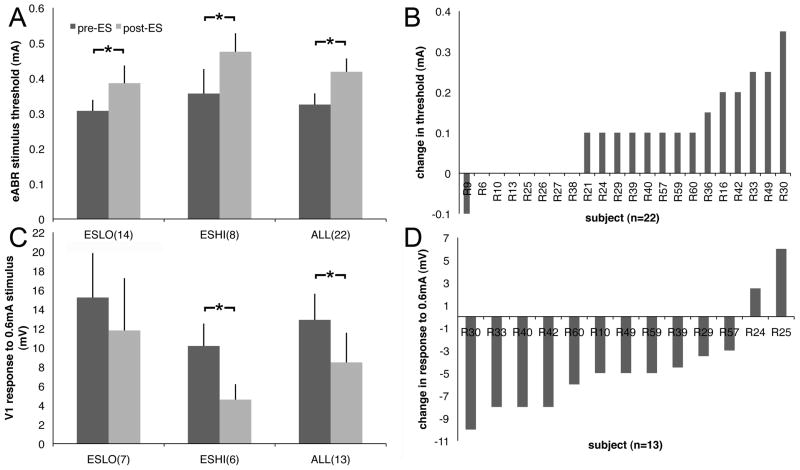
Comparison of eABR threshold before and after stimulation of the same rat reveals a significant (n=22, Wilcoxon matched-pairs signed-ranks, p=0.001, η2=0.65) increase in threshold after high (n=6, Wilcoxon matched-pairs signed-ranks, p=0.016, η2=0.91) or low rate (n=7, Wilcoxon matched-pairs signed-ranks, p=0.047, η2=0.62) ES with no significant difference between them (Fig. 8A,B). Wave 1 amplitude in response to a 0.6 mA stimulus decreased significantly after high rate ES (Fig. 8C,D, n=6, Wilcoxon matched-pairs signed-ranks, p=0.031, η2=0.96). Of the 18 ES subjects used for the pJun qIF determinations, one subject for which there was no growth function measurement at 0.6 mA was excluded. Five additional subjects not used for pJun qIF study were included for a total of 22 subjects.
Figure 8.
Effect of ES on the eABR. (A) Mean thresholds. (B) Individual subjects used in A. (C) Wave 1 amplitude. (D) Individual subjects used in C. ESLO=100 Hz, ESHI=5 kHz, *p<0.01.
Conclusions
The MAP kinase JNK plays an essential role in neuronal apoptosis by phosphorylating Jun28–31. Jun phosphorylation occurs in apoptotic SGNs21 after aminoglycoside exposure during the period of SGN death32. Four hours of acute intracochlear ES decreased pJun immunoreactivity, supporting a hypothesis that ES inhibits apoptosis. This effect was significant in SGNs peri-apical to the stimulus (RC3), but not in basal regions (RC1,2). While insertion trauma may affect SGN survival33, lack of suppression of Jun phosphorylation adjacent to the electrode is unlikely to be due to surgical trauma because pJun levels were not significantly increased in control operated but unstimulated rats. Lack of suppression of Jun phosphorylation in basal turn SGNs, in which Jun phosphorylation is highest, is consistent with the possibility that the ability of ES to suppress Jun phosphorylation in SGNs declines as SGN death becomes more advanced.
The ability of ES to promote SGN viability may also be affected by duration and etiology of deafness5,15,34 and the closely related variable, number of surviving SGNs. Pre-implantation duration of deafness has been shown to be an important predictor of SGN viability in animals35,36, auditory cortex activation in animals37 and humans38, and implant performance in human subjects39. Here, pJun qIF was unaffected by duration of deafness in unstimulated cochleae but ES was effective in suppressing Jun phosphorylation in rats deafened for 2–3 weeks but not in rats deafened for 6–7 weeks. It is unclear whether this is due to the smaller number of SGNs or to another consequence of longer post-deafening duration. Experiments in which deafening procedures result in substantial loss of SGNs prior to electrode implantation show no significant survival-promoting effect of ES16,40. Possibly, increased paracrine neurotrophic support from more ganglion cells may facilitate potential anti-apoptotic effects of ES as does exogenous neurotrophic factor18.
Stimulus parameters such as pulse rate, amplitude and stimulus configuration have been varied clinically to achieve maximum effectiveness in transmitting discriminable auditory signals41, as well as experimentally to assess effects on cortical activation42. High pulse rate stimulation has been proposed as a means of causing a more stochastic activation of SGNs43 and increasing neural excitability44. However 200 Hz ES is more effective than 1,000 Hz ES in preventing SGN death after hair cell loss45. Moreover, here, as in previous studies46, high rate ES tends to suppress eABR more than low rate. These findings are consistent with our observation that low rate ES is more effective than is high rate in suppressing Jun phosphorylation.
Some studies have found that ES in vivo reduces SGN death after hair cell loss33,34,45,47. Other studies have not supported a survival-promoting effect of ES but have found that ES potentiates the survival-promoting effect of the neurotrophic factor BDNF48. Consistent with these observations, we show here that ES can suppress a known proapoptotic intracellular signaling pathway, the JNK-Jun pathway. The effectiveness of such suppression, however, depends on proximity to the electrode, pre-existing level of Jun phosphorylation, duration of deafness, and stimulus rate. These observations may help explain why different studies have come to different conclusions regarding the efficacy of ES in preventing SGN death in vivo.
Summary
Acute patterned intracochlear electrical stimulation (ES) suppresses phosphorylation of Jun, a pro-apoptotic transcription factor in rat spiral ganglion neurons (SGNs). This is consistent with studies showing that ES can, alone or with other trophic factors, slow the death of deafferented SGNs. We show that this effect is significant only for ES administered relatively early after deafening and for low rate (100 Hz) rather than high rate (5 kHz) ES.
Acknowledgments
Funding: This study was supported by R01 DC002961 (SHG), P30 DC010362 (University of Iowa) and a CORE resident research grant from the AAO-HNS (JCK); JCK was also supported by T32 DC000040 (University of Iowa)
The authors would like to thank Prof. Jacob J. Oleson of the University of Iowa Dept. of Biostatistics for essential assistance with the statistical analysis, Erin Bailey for assistance in deafening and testing hearing, Penny Harding for assistance with histology preparation, and Steven Sloan for assistance with microscopy.
Footnotes
This paper was presented as an oral presentation at the AAO-HNS Annual Meeting 2012 and prior to completion was presented as a poster at the ARO MidWinter Meeting 2009.
Conflicts of Interest: None
References
- 1.Bichler E, Spoendlin H, Rauchegger H. Degeneration of cochlear neurons after amikacin intoxication in the rat. Arch Otorhinolaryngol. 1983;237:201–8. doi: 10.1007/BF00453725. [DOI] [PubMed] [Google Scholar]
- 2.Lenoir M, Daudet N, Humbert G, et al. Morphological and molecular changes in the inner hair cell region of the rat cochlea after amikacin treatment. J Neurocytol. 1999 Oct-Nov;28(10–11):925–37. doi: 10.1023/a:1007034508547. [DOI] [PubMed] [Google Scholar]
- 3.Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- 4.Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 5.Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 6.Nadol JB., Jr Degeneration of cochlear neurons as seen in the spiral ganglion of man. Hear Res. 1990;49:141–54. doi: 10.1016/0378-5955(90)90101-t. [DOI] [PubMed] [Google Scholar]
- 7.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants; relation of histopathology to performance. Laryngoscope. 2006;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 8.Pfingst BE, Bowling SA, Colesa DJ, et al. Cochlear infrastructure for electrical hearing. Hear Res. 2011 Nov;281(1–2):65–73. doi: 10.1016/j.heares.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano A, Seldon HL, Clark GM, et al. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998;118:313–26. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- 10.Khan AM, Whiten DM, Nadol JB, Jr, et al. Histopathology of human cochlear implants: correlation of psychophysical and anatomical measures. Hear Res. 2005;205:83–92. doi: 10.1016/j.heares.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Goldwyn JH, Bierer SM, Bierer JA. Modeling the electrode-neuron interface of cochlear implants: effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res. 2010 Sep 1;268(1–2):93–104. doi: 10.1016/j.heares.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997 Mar 15;17(6):1959–70. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen I, Limb CJ, Ryugo DK. The effect of cochlear-implant-mediated electrical stimulation on spiral ganglion cells in congenitally deaf white cats. J Assoc Res Otolaryngol. 2010 Dec;11(4):587–603. doi: 10.1007/s10162-010-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–62. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Leake PA, Stakhovskaya O, Hradek GT, et al. Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hear Res. 2008;242:86–99. doi: 10.1016/j.heares.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki S, Kawano A, Seldon L, et al. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–695. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell A, Miller JM, Finger PA, et al. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd RK, Coco A, Epp SB, et al. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ham J, Eilers A, Whitfield J, et al. c-Jun and the transcriptional control of neuronal apoptosis. Biochem Pharmacol. 2000 Oct 15;60(8):1015–21. doi: 10.1016/s0006-2952(00)00372-5. [DOI] [PubMed] [Google Scholar]
- 20.Eilers A, Whitfield J, Babij C, et al. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998 Mar 1;18(5):1713–24. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam SA, Robinson BK, Huang J, et al. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007 Aug 20;503(6):832–52. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- 22.Onejeme AU, Khan KM. Morphologic study of effects of kanamycin on the developing cochlea of the rat. Teratology. 1984;29:57–71. doi: 10.1002/tera.1420290108. [DOI] [PubMed] [Google Scholar]
- 23.Judkins RF, Li H. Surgical anatomy of the rat middle ear. Otolaryngol Head Neck Surg. 1997;117:438–47. doi: 10.1016/S0194-59989770011-1. [DOI] [PubMed] [Google Scholar]
- 24.Zha XM, Bishop JF, Hansen MR, et al. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hear Res. 2001 Jun;156(1–2):53–68. doi: 10.1016/s0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 25.Hu N, Abbas PJ, Miller CA, et al. Auditory response to intracochlear electric stimuli following furosemide treatment. Hear Res. 2003 Nov;185(1–2):77–89. doi: 10.1016/s0378-5955(03)00261-2. [DOI] [PubMed] [Google Scholar]
- 26.Whitlon DS, Szakaly R, Greiner MA. Cryoembedding and sectioning of cochleas for immunocytochemistry and in situ hybridization. Brain Res Protocols. 2001;6:159–166. doi: 10.1016/s1385-299x(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 27.Choung YH, Taura A, Pak K, et al. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience. 2009 Jun 16;161(1):214–26. doi: 10.1016/j.neuroscience.2009.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 29.Ham J, Babij C, Whitfield J, et al. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–39. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 30.Bogoyevitch MA, Boehm I, Oakley A, et al. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Bruckner SR, Tammariello SP, Kuan CY, et al. JNK3 contributes to c-Jun activation and apoptosis but not oxidative stress in nerve growth factor-deprived sympathetic neurons. J Neurochem. 2001;78:298–303. doi: 10.1046/j.1471-4159.2001.00400.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeong SW, Kim LS, Hur D, et al. Gentamicin-induced spiral ganglion cell death: apoptosis mediated by ROS and the JNK signaling pathway. Acta Otolaryngol. 2010 Jun;130(6):670–8. doi: 10.3109/00016480903428200. [DOI] [PubMed] [Google Scholar]
- 33.Leake PA, Rebscher SJ. Anatomical considerations and long-term effects of electrical stimulation. In: Zeng F-G, Popper AN, Fay RR, editors. Auditory Prostheses, Springer Handbook of Auditory Research. Springer Verlag; New York, NY: 2004. [Google Scholar]
- 34.Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001 Jan;151(1–2):1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 35.Sly DJ, Heffer LF, White MW, et al. Deafness alters auditory nerve fibre responses to cochlear implant stimulation. Eur J Neurosci. 2007 Jul;26(2):510–22. doi: 10.1111/j.1460-9568.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise AK, Tu T, Atkinson PJ, et al. The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection. Hear Res. 2011 Aug;278(1–2):69–76. doi: 10.1016/j.heares.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallon JB, Shepherd RK, Brown M, et al. Effects of neonatal partial deafness and chronic intracochlear electrical stimulation on auditory and electrical response characteristics in primary auditory cortex. Hear Res. 2009 Nov;257(1–2):93–105. doi: 10.1016/j.heares.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green KM, Julyan PJ, Hastings DL, et al. Auditory cortical activation and speech perception in cochlear implant users: effects of implant experience and duration of deafness. Hear Res. 2005 Jul;205(1–2):184–92. doi: 10.1016/j.heares.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996 Sep-Oct;1(5):293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res. 1999 Jul;133(1–2):27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- 41.Rubinstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):444–8. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- 42.Middlebrooks JC, Bierer JA, Snyder RL. Cochlear implants: the view from the brain. Curr Opin Neurobiol. 2005;15(4):488–93. doi: 10.1016/j.conb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein JT, Hong R. Signal coding in cochlear implants: exploiting stochastic effects of electrical stimulation. Ann Otol Rhinol Laryngol Suppl. 2003;191:14–9. doi: 10.1177/00034894031120s904. [DOI] [PubMed] [Google Scholar]
- 44.Heffer LF, Sly DJ, Fallon JB, et al. Examining the auditory nerve fiber response to high rate cochlear implant stimulation: chronic sensorineural hearing loss and facilitation. J Neurophysiol. 2010 Dec;104(6):3124–35. doi: 10.1152/jn.00500.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller JM, Altschuler RA. Effectiveness of different electrical stimulation conditions in preservation of spiral ganglion neurons following deafness. Annals Otol Rhinol & Laryngol Suppl. 1995;166(104):57–60. [PubMed] [Google Scholar]
- 46.Huang CQ, Shepherd RK. Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates: V. Effects of electrode surface area. Hear Res. 2000;146(1–2):57–71. doi: 10.1016/s0378-5955(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 47.Leake PA, Stakhovskaya O, Hradek GT, et al. Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hear Res. 2008;242:86–99. doi: 10.1016/j.heares.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepherd RK, Coco A, Epp SB, et al. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]