Abstract
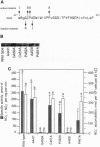
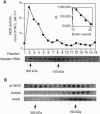
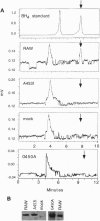
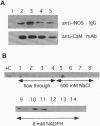
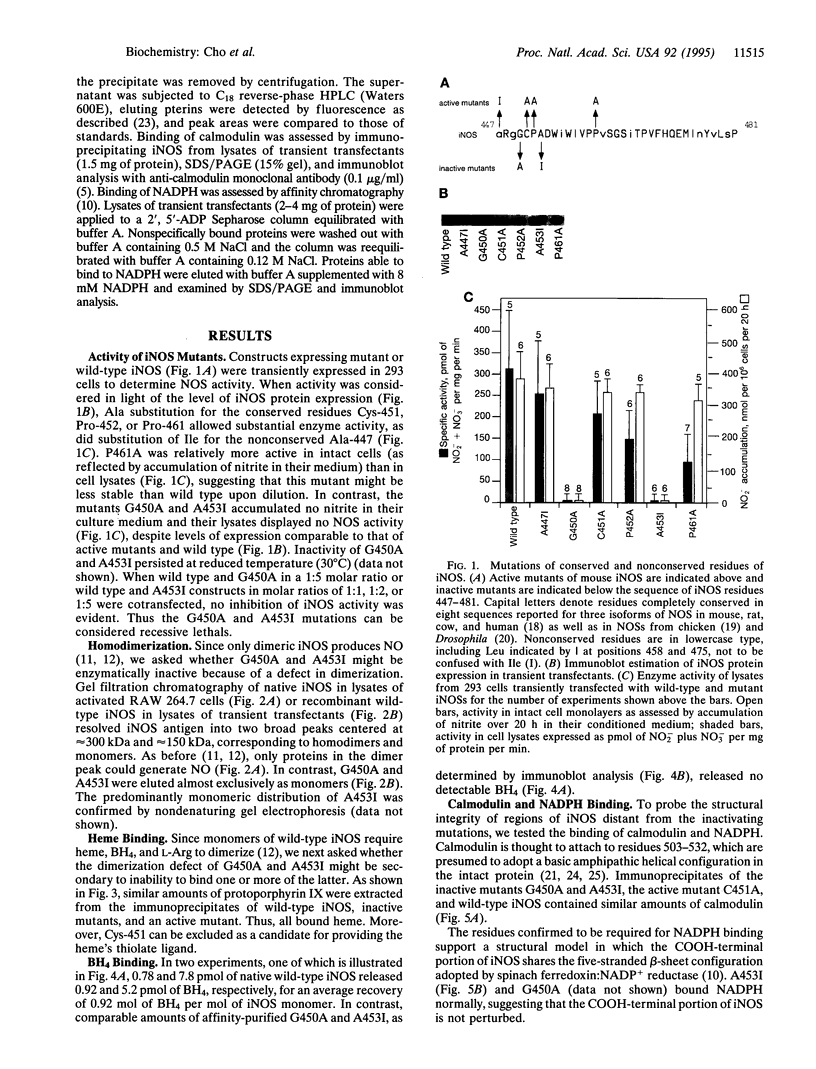
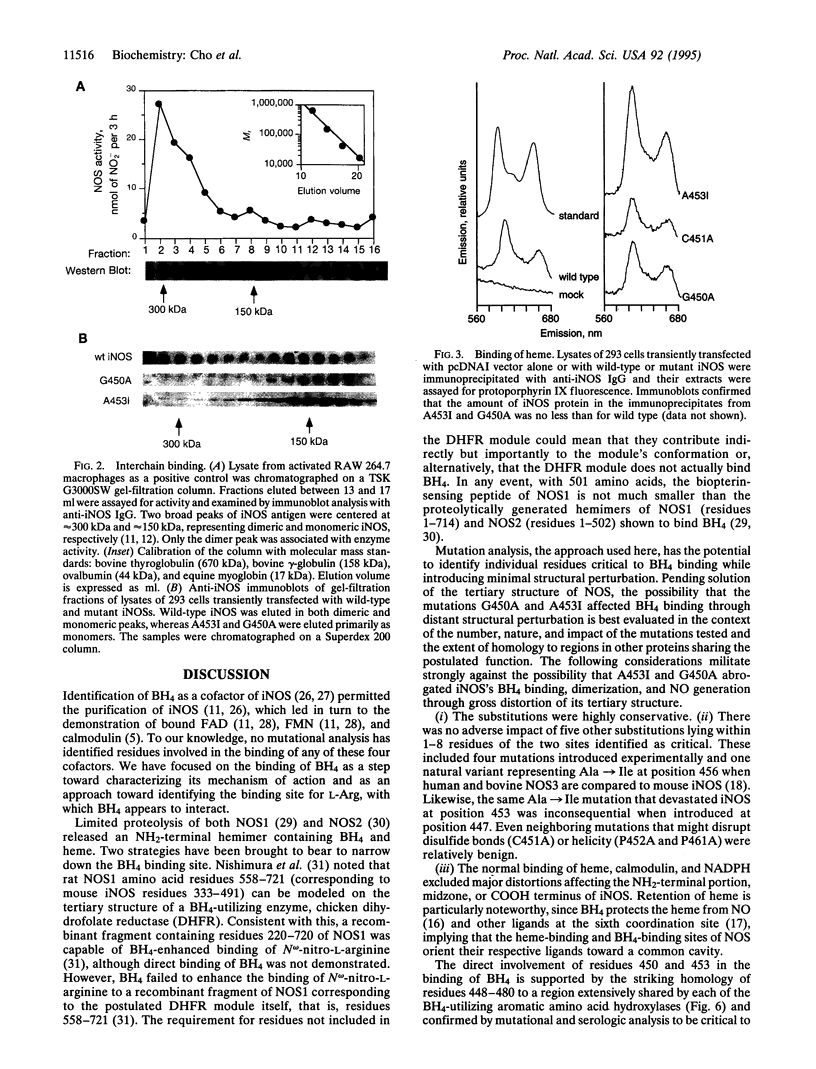
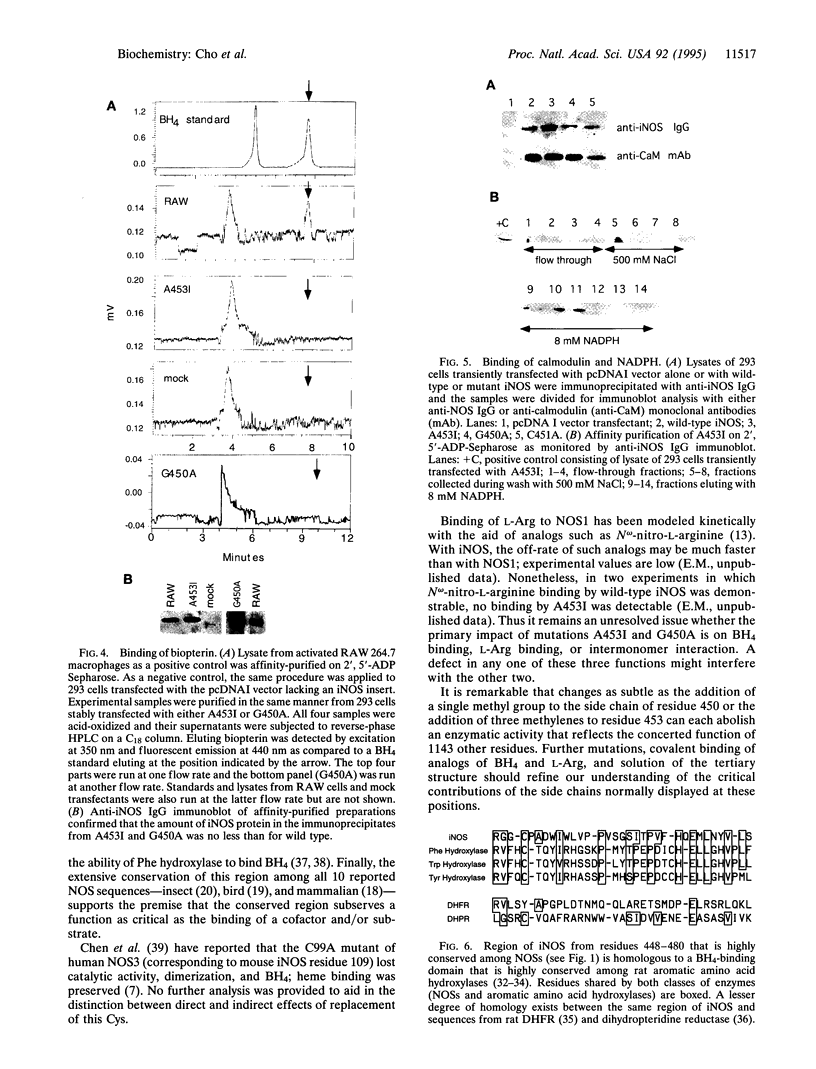
Nitric oxide synthases (NOSs) require tetrahydrobiopterin (BH4) for dimerization and NO production. Mutation analysis of mouse inducible NOS (iNOS; NOS2) identified Gly-450 and Ala-453 as critical for NO production, dimer formation, and BH4 binding. Substitutions at five neighboring positions were tolerated, and normal binding of heme, calmodulin, and NADPH militated against major distortions affecting the NH2-terminal portion, midzone, or COOH terminus of the inactive mutants. Direct involvement of residues 450 and 453 in the binding of BH4 is supported by the striking homology of residues 448-480 to a region extensively shared by the three BH4-utilizing aromatic amino acid hydroxylases and is consistent with the conservation of these residues among all 10 reported NOS sequences, including mammalian NOSs 1, 2, and 3, as well as avian and insect NOSs. Altered binding of BH4 and/or L-arginine may explain how the addition of a single methyl group to the side chain of residue 450 or the addition of three methylenes to residue 453 can each abolish an enzymatic activity that reflects the concerted function of 1143 other residues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baek K. J., Thiel B. A., Lucas S., Stuehr D. J. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem. 1993 Oct 5;268(28):21120–21129. [PubMed] [Google Scholar]
- Chen P. F., Tsai A. L., Wu K. K. Cysteine 184 of endothelial nitric oxide synthase is involved in heme coordination and catalytic activity. J Biol Chem. 1994 Oct 7;269(40):25062–25066. [PubMed] [Google Scholar]
- Cho H. J., Xie Q. W., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992 Aug 1;176(2):599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B. A., Milstien S., Gutierrez J. C., Levine R. A., Yanak B. L., Kaufman S. Isolation and expression of rat liver sepiapterin reductase cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6436–6440. doi: 10.1073/pnas.87.16.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Mercer J. F. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. Structural homology with tyrosine hydroxylase, glucocorticoid regulation, and use of alternate polyadenylation sites. J Biol Chem. 1986 Mar 25;261(9):4148–4153. [PubMed] [Google Scholar]
- Darmon M. C., Guibert B., Leviel V., Ehret M., Maitre M., Mallet J. Sequence of two mRNAs encoding active rat tryptophan hydroxylase. J Neurochem. 1988 Jul;51(1):312–316. doi: 10.1111/j.1471-4159.1988.tb04871.x. [DOI] [PubMed] [Google Scholar]
- Dickson P. W., Jennings I. G., Cotton R. G. Delineation of the catalytic core of phenylalanine hydroxylase and identification of glutamate 286 as a critical residue for pterin function. J Biol Chem. 1994 Aug 12;269(32):20369–20375. [PubMed] [Google Scholar]
- Ghosh D. K., Stuehr D. J. Macrophage NO synthase: characterization of isolated oxygenase and reductase domains reveals a head-to-head subunit interaction. Biochemistry. 1995 Jan 24;34(3):801–807. doi: 10.1021/bi00003a013. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevel J. M., White K. A., Marletta M. A. Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J Biol Chem. 1991 Dec 5;266(34):22789–22791. [PubMed] [Google Scholar]
- James P., Vorherr T., Carafoli E. Calmodulin-binding domains: just two faced or multi-faceted? Trends Biochem Sci. 1995 Jan;20(1):38–42. doi: 10.1016/s0968-0004(00)88949-5. [DOI] [PubMed] [Google Scholar]
- Jennings I. G., Kemp B. E., Cotton R. G. Localization of cofactor binding sites with monoclonal anti-idiotype antibodies: phenylalanine hydroxylase. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5734–5738. doi: 10.1073/pnas.88.13.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P., Schmid M., Leopold E., Schmidt K., Werner E. R., Mayer B. The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem. 1994 May 13;269(19):13861–13866. [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–12234. [PubMed] [Google Scholar]
- Matsuoka A., Stuehr D. J., Olson J. S., Clark P., Ikeda-Saito M. L-arginine and calmodulin regulation of the heme iron reactivity in neuronal nitric oxide synthase. J Biol Chem. 1994 Aug 12;269(32):20335–20339. [PubMed] [Google Scholar]
- McMillan K., Masters B. S. Optical difference spectrophotometry as a probe of rat brain nitric oxide synthase heme-substrate interaction. Biochemistry. 1993 Sep 28;32(38):9875–9880. doi: 10.1021/bi00089a001. [DOI] [PubMed] [Google Scholar]
- McMillan K., Masters B. S. Prokaryotic expression of the heme- and flavin-binding domains of rat neuronal nitric oxide synthase as distinct polypeptides: identification of the heme-binding proximal thiolate ligand as cysteine-415. Biochemistry. 1995 Mar 21;34(11):3686–3693. doi: 10.1021/bi00011a025. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nishimura J. S., Martasek P., McMillan K., Salerno J., Liu Q., Gross S. S., Masters B. S. Modular structure of neuronal nitric oxide synthase: localization of the arginine binding site and modulation by pterin. Biochem Biophys Res Commun. 1995 May 16;210(2):288–294. doi: 10.1006/bbrc.1995.1659. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Regulski M., Tully T. Molecular and biochemical characterization of dNOS: a Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9072–9076. doi: 10.1073/pnas.92.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M. K., Marletta M. A. Characterization of neuronal nitric oxide synthase and a C415H mutant, purified from a baculovirus overexpression system. Biochemistry. 1994 Dec 13;33(49):14723–14732. doi: 10.1021/bi00253a010. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaz M., Hoch J. A., Trach K. A., Hural J. A., Webber S., Whiteley J. M. Structural studies and isolation of cDNA clones providing the complete sequence of rat liver dihydropteridine reductase. J Biol Chem. 1987 Dec 5;262(34):16412–16416. [PubMed] [Google Scholar]
- Sheta E. A., McMillan K., Masters B. S. Evidence for a bidomain structure of constitutive cerebellar nitric oxide synthase. J Biol Chem. 1994 May 27;269(21):15147–15153. [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Wang J., Stuehr D. J., Rousseau D. L. Tetrahydrobiopterin-deficient nitric oxide synthase has a modified heme environment and forms a cytochrome P-420 analogue. Biochemistry. 1995 May 30;34(21):7080–7087. doi: 10.1021/bi00021a020. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H., Kashiwabara Y., Baum M., Weidner J. R., Elliston K., Mumford R., Nathan C. Carboxyl terminus of inducible nitric oxide synthase. Contribution to NADPH binding and enzymatic activity. J Biol Chem. 1994 Nov 11;269(45):28500–28505. [PubMed] [Google Scholar]