Abstract
During the last two decades the fields of molecular and cellular cardiology, and more recently molecular cardiac surgery, have developed rapidly. The concept of delivering cDNA encoding a therapeutic gene to cardiomyocytes using a vector system with substantial cardiac tropism, allowing for long-term expression of a therapeutic protein, has moved from hypothesis to bench to clinical application. However, the clinical results to date are still disappointing. The ideal gene transfer method should be explored in clinically relevant animal models of heart disease to evaluate the relative roles of specific molecular pathways in disease pathogenesis, helping to validate the potential targets for therapeutic intervention. Successful clinical cardiovascular gene therapy also requires the use of nonimmunogenic cardiotropic vectors capable of expressing the requisite amount of therapeutic protein in vivo and in situ. Depending on the desired application either regional or global myocardial gene delivery is required. Cardiac-specific delivery techniques incorporating mapping technologies for regional delivery and highly efficient methodologies for global delivery should improve the precision and specificity of gene transfer to the areas of interest and minimize collateral organ gene expression.
“Advances in science are a matter of time and courage of the mind.”
– Voltaire
Gene transfer may become a preferred option for the treatment of many categories of heart disease in the near future. The growth in the importance of genetic approaches is based on a greater depth of understanding of the cellular and molecular alterations associated with cardiovascular disease states and advances in recombinant DNA technology.
Over the past few years, a key focus in cardiac gene therapy research has been to develop methods to enhance the efficiency of gene delivery [1–3]. Achieving success in this regard requires the application and synergistic interaction of several essential elements. First, a packaging system for genetic material: gene vectors. Vectors should provide transduction of the gene of interest to postmitotic cells such as cardiomyocytes, and ensure sufficient cellular transduction and sufficient duration of transgene expression to achieve clinical efficacy. For some applications such as heart failure (HF), long-term gene expression is required whereas for coronary ischemia, short-term expression may suffice. Second, the vector must be delivered to the myocardium via a transport system (delivery route), which should make the most effective transition possible through biological barriers with minimal losses into the systemic circulation. Third, the therapeutic gene must be expressed in the target tissue. The required synergy relies on the simultaneous identification of targeted molecular pathways of relevance to disease pathogenesis in potential target cells. The successful utilization of all three of the above elements should ultimately result in the transduction of the required number of targeted myocytes with the sufficient gene expression to achieve therapeutic efficacy. The solution of these fundamental problems will find broad application in gene therapies that are rate-limiting for implementation in clinical practice including overcoming biological barriers to gene transfer, mitigation of host immune response and maintenance of sustained transgene expression.
Vectors
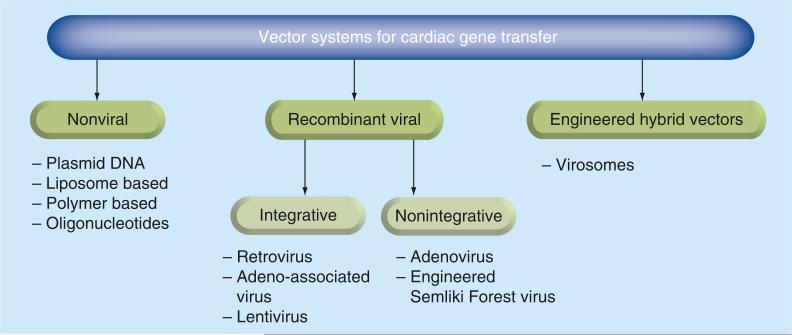
The concept of gene therapy was initially considered to be the simple introduction of a copy of a deficient gene into cells to restore their function. The achievement of this objective is linked with the creation of special vehicles, vectors, which attempt to solve the main technical issues associated with the efficiency of gene delivery to the appropriate cells, expression of the deficient gene for an extended time and the possibility of multiple re-administrations for therapeutic purposes (Figure 1). BOx I lists the major milestones in vector discoveries in heart gene therapy.
Viral vectors
Viruses represent a natural component of human life and therefore they are very attractive as vectors for gene therapy. However, they have to overcome the physiological barriers and the physiological response that protects cells from viral infection. This tissue protection is associated with immunological, inflammatory, proliferative and neurohormonal responses that restrict the viral binding to cell receptors, prevent mRNA translation, and eventually decrease the efficacy of gene transfer and expression of the transferred gene. Furthermore, the engineering of viral genomes does not preclude cytotoxicity in infected cells and muting the effectiveness of the therapeutic intervention [4].
Viral vectors are derived from viruses by replacing their genetic components with the therapeutic or marker gene. Interaction between the vectors and the viral genes can result in the generation of infectious viruses. Considering this, the basic principle used in vector design is the removal of genes encoding viral components essential for viral replication. The viral vectors can be divided into integrating and non-integrating [5]. The integrating vectors can be incorporated into recipient cells and hold the promise of life-long expression. Non-integrating vectors are maintained in an episomal location.
The main challenges facing the vector are as follows:
■ Escaping the neutralizing effects of specific antibodies and nonspecific adsorption to other blood components;
■ Overcoming the endothelial barrier and penetrating the vascular wall for diffusion through the extracellular matrix;
■ Uptake into the cell at the level of the plasma membrane and efficient trafficking to the nucleus;
■ Synthesis by the host of the complementary DNA strand for single-stranded delivery vectors followed by transcription and translation of the transgene [6].
No single vector system is likely to be optimal for all potential cardiac gene therapy applications. A perfect vector should be administered by non-invasive delivery routes, target the optimal number of cells and express a therapeutic amount of transgene product with the desired regulation for a defined length of time [7].
Nonviral vectors
Nonviral gene transfer with plasmid DNA has been extensively used in cardiovascular clinical trials [8–10]. Plasmids are easy to isolate, can be produced in large quantities and generally contain no antigenic proteins. Two decades ago it was demonstrated that plasmid-mediated myocardial gene transfer was detectable in 100% of rats up to 7 days and in 30% up to 60 days after injection [11]. However, many limitations of the plasmid were subsequently identified such as low levels of transduction efficiency, short biological half-life, fast intracellular degradation, non specific binding to unintended targets, low transfer from the cytoplasm into the nucleus, and so forth. Considering these deficiencies, scientists began to combine plasmid delivery with various physical factors such as electroporation, sonoporation, laser-based transfection and magnetic transfection [12–14]. In addition, chemical carriers such as polyplexes and lipoplexes were used, which protect DNA from degradation and facilitate DNA transfer to the cytosol and nucleus [3]. Moreover, promising results with minicircle DNA proved the necessity to continue further studies in this direction [15]. The characteristics of the ideal heart gene therapy vector are listed in BOx 2.
Targets
A large number of potential therapeutic targets in cardiac myocytes have been identified, including cell membrane receptors and their ligands, calcium handling proteins and their modulators, intracellular signaling molecules, transcription factors, contractile proteins in myocytes and their interaction, and so forth. [16,17]. It should be noted that the identification of targets and advancements in experimental models or clinical practice are two completely distinct phases of a single process, which sometimes do not translate from one to another. For example, the βARKct gene was discovered in 1995 [18], and despite the approximately 50 publications demonstrating its efficacy in models of HF it has yet to be used in a clinical trial. Moreover new evidence obtained about the pathophysiological mechanisms of genetic, molecular, neurohormonal and hemodynamic changes related to the development of disparate cardiovascular diseases reveal that cardiac pathology is not an isolated process [19]. It is a complex state simultaneously consisting of both causative and compensatory alterations in multiple signaling pathways. Therefore, the study of potential targets should be conducted through applications of genomics, proteomics and metabolomics acting on specific elements within cardiomyocytes: including myofibrils, sarcoplasmic reticulum and mitochondria. For example, current investigations of HF targets are carried out through the assessment of the impacts on adverse remodeling, contractility, arrhythmias, apoptosis and oxidative stress [20]. However for specific mechanisms of HF such as pressure or volume overload, ischemia, or genetic cardiomyopathy the appropriate therapeutic targets of gene therapy will predictably differ. Therefore, specific molecular targets should be found and investigated depending on the etiology of HF. In this article, the authors discuss the current progress in identifying molecular targets most relevant to ischemic heart disease (IHD) and in HF.
Targets in IHD
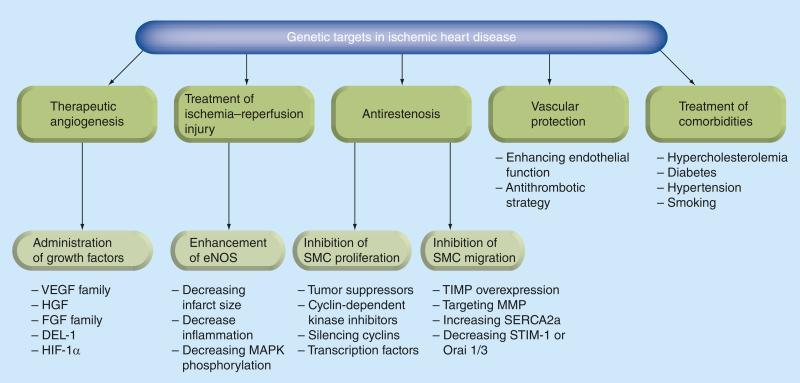
Current gene therapy research efforts in ischemic heart disease should aim to prevent coronary restenosis after angioplasty and graft atherosclerosis after coronary artery bypass graft procedures, stabilize coronary plaques, stimulate angiogenesis, limit reperfusion injury, and provide cardioprotection against apoptosis and fibrosis (Figure 2). However, much of the research is devoted to the study of stimulation of angiogenesis and neovascularization of fibrous post-infarct or poorly perfused (hibernating) myocardium. Therapeutic angiogenesis can be achieved by gene transfer of transforming growth factors such as VEGF [21], HGF [22], FGF [23], HIF-1a [24], angiopoietin-1 [25], IGF-1 [26] and SDF-1 α [27,28]. VEGF has five isoforms that act on tyrosine kinase receptors. This protein factor has been demonstrated to stimulate endothelial cell proliferation, migration and vascular permeability, and to affect fibroblast and smooth muscle growth. Pre clinical gene therapy studies with VEGF in various large animal models of myocardial ischemia have demonstrated stimulation of angiogenesis and improvement in fractional shortening, and also reduction of infarct size and peri-infarct fibrosis [29]. In addition, it has been noted that there is an appearance of apoptosis-resistant cardiomyocytes in the border zone and improvement of myocardial viability [30].
Targets in HF
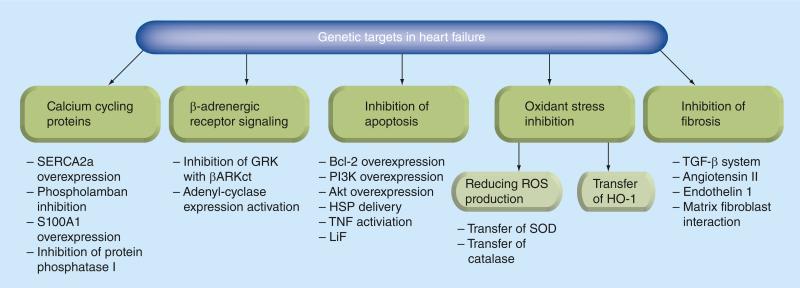
HF is not only a constellation of clinical signs and symptoms indicative of poor performance of the heart as a pump. It is in fact a disorder of structural cellular and molecular processes and the mediators that control them. The myocardial injury might be caused by acute or chronic diseases including myocardial infarction, systemic hypertension, valvular disorders, or genetic cardiomyopathies. An initial pathological insult prompts adaptive and primary compensatory hemodynamic alterations, which in turn lead to activation of the rennin–angiotensin–aldosterone system and a sympathetic nervous system response. These, and other mechanisms, contribute to increasing oxygen consumption and to a decrement in energy efficiency. Eventually these events result in myocyte hypertrophy, fibrosis and apoptosis. Therefore effective gene therapy in HF should impact specific signaling pathways depending on the etiology, stage in the evolution of the process, hemodynamic and structural changes (Figure 3). Up to this point the main achievements have been associated with the efforts of two labs targeting proteins involved in cardiomyocyte calcium handling during excitation–contraction coupling and targeting the cardiac b-adrenergic receptor signaling system. Published results have evaluated SERCA2a overexpression, enhancing activity of SERCA2a through modulation of phospholamban and targeting S100A1, protein phosphatase 1 and ryanodine receptors. Moreover the importance of targeting inhibition of GRK2 and overexpression of β-adrenoreptors, and adenylyl cyclase overexpression has been carefully studied [31–34]. Nonetheless, it is clear that the development and assessment of new potential targets in HF is a very fruitful area for continued investigation.
Gene delivery
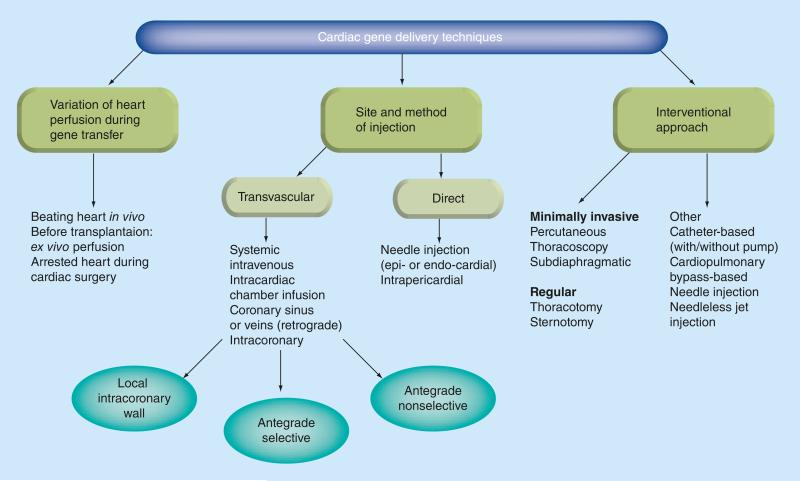
During the dawn of cardiac gene therapy research, the pivotal role of effective gene delivery techniques was underappreciated and delivery efficacy was not quantitatively defined or evaluated. Furthermore, investigators generally considered that only improving the vector tropism could increase cellular transduction. More recently there has been an increased appreciation of the importance of vector delivery. Aside from the previously tested simple intramyocardial and antegrade intracoronary injection methods, a very large number of delivery approaches have appeared including interventional approaches, site-specific injection using mapping techniques, methods of isolated cardiac perfusion and devices to enhance transfection. The authors have tried to organize the most prevalent techniques of gene delivery here for a better understanding of the scope of the problem (Figure 4). The major milestones in the development of gene delivery techniques are represented in BOx 3.
Intramyocardial delivery
There is still no consensus on which method of gene transfer is better: intramyocardial or intra-coronary. Each of these methods has its positive and negative aspects. In our view, both of them will be used in future, though perhaps for differing applications. Intramyocardial transfer was first used in 1990. Histochemical ana lysis of rats’ heart sections injected with a β-galactosidase/plasmid DNA construct demonstrated marker gene activity 3–4 weeks after delivery in vivo [35]. Use of adenovirus-mediated gene transfer in swine resulted in a 140,000-fold increase in the ratio of recombinant protein produced to the number of genomes injected compared with plasmid DNA, and it was also demonstrated that the amount of recombinant protein increases with the amount of virus [36]. The next important step was the finding that the percutaneous endomyocardial injection is feasible and associated with almost three-times greater microsphere retention than epicardial injection [37]. Later studies illustrated the significance of fluoroscopic guidance [38], and especially electroanatomical mapping with the Noga® system to provide gene transfer at the specific and mapped myocardial site [39]. Furthermore, the utilization of this method induced therapeutic angiogenesis [40], modulated cardiac pacemaking activity [41], improved contractility and reversed left ventricular (LV) remodeling [34].
Transvascular delivery
Most heart diseases have diffuse cardiac tissue involvement. Therefore, gene therapy will likely require global transduction of the myocardium that can only be achieved with intravascular transfer. However, simple intravenous gene injection is ineffective probably because of the first-pass pulmonary uptake, and intracavitary (left atrium) delivery provides worse results than intracoronary delivery due to systemic spillage [42]. So the main efforts of scientists have focused on intracoronary transfer. Today it is impossible to imagine the development of modern cardiology without coronary catheterization procedures, performed millions of times per year in the USA. Unfortunately, simple antegrade intracoronary delivery resulted in limited transfection with variable efficacy across animal species and humans. This inefficiency is largely explained by the dilution of gene construct concentration in blood circulation with systemic leakage leading to collateral organ uptake. In an effort to achieve increased efficiency, investigators began to use transient coronary occlusion [43], concomitant coronary venous blockade [44] and cardiac arrest with obstruction of venous return [45]. Although these methods have contributed to improving gene expression in animal models, they will probably achieve limited application in the clinic. Use of retrograde intracoronary transfer through the coronary sinus is undoubtedly a step forward compared with a simple selective intracoronary technique. First, it can be easily applied to clinical practice, particularly in patients with severe coronary artery disease. Second, it allows for prolonged adhesion time of the vector to the cardiac endothelium [46], and overcomes the resistance of precapillary sphincters proximally located on the arterial side of the capillary bed [47]. Finally, it can reduce myocardial reperfusion injury [48].
Gene-eluting stents represent a promising new platform for localized treatment of chronic atherosclerotic occlusion of coronary arteries, restenosis after percutaneous transluminal coronary angioplasty, vein graft disease after coronary artery bypass graft, and so forth. In recent years efforts have been made to increase the incorporation of viral and nonviral vectors in the coating matrix of stents, increase the elution of the target gene from the stent, and enhance transfer to the coronary endothelium [49]. Further research studies are required to assess the feasibility of this technique in clinical trials.
More than one million cardiac procedures with extracorporeal circulation are carried out in the world annually. The successful use of cardio-pulmonary bypass for gene transfer was demonstrated in a preclinical large animal model. The proposed technique allows multiple-pass recirculation of vector through the heart and wash out of the system after gene transfer, which limits extracardiac gene expression and minimizes the potential for an immune response against the vector capsid [50].
Cell-based gene delivery
Cell-based gene delivery provides an alternative method of gene transfer to viral vectors and nonviral vectors. Combining cell transplantation with cardiac gene delivery can improve cell survival, reduce apoptosis, promote angiogenesis and improve LV contractile function. The genetically modified human skeletal myoblasts have been transplanted into a rat heart model of acute myocardial infarction. The study demonstrates that although gene transfection efficiency using liposome-mediated VEGF(165) gene transfer with human skeletal myoblasts was low; hVEGF(165) gene expression efficiency was sufficient to induce neovascularization, improve blood flow and improve heart function [51]. Cell-based transgenic overexpression of netrin-1 attenuated infarct size expansion and promoted angiogenesis in the infarcted heart with concomitant preservation of heart function indices [52]. It was also reported that the transplantation of VEGF-expressing skeletal myoblasts by HVJ-liposome-mediated gene transfection results in transient, high-level VEGF expression within rat myocardium suffering from acute infarction. This expression leads to successful angiogenesis, which is associated with a reduction in infarct size and an improvement in cardiac function without tumor formation [53]. The ideal gene delivery technique model is outlined in BOx 4.
Clinical trials
The number of cardiovascular gene therapy clinical trials was 144 by 2011, accounting for 8.5% of total trials and becoming the second most investigated application after cancer diseases [54,55]. The vast majority of cardiac trials have addressed therapeutic angiogenesis.
However, the lack of available cardiotropic vectors, the absence of knowledge about basic principles of gene transfer techniques and the difficulty in finding effective potential cellular targets for intervention has hampered the development of effective genetic treatments for heart diseases. On the other hand, in spite of the fact that cardiovascular disease remains the leading cause of mortality, morbidity and health-care expenditure around the world, and in spite of the evidence of the benefits and feasibility of cardiac gene therapy in preclinical models, the number of oncological human trials is 7.6-times higher than cardiovascular. No doubt the insufficient number of trials is a major reason for the lack of answers to such questions as: which category of patients will benefit from gene therapy; how do we improve myocyte transduction and gene transfer efficiency; and what are the primary targets for HF patients? Only the identification of clear indications for gene therapy combined with the optimization of delivery routes and targets are expected to generate new genetic treatments for ischemic heart disease and HF.
IHD
Based on the promising results of experiments on small and large animals, several clinical trials were carried out using specific isoforms of VEGF mainly in patients with no other therapeutic options [56]. Although there have been some improvements in angina class and stress sestamibi scans, none of the randomized controlled Phase II/III trials have demonstrated clinically relevant positive effects [56,57]. The most likely reason for this apparent discrepancy may be related to the placebo effect, patient selection and ineffective gene expression [57]. The efficacy of FGF to promote angiogenesis has been well established in an animal model of coronary ischemia. Intracoronary delivery of adenovirus vector encoding FGF4 in pigs with myocardial ischemia increased regional perfusion. In addition to angiogenesis, FGF5 overexpression can stimulate adaptive hypertrophy and improve wall thickening in hibernating myocardium [58].
The AGENT (angiogenic gene therapy) 3 and AGENT 4 trials using a low and high dose of adenoviral-mediated intracoronary administration of FGF4 were initiated and enrolled 532 patients. The authors of the work found a beneficial effect on total exercise treadmill test, time to ST-segment depression and angina [59]. Perhaps the most important indicator of the more than 15-year history of clinical trials in IHD is the fact that no adverse safety events have been detected. Long-term follow-up times up to 10 years in the Kuopio Angiogenesis Trial in 103 patients did not demonstrate increases in the incidence of cancer, diabetes, arrhythmias or any other disease compared with the control group [60]. This promising safety profile certainly encourages the development of further clinical trials.
HF
Specifically in HF, there are currently a number of ongoing trials targeting various pathways for rescuing the failing myocardium. The first clinical trial of gene therapy in patients with HF (CUPID) was launched in 2008 [31]. The goal of this trial was to evaluate the safety profile and effect of SERCA2a cDNA by delivering a recombinant adeno-associated virus type 1. Participants in this trial were administered a single intracoronary infusion of adeno-associated virus type 1/SERCA2a. A 12-month follow-up demonstrated an acceptable safety profile. Improvement was detected in several patients. Phase II of the CUPID trial enrolled 39 patients to receive one of three escalating doses of intra-coronary delivery of SERCA2a versus placebo. At 6 months, patients reported improvement or stabilization of clinical symptoms and functional tests as well as LV function (ejection fraction and LV end-systolic volume). Currently the effects of SERCA2a are being investigated in two other clinical trials in the UK and France. In a separate clinical study, adenovirus-5 encoding human adenylyl cyclase type 6 is being delivered through intracoronary injection to patients with HF. The trial is currently enrolling patients.
Preclinical studies indicate that adult stem cells induce tissue repair by activating endogenous stem cells through SDF-1 [61]. A first-inhuman dose-escalation study with 12 months of follow-up in 17 subjects with ischemic cardiomyopathy was performed with endocardial delivery of DNA plasmid encoding human SDF-1. Therapeutic efficacy was evaluated by cardiac perfusion via computed tomography imaging, New York Heart Association classification, 6-min walk distance, and quality of life after 4 and 12 months. The clinical data suggest that re-establishment of SDF-1 expression is safe. Data from middle- and high-dose groups report statistically significant improvements in quality of life, and New York Heart Association classification [62]. The major milestones in cardiac gene therapy clinical trials are outlined in BOx 5.
Main obstacles delaying progress in effective clinical translation of cardiac gene therapy
It is well known that the process of moving to human clinical trials requires establishing proof of principle in vitro, then in transgenic, murine and/or rodent models, and eventually in large animal preclinical models. The experience obtained from animal models demonstrates that distinct cardiac diseases require specific genetic treatment; namely ischemic, valvular or hypertensive pathological conditions need to employ different gene therapy strategies and targets [33]. Moreover, even the large animals used in preclinical studies differ considerably from the human patients in terms of age, physiology and concomitant diseases that are difficult to replicate in animals. Therefore, not surprisingly, animal experiments with recombinant growth factors for angiogenesis were very reassuring but the clinical studies demonstrated limited benefits [60]. Moreover most of the clinical trials in IHD involve the use of gene therapy for ‘no-option’ patients with multiple comorbidi-ties that limit the efficacy of gene transfer. In these conditions a single dose of therapy may not cause measurable improvement. Thus, it is very important to choose the correct group of cardiac patients for gene therapy. It is understandable that a group of selected patients should be standardized. In particular this applies to the stage of disease, pharmacological treatment, angiographic findings and comorbidities.
In addition, many trials have used variable and often subjective parameters for endpoints of therapy such as duration of exercise before angina, ejection fraction determined by echocardiogram, perfusion measured by single-photon emission computed tomography, exercise treadmill time, and so forth. It is necessary to apply techniques such as cardiac magnetic resonance perfusion imaging and positron emission tomography that are much more sensitive and precise in determining parameters such as LV systolic and diastolic volume, infarct size, and perfusion in different ischemic areas. Moreover, the optimal dose and timing of gene therapy should be standardized.
One of the main factors limiting the positive effect of gene therapy is transient therapeutic gene expression. This problem is multifaceted. First, many clinical trials were carried out with nonviral or short-term efficacy virus vectors, and the protocols used a single-dose method of delivery [57]. Second, techniques of gene delivery applied in clinical trials were simple without attempts to limit collateral expression or to extend vector residence time, overcome the endothelial barrier or to utilize retrograde transcoronary transfer [3]. Evaluation of gene expression was commonly performed on the basis of functional cardiac parameters and not on sensitive techniques such as reverse transcriptase real-time PCR. Finally, many authors believe that a strong placebo effect with reported clinical improvement in several clinical trials complicates the true assessment of gene therapy advantages. Collectively, these concerns underscore the need to conduct randomized, blinded, placebo-controlled studies in large-scale trials [57,60,63]. Also, identification of ischemic areas via electromechanical mapping systems, single-photon emission computed tomography or MRI is needed for more effective use of direct intramyocardial gene injections. Gene therapy should also be tested as an adjunctive treatment to conventional therapy.
Future perspective
Over the last two decades genetic approaches have undergone substantial changes, mainly due to the emergence of new data contributing to the understanding of normal cardiovascular function and the pathophysiologic basis of cardiovascular diseases at the molecular level. Gene therapy holds promise for correcting key molecular defects. Currently being researched using different forms of gene manipulation include gene overexpression, gene augmentation designed to overexpress a protein, gene replacement and correction of a missing of defective or nonactive gene, gene knockdown-inhibition of host cell gene expression, and miRNA upregulation with the potential to affect large numbers of interrelated mRNAs. For gene therapy to be successful, the requisite amount of a therapeutic gene must be delivered into the target tissue to alter the cellular pattern of gene expression to produce a therapeutic effect. Still, there are many questions that need to be answered. One of them is: what is the threshold for phenotypic correction in myocytes? For example, creation of a biological pacemaker requires the focal genetic modification of only a modest number of cells. By contrast, restoring myocardial contractility in HF has a higher threshold and may require successful gene transfer to a majority of the cells. Future gene therapy approaches will likely use a combined approach that targets diverse mechanisms of the disease and their relevant signaling pathways. For these goals researchers may use a multigene approach. For example, supportive interaction between angiopoietin-1 and Akt during mesenchymal stem cell transplantation enhanced cell survival, improved angiomyogenesis and restored global cardiac function [64]; and simultaneous transgenic overexpression of different growth factors activates diverse signaling pathways for improving cellular repair of the infarcted heart [65]. Another promising approach is the induction of miRNAs with gene or cell therapy that act as intracellular and intercellular mediators in many cardiac diseases and HF. Using miRNA has been considered as a potential novel therapeutic strategy that can provide functional recovery of the ischemic heart [66,67]. Moreover, gene targeting of cellular organelles that have their own genome, such as mitochondria, can simulate the effects of preconditioning for improved donor stem cell survival in the infarcted heart and provide an alternative therapeutic option for acute myocar-dial infarction [68]. Finally, gene therapy may be an adjunct to conventional therapies including percutaneous coronary intervention and standard or minimally invasive cardiac surgical procedures.
Box 1. Milestones in vector discoveries in cardiac gene therapy.
■ Cardiac gene therapy research intensified after it was demonstrated that the b-galactosidase gene under the control of the Rous sarcoma virus promoter could be introduced and expressed in cardiac myocytes after direct injection of plasmid DNA into the rat left ventricle [35]
■ Establishment of the first infectious clone of adeno-associated virus serotype 2 as a gene therapy vector [69]
■ Direct myocardial injection of a recombinant vector based on adenovirus serotype 5 was reported to program gene expression in the cardiomyocytes of a large animal species [36]
■ It was demonstrated that viral vectors have several orders of magnitude more efficiency than plasmid DNA [70]
■ The ability to genetically modify the myocardial cells with adeno-associated virus was shown in a rat's heart after intramyocardial injection and in a pig's heart after selective coronary catheterization [71]
■ Isolation of adeno-associated virus serotype 9 as a vector with a high natural affinity for rodent cardiac tissue was achieved [72]
Box 2. Ideal vector model.
■ Cardiotropism with homogeneous delivery to cardiomyocytes
■ Minimal direct toxicity, and minimal immune and inflammatory responses after delivery
■ A large coding capacity: sufficient to deliver the therapeutic gene
■ Ability to transduce non-dividing cells such as myocytes
■ Efficient production at high titers
■ Ability to express its genetic cargo over a time period sufficient to achieve therapeutic efficacy
■ Ability to replicate and integrate into the human genome
■ Interaction with regulatory elements augmenting communications between transcription and cell action
■ Capacity for site-specific integration, allowing for long-term expression
Box 3. Major milestones of development of gene delivery methods.
■ Application of intramyocardial delivery with plasmid DNA [35]
■ The possibility of antegrade intracoronary approach in vitro was demonstrated [73]
■ The benefits of retrograde transfer through the coronary sinus were found [46]
■ Use of gene-eluting stents in ananimal model [74]
■ Development of ‘closed-loop’ recirculatory system with extracorporeal circulation [75]
Box 4. Ideal gene delivery technique model.
■ Simple and reproducible
■ Promotes a homogeneous gene expression profile
■ Ability to have repeat administration
■ Low incidence of collateral organ expression
■ Low inflammatory reaction
■ Includes closed-loop circulatory system with multiple-pass kinetics
Key Terms.
Gene delivery: Various methods used to transfer the gene construct, (including viral or nonviral systems) selected to target the cells.
Gene vectors: Special vehicles providing the delivery of DNA or other genetic material into cells. This process can be performed in vivo and in vitro. Delivery of genes by a virus is termed transduction. Viral vectors are derived from viruses that use molecular mechanisms to transport their genomes inside the cells they infect. The genetic components of the virus are replaced with the gene of interest when constructing the vector.
Gene therapy clinical trials: Sets of molecular, genetic and clinical tests for human health intervention. Clinical trials involve four phases, each with a different purpose.
Gene targeting: Diseases or signaling pathways of diseases caused by single or multiple genetic defects that are treated with the introduction of genes directly into cells.
Box 5. Major milestones in clinical trials.
■ Basic fibroblast growth factor-1 was injected intramyocardially close to bypassed vessels during cardiac surgery in 20 patients [76]
■ Clinical trial in five patients with ischemic heart disease (refractory angina). The authors of the work injected naked plasmid DNA encoding VEGF (phVEGF165) directly into the ischemic myocardium via a thoracotomy [77]
■ Cardiac gene transfer (21 patients) was optimized by direct intramyocardial delivery of adenovirus containing VEGF cDNA (AdVEGF121) as an adjunct to coronary artery bypass grafting [78]
■ Catheter-based intracoronary approach after angioplasty was first evaluated as a method of clinical gene transfer [79]
■ First-in-human clinical trial with single intracoronary infusion of adeno-associated virus type 1/SERCA2a in advanced heart failure in nine patients [31]
Executive summary.
Vectors for cardiac gene transfer
■ Adeno-associated viruses are nonpathogenic, have a long-term expression profile, have low immunogenicity, and serotypes 6 and 9 have significant cardiac tropism. However, they have a small insert capacity, are complex to produce and low titers limit their use.
■ Adenoviruses are readily produced in high titers and have high transduction performance without integration into the host genome. They induce inflammation and a potent immune response, and have short-term expression and nonspecific cellular tropism.
■ Lentiviruses have high transduction efficiency, good long-term expression and induce a low immune response. They provide the ability to integrate into the host cells’ genomes, but they increase the risk of oncogenesis and demonstrate limited cardiac tropism.
■ Naked plasmid DNA is constructed based on simple methodology, has a large DNA insert capacity and minimal safety risks. Low transduction efficiency and a transient expression profile are the main limitations.
Gene targets in ischemic heart disease
■ Creation of gene therapy targets in ischemic heart disease should be directed to prevent coronary restenosis after angioplasty and graft atherosclerosis after coronary artery bypass procedures, stabilize arterial plaques, stimulate angiogenesis, limit reperfusion injury, and provide cardioprotection. Currently, much of the research is devoted to the study of stimulation of angiogenesis and neovascularization of the myocardium. Therapeutic angiogenesis can be achieved by gene transfer of transforming growth factors such as VEGF, HGF, FGF and HIF-1a.
Gene targets in heart failure
■ Effective gene therapy in heart failure should impact different signaling pathways depending on the etiology and stage of the process, as well as hemodynamic and structural changes. Currently, primary targets in heart failure include increasing contractility and reducing adverse remodeling by affecting calcium cycling proteins and b-adrenergic receptor signaling. Other promising goals are inhibition of apoptosis, fibrosis, myocardial hypertrophy and oxidant stress depression.
Intramyocardial cardiac gene delivery methods
■ This technique can be performed using catheter-based and minimally invasive surgical approaches. It allows a high local vector concentration in cardiac tissue and is good for focal treatment of regional ischemia and conduction abnormalities. A big advantage is the lack of a need to traverse the endothelial barrier. Shortcomings include the transgene expression being limited to the injection site, no prevention of systemic viral escape and non-homogeneous distribution, which is not desirable for many diseases.
Transvascular cardiac gene delivery methods
■ This route usually demonstrates efficient gene transfer and allows for the vector's delivery to the whole myocardium. Additionally, it offers the ability to perform repeat administrations and create ‘closed loop’ recirculation systems for enhanced transfer and minimized collateral exposure. The disadvantages include dilution of the vector in systemic blood, which leads to rapid washout from the heart, poor performance in atherosclerotic vessels, high incidence of cell-mediated immune response and exposure of the vector to neutralizing antibodies.
Reasons delaying progress in effective clinical translation of cardiac gene therapy
■ Insufficient therapeutic gene expression limits the positive effect of gene therapy in clinical trials. This is due to:
■ Single-dose methods of delivery with nonviral or non-integrating virus vectors;
■ The use of simple techniques of gene transfer without methods limiting collateral expression and extending vector residence time;
■ A strong placebo effect complicates the real assessment of gene therapy advantages;
■ The lack of standardized dose and timing of transfer, correct selection of patients, and evaluations of molecular and clinical criteria.
Figure 1.
Vector systems for cardiac gene transfer.
Figure 2. Gene targets in ischemic heart disease.
DEL-1: Developmental endothelial locus-1; eNOS: Endothelial nitric oxide synthase; SMC: Smooth muscle cells.
Figure 3. Genetic targets in heart failure.
βARKct: β-ARK carboxyl-terminus; Bcl-2: B-cell lymphoma 2; HSP: Heat shock proteins; ROS: Reactive oxygen species.
Figure 4.
Cardiac gene delivery techniques.
Acknowledgements
The authors acknowledge the National Heart, Lung, and Blood Institute Gene Therapy Resource Program.
The preparation of this article was supported by the NIH grant 1-R01 HL083078–01A2.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Rapti K, Chaanine AH, Hajjar RJ. Targeted gene therapy for the treatment of heart failure. Can. J. Cardiol. 2011;27(3):265–283. doi: 10.1016/j.cjca.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleger ST, Brinks H, Ritterhoff J, et al. Heart failure gene therapy: the path to clinical practice. Circ. Res. 2013;113(6):792–809. doi: 10.1161/CIRCRESAHA.113.300269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz MG, Fargnoli AS, Bridges CR. Myocardial gene transfer: routes and devices for regulation of transgene expression by modulation of cellular permeability. Hum. Gene Ther. 2013;24(4):375–392. doi: 10.1089/hum.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzau VJ, Beatt K, Pompilio G, Smith K. Current perceptions of cardiovascular gene therapy. Am. J. Cardiol. 2003;92(9B):18N–23N. doi: 10.1016/s0002-9149(03)00964-0. [DOI] [PubMed] [Google Scholar]
- 5.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat. Rev. Genet. 2000;1(2):91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 6.Muller OJ, Ksienzyk J, Katus HA. Gene-therapy delivery strategies in cardiology. Future Cardiol. 2008;4(2):135–150. doi: 10.2217/14796678.4.2.135. [DOI] [PubMed] [Google Scholar]
- 7.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 8.Henry TD, Annex BH, Mckendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 9.Makinen K, Manninen H, Hedman M, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol. Ther. 2002;6(1):127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 10.Reilly JP, Grise MA, Fortuin FD, et al. Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients. J. Interv. Cardiol. 2005;18(1):27–31. doi: 10.1111/j.1540-8183.2005.04026.x. [DOI] [PubMed] [Google Scholar]
- 11.Buttrick PM, Kass A, Kitsis RN, Kaplan ML, Leinwand LA. Behavior of genes directly injected into the rat heart in vivo. Circ. Res. 1992;70(1):193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- 12.Ayuni EL, Gazdhar A, Giraud MN, et al. In vivo electroporation mediated gene delivery to the beating heart. PLoS ONE. 2010;5(12):e14467. doi: 10.1371/journal.pone.0014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13■■.Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108(8):1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [Ultrasound-targeted microbubble destruction directs plasmid transgene expression with greater specificity than viral vectors.] [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Jena PK, Behera S, Lockey RF, Mohapatra S, Mohapatra S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine. 2010;6(1):64–69. doi: 10.1016/j.nano.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayrhofer P, Schleef M, Jechlinger W. Use of minicircle plasmids for gene therapy. Methods Mol. Biol. 2009;542:87–104. doi: 10.1007/978-1-59745-561-9_4. [DOI] [PubMed] [Google Scholar]
- 16.Lavu M, Gundewar S, Lefer DJ. Gene therapy for ischemic heart disease. J. Mol. Cell Cardiol. 2011;50(5):742–750. doi: 10.1016/j.yjmcc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ. Res. 2012;110(5):777–793. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch WJ, Rockman HA, Samama P, et al. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a β ARK inhibitor. Science. 1995;268(5215):1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 19.Nabel EG. Principles of Cardiovascular Molecular Biology and Genetics. In: Bonow RO, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier Health Sciences; MO, USA: 2011. pp. 57–70. [Google Scholar]
- 20.Yerevanian A, Yerevanian A, Hajjar RJ. Progress in gene therapy for heart failure. J. Cardiovasc. Pharmacol. 2013 doi: 10.1097/FJC.0b013e3182a2e8b8. doi:10.1097/FJC.0b013e3182a2e8b8 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Taimeh Z, Loughran J, Birks EJ, Bolli R. Vascular endothelial growth factor in heart failure. Nat. Rev. Cardiol. 2013;10(9):519–530. doi: 10.1038/nrcardio.2013.94. [DOI] [PubMed] [Google Scholar]
- 22.Jayasankar V, Woo YJ, Bish LT, et al. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation. 2003;108(Suppl. 1):II230–II236. doi: 10.1161/01.cir.0000087444.53354.66. [DOI] [PubMed] [Google Scholar]
- 23.Vatner SF. FGF induces hypertrophy and angiogenesis in hibernating myocardium. Circ. Res. 2005;96(7):705–707. doi: 10.1161/01.RES.0000164184.63158.6c. [DOI] [PubMed] [Google Scholar]
- 24.Ytrehus K. Hypoxia-inducible factor 1 a: a new piece in the preconditioning puzzle. Cardiovasc. Res. 2008;77(3):443–444. doi: 10.1093/cvr/cvm104. [DOI] [PubMed] [Google Scholar]
- 25.Ye L, Haider H, Jiang S, et al. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. Eur. J. Heart Fail. 2007;9(1):15–22. doi: 10.1016/j.ejheart.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008;103(11):1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 27.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1α promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J. Mol. Cell. Cardiol. 2007;42(4):792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penn MS. Importance of the SDF-1. CXCR4 axis in myocardial repair. Circ. Res. 2009;104(10):1133–1135. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera Janavel G, Crottogini A, Cabeza Meckert P, et al. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Ther. 2006;13(15):1133–1142. doi: 10.1038/sj.gt.3302708. [DOI] [PubMed] [Google Scholar]
- 30.Ferrarini M, Arsic N, Recchia FA, et al. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ. Res. 2006;98(7):954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 31.Hajjar RJ, Zsebo K, Deckelbaum L, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J. Card. Fail. 2008;14(5):355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Kairouz V, Lipskaia L, Hajjar RJ, Chemaly ER. Molecular targets in heart failure gene therapy: current controversies and translational perspectives. Ann. NY Acad. Sci. 2012;1254:42–50. doi: 10.1111/j.1749-6632.2012.06520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz MG, Fargnoli AS, Tomasulo CE, Pritchette LA, Bridges CR. Model-specific selection of molecular targets for heart failure gene therapy. J. Gene Med. 2011;13(10):573–586. doi: 10.1002/jgm.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34■■.Rengo G, Lymperopoulos A, Zincarelli C, et al. Myocardial adeno-associated virus serotype 6-βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119(1):89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [Demonstrates that long-term cardiac AAV6-BARKct gene therapy results in improvement of cardiac function and reverses remodeling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin H, Parmacek MS, Morle G, Bolling S, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- 36■■.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90(5):2414–2424. doi: 10.1161/01.cir.90.5.2414. [Demonstrates that intramyocardial injection of adenovirus can program recombinant gene expression in cardiomyocytes.] [DOI] [PubMed] [Google Scholar]
- 37■■.Grossman PM, Han Z, Palasis M, Barry JJ, Lederman RJ. Incomplete retention after direct myocardial injection. Catheter Cardiovasc. Interv. 2002;55(3):392–397. doi: 10.1002/ccd.10136. [Catheter-based needle endomyocardial injection is associated with superior injectate retention and more injectate may be retained at lower volumes.] [DOI] [PubMed] [Google Scholar]
- 38■■.Gwon HC, Jeong JO, Kim HJ, et al. The feasibility and safety of fluoroscopy-guided percutaneous intramyocardial gene injection in porcine heart. Int. J. Cardiol. 2001;79(1):77–88. doi: 10.1016/s0167-5273(01)00410-7. [DOI] [PubMed] [Google Scholar]
- 39.Kornowski R, Leon MB, Fuchs S, et al. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy. Results in normal and ischemic porcine models. J. Am. Coll. Cardiol. 2000;35(4):1031–1039. doi: 10.1016/s0735-1097(99)00642-7. [DOI] [PubMed] [Google Scholar]
- 40.Koransky ML, Robbins RC, Blau HM. VEGF gene delivery for treatment of ischemic cardiovascular disease. Trends Cardiovasc. Med. 2002;12(3):108–114. doi: 10.1016/s1050-1738(01)00158-x. [DOI] [PubMed] [Google Scholar]
- 41.Edelberg JM, Huang DT, Josephson ME, Rosenberg RD. Molecular enhancement of porcine cardiac chronotropy. Heart. 2001;86(5):559–562. doi: 10.1136/heart.86.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarous DF, Shou M, Stiber JA, et al. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc. Res. 1997;36(1):78–85. doi: 10.1016/s0008-6363(97)00142-9. [DOI] [PubMed] [Google Scholar]
- 43.Logeart D, Hatem SN, Rucker-Martin C, et al. Highly efficient adenovirus-mediated gene transfer to cardiac myocytes after single-pass coronary delivery. Hum. Gene Ther. 2000;11(7):1015–1022. doi: 10.1089/10430340050015329. [DOI] [PubMed] [Google Scholar]
- 44.Hayase M, Del Monte F, Kawase Y, et al. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am. J. Physiol. Heart Circ. Physiol. 2005;288(6):H2995–H3000. doi: 10.1152/ajpheart.00703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Z, Fach C, Sasse A, Godecke A, Schrader J. A minimally invasive approach for efficient gene delivery to rodent hearts. Gene Ther. 2004;11(3):260–265. doi: 10.1038/sj.gt.3302167. [DOI] [PubMed] [Google Scholar]
- 46.Boekstegers P, von Degenfeld G, Giehrl W, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7(3):232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 47.Katz MG, Fargnoli AS, Pritchette LA, Bridges CR. Gene delivery technologies for cardiac applications. Gene Ther. 2012;19(6):659–669. doi: 10.1038/gt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Degenfeld G, Raake P, Kupatt C, et al. Selective pressure-regulated retroinfusion of fibroblast growth factor-2 into the coronary vein enhances regional myocardial blood flow and function in pigs with chronic myocardial ischemia. J. Am. Coll. Cardiol. 2003;42(6):1120–1128. doi: 10.1016/s0735-1097(03)00915-x. [DOI] [PubMed] [Google Scholar]
- 49.Sharif F, Hynes SO, Mcmahon J, et al. Gene-eluting stents: comparison of adenoviral and adeno- associated viral gene delivery to the blood vessel wall in vivo. Hum. Gene Ther. 2006;17(7):741–750. doi: 10.1089/hum.2006.17.741. [DOI] [PubMed] [Google Scholar]
- 50.Fargnoli AS, Katz MG, Yarnall C, et al. Cardiac surgical delivery of the sarcoplasmic reticulum calcium ATPase rescues myocytes in ischemic heart failure. Ann. Thorac. Surg. 2013;96(2):586–595. doi: 10.1016/j.athoracsur.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye L, Haider H, Tan R, et al. Angiomyogenesis using liposome based vascular endothelial growth factor-165 transfection with skeletal myoblast for cardiac repair. Biomaterials. 2008;29(13):2125–2137. doi: 10.1016/j.biomaterials.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Durrani S, Haider KH, Ahmed RP, Jiang S, Ashraf M. Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury. Stem. Cells Dev. 2012;21(10):1769–1778. doi: 10.1089/scd.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki K, Murtuza B, Smolenski RT, et al. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104(12 Suppl. 1):I207–I212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 54.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007 – an update. J. Gene Med. 2007;9(10):833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 55.Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J. Control. Release. 2012;161(2):377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol. Ther. 2007;15(7):1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 57.Hedman M, Hartikainen J, Yla-Herttuala S. Progress and prospects: hurdles to cardiovascular gene therapy clinical trials. Gene Ther. 2011;18(8):743–749. doi: 10.1038/gt.2011.43. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ. Res. 2005;96(7):767–775. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- 59.Henry TD, Grines CL, Watkins MW, et al. Effects of Ad5FGF-4 in patients with angina: an ana lysis of pooled data from the AGENT-3 and AGENT-4 trials. J. Am. Coll. Cardiol. 2007;50(11):1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res. Cardiol. 2011;106(6):897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 61.Askari AT, Penn MS. Stromal cell-derived factor-1 mediates stem cell homing and tissue regeneration. Discov. Med. 2003;3(18):46–47. [PubMed] [Google Scholar]
- 62■■.Penn MS, Mendelsohn FO, Schaer GL, et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ. Res. 2013;112(5):816–825. doi: 10.1161/CIRCRESAHA.111.300440. [Describes how overexpression of SDF-1 via gene therapy is a strategy for improving clinical symptoms in patients with ischemic cardiomyopathy.] [DOI] [PubMed] [Google Scholar]
- 63.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ. Res. 2009;105(8):724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ. Res. 2006;99(7):776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 65.Konoplyannikov M, Haider KH, Lai VK, Ahmed RP, Jiang S, Ashraf M. Activation of diverse signaling pathways by ex vivo delivery of multiple cytokines for myocardial repair. Stem Cells Dev. 2013;22(2):204–215. doi: 10.1089/scd.2011.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66■■.Kim HW, Jiang S, Ashraf M, Haider KH. Stem cell-based delivery of Hypoxamir-210 to the infarcted heart: implications on stem cell survival and preservation of infarcted heart function. J. Mol. Med. (Berl.) 2012;90(9):997–1010. doi: 10.1007/s00109-012-0920-1. [Reports on transgenic induction of miR-210 in mesenchymal stem cells helps in functional recovery of ischemic hearts.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol. Pharmacol. 2011;80(4):558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu G, Jiang S, Ashraf M, Haider KH. Subcellular preconditioning of stem cells: mito-Cx43 gene targeting is cytoprotective via shift of mitochondrial Bak and Bcl-xL balance. Regen. Med. 2012;7(3):323–334. doi: 10.2217/rme.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl Acad. Sci. USA. 1982;79(6):2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ. Res. 1993;73(6):1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 71.Kaplitt MG, Xiao X, Samulski RJ, et al. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann. Thorac. Surg. 1996;62(6):1669–1676. doi: 10.1016/s0003-4975(96)00946-0. [DOI] [PubMed] [Google Scholar]
- 72■■.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006;99(4):e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [Demonstrates that adeno-associated virus type 2/9 is able to transduce myocardium at a 200-fold higher level than adeno-associated virus type 2/1.] [DOI] [PubMed] [Google Scholar]
- 73.Donahue JK, Kikkawa K, Johns DC, Marban E, Lawrence JH. Ultrarapid, highly efficient viral gene transfer to the heart. Proc. Natl Acad. Sci. USA. 1997;94(9):4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klugherz BD, Jones PL, Cui X, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat. Biotechnol. 2000;18(11):1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 75■■.Bridges CR, Burkman JM, Malekan R, et al. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann. Thorac. Surg. 2002;73(6):1939–1946. doi: 10.1016/s0003-4975(02)03509-9. [Reports the efficiency of separating the cardiac circuit from the systemic during gene transfer.] [DOI] [PubMed] [Google Scholar]
- 76.Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97(7):645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 77.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98(25):2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 78.Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: Phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100(5):468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 79.Hedman M, Hartikainen J, Syvanne M, et al. safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: Phase II results of the kuopio angiogenesis trial (KAT). Circulation. 2003;107(21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]