Abstract
Spontaneous tumors often contain heterogeneous populations of tumor cells with different tumor-initiating potentials or cancer cell “stemness.” Clonal heterogeneity can be traced to specific locations inside a tumor where clones with different metastatic capabilities are identified, suggesting that the tumor microenvironment can exert a significant effect on the evolution of different clonal populations. Hypoxia is a common feature of tumor microenvironments and has the potential to facilitate malignant progression. This chapter provides a synopsis of hypoxia-regulated pathways implicated in the maintenance of cancer stem cells.
Keywords: Cancer stem cells, Differentiation, Hypoxia, Progenitor cells, Tumor microenvironment
2.1 Introduction
In primary tumors, there are often functional and phenotypical heterogeneities among tumor cell populations (Marusyk et al. 2012) although they share the same clonal origin. As was elegantly shown by Yachida et al. (2010), clonal populations with variable metastatic potentials are found in distinct regions within the primary carcinoma of patients with pancreatic cancer, although these clones are genetically evolved from the original parental, nonmetastatic clone. The mechanisms underlying clonal heterogeneity, however, remain to be investigated. Nonetheless, it is highly possible that the heterogeneous nature of tumor microenvironments plays a critical role in the evolution and selection of aggressive clones.
One of the most commonly recognized features of tumor microenvironment is hypoxia, that is, insufficient oxygenation to meet the metabolic demands of viable tumor cells (Vaupel and Mayer 2007). Hypoxic (oxygen partial pressure pO2 < 10 mmHg) regions have been directly detected using a needle-based, polarographic pO2 electrode in many types of human cancers (Vaupel et al. 2007). The presence of tumor hypoxia also has been indirectly analyzed by immunohistochemistry using hypoxia-activated compounds (Evans and Koch 2003), such as EF5 and pimonidazole, or endogenous hypoxia-induced molecules (Moon et al. 2007), such as hypoxia-inducible factor (HIF)-1α, glucose transporter 1, and carbonic anhydrase 9. However, the staining patterns of different hypoxia markers are not identical (Li et al. 2007b; Vukovic et al. 2001), likely because of their different modes of activation and/or regulation by hypoxia. Nevertheless, it has been shown that hypoxic regions are randomly distributed within the tumor proper (Horsman et al. 2012), suggesting that hypoxia may play a critical role in the clonal evolution of tumor cells in different tumor microenvironments.
Tumor hypoxia is, clinically, an independent prognostic factor for poor patient survival (Nordsmark and Overgaard 2004; Brizel et al. 1996, 1999; Hockel et al. 1996 ; Young et al. 1988). Hypoxic tumor cells seem to be more aggressive, with reduced apoptosis (Graeber et al. 1996), increased drug-resistance (Wartenberg et al. 2003; Comerford et al. 2002), and increased metastatic potential (Rofstad 2000; Subarsky and Hill 2003). Hypoxia can also increase genomic instability by down-regulating the expression of DNA repair genes (Koshiji et al. 2005; Mihaylova et al. 2003; Bindra et al. 2004, 2005). These observations strongly suggest that hypoxia exerts a powerful selection pressure for the emergence of aggressive tumor clones.
The malignant progression of a benign growth is a slow process. It often takes more than a decade for metastatic clones to emerge in spontaneous human tumors (Luebeck 2010; Yachida et al. 2010; Jones et al. 2008; Beerenwinkel et al. 2007). Malignant progression often results from cumulative genetic mutations in oncogenes and tumor suppressor genes, as well as epigenetic changes (Hanahan and Weinberg 2000; Vogelstein and Kinzler 2004). It is imperative to note that these seemingly random and independent mutational events must take place in a single tumor cell originating from the initial oncogenic transformation and that this tumor cell must be able to copy itself so that previously acquired mutations can be inherited in the subsequent daughter cell stages. Therefore, only a stem cell–like cancer cell can complete this protracted journey of change from a benign cell to a metastatic tumor cell.
A number of recent studies have shown that hypoxia can inhibit differentiation of embryonic stem cells and progenitor cells (Ezashi et al. 2005; Gustafsson et al. 2005; Lin et al. 2006; Yun et al. 2002). Hypoxic tumor cells seem to be poorly differentiated and express stem cell markers (Das et al. 2008; Jogi et al. 2002). Under hypoxic conditions, tumor cells show increased clonogenic potential (Desplat et al. 2002; Kim et al. 2009; Schmaltz et al. 1998). Exposure to hypoxia in vitro also results in enhanced tumorigenic potential in vivo (Jogi et al. 2002). These interesting observations lead to a new paradigm that tumor hypoxia may facilitate the emergence of malignant clones by maintaining cancer stem cells in their undifferentiated stem cell state, which permits self-renewal and uninterrupted accumulation of genetic and epigenetic changes over a protracted period of time. This chapter briefly reviews the current advances in the understanding of hypoxia and its role in stem cell maintenance. The field of cancer stem cell research is witnessing an explosive expansion. We apologize to those authors whose work is not cited because of space limitations.
2.2 Mechanisms of Hypoxia-Dependent Stemness Regulation
2.2.1 Hypoxia-Inducible Factors and Cancer Cell Stemness
In addition to being an essential molecule for oxidative phosphorylation in mitochondria, oxygen (O2) also functions as an important signaling molecule and regulates a wide range of biological processes, including erythropoiesis, angiogenesis, and cellular differentiation. O2 cannot be stored in cells and needs to be constantly supplied to support cellular functions and maintain cell viability. Because of the limited supply, specific O2-sensing pathways have evolved in higher-order organisms, especially mammals, to deal with potential O2 deficiency.
The most prominent and best understood hypoxia-induced signaling pathways currently are anchored by HIF-1 and HIF-2, heterodimeric transcription factors consisting of an O2-regulated alpha subunit (HIF-1α or -2α), and the O2-insensitive HIF-1β (Semenza 2003). Although they share similar structures and functions, HIF-1α is ubiquitously expressed, whereas HIF-2α has relatively limited tissue distribution; they also each have nonoverlapping functions (Hu et al. 2006). Furthermore, the expression of HIF-1α and HIF-2α is differentially regulated under conditions of acute and chronic hypoxia, respectively (Lin and Yun 2010).
The O2-sensing ability of HIF-α subunits is realized via O2-dependent hydroxylation of two proline residues located in the O2-dependent degradation domain (Ivan et al. 2001; Jaakkola et al. 2001). The hydroxylated HIF-α interacts with the von Hippel-Lindau (VHL) protein in the E3 ligase complex for ubiquitination and proteasome-mediated degradation (Maxwell et al. 1999; Ohh et al. 2000). Under hypoxia (generally at pO2 levels < 2 %), proline hydroxylation is impaired and the unhydroxylated HIF-α translocates into the nucleus, where it dimerizes with the O2-insensitive HIF-1β. The enhancer regions of hypoxia-induced genes typically contain one or more of the consensus sequence 5′-ACGTG-3′, dubbed the hypoxia-responsive enhancer element (HRE), which is directly bound by HIF-1 or -2 (Semenza 2000; Harris 2002).
In general, increased HIF accumulation and activity facilitate tumor development, which is perhaps best illustrated by renal cell carcinomas (RCCs). Genetic mutations of the VHL tumor suppressor gene result in loss of function of the VHL tumor suppressor protein and consequent activation of the HIF pathway under normoxia, which promotes RCC development (Ohh et al. 2000; Maxwell et al. 1999). However, it should be noted that Vhl deletion in murine renal proximal tubule cells does not lead to the development of renal cancers (Rankin et al. 2006), suggesting that additional pathways also are required for RCC development. Nevertheless, solid tumors often show elevated levels of the HIF-1α protein compared to adjacent normal tissues (Harris 2002; Semenza 2003; Vaupel and Mayer 2007). Elevated levels of HIF-1α protein (Aebersold et al. 2001; Burri et al. 2003) or HIF-2α protein (Holmquist-Mengelbier et al. 2006) are significantly correlated with poor patient survival. Furthermore, studies have shown that HIF-1α and HIF-2α can synergize with different protooncogenes, such as Akt and c-Myc, to facilitate tumor cell survival and growth (Bedogni et al. 2005; Gordan et al. 2007).
However, it is worth noting that, under certain circumstances, increased HIF expression or activity seems to have a negative effect on tumor growth. Teratomas derived from HIF-1α-deficient murine embryonic stem (ES) cells grow faster than those from the wild-type ES cells, in part because apoptosis occurs less often in HIF-1α-deficient, ES-derived tumors (Carmeliet et al. 1998). On the other hand, overexpression of HIF-2α in rat glioma tumors increases tumor cell apoptosis and reduces the growth of these tumors, despite enhanced angiogenesis (Acker et al. 2005). These inconsistent observations suggest that the dichotomous functions of HIFs may depend on interactions between the HIF and other pathways in different tumor cell types, their microenvironments, or both.
Several lines of evidence have demonstrated that HIF activation is associated with an undifferentiated phenotype. In primary pancreatic cancers, nuclear accumulation of the HIF-1α protein is primarily found in poorly differentiated tumor cells (Couvelard et al. 2005). Increased levels of HIF-1α and HIF-2α have been found in the stem cell–like populations of neuroblastomas (Pietras et al. 2008, 2009) and gliomas (Li et al. 2009). Downregulation of HIF-1α or HIF-2α by RNA interference results in reduced growth of the tumor sphere, an in vitro assay of self-renewal, and survival of glioma stem cells (Li et al. 2009). It is interesting to note that HIF-2α expression can be easily detected in glioma stem cells under hypoxic conditions (Li et al. 2009). In a similar way, HIF-2α is also preferentially expressed in immature neural crest-like neuroblastoma cells in vivo and seems to be required for maintenance of the undifferentiated neuroblastoma cells (Pietras et al. 2008, 2009). These data strongly support a role of HIF-1 and/or HIF-2 in the maintenance of cancer stem cells. These studies also point out the functional differences between HIF-1 and HIF-2 in the maintenance of undifferentiated cancer stem cell phenotypes.
2.2.2 Hypoxia-Inducible Factors and Stem Cell Gene Expression
Studies have shown that the HIF pathway is involved in upregulating the expression of several stem cell genes. The pluripotency gene POU5F1 (Oct3/4) is one of the four or five critical genes that collectively transform adult somatic cells into pluripotent stem cells (Meissner et al. 2007; Takahashi et al. 2007; Yu et al. 2007). In transgenic mice with doxycycline-inducible expression of POU5F1, induced POU5F1 expression results in inhibition of cellular differentiation and dysplastic growths in epithelial tissues (Hochedlinger et al. 2005), thus demonstrating a direct role of POU5F1 in tumorigenesis. Consistent with this notion, it has been found that germ cell cancers and several types of somatic cancers – including human cervical carcinomas, breast carcinomas, and pancreatic cancers – express elevated levels of POU5F1 (Cheng 2004; Gidekel et al. 2003; Jones et al. 2004; Tai et al. 2005).
Using a genetic “knock-in” mouse model, Covello et al. (2006) replaced the endogenous Hif1a gene locus with the Hif2a locus. The increased Hif2a gene dosage and the absence of Hif1a resulted in increased expression of HIF-2α-specific genes including POU5F1 in mouse embryonic tissues (Covello et al. 2006). HIF-2α, but not HIF-1α, directly binds to the POU5F1 promoter/enhancer. Loss of HIF-2α reduces the number of embryonic primordial germ cells that require POU5F1 for survival and maintenance. Furthermore, the loss of POU5F1 results in decreased growth of mouse ES cell–derived teratomas (Covello et al. 2006). Reduced HIF-2α expression similarly results in decreased expression of POU5F1 and other stem cell genes in human ES cells cultured at 5 % O2 (Forristal et al. 2010). These observations strongly suggest that HIF-2α plays a significant role in stem cell maintenance. It will be interesting to see whether hypoxia increases POU5F1 expression in common types of tumors.
Delta-like 1 homolog (Drosophila), or DLK1, is a type I transmembrane protein with abundant expression in embryonic tissues and immature cells, but not in differentiated adult tissues (Floridon et al. 2000), suggesting a role for DLK1 in the regulation of stem cells and progenitor cells. Elevated expression of DLK1 has been reported in several tumor types (Jensen et al. 1994; Tornehave et al. 1996; Yin et al. 2006; Sakajiri et al. 2005; Van Limpt et al. 2003; Li et al. 2005). Studies have shown that DLK1 is robustly expressed in undifferentiated, but not differentiated, neuroblastoma cells (Begum et al. 2012; Kim et al. 2009). Downregulation of DLK1 by RNA interference sensitizes neuroblastoma cells to spontaneous neuronal differentiation, decreases clonogenicity or colony-forming potential, and suppresses tumorigenicity (Begum et al. 2012; Kim et al. 2009). Overexpression of DLK1, on the other hand, inhibits differentiation, enhances clonogenicity, and increases tumorigenicity (Kim et al. 2009). The DLK1 cytoplasmic domain, especially tyrosine-339 and serine-355, is required for maintaining both clonogenicity and tumorigenicity (Kim et al. 2009). The HIF pathway directly regulates DLK1 transcription as both HIF-1α and HIF-2α can bind to the HRE in the upstream DLK1 promoter/enhancer region under hypoxic conditions (Kim et al. 2009). In neuroblastoma xenografts, the DLK1-positve neuroblastoma cells seem to be preferentially localized in the pimonidazole-positive hypoxic region (Begum et al. 2012). These observations demonstrate that the HIF-DLK1 pathway has the potential to maintain cancer stem cells in the hypoxic tumor microenvironment.
The pentaspan transmembrane glycoprotein prominin-1 (CD133), a widely used marker for isolating perspective cancer stem cells from a variety of tumors (Visvader and Lindeman 2008), experiences increased expression in hypoxia -treated (1 % O2) human glioma cells and can promote the expansion of the CD133 + tumor cell population (Griguer et al. 2008; Seidel et al. 2010; Soeda et al. 2009). Both HIF-1α and HIF-2α seem to be involved in the hypoxia-dependent induction of CD133 expression because knocking down either HIF-1α (Soeda et al. 2009) or HIF-2α (Seidel et al. 2010) reduces the hypoxia-induced CD133 expression in glioma cells. However, it remains to be determined how HIF enhances CD133 transcription. On the other hand, severe hypoxia (0.1 % O2) seems to downregulate CD133 expression in several gastric, colorectal, and lung cancer cell lines (Matsumoto et al. 2009). These seemingly contradictory findings nonetheless suggest that CD133 may be more involved in cancer stem cell maintenance under moderate (1 % O2) rather than severe (0.1 % O2) hypoxia. Investigation of the transcriptional regulation of CD133 expression by HIF at different pO2 levels may provide mechanistic insights into the O2 concentration–dependent regulation of CD133 expression.
The CD44+/CD24−/low signature has been used to identify breast cancer stem cells (Al-Hajj et al. 2003). As shown by global gene expression and genetic profiles, CD24 + and CD44 + breast cancer cells from the same tumor are clonally related but genetically different (Shipitsin et al. 2007). Elevated CD24 levels have been found to significantly – but counterintuitively – correlate with advanced disease stages in several types of human epithelial cancers, including breast cancer, ovarian cancer, and prostate cancer (Kristiansen et al. 2004). Large-scale immunohistochemical analyses of CD24 and CD44 protein levels in human breast cancer tumor samples have found that the combined CD44 +/CD24− phenotype is associated with the most favorable prognosis, whereas the CD44 −/CD24+ phenotype predicts the worst outcome (Mylona et al. 2008 ; Ahmed et al. 2012). In addition, CD24 + tumor-initiating populations also have been found in pancreatic cancers (Ishizawa et al. 2010; Li et al. 2007a), liver cancers (Lee et al. 2011), and colorectal cancers (Vermeulen et al. 2008; Ke et al. 2012). An interesting recent study has shown that CD24 expression is strongly induced by hypoxia in a human bladder cancer cell line (Thomas et al. 2012). Promoter analysis has demonstrated that an HRE in the upstream promoter/enhancer region is required for both hypoxia-induced and HIF-1α-dependent CD24 expression (Thomas et al. 2012). Combined HIF-1α + and CD24 + immunostaining in a cohort of 101 human urothelial cancer samples showed a statistically significant association with reduced overall survival (Thomas et al. 2012). These data suggest that HIF and/or hypoxia may play an important role in the clonal maintenance or evolution of the aggressive CD24 + tumor stem populations in the tumor microenvironment.
2.2.3 Other Hypoxia-Regulated Genes and Cancer Stemness
Structures of chromosomes dynamically change during DNA replication and gene transcription and are accompanied by posttranslational modifications of histones, including acetylation of lysine residues and methylation of lysine or arginine residues. Histone demethylases are members of the JmjC domain-containing 2-oxoglutarate oxygenases and catalyze the removal of Nε-methyl groups from lysine residues via O2-dependent hydroxylation (Loenarz and Schofield 2011). They play an important role in both normal embryonal development and cancer (Yamane et al. 2007; Klose et al. 2007; Lan et al. 2007; Iwase et al. 2007). Using the histone 3 lysine 4 (H3K4) demethylase JARID1B (KDM5B/PLU-1/RBP2-H1) as a biomarker, a small subpopulation of slow-cycling melanoma cells that are essential for continuous tumor growth has been identified in patients with advanced tumors (Roesch et al. 2008, 2010). It is interesting that JARID1B expression in melanoma cells increases rapidly under hypoxia (1 % pO2) and gradually returns to normal levels after extended culture under atmospheric conditions (Roesch et al. 2010). However, it is not yet clear how JARID1B expression and its enzymatic activity are regulated under hypoxic conditions. Nonetheless, because melanoma cells can easily transition between JARID1B+ and JARID1B− states, these data suggest that the hypoxic microenvironment may play a significant role in maintaining a population of melanoma cells with long-term repopulating potential, at least in part by augmenting JARID1B expression.
Krieg et al. (2010) also have reported that histone demethylase genes JMJD1A, JMJD2B, and JARID1B are induced by hypoxia in human RCCs. Interestingly, their hypoxia-dependent expression is abolished in HIF-1α-knockout mouse embryonic fibroblasts, suggesting that HIF-1 is necessary for hypoxic induction. Furthermore, downregulation of JMJD1A reduces xenograft tumor growth in vivo (Krieg et al. 2010). These data indicate that hypoxia can facilitate tumor growth via histone demethylase–mediated chromatin remodeling.
The histone methyltransferase mixed-lineage leukemia 1 (MLL1), also known as human trithorax or acute lymphocytic leukemia-1, is a member of the trithorax family of global transcription activators. MLL1 is preferentially expressed in glioma stem cells and is necessary for maintaining their self-renewal (Heddleston et al. 2012). Hypoxia significantly increases MLL1 expression in both stem and non–stem cell populations. Both HIF-1α and HIF-2α seem to be involved in the regulation of MLL1 expression (Heddleston et al. 2012), although the mechanism of regulation remains to be determined. It is interesting to note that inhibition of MLL1 expression decreases the expression of HIF-2α as well as that of hypoxia-induced genes (Heddleston et al. 2012). These data suggest a positive feedback between HIF-2α and MLL1 for the induction and maintenance of glioma stem cells.
2.3 Summary
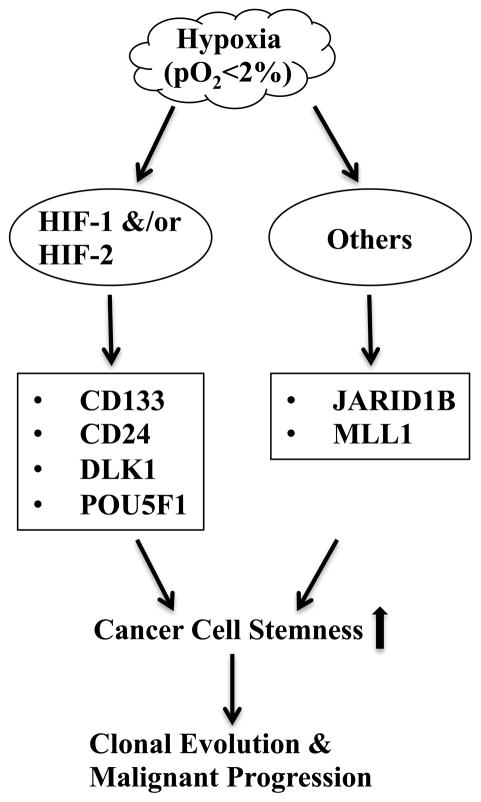
As discussed earlier, hypoxia clearly has the potential to exert significant effect on the maintenance and evolution of cancer stem cells via both HIF-dependent transcriptions and chromatin remodeling in cancer cells (Fig. 2.1). Hypoxia also inhibits differentiation of mesenchymal stem/progenitor cells (Lin et al. 2006, 2008, 2009; Yun et al. 2002, 2005), thus creating a niche wherein cancer stem cells could be arrested in an undifferentiated state via interactions with their surrounding immature stromal cells (Lin and Yun 2010). However, it is worth noting that because of the plasticity of stemness and the expression of a heterogeneous array of stem cell markers (Magee et al. 2012 ; Shipitsin et al. 2007 ; Visvader and Lindeman 2012), cancer stem cells that are localized in or emerge from a hypoxic microenvironment may exist in a different stem cell state or express different sets of stem cell markers compared to developmentally similar cancer stem cells localized in nonhypoxic regions. Nonetheless, the hypoxia-stemness paradigm offers a new perspective on the role of hypoxia in facilitating malignant progression and therapy resistance. Since hypoxic regions are heterogeneously located throughout the tumor proper (Horsman et al. 2012), it is highly probable that hypoxic cancer stem cell niches may contribute to the microenvironment-specific emergence of metastatic clones (Yachida et al. 2010). Therefore, targeting the hypoxic cancer stem cell niche would be highly effective for controlling tumor growth, as well as for preventing metastasis.
Fig. 2.1.
Hypoxia-Activated Pathways Leading to Cancer Stem Cell Maintenance. Multiple stem cell-related genes encoding cell surface proteins, transcription factors, or chromatin modifying enzymes are up-regulated under hypoxic conditions either directly by the HIF transcription factor pathway or by other yet unknown mechanisms. These different pathways may function either synergistically or additively to maintain cancer stem cells by enhancing their self-renewal and blocking their differentiation. Increased lifespan of cancer stem cells allows inheritable accumulation of multiple genetic mutations and epigenetic changes that are crucial for clonal evolution and malignant progression.
Acknowledgments
The authors are grateful to the members of the Yun Laboratory for their contribution to the understanding of tumor hypoxia and stemness regulation. The authors also thank Lisa Cabral for excellent assistance in the preparation of this manuscript. Z.Y. is supported in part by R01CA125021 and R01CA148996 from the National Institutes of Health, Bethesda, MD, USA.
References
- Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, Fukumura D, Moreno-Murciano MP, Herbert JM, Burger A, Riedel J, Elvert G, Flamme I, Maxwell PH, Collen D, Dewerchin M, Jain RK, Plate KH, Carmeliet P. Genetic evidence for a tumor suppressor role of HIF-2α. Cancer Cell. 2005;8:131–141. doi: 10.1016/j.ccr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia-inducible factor-1α: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, Green AR, Ilyas M, Ellis IO. A CD44(−)/CD24(+) phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat. 2012;133:979–995. doi: 10.1007/s10549-011-1865-8. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Beerenwinkel N, Antal T, Dingli D, Traulsen A, Kinzler KW, Velculescu VE, Vogelstein B, Nowak MA. Genetic progression and the waiting time to cancer. PLoS Comput Biol. 2007;3:e225. doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum A, Kim Y, Lin Q, Yun Z. DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo. Cancer Lett. 2012;318:26–33. doi: 10.1016/j.canlet.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, Lizardi P, Hedley DW, Bristow RG, Glazer PM. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, Bristow RG, Classon MK, Glazer PM. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, Mazzucchelli L, Greiner RH, Gruber G. Significant correlation of hypoxia-inducible factor-1α with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:494–501. doi: 10.1016/s0360-3016(02)04579-0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Cheng L. Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer. 2004;101:2006–2010. doi: 10.1002/cncr.20566. [DOI] [PubMed] [Google Scholar]
- Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- Desplat V, Faucher JL, Mahon FX, Dello Sbarba P, Praloran V, Ivanovic Z. Hypoxia modifies proliferation and differentiation of CD34(+) CML cells. Stem Cells. 2002;20:347–354. doi: 10.1634/stemcells.20-4-347. [DOI] [PubMed] [Google Scholar]
- Evans SM, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195:1–16. doi: 10.1016/s0304-3835(03)00012-0. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR, Jr, Gillespie GY. CD133 is a marker of bioenergetic stress in human glioma. PLoS One. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Heddleston JM, Wu Q, Rivera M, Minhas S, Lathia JD, Sloan AE, Iliopoulos O, Hjelmeland AB, Rich JN. Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ. 2012;19:428–439. doi: 10.1038/cdd.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Pahlman S. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9:674–687. doi: 10.1038/nrclinonc.2012.171. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in stem cells. Mol Cell Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, Hidalgo M, Tsao M, Ailles L, Waddell TK, Maitra A, Neel BG, Matsui W. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7:279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jensen CH, Krogh TN, Hojrup P, Clausen PP, Skjodt K, Larsson LI, Enghild JJ, Teisner B. Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur J Biochem. 1994;225:83–92. doi: 10.1111/j.1432-1033.1994.00083.x. [DOI] [PubMed] [Google Scholar]
- Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural. 2002 doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TD, Ulbright TM, Eble JN, Cheng L. OCT4: a sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–8547. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE, Kinzler KW, Vogelstein B, Willis J, Markowitz SD. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J, Wu X, Wu X, He X, Lian L, Zou Y, He X, Wang H, Luo Y, Wang L, Lan P. A subpopulation of CD24(+) cells in colon cancer cell lines possess stem cell characteristics. Neoplasma. 2012;59:282–288. doi: 10.4149/neo_2012_036. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009;69:9271–9280. doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Yan Q, Tothova Z, Yamane K, Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y, Kaelin WG., Jr The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. HIF-1α induces genetic instability by transcriptionally downregulating MutSα expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1α enhances hypoxic gene expression and tumor growth. Mol Cell Biol. 2010;30:344–353. doi: 10.1128/MCB.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/b:hijo.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, Shi Y. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–694. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–4476. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007a;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li XF, Carlin S, Urano M, Russell J, Ling CC, O’Donoghue JA. Visualization of hypoxia in microscopic tumors by immunofluorescent microscopy. Cancer Res. 2007b;67:7646–7653. doi: 10.1158/0008-5472.CAN-06-4353. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, Mclendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Yun Z. Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics. Cancer Biol Ther. 2010;9:949–956. doi: 10.4161/cbt.9.12.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- Lin Q, Kim Y, Alarcon RM, Yun Z. Oxygen and cell fate decisions. Gene Regul Syst Biol. 2008;2:1–9. [Google Scholar]
- Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276:2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenarz C, Schofield CJ. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem Sci. 2011;36:7–18. doi: 10.1016/j.tibs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Luebeck EG. Cancer: genomic evolution of metastasis. Nature. 2010;467:1053–1055. doi: 10.1038/4671053a. [DOI] [PubMed] [Google Scholar]
- Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Arao T, Tanaka K, kaneda H, Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, Saijo N, Nishio K. mTOR signal and hypoxia-inducible factor-1α regulate CD133 expression in cancer cells. Cancer Res. 2009;69:7160–7164. doi: 10.1158/0008-5472.CAN-09-1289. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibro-blasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Mihaylova VT, Bindra RS, Yuan J, Campisi D, Narayanan L, Jensen R, Giordano F, Johnson RS, Rockwell S, Glazer PM. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–3273. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EJ, Brizel DM, Chi JT, Dewhirst MW. The potential role of intrinsic hypoxia markers as prognostic variables in cancer. Antioxid Redox Signal. 2007;9:1237–1294. doi: 10.1089/ars.2007.1623. [DOI] [PubMed] [Google Scholar]
- Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L. The clinicopathologic and prognostic significance of CD44+/CD24(−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39:1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43:396–403. doi: 10.1080/02841860410026189. [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, Pahlman S. High levels of HIF-2α highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–488. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, Rehn M, Beckman S, Noguera R, Navarro S, Cammenga J, Fredlund E, Kaplan DR, Pahlman S. HIF-2α maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:16805–16810. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Mueller AM, Stempfl T, Moehle C, Landthaler M, Vogt T. RBP2-H1/JARID1B is a transcriptional regulator with a tumor suppressive potential in melanoma cells. Int J Cancer. 2008;122:1047–1057. doi: 10.1002/ijc.23211. [DOI] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- Sakajiri S, O’Kelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, Shih LY, Kim KH, Sul HS, Jensen CH, Teisner B, Kawamata N, Koeffler HP. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005;19:1404–1410. doi: 10.1038/sj.leu.2403832. [DOI] [PubMed] [Google Scholar]
- Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S, Garvalov BK, Wirta V, Von Stechow L, Schanzer A, Meletis K, Wolter M, Sommerlad D, Henze AT, Nister M, Reifenberger G, Lundeberg J, Frisen J, Acker T. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2α. Brain. 2010;133:983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, Mckay RD, Engh J, Iwama T, Kunisada T, Kassam AB, Pollack IF, Park DM. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomas S, Harding MA, Smith SC, Overdevest JB, Nitz MD, Frierson HF, Tomlins SA, Kristiansen G, Theodorescu D. CD24 is an effector of HIF-1-driven primary tumor growth and metastasis. Cancer Res. 2012;72:5600–5612. doi: 10.1158/0008-5472.CAN-11-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996;106:535–542. doi: 10.1007/BF02473268. [DOI] [PubMed] [Google Scholar]
- Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003;105:61–69. doi: 10.1002/ijc.11047. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Todaro M, De Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Vukovic V, Haugland HK, Nicklee T, Morrison AJ, Hedley DW. Hypoxia-inducible factor-1α is an intrinsic marker for hypoxia in cervical cancer xenografts. Cancer Res. 2001;61:7394–7398. [PubMed] [Google Scholar]
- Wartenberg M, Ling FC, Muschen M, Klein F, Acker H, Gassmann M, Petrat K, Putz V, Hescheler J, Sauer H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003;17:503–505. doi: 10.1096/fj.02-0358fje. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, Said JW, Black KL, Koeffler HP. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–1861. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988;85:9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040–3055. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]