Abstract
Background Little is known about agreement between patients and physicians on content and outcomes of clinical discussions. A common perception of content and outcomes may be desirable to optimize decision making and clinical care.
Objective To determine patient–physician agreement on content and outcomes of coronary heart disease (CHD) prevention discussions.
Design Cross‐sectional survey nested within a randomized CHD prevention study.
Setting and participants University internal medicine clinic; 24 physicians and 157 patients.
Methods Following one clinic visit, we surveyed patients and physicians on discussion content, decision making and final decisions about CHD prevention. For comparison, we audio‐recorded, transcribed and coded 20 patient–physician visits. We calculated percent agreement between patient/physician reports, patient/transcription reports and physician/transcription reports. We calculated Cohen’s kappas to compare patient/physician perspectives.
Results Patients and physicians agreed on whether CHD was discussed in 130 visits (83%; kappa = 0.55; 95% CI 0.40–0.70). When discussions occurred, they agreed about discussion content (pros versus cons) in 53% of visits (kappa = 0.15; 95% CI −0.01–0.30) and physicians’ recommendations in 73% (kappa = 0.44; 95% CI 0.28–0.66). Patients and physicians agreed on final decisions to take medication in 78% (kappa = 0.58; 95% CI 0.45–0.71) and change lifestyle in 69% (kappa = 0.38; 95% CI 0.24–0.53). They agreed less often, 43% (kappa = 0.13; 95% CI −0.11–0.37) about degree of involvement in decision making. Audio‐recorded results were similar, but showed very low agreement between transcripts and patients’ and physicians’ self‐report on discussion content and decision making.
Conclusions Disagreements about clinical discussions and decision making may be common. Future work is needed to determine: how widespread such agreements are; whether they impact clinical outcomes; and the relative importance of the subjective experience versus objective steps of shared decision making.
Keywords: patient–physician agreement, shared decision making
Background
Shared decision making (SDM) is increasingly popular in clinical practice and even advocated as an ideal decision making model for preference‐sensitive medical decisions. 1 It is a process that involves at least two parties (patient and physician) who each participate in decision making through sharing information, expressing preferences and agreeing on a treatment decision. 2 SDM is important and distinct from other decision‐making models, because it integrates the clinical expertise of the physician with the values and preferences of the patient.
To facilitate the process of shared decision making, several frameworks have explicitly described its components. 3 , 4 , 5 , 6 As outlined in a recent systematic review, these frameworks agree that shared decision making includes discussion of patients’ treatment options and their pros and cons; expression and clarification of patients’ values and preferences; and an explicit process of making or deferring a decision. 7 Frameworks, however, differentially endorse other components (e.g. defining the problem, physician check for understanding, arranging for follow‐up), suggesting these are less essential to the SDM process.
Building on common conceptions of shared decision making, researchers have set about measuring shared decisions and their impact on clinical outcomes. Most work has focused on patients’ or physicians’ perceptions that shared decision making occurred 8 , 9 , 10 , 11 , 12 ; and how decision aids (i.e. multi‐media tools designed to promote shared decision making) and patients’ involvement in decision making affect outcomes. 13 , 14 By contrast, little work has focused on the joint patient–physician experience of shared decision making or how that might impact outcomes. Indeed, it is generally assumed that if a core set of components are observed to be present by third party review, 15 , 16 the process of decision making is sufficient to be ‘shared’. While several studies reporting discordant patient–provider perceptions of the clinical encounter support this assumption, 17 , 18 , 19 , 20 other recent work challenges it. 21 , 22 , 23 , 24
Several studies suggest that SDM may result from more than a discussion about patients’ options and values, and an apparent decision. 19 , 20 One study concluded that both patients and physicians frequently make assumptions about the others’ understanding and do not fully engage in the SDM process. 9 Other studies show that patients and physicians conceptualize shared decision making differently than the research community 21 , 23 , 24 with patients, in particular, conceptualizing SDM more in terms of gaining respect, building trust and receiving empathy than on information exchange or expression of preferences. 22 Studies such as these suggest that querying both patients and physicians about key information may be necessary to capture a truly informed decision, and querying them about their respective perceptions of involvement may be necessary to understand when decisions are truly shared.
Given this evidence, we propose that further study of patients’ and physicians’ perceptions of clinical encounters (and how they relate to an observational comparison) is warranted. Such an analysis might help identify areas for future improvements in the shared decision making process and guide future measurement of shared decision making.
Our objectives for this study, therefore, were to examine the extent of agreement between patients and physicians regarding the content and outcomes of one common clinical discussion, coronary heart disease (CHD) prevention, and subsequently to compare patient and physician reports of visit content and outcomes to coded transcriptions of their clinic visit.
Methods
Overview
To examine patient–physician agreement, we performed a cross‐sectional survey nested within a randomized trial of heart disease prevention (see Fig. 1). In the larger trial, after collecting baseline data on demographics, CHD risk and attitudes, we centrally randomized patients to either a multi‐component intervention or usual care, and saw them for two additional study visits over several months. The intervention in this trial consisted of a computerized decision aid and coaching tool administered at the second study visit and a series of tailored adherence reminders delivered between the second and third study visits.
Figure 1.
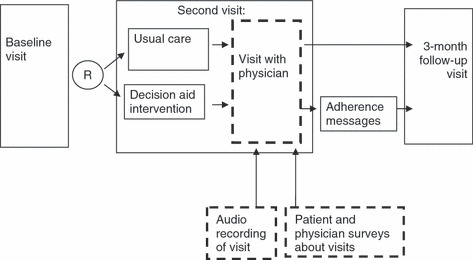
Study design. Solid lines indicate design of larger randomized trial. Dashed lines indicate design of current nested study.
This paper focuses on data collected at the second study visit, between June 2007 and December 2009, when we surveyed both patients and their physicians about the content and outcomes of a clinic visit and audio‐recorded a sub‐sample of visits to provide an observational comparison of what actually happened. We report the main outcomes of our trial (e.g. data collected at the third study visit on adherence and predicted CHD risk) and other secondary outcomes (e.g. data from the second study visit on intent to start specific therapies) in a separate paper (Sheridan SL, Behrend L, Pignone MP et al. under review). The University of North Carolina at Chapel Hill’s Biomedical Institutional Review Board (IRB) approved all study procedures.
Setting
We conducted our study in one university internal medicine clinic. This clinic employs a total of 93 physicians, including 17 attendings (physicians‐in‐practice) and 76 residents (physicians‐in‐training), who were not part of our research team and were thus eligible to participate in the study. Forty of these physicians agreed to participate in the larger trial (including 16 attendings and 24 residents), but only 24 (all 16 attendings and 8 residents) had patients enrolled in this study. All participating physicians attended a 1‐h educational session designed to provide information about global CHD risk and risk reduction; highlight the value of patient choice and involvement in decision making; and provide resources to support patient adherence, including action plans, pillboxes and smoking cessation quitlines.
Participants
The sample for this study included all 24 physicians and their 157 patients (range 1–20 patients per physician) who participated in the larger trial and had complete participation through the second study visit (100% of physicians, 96% of all patients from the larger trial). Patients were eligible to participate in both the larger trial and this sub‐study if they were presenting for care with an enrolled physician, were between 40 and 79 years old, had no prior history of cardiovascular disease, diabetes mellitus, or other serious medical condition that limited their life expectancy to less than 5 years, and were at moderate (6–10%) to high risk (>10%) of heart disease over 10 years based on a Framingham risk equation. 25 We excluded patients if they were presenting for their first visit, had no cholesterol check within the past 18 months, were unable to speak or read English, or had extreme elevations of systolic blood pressure (>180 mmHg) or cholesterol (>300 mg/dl).
Procedure
This analysis focuses on data collected at the second study visit of the larger randomized trial. Patients randomized to the intervention group presented 45 min early to a previously scheduled clinic visit and viewed a computerized CHD prevention decision aid with coaching component. The decision aid educated patients about their global CHD risk and the best available treatment options (aspirin, blood pressure and cholesterol medications, and smoking cessation), and facilitated values clarification and a choice among treatments. The coaching component provided suggestions for overcoming barriers to talking with their physician (e.g. ‘the doctor decides what we talk about’; ‘my doctor uses words I don’t understand’) and for gaining support for treatment (in the form of prescriptions, referrals, or encouragement). Both had undergone formative testing, including pilot testing of the decision aid alone 26 and cognitive and usability testing of a revised decision aid with the coaching component (Sheridan SL, Turner AL, Pignone MP et al. available from authors upon request). After viewing the decision aid, patients proceeded to a previously scheduled visit with their regular physician. Patients randomized to the control group did not present early and received usual care (physician‐provided risk factor management) from their physician.
Following the clinic visit, a research assistant gave self‐administered post‐visit surveys to all patients to complete via pen‐and‐paper. To reduce burden, the research assistant dropped off post‐visit surveys for physicians to complete as time allowed during or after their clinic schedule. Most (>80%) physicians filled out surveys on the same day. Surveys asked patients and their physicians about visit content and outcomes using the same questions. To provide an observational comparison to patient and physician report, we also audio‐recorded the first consecutive 20 visits (including nine different physicians with a range of one to five patients per physician); half of these visits were for patients in the intervention group and half for patients in the control group. All audio‐tapes were transcribed verbatim and coded by two independent coders for visit content.
Survey content
We structured the patient and physician surveys for this analysis to capture agreement on visit content central to SDM. 7 At a minimum, SDM requires the effective integration of the knowledge held by the physician and the values and preferences held by the patient. Ideally, then, both parties should agree on the following: that a discussion occurred; whether the pros and cons of treatment options were discussed; that the patient expressed his or her preferences regarding next steps and decisions; and that a final decision was made or actively deferred. We adapted survey content from a prior study of prostate cancer screening, 27 which derived questions based on key SDM concepts 2 , 7 , 28 and expert opinion, and cognitively tested content to ensure quality and understanding.
In completing surveys, participants first reported whether or not they had a CHD prevention discussion during their clinic visit. Participants who did not report having a CHD discussion did not continue to answer questions. If they did report having a CHD discussion, they continued to report on details of the discussion. They reported on discussion content in two ways: by indicating whether they discussed mostly pros, pros and cons, or mostly cons about changing risk factors (such as high blood pressure or cholesterol) or reducing overall chances of heart disease; and by whether and how much patients expressed their preferences about risk factor treatment options (a lot, a little, or not really). They reported on involvement in decision making by indicating who made the final decision (physician alone; physician with patient opinion; shared decision; patient with physician opinion; patient alone). 28 Finally, participants reported on physician recommendations and final decisions to take medicines (including aspirin, blood pressure medication, cholesterol medication, or smoking cessation medication) or change their lifestyle (including diet and exercise changes and smoking cessation).
When both physician and patient participants did not report having a CHD discussion and did not continue to answer questions, data were missing. When data were missing for only one (either physician or patient) participant, we assumed a ‘no’ answer for questions with dichotomous yes/no outcomes (including physician recommendations and final decisions) because no recommendations or final decisions regarding CHD prevention could be made without a CHD prevention discussion. For nominal outcomes (discussion of pros or cons, patient expression of preferences, and who made the final decision), however, we made no assumptions and report agreement only on data that were available for both parties.
Coding visit transcript content
We coded visit transcripts to correspond with survey content, similar to methods employed by many observational studies of SDM. 6 , 16 , 29 , 30 When determining the presence of a CHD prevention discussion, we required a specific statement about lowering chances of heart disease. When coding for discussion content, we counted the number of pros and cons discussed and then categorized this into mostly pros, pros and cons, or mostly cons. To code patient expression of preferences, we counted the number of preferences patients expressed regarding treatment features (such as cost) or regarding a course of action (such as taking a blood pressure medication) and categorized them into a lot (expressed three or more preferences), a little (expressed one to two preferences), and not really (did not express any preferences). To code for who made the final decision, we used Charles’(1997) definition of shared decision making, 2 which requires both patient and physician to share information, express preferences, and negotiate a decision. We counted whether prevention options were discussed, if the physician and patient both expressed their preferences about risk factor treatment options, and whether a decision was made. We then combined these factors into five categories (see Appendix 1 in supporting information) of decision making to match the answer options on participant surveys: physician alone; physician with patient opinion; shared decision; patient with physician opinion; patient alone. Finally, to code physician recommendations and final decisions, we required a specific statement of recommendation or plan to take medication or make lifestyle changes. Our two independent coders had very high agreement for coding across content questions (kappa = 0.88–1.0).
Analysis
For each of the outcomes described above, we calculated the percent agreement between (i) patient and physician report (full sample), (ii) patient and transcription report (sub‐sample only), and (iii) physician and transcription report (sub‐sample only) after accounting for the effects of clustering of patients within physicians. We also compared patient and physician report by calculating simple and weighted kappa values, which measure the agreement beyond chance between two variables and range from almost perfect agreement (0.80–1.00) to poor or no agreement (0.00–0.19), with negative values indicating worse agreement than expected by chance. 31 To calculate standard errors for kappa values, we used a bootstrap method, sampling physicians with replacement and including in the bootstrap sample all patients seen by the sampled physician. This method preserved the correlation structure of the patients within physicians and allowed us to account for the effect of clustering of patients within physicians. For outcomes with low kappa values or low percentage agreement, we examined and report on patterns of disagreement.
We initially analysed our results by the intervention and control groups of the larger randomized trial. However, finding no difference across groups, we combined groups into our single full sample for reporting in this manuscript.
Results
Participant characteristics
In the full analysis, we included all 157 patients and 24 physicians who were eligible for and participated in our larger CHD prevention trial (see 1, 2). The sub‐sample analysis of 20 clinic visits involved 20 patients and 9 different physicians. See Fig. 2 for a flow diagram of these samples.
Table 1.
Patient characteristics
| Characteristic | Full sample (N = 157) | Reporting CHD discussions (N = 103) | Sub‐sample (N = 20) | Reporting CHD discussions (N = 17) |
|---|---|---|---|---|
| Mean age (range) | 63 (40–79) | 62 (40–78) | 64 (45–77) | 62 (45–75) |
| Female (%) | 27 | 22 | 20 | 12 |
| Race | ||||
| White (%) | 85 | 87 | 90 | 100 |
| Black (%) | 10 | 9 | 10 | 0 |
| At least some college education (%) | 90 | 90 | 95 | 100 |
| Good self‐perceived health status (%) | 90 | 89 | 80 | 82 |
| Mean CHD risk | 11.3 | 11.3 | 13.3 | 13.8 |
| Preferred participation in decision making about CHD prevention | ||||
| Patient decides alone (%) | 3 | 3 | 5 | 6 |
| Patient decides with physician opinion (%) | 31 | 37 | 25 | 29 |
| Share decision (%) | 44 | 40 | 50 | 47 |
| Physician decides with patient opinion (%) | 11 | 8 | 10 | 6 |
| Physician decides alone (%) | 12 | 12 | 10 | 12 |
Table 2.
Physician characteristics
| Characteristic | Full sample (N = 24) | Reporting CHD discussions (N = 23) | Sub‐sample (N = 9) | Reporting CHD discussions (N = 7) |
|---|---|---|---|---|
| Mean age (range) | 38 (28–75) | 38 (27–75) | 46 (29–75) | 44 (29–57) |
| Female (%) | 29 | 30 | 11 | 0 |
| Current standing | ||||
| Resident (%) | 33 | 30 | 11 | 14 |
| Attending physician (%) | 67 | 60 | 89 | 86 |
| Preferred participation in decision making about CHD prevention | ||||
| Patient decides alone (%) | 4 | 4 | 11 | 0 |
| Patient decides with physician opinion (%) | 21 | 17 | 22 | 29 |
| Share decision (%) | 67 | 70 | 56 | 57 |
| Physician decides with patient opinion (%) | 8 | 9 | 11 | 14 |
| Physician decides alone (%) | 0 | 0 | 0 | 0 |
Figure 2.
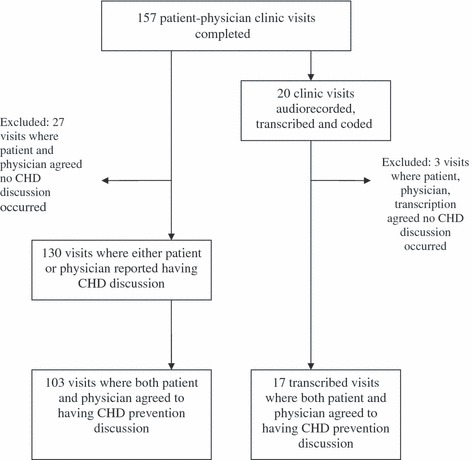
Study flow diagram.
Patients in our full sample were mostly white, male, had at least some college education and a good self‐perceived health status. Their mean predicted CHD risk over 10 years was 11.3%. Most patients expressed a preference for shared decision making about CHD prevention. Characteristics of patients who agreed with physicians about having CHD discussions (N = 103) were similar to those of the full sample. Characteristics of patients in the sub‐sample were also similar to those in the full sample, although those in the sub‐sample who reported discussions were slightly more likely to be male.
Physicians in our full sample were mostly attendings (physicians‐in‐practice) and most also indicated a preference for shared decision making about CHD prevention. The sub‐sample of physicians was slightly older and contained a higher proportion of males and attendings than the full sample. Those physicians reporting CHD discussions in both the full sample and sub‐sample had similar characteristics to their respective full groups.
Full sample analysis: patient–physician agreement on visit content
CHD discussions
Patients and physicians agreed 83% of the time (95% CI: 72–86%) about whether they had a CHD prevention discussion during their clinic visit (see Table 3), with a kappa value of 0.55 (95% CI: 0.40–0.70) indicating moderate agreement. We found 27 cases where both patient and physician agreed that no CHD prevention discussion occurred, and excluded these cases from the remainder of the analysis. In 103 visits, both the patient and physician agreed a discussion occurred and in 27 visits, only one reported having a CHD prevention discussion. In the following sections, we report on the remaining visits where either the patient or physician reported that a discussion occurred.
Table 3.
Patient and physician agreement on CHD prevention discussions
| Number of patients and physicians agreeing | Percent agreement (95% CI) | Kappa (95% CI) | |
|---|---|---|---|
| Presence of CHD discussion (N = 157) | |||
| Reported they talked about CHD | |||
| Yes | 103 | 83% | 0.55 (0.40–0.70) |
| No | 27 | (72–86%) | |
| Content of discussion (N = 103) | |||
| Reported they talked about | |||
| Mostly pros | 31 | 53% (45–64%) | 0.15 (−0.01–0.30) |
| Pros and cons | 24 | ||
| Mostly cons | 0 | ||
| Patient expressed preferences (N = 103) | |||
| A lot | 16 | 41% (33–49%) | 0.16 (0.03–0.28) |
| A little | 19 | ||
| Not really | 8 | ||
| Who made final decision (N = 103) | |||
| Patient alone | 0 | 43% (32–54%) | 0.13 (−0.11–0.37) |
| Patient with physician opinion | 5 | ||
| Shared decision | 34 | ||
| Physician with patient opinion | 3 | ||
| Physician alone | 2 | ||
| Physician recommendation (N = 130) | |||
| Take medicine | 94 | 72% (66–79%) | 0.40 (0.28–0.53) |
| Change lifestyle | 97 | 75% (65–84%) | 0.49 (0.32–0.66) |
| Final decision (N = 130) | |||
| Take medicine | 102 | 78% (71–86%) | 0.58 (0.45–0.71) |
| Change lifestyle | 90 | 69% (62–77%) | 0.38 (0.24–0.53) |
| Discuss at next visit | 87 | 67% (55–79%) | 0.12 (−0.09–0.34) |
Discussion content
Patients and their physicians agreed 53% of the time (95% CI: 45–64%) about whether they discussed mostly pros, pros and cons, or mostly cons about changing risk factors or lowering overall chances of heart disease (kappa = 0.15; 95% CI: −0.01–0.30). Table 4 illustrates the pattern of agreement, with shaded boxes indicating agreement. On 22% of visits the physician reported discussing mostly pros while the patient reported discussing both pros and cons. Similarly, on 21% of visits the patient reported discussing only pros while the physician reported discussing pros and cons. We found very little extreme disagreement in the form of one participant indicating discussion of only pros and the other indicating discussion of only cons (1% on either side).
Table 4.
Patient and physician agreement on discussion of pros and cons
| Patient report | Physician report | ||
|---|---|---|---|
| Mostly pros | Pros and cons | Mostly cons | |
| Mostly pros (%) | 30 | 21 | 1 |
| Pros and cons (%) | 22 | 23 | 0 |
| Mostly cons (%) | 1 | 0 | 0 |
Total percent agreement: 53% (45–64%).
Kappa: 0.15 (−0.01–0.30).
Patients and physicians agreed on only 41% of visits (95% CI: 33–49%) about whether the patient expressed preferences about risk factor treatment options a lot, a little, or not really at all during the clinic visit (see Table 5). The kappa for this outcome (=0.16; 95% CI: 0.03–0.28) was low and indicated poor agreement beyond chance. The majority of disagreement favored physician over‐reporting of patient participation, with 9% of visits in which the physician reported a lot of patient participation though the patient reported not really any participation.
Table 5.
Patient and physician agreement on patient expression of preferences
| Physician report | |||
|---|---|---|---|
| A lot | A little | Not really | |
| Patient report | |||
| A lot (%) | 15 | 10 | 2 |
| A little (%) | 18 | 18 | 9 |
| Not really (%) | 9 | 12 | 8 |
Total percent agreement: 41% (33–49%).
Kappa: 0.16 (0.03–0.28).
Involvement in decision making
Patients and physicians agreed during 43% of visits (95% CI: 32–54%) about who made the final decision (kappa = 0.13; 95% CI: −0.11 to 0.37). Disagreements were variable (see Table 6), with both some patients and some physicians reporting shared decisions when the other party reported no role or an independent role in the decision. Overall, as with the preference analysis, there was a trend indicating that physicians are more likely to perceive higher patient involvement in decision making than the patients themselves report. In sensitivity analysis, collapsing the middle three categories of decision making (for comparison of physician alone, any sharing of decision, patient alone; data not in tables) increased percent agreement to 78% (CI 66–88%) with little change in kappa (kappa = 0.05; 95% CI: −0.11 to 0.21).
Table 6.
Patient and physician agreement on involvement in decision making
| Physician report | |||||
|---|---|---|---|---|---|
| Physician | Physician with patient input | Shared | Patient with physician input | Patient | |
| Patient report | |||||
| Physician (%) | 2 | 0 | 7 | 1 | 1 |
| Physician with patient input (%) | 1 | 3 | 7 | 0 | 0 |
| Shared (%) | 5 | 6 | 33 | 13 | 4 |
| Patient with physician input (%) | 0 | 2 | 9 | 5 | 1 |
| Patient (%) | 0 | 0 | 1 | 0 | 0 |
Total percent agreement: 43% (32–54%).
Kappa: 0.13 (−0.11–0.37).
Physician recommendations and final decisions
Patients and physicians agreed more frequently regarding physician recommendations and final decisions. They agreed 72% of the time (95% CI: 66–79%) about recommendations to take medicine and 75% of the time (95% CI: 65–84%) about recommendations to change lifestyle. The kappa values for both outcomes indicate moderate agreement beyond chance [kappa = 0.40 (95% CI: 0.28–0.53) and 0.49 (95% CI: 0.32–0.66), respectively]. Patients and physicians agreed 78% (95% CI: 71–86%) and 69% (95% CI: 62–77%) of the time about final decisions to take medication and change lifestyle. However, there was somewhat less agreement (67%; 95% CI: 55–79%; kappa = 0.12; 95% CI: −0.09 to 0.34) about final decisions to discuss again at a later visit.
Sub‐sample analysis: patient and physician agreement with observed visit content
CHD discussions
A majority of the time, coded transcriptions agreed with both physicians (75%; N = 15 out of 20) and patients (65%; N = 13 out of 20) that a CHD discussion occurred (see Table 7). For the remainder of the sub‐sample analysis, we excluded three visits during which patient, physician, and transcription agreed that no CHD prevention discussion occurred. Average visit length in the remaining 17 visits was 22 min (range 13–37 min).
Table 7.
Comparison of patient and physician report to coded transcriptions of clinic visits
| Physician/transcription (N = 17) | Patient/transcription (N = 17) | |||
|---|---|---|---|---|
| Number agreeing | Percent agreement (%) | Number agreeing | Percent agreement (%) | |
| Presence of CHD discussion | ||||
| Reported they talked about CHD | 15 | 75 | 13 | 65 |
| Content of discussion | ||||
| Mostly pros, pros and cons, mostly cons | 7 | 44 | 4 | 24 |
| Patient expressed preferences | ||||
| A lot | 0 | 27 | 1 | 25 |
| A little | 0 | 2 | ||
| Not really | 4 | 1 | ||
| Who made final decision | ||||
| Patient alone | 0 | 33 | 0 | 32 |
| Patient with physician opinion | 0 | 1 | ||
| Shared decision | 2 | 2 | ||
| Physician with patient opinion | 3 | 1 | ||
| Physician alone | 0 | 0 | ||
| Physician recommendation | ||||
| Take medicine | 12 | 71 | 15 | 88 |
| Change lifestyle | 13 | 76 | 14 | 82 |
| Final decision | ||||
| Take medicine | 15 | 88 | 15 | 88 |
| Change lifestyle | 14 | 82 | 13 | 76 |
| Discuss at next visit | 11 | 65 | 13 | 76 |
Discussion content
We found fairly low agreement between coded transcriptions and both physician and patient report regarding whether the discussion covered mostly pros, pros and cons, or mostly cons of risk factor treatment options. Only seven out of 17 physician/transcription comparisons (44%) and four out of 17 patient/transcription comparisons (24%) agreed on this outcome.
We found very low agreement when comparing transcriptions and surveys regarding whether and how much the patient expressed preferences. Four out of 17 physician/transcription comparisons (27%) and four out of 17 patient/transcription comparisons (25%) agreed on patient expression of preferences during the clinic discussion.
Involvement in decision making
Agreement was also low with regard to involvement in decision making. Only five physician/transcription comparisons (33%) and four patient/transcription comparisons (32%) agreed on who made the final treatment decision. Collapsing the middle three categories (for comparison of physician alone, any sharing of decision, patient alone; data not in tables) increased this agreement to 15 physician/transcription comparisons (88%) and 13 patient/transcription comparisons (76%).
Physician recommendations and final decisions
We found high agreement between transcriptions and physician and patient report regarding physician recommendations. Twelve physician/transcription comparisons (71%) agreed on recommendations to take medicine and 13 (76%) agreed on recommendations to change lifestyle. Fifteen (88%) and 14 (82%) patient/transcription comparisons agreed on these same outcomes, respectively.
Agreement was also high regarding final decisions. Fifteen (88%) of both physician and patient/transcription comparisons agreed about final decisions to take medicine. Fourteen physician/transcription comparisons (82%) and 13 patient/transcription comparisons (76%) agreed about final decisions to change lifestyle. Finally, 11 physician/transcription comparisons (65%) and 13 patient/transcription comparisons (76%) agreed on final decisions to discuss at a later visit.
Discussion
Our analysis of patient–physician agreement on the content and outcomes of CHD prevention discussions indicated fair to moderate agreement about presence of discussions, physician recommendations, and final decisions to either take medicine or change lifestyle. We found poor agreement regarding discussion content (mostly pros, pros and cons, or mostly cons; patient expression of preferences) and involvement in decision making. Coded transcriptions agreed with patient and physician report on most outcomes. However, it was clear that patient and physician reports each did not agree well with coded transcriptions on content of CHD discussions and involvement in decision making.
We are encouraged that patients and physicians agreed in 83% of visits about presence of CHD discussions and in 78% and 69% of clinic visits, respectively, about final decisions to either take medicine or change lifestyle. The lack of agreement on discussions [and the lack of actual discussions (n = 27 visits)] is perhaps not unexpected given that we recruited patients who were already scheduled for routine clinic appointments in which other clinical matters may have taken priority over CHD prevention. 32 However, the agreement we found about final decisions is slightly lower than a previous study reported regarding actions taken during consultations, 33 and leaves roughly one quarter of clinic encounters where patients and physicians did not agree about their final decisions. Disagreement on this outcome could have important clinical implications if physicians are unable to provide the necessary support (such as prescriptions for medication, diet counselling, or referral to smoking cessation programs) to carry out decisions. It could also have important implications if physicians believe that their patients have agreed to treatment regimens that they have no real intention of following; prior studies suggest this is an important concern. 34 Although we are unclear what might have contributed to this disagreement in our sample, previous studies have found similar misunderstandings to result from a lack of clear patient communication of treatment preferences and expectations. 33 , 34 Other possible contributors are patient factors such as literacy or educational level (though both are high overall in our sample) or the communication style of the various physicians in the sample.
A result of greater concern is the finding of poor agreement between patients and physicians on measures of discussion content (discussion of mostly pros, pros and cons, or mostly cons and expression of patient preferences) and involvement in decision making. Though we are unaware of other studies examining patient–physician agreement on these exact outcomes, this is consistent with literature describing physicians’ tendencies to overestimate information exchange 35 , 36 , 37 and the extent of patient participation. 9 , 38 Discussion of pros and cons and patients’ expression of preferences are important when considering the balance and depth of a clinic discussion, and both are recognized as essential components of the shared decision making process. 7 Disagreement about discussion of pros and cons about treatment options is concerning as it could indicate an imbalanced discussion, one that does not include all relevant information, or one in which information is not presented in ways that are accessible to the other participant. Disagreement about expression of patient preferences raises many of the same concerns. Disagreement about who made the final decision, on the other hand, is concerning to the extent that physicians believe they are allowing patients to participate in decision making, but are not; this is the very pattern we and others 9 , 38 have observed. In addition, prior research suggests that patients may value the process of involvement even more than who actually makes the final decision. 21 , 24 This raises the possibility that disagreements which suppress patient involvement may adversely affect clinical outcomes such as satisfaction with care, self‐efficacy, or trust. 13 , 39 , 40
Our sub‐sample provides important insights into the disagreements between patients and physicians in our full sample and raises questions about how to best measure SDM. In the sub‐sample, like the full sample, there was poor agreement on discussion of pros and cons, discussion of patient preferences, and involvement in decision making. Here, however, the disagreement was between patient and transcript or physician and transcript. These findings have different implications depending on the outcome measure.
Poor patient‐transcription or physician‐transcription agreement on discussion content (including pros and cons and patient preferences) is consistent with other observational studies reporting routine clinical decisions are often made without a great deal of information sharing 7 , 36 , 37 and suggests that communication within the clinical encounter needs improvement. One potential way for improvement is for physicians to tailor their communication style based on patient factors such as literacy or education level. 41 , 42 In addition, physicians should check patient understanding to help improve agreement about discussion content 6 , 43 or follow‐up via mail or email with summaries of key information and decisions. Alternately, physicians (or patients) might consider participation in programs that teach effective ways to elicit (or discuss) patient preferences. 44 , 45
Poor patient‐transcription or physician‐transcription agreement on involvement in decision making has different implications. This finding is consistent with previous research indicating that patients have trouble determining who made the final clinical decisions and to what extent involved parties participated. 21 , 23 , 24 These same studies suggest that patients and physicians conceptualize SDM differently than the research community. These findings suggest that our brief, objective SDM assessment (and more recent and extensive measures) 46 may not align with patients’ and physicians’ general perceptions of a shared decision. Some work suggests that patients may conceptualize SDM more in terms of gaining respect, building trust, and receiving empathy than on information exchange or expression of preferences. 22 To move the field forward, it will therefore be important not only to better define patients’ and physicians’ notions of shared decision making, but also to determine what most impacts clinical outcomes: the patients’ experience of SDM, physicians’ experience of SDM, or external and observational documentation of SDM. Whichever of these outcomes has the biggest impact should then be incorporated as part of the core SDM measure.
In considering such conclusions, we must acknowledge the following limitations. First, our sample size overall, along with wide confidence intervals, limits the precision of our results. Moreover, the results from comparisons to 20 coded transcriptions are hypothesis‐generating only. Because we recorded consecutive visits, these might not be representative of consultations in general at this practice. Second, our sample was not powered to detect differences in agreement among those in the intervention and control groups of our larger trial. It is possible that such differences exist and could be detected if a larger sample were examined. Third, there is the potential for recall error as physicians may not have had time to complete surveys immediately following the clinic visit. However, most physicians returned surveys on the same day they were delivered and were able to use visit notes in completing surveys, minimizing our concern. Fourth, there are potential issues with interpretation of survey questions, and alternate measures may produce different results. We designed our surveys to ask identical questions to patients and physicians and used questions that would explore the nature of and participation in the discussion. After we conducted this study, Melbourne et al. 46 published their adaptation of the 12‐item OPTION scale to measure joint perceptions of SDM, providing data to suggest that some individuals have problems understanding ‘pros and cons’ language and appear to better understand the meaning of ‘advantages and disadvantages.’ Furthermore, different questions about expression of patient preferences may change agreement; our responses for expression of patient preferences ranged from ‘a lot’ to ‘not really’ and did not include a hard zero, which could have affected interpretation. Additionally, the new dyadic OPTION scale asks about ‘concerns’, ‘worries’, ‘expectations’, and ‘ideas’ to explore preferences and values and may yield different results. In addition, we did not specify a time frame for patient involvement, which might have affected our comparisons with visit transcriptions if participants perceived involvement in decision making over time. Furthermore, because CHD prevention allows for decisions to be made over time, we may have underestimated patient involvement with our measures, which we intended to focus on one clinical visit. Fifth, the firm criteria we used for coding visit transcripts could have contributed to the disagreement between transcripts and patient and physician surveys; for example, coding the number of preferences expressed did not allow us to consider the ‘strength’ of a preference, which could underestimate the extent of participation when a patient expresses fewer preferences but with greater emphasis. However, such criteria are necessary for any coding scheme and have been widely employed. 6 , 16 , 29 , 30 Finally, it is possible that participants in the study were already sensitized to SDM, and our results may not generalize to other populations that had not been exposed to a similar intervention. Our results also may not generalize to more diverse patient populations, those with less education, or those with less desire for shared decision making about CHD prevention.
Limitations aside, we feel the clinical and research community should be aware that disagreements about the content of and participation in clinical discussions may be common. Although we found reasonably good agreement on whether discussions occur, what recommendations physicians made, and what final decision was made, there was poor agreement on content of discussions and involvement in decision making. Future work is needed not only to determine how widespread such disagreements are in a more diverse population, but also to what extent these disagreements impact clinical outcomes such as satisfaction, trust and adherence. Future research should also continue to improve on the measurement of shared decision making and its components and investigate the relative importance of the subjective experience versus objective steps of SDM. It could be that combining the subjective assessment of involvement with objective verification of adequate information exchange is key to ensuring quality decision making. Further, the disagreements we have described indicate the need to intervene and improve physician–patient communication and SDM. Most often it is physicians who have the power to set the tone of a clinic visit and give patients the opportunity to express treatment preferences; 47 yet they tend to overestimate information exchange 35 , 36 , 37 and the extent of patient participation. 9 , 38 Given these findings, investigators should continue to explore how best to facilitate clearer communication between patients and their physicians.
Conflict of interest
The authors do not have any conflicts of interest to declare.
Source of funding
Financial support for this study was provided by a grant from the National Heart Lung and Blood Institute (grant number 1 K23 HL074375). Dr Cai’s time was supported in part by the National Center for Research Resources (grant number UL1 RR025747).
Supporting information
Appendix 1. Transcription definition of decision‐making.
Supporting info item
Acknowledgements
The authors would like to thank the Working Group on Risk, Communication, and Healthcare Decision‐making for their feedback and input on prior versions of this manuscript.
References
- 1. O’Connor AM, Legare F, Stacey D. Risk communication in practice: the contribution of decision aids. BMJ, 2003; 327: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science and Medicine, 1997; 44: 681–692. [DOI] [PubMed] [Google Scholar]
- 3. Charles C, Gafni A, Whelan T. Decision‐making in the physician–patient encounter: revisiting the shared treatment decision‐making model. Social Science and Medicine, 1999; 49: 651–661. [DOI] [PubMed] [Google Scholar]
- 4. Elwyn G, Edwards A, Kinnersley P, Grol R. Shared decision making and the concept of equipoise: the competences of involving patients in healthcare choices. British Journal of General Practice, 2000; 50: 892–899. [PMC free article] [PubMed] [Google Scholar]
- 5. Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ, 1999; 319: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA, 1999; 282: 2313–2320. [DOI] [PubMed] [Google Scholar]
- 7. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Education Counseling, 2006; 60: 301–312. [DOI] [PubMed] [Google Scholar]
- 8. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. Health Expectations, 2006; 9: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saba GW, Wong ST, Schillinger D et al. Shared decision making and the experience of partnership in primary care. Annals of Family Medicine, 2006; 4: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGuire AL, McCullough LB, Weller SC, Whitney SN. Missed expectations? Physicians’ views of patients’ participation in medical decision‐making. Medical Care, 2005; 43: 466–470. [DOI] [PubMed] [Google Scholar]
- 11. Murray E, Pollack L, White M, Lo B. Clinical decision‐making: physicians’ preferences and experiences. BMC Family Practice, 2007; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray E, Pollack L, White M, Lo B. Clinical decision‐making: Patients’ preferences and experiences. Patient Education Counseling, 2007; 65: 189–196. [DOI] [PubMed] [Google Scholar]
- 13. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Annals of Internal Medicine, 1985; 102: 520–528. [DOI] [PubMed] [Google Scholar]
- 14. O’Connor AM, Bennett CL, Stacey D et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2009:CD001431. [DOI] [PubMed] [Google Scholar]
- 15. Elwyn G, Edwards A, Mowle S et al. Measuring the involvement of patients in shared decision‐making: a systematic review of instruments. Patient Education Counseling, 2001; 43: 5–22. [DOI] [PubMed] [Google Scholar]
- 16. Elwyn G, Hutchings H, Edwards A et al. The OPTION scale: measuring the extent that clinicians involve patients in decision‐making tasks. Health Expectations, 2005; 8: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bensing JM, Tromp F, van Dulmen S, van den Brink‐Muinen A, Verheul W, Schellevis FG. Shifts in doctor–patient communication between 1986 and 2002: a study of videotaped general practice consultations with hypertension patients. BMC Family Practice, 2006; 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Donnell H, Phillips RS, Wenger N, Teno J, Davis RB, Hamel MB. Preferences for cardiopulmonary resuscitation among patients 80 years or older: the views of patients and their physicians. Journal of the American Medical Directors Association, 2003; 4: 139–144. [DOI] [PubMed] [Google Scholar]
- 19. Montgomery AA, Fahey T. How do patients’ treatment preferences compare with those of clinicians? Quality in Health Care, 2001; 10 (Suppl. 1): i39–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cotler SJ, Patil R, McNutt RA et al. Patients’ values for health states associated with hepatitis C and physicians’ estimates of those values. American Journal of Gastroenterology, 2001; 96: 2730–2736. [DOI] [PubMed] [Google Scholar]
- 21. Edwards A, Elwyn G. Inside the black box of shared decision making: distinguishing between the process of involvement and who makes the decision. Health Expectations, 2006; 9: 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lown BA, Hanson JL, Clark WD. Mutual influence in shared decision making: a collaborative study of patients and physicians. Health Expectations, 2009; 12: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Entwistle VA, Watt IS, Gilhooly K, Bugge C, Haites N, Walker AE. Assessing patients’ participation and quality of decision‐making: insights from a study of routine practice in diverse settings. Patient Education Counseling, 2004; 55: 105–113. [DOI] [PubMed] [Google Scholar]
- 24. Peek ME, Quinn MT, Gorawara‐Bhat R, Odoms‐Young A, Wilson SC, Chin MH. How is shared decision‐making defined among African‐Americans with diabetes? Patient Education Counseling, 2008; 72: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837–1847. [DOI] [PubMed] [Google Scholar]
- 26. Sheridan SL, Shadle J, Simpson RJ Jr, Pignone MP. The impact of a decision aid about heart disease prevention on patients’ discussions with their doctor and their plans for prevention: a pilot randomized trial. BMC Health Services Research, 2006; 6: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Driscoll DL, Rupert DJ, Golin CE et al. Promoting prostate‐specific antigen informed decision‐making. Evaluating two community‐level interventions. American Journal of Preventive Medicine, 2008; 35: 87–94. [DOI] [PubMed] [Google Scholar]
- 28. Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. The Canadian Journal of Nursing Research, 1997; 29: 21–43. [PubMed] [Google Scholar]
- 29. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. Journal of General Internal Medicine, 1997; 12: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roter D, Larson S. The Roter interaction analysis system (RIAS): utility and flexibility for analysis of medical interactions. Patient Education Counseling, 2002; 46: 243–251. [DOI] [PubMed] [Google Scholar]
- 31. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics, 1977; 33: 159–174. [PubMed] [Google Scholar]
- 32. Stange KC, Zyzanski SJ, Jaen CR et al. Illuminating the ‘black box’. A description of 4454 patient visits to 138 family physicians. Journal of Family Practice, 1998; 46: 377–389. [PubMed] [Google Scholar]
- 33. Hooper R, Rona RJ, French C, Jones M, Wessely S. Unmet expectations in primary care and the agreement between doctor and patient: a questionnaire study. Health Expectations, 2005; 8: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Britten N, Stevenson FA, Barry CA, Barber N, Bradley CP. Misunderstandings in prescribing decisions in general practice: qualitative study. BMJ, 2000; 320: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makoul G, Arntson P, Schofield T. Health promotion in primary care: physician–patient communication and decision making about prescription medications. Social Science and Medicine, 1995; 41: 1241–1254. [DOI] [PubMed] [Google Scholar]
- 36. Waitzkin H. Information giving in medical care. Journal of Health and Social Behavior, 1985; 26: 81–101. [PubMed] [Google Scholar]
- 37. Waitzkin H. Doctor‐patient communication. Clinical implications of social scientific research. JAMA, 1984; 252: 2441–2446. [DOI] [PubMed] [Google Scholar]
- 38. Stevenson FA, Barry CA, Britten N, Barber N, Bradley CP. Doctor‐patient communication about drugs: the evidence for shared decision making. Social Science and Medicine, 2000; 50: 829–840. [DOI] [PubMed] [Google Scholar]
- 39. Golin C, DiMatteo MR, Duan N, Leake B, Gelberg L. Impoverished diabetic patients whose doctors facilitate their participation in medical decision making are more satisfied with their care. Journal of General Internal Medicine, 2002; 17: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mead N, Bower P, Hann M. The impact of general practitioners’ patient‐centredness on patients’ post‐consultation satisfaction and enablement. Social Science and Medicine, 2002; 55: 283–299. [DOI] [PubMed] [Google Scholar]
- 41. Pignone M, DeWalt DA, Sheridan S, Berkman N, Lohr KN. Interventions to improve health outcomes for patients with low literacy. A systematic review. Journal of General Internal Medicine, 2005; 20: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiss B. Health literacy and patient safety: Help patients understand. Manual for clinicians, 2nd edn American Medical Association Foundation and American Medical Association, 2007. Available at: http://www.ama‐assn.org/ama1/pub/upload/mm/healthlitclinicians.pdf. [Google Scholar]
- 43. Schillinger D, Piette J, Grumbach K et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Archives of Internal Medicine, 2003; 163: 83–90. [DOI] [PubMed] [Google Scholar]
- 44. Elwyn G, Edwards A, Hood K et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Family Practice, 2004; 21: 337–346. [DOI] [PubMed] [Google Scholar]
- 45. Bieber C, Muller KG, Blumenstiel K et al. A shared decision‐making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial. Journal of Psychosomatic Research, 2008; 64: 13–20. [DOI] [PubMed] [Google Scholar]
- 46. Melbourne E, Sinclair K, Durand MA, Legare F, Elwyn G. Developing a dyadic OPTION scale to measure perceptions of shared decision making. Patient Education Counseling, 2009; 78: 177–183. [DOI] [PubMed] [Google Scholar]
- 47. Collins S, Drew P, Watt I, Entwistle V. ‘Unilateral’ and ‘bilateral’ practitioner approaches in decision‐making about treatment. Social Science and Medicine, 2005; 61: 2611–2627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Transcription definition of decision‐making.
Supporting info item


