Abstract
Glial cells are major components of the nervous system. The roles of these cells are not fully understood, however. We have now identified a secreted protein, designated Meteorin, that is expressed in undifferentiated neural progenitors and in the astrocyte lineage, including radial glia. Meteorin selectively promoted astrocyte formation from mouse cerebrocortical neurospheres in differentiation culture, whereas it induced cerebellar astrocytes to become radial glia. Meteorin also induced axonal extension in small and intermediate neurons of sensory ganglia by activating nearby satellite glia. These observations suggest that Meteorin plays important roles in both glial cell differentiation and axonal network formation during neurogenesis.
Keywords: axonal extension, glial differentiation, secreted protein
Introduction
Formation of the vertebrate nervous system is a complex process that involves multiple steps. One important step is cell diversification, the generation of neurons and glia, from a homogeneous sheet of neural progenitors. Two distinct mechanisms are responsible for this cell diversification: cell-intrinsic mechanisms are reflected by the fact that stem cells isolated from the mouse embryonic cerebral cortex produce different cell types in a predefined order, neuroblasts before glioblasts, that resembles the order apparent in situ (Qian et al, 2000). In contrast, non-cell-autonomous mechanisms require environmental cues. For example, grafts of adult hippocampus-derived progenitors differentiate into neuronal types appropriate for their ectopic location (Suhonen et al, 1996; Takahashi et al, 1998). Various factors that contribute to such environmental cues have been identified and shown to influence differentially the generation of the three major cell types of the central nervous system (CNS): neurons, oligodendrocytes, and astrocytes (Johe et al, 1996). Cytokines such as the leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) are important cues for astrocyte generation. These proteins promote astrocyte differentiation by binding to their receptors, including LIF-R and gp130, and thereby activating the JAK-STAT signaling pathway (Richards et al, 1996; Bonni et al, 1997). The Notch signaling pathway also contributes to astrocyte generation. Activated Notch1 and Notch3 promote astrocyte differentiation from adult hippocampus-derived progenitors without affecting STAT3 phosphorylation (Tanigaki et al, 2001). Bone morphogenetic protein 2 also both acts synergistically with LIF to induce astrocyte generation by triggering the formation of a complex of Smads and STAT3 bridged by p300 (Nakashima et al, 1999) as well as engages in crosstalk with Notch signaling by inducing the expression of the Notch effector Hes-5 (Nakashima et al, 2001).
Radial glia emerge at the peak of neurogenesis and have been thought to belong to the astrocyte lineage because of their similarity to astrocytes in terms of organellar structure and expression of molecular markers, as well as their ability to generate astrocytes (Campbell and Gotz, 2002). These cells are also able to generate neurons, however, and act as bipotential progenitors that produce both neurons and glia in a spatiotemporally regulated manner (Malatesta et al, 2000; Hartfuss et al, 2001), rendering their identity ambiguous. Several environmental cues have been shown to influence the formation of radial glia. Activated Notch, for example, promotes the generation of these cells in vivo (Gaiano et al, 2000). In the developing cerebellum, granule cell-derived Neuregulin and Purkinje cell-derived Sonic hedgehog (Shh) also influence radial glial differentiation (Rio et al, 1997; Dahmane and Ruiz-i-Altaba, 1999).
Neuronal connections are established through communication between developing neural cells. During this process, neurons extend axons to their targets, adjust their numbers, and refine their terminal branches and synapses. The timing of these events is determined in part by environmental cues, one important source of which is glial cells. Schwann cells and their precursors play an important role in the development of peripheral nerves (Jessen and Mirsky, 1999). These cells produce neurotrophic factors, including members of the neurotrophin and glial cell line-derived neurotrophic factor (GDNF) families (Airaksinen and Saarma, 2002), as well as adhesion molecules. The precise roles of glial cells in the establishment of neural connections remain largely unknown, however.
We have now identified a novel secreted protein (Meteorin) that is expressed in undifferentiated neural progenitors and in the astrocyte lineage including radial glia. In culture, this factor acts on cells of the glial lineage to influence their differentiation. Our data suggest that Meteorin regulates glial cell differentiation and the formation of axonal networks.
Results
Identification of meteorin as a novel secreted protein
Cells of the mouse P19 pluripotent embryonal carcinoma line differentiate into neural cells, including neurons and astrocytes, on exposure to all-trans retinoic acid (RA). Using an RNA subtraction procedure, we isolated several cDNA clones corresponding to genes whose expression was induced after incubation of these cells with 1 μM RA for 2 days. One such clone was analyzed in the present study. In situ hybridization analysis confirmed that the gene corresponding to this clone, which we have designated Meteorin (named after meteor, because this gene product can transform glial cells into cells with an elongated trail), is not expressed in undifferentiated P19 cells but is expressed at a high level after treatment of the cells with RA (Figure 1B).
Figure 1.
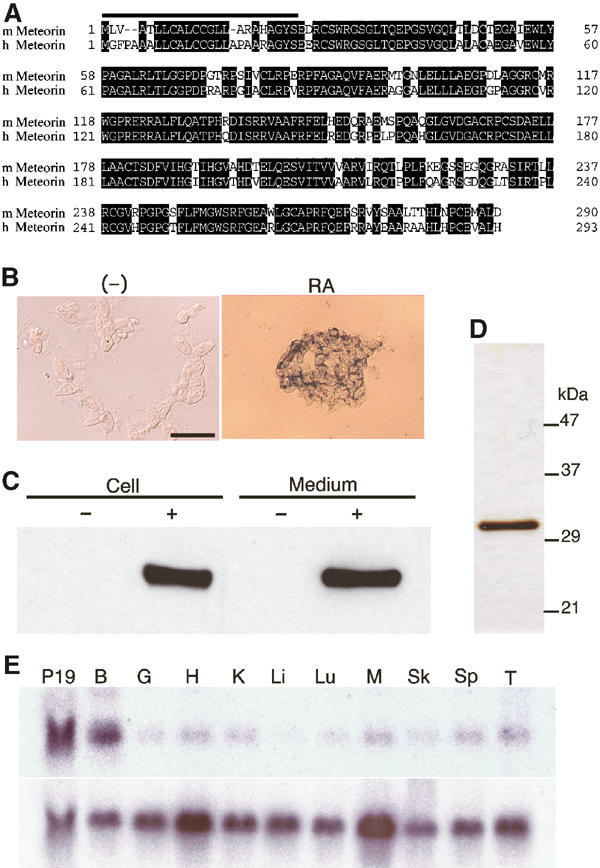
Meteorin is a novel secreted protein expressed in neural tissues. (A) Amino-acid sequences of mouse (m) and human (h) Meteorin. Identical residues are shaded, and the putative NH2-terminal signal sequences are indicated by the solid overline. The nucleotide sequences of mouse and human Meteorin cDNAs are available in NCBI under accession nos. XM128551 and NM024042, respectively. (B) P19 cells were left untreated or incubated for 2 days with 1 μM RA. They were then subjected to in situ hybridization with a probe specific for Meteorin mRNA. Scale bar, 100 μm. (C) Cell lysates and CM of parental CHO cells (−) or of CHO cells stably expressing Meteorin-His6 (+) were subjected to immunoblot analysis with antibodies to Meteorin. The region of the blot containing the immunoreactive protein is shown. (D) SDS–polyacrylamide gel electrophoresis and silver staining of Meteorin-His6 purified from the CM of CHO cells stably expressing the recombinant protein. The positions of molecular size standards are indicated. (E) Northern blot analysis of Meteorin expression in adult mouse organs. Polyadenylated RNA was probed with cDNAs specific for Meteorin (upper panel) or G3PDH (lower panel) mRNAs. P19, P19 cells treated with 1 μM RA for 2 days; B, brain; G, gut; H, heart; K, kidney; Li, liver; Lu, lung; M, skeletal muscle; Sk, skin; Sp, spleen; T, testis.
Mouse Meteorin mRNA contains an open reading frame that encodes a protein of 290 amino acids (Figure 1A). The encoded amino-acid sequence showed no significant homology to the sequence of any previously described protein, suggesting that Meteorin is a novel protein. The predicted protein contains a putative signal peptide in its NH2-terminal region, but otherwise possesses no conserved motifs. The cDNA for a human ortholog of mouse Meteorin encodes a protein of 293 amino acids (Figure 1A). A total of 238 amino acids (82%) are identical in the mouse and human proteins, and all cysteine residues are conserved. According to the NCBI database, Meteorin is located on mouse chromosome 17 (A3.3) and human chromosome 16 (p13.3), and there was no corresponding human diseases reported on this locus.
The presence of a putative signal peptide in the NH2-terminal region of the predicted protein suggested that Meteorin is secreted. To examine the subcellular localization of Meteorin, we established stable CHO cell lines that express the protein tagged with the hexahistidine (His6) epitope at its COOH-terminus. We also prepared rabbit polyclonal antibodies to synthetic peptides based on the amino-acid sequence of Meteorin. Immunoblot analysis with these antibodies detected the Meteorin-His6 fusion protein in both the lysate and culture supernatant of the CHO cells expressing it (Figure 1C), indicating that Meteorin indeed encodes a secreted protein. The immunoreactive protein migrated at a position corresponding to a molecular size of ∼30 kDa, as did the fusion protein purified from the culture medium conditioned by these cells (Figure 1D).
Meteorin is expressed in neural progenitors and the glial cell lineage
The expression pattern of Meteorin was first examined by Northern blot analysis. Meteorin mRNA was most abundant in the brain, but was also detected in smaller amounts in other organs (Figure 1E). We examined Meteorin expression during mouse development by in situ hybridization. Meteorin transcripts appeared largely restricted to the central and peripheral nervous systems. They were prominent during neurogenesis in regions of the CNS that contain neural progenitors (progenitor cells able to generate neurons, astrocytes, and oligodendrocytes). In the developing retina, for example, Meteorin expression was detected in all cell layers at embryonic day (E) 10.5 (Figure 2A) but was restricted to the external layer, where the retinal progenitor cells are located, at E14.5 (Figure 2B and C); the inner layer, which contains postmitotic neurons, was devoid of Meteorin transcripts at this later time. In the spinal cord, Meteorin expression was apparent in all layers at E9.5 (Figure 2D), but became limited to the ventricular zone (being lost from the mantle layer, the location of newly generated neurons) just 1 day later (Figure 2E). In the PNS, Meterorin expression was detected in migrating neural crest (Figure 2F).
Figure 2.

Expression of Meteorin in developing neural tissues of the mouse. Developing mouse neural tissues were sectioned and subjected to in situ hybridization with probes specific for Meteorin (A–G, I, K, M, O, Q), Protein zero (H, J), or GLAST (L, N, P, R) mRNAs. (A–C) Developing retina at E10.5 (A) and E14.5 (B, C). A higher magnification view of (B) is shown in (C). Meteorin expression becomes restricted to the undifferentiated outer cell layer during this period, with the inner cell layer, indicated by two-headed arrow in (C), being devoid of Meteorin expression at E14.5. (D, E) Developing spinal cord at E9.5 (D) and (the ventral side) at E10.5 (E). Meteorin expression is restricted to the ventricular zone, being absent from the mantle layer, at E10.5. (F–J) Migrating neural crest at E9.5 (F) and developing DRG at E11.5 (G, H) and E12.5 (I, J). Meteorin expression is apparent in migrating neural crest cells (arrowhead in F), and newly formed glial cells (F, G, I) that express Protein zero (H, J). Schwann cell progenitors and satellite glial cells are indicated by arrowheads (G, H) and arrows (I, J), respectively. (K, L) Coronal sections of the brain at P6 (dorsal, up; lateral, left). Meteorin is expressed in the subventricular zone of the lateral ventricle (LV) and the corpus callosum (CC). Many cells in the parenchyma express GLAST but not Meteorin. (M, N) Cerebellum at P8. Meteorin (M) and GLAST (N) are expressed in the Purkinje cell layer, most likely in radial glia. Purkinje cells (arrows) are devoid of Meteorin mRNA. Young astrocytes in the inner granule cell layer express GLAST (arrowhead) but not Meteorin. (O–R) Developing spinal cord at E12.5 (O, P) and E14.5 (Q, R). The pattern of Meteorin expression (O, Q) is similar to that of GLAST expression (P, R). Arrows indicate migrating young astrocytes (Q, R). Scale bar (shown in (A)): 25 μm (A, M, N), 250 μm (B), 50 μm (C, E), 75 μm (D, O–R), 100 μm (F–J), and 200 μm (K, L).
Meteorin expression became restricted to glial cells as neural tissues developed. In the developing dorsal root ganglion (DRG), the pattern of Meteorin expression was similar to that of the gene for protein zero, a glial cell marker (Figure 2G–J). Meteorin mRNA was thus detected in nerve roots, the site of Schwann cell progenitors, but not in ganglia, at E11.5 (Figure 2G and H). Meteorin expression was detected in the newly emergent satellite glial cells in ganglia at E12.5 (Figure 2I and J) and was maintained in Schwann cells, but was not apparent in neurons, at postnatal day (P) 2 (Supplementary Figure 3M–O). In the postnatal telencephalon, Meteorin transcripts were detected in the subventricular zone of the lateral ventricle and in the corpus callosum (Figure 2K and L), both of which contain glial progenitors (progenitor cells able to generate both astrocytes and oligodendrocytes) as well as astrocyte precursors.
Meteorin expression was also detected in radial glia. In the cerebellum, Meteorin mRNA appeared first in the ventricular zone at E14.5. At P8, the time of granule cell migration, Meteorin expression was apparent in the Purkinje cell layer; it was restricted to the region between Purkinje cell bodies, where Bergmann glia are located (Figure 2M). This pattern of expression was similar to that of GLAST (Figure 2N), a radial glial marker, suggesting that Bergmann glia express Meteorin. Radial glia are also present in the ventricular zone of the spinal cord during neurogenesis; at E12.5, the expression of both Meteorin and GLAST was detected in these cells (Figure 2O and P). After neurogenesis is completed, these radial glia transform into astrocytes and migrate into the mantle layer. Again, Meteorin and GLAST expression overlapped in these cells at E14.5 (Figure 2Q and R). In the postnatal retina, Meteorin expression was detected in Muller glia, which are positive for GLAST (Supplementary Figure 3J–L). Together, these observations suggest that Meteorin is expressed in neural progenitors as well as in the glial cell lineage, including radial glia.
Meteorin promotes axon extension and alters satellite glial morphology in sensory ganglia
Given that Meteorin is a secreted protein and is expressed in neural progenitors and glia, we investigated whether it might act on neighboring neurons. We first examined whether Meteorin possesses neuritogenic activity with sensory ganglia. Meteorin indeed stimulated neurite outgrowth in cultured DRG explants. Whereas the explants did not give rise to 2H3-immunopositive neurites (axons) in the absence of Meteorin, many such neurites were generated in its presence (Figure 3A and B). Dose–response analysis of this activity revealed an EC50 of ∼20 ng/ml (Figure 3I). Meteorin manifested similar neuritogenic activity with trigeminal sensory ganglia (data not shown).
Figure 3.
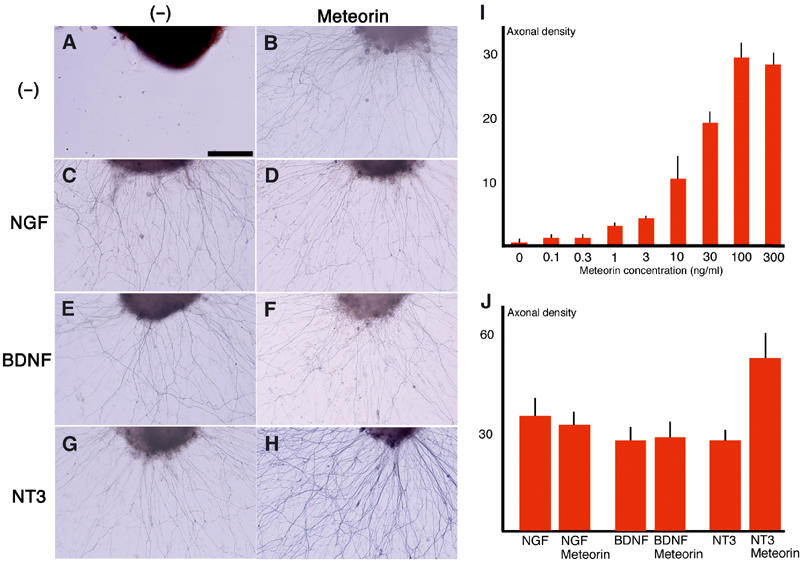
Meteorin promotes axonal extension in DRG explants. DRG explants obtained from E12.5 mouse embryos were cultured for 24 h in the absence (A, C, E, G) or presence (B, D, F, H) of Meteorin (80 ng/ml), and were stained with the 2H3 antibody to identify axons. (A, B) In the absence of other neurotrophic factors, neurite extension was not observed in the absence of Meteorin (A), but was apparent in its presence (B). (C–H) Culture of DRG explants for 48 h with NGF (10 ng/ml), BDNF (10 ng/ml), or NT3 (10 ng/ml) in the absence or presence of Meteorin, as indicated, revealed that Meteorin exhibited an additive effect on neurite outgrowth with NT3 but not with NGF or BDNF. Scale bar, 100 μm (A–H). (I) Dose–response analysis of axonal extension activity of Meteorin. The DRGs obtained from E12.5 mouse embryos were incubated with various concentrations of Meteorin. The density of axons was quantitatively estimated and is shown in this histogram. The mean and s.e.m. are shown (three experiments (N=13–16) for each concentration). (J) Density of axons generated by the indicated factor(s) was quantitatively estimated and is shown in the histogram. Meteorin showed additive effects with NT-3, but not with NGF and BDNF. The mean and s.e.m. are shown (three experiments (N=12–16) for each combination).
We next examined whether Meteorin cooperates with other neuritogenic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT3). The combination of Meteorin and NT3 resulted in the generation of neurites from DRG explants that were denser than those observed with NT3 alone (Figure 3G and H). This additive effect was not observed with Meteorin and either NGF (Figure 3C and D) or BDNF (Figure 3E and F). On the other hand, the length of axons did not change significantly (382.3±25.1 μm (N=13) with Meteorin alone; 426.5±31.5 μm (N=13) with NGF; 403.0±42.0 μm (N=14) with NGF+Meteorin; 391.8±33.2 μm (N=13) with BDNF; 380.7±29.3 μm (N=14) with BDNF+Meteorin; 394.5±22.5 μm (N=12) with NT-3; and 418.6±31.4 μm (N=15) with NT-3+Meteorin). To determine whether the promotion of neurite outgrowth by Meteorin reflects a direct effect on neurons, we cultured dissociated DRG cells at low or high density in the absence or presence of this protein. Meteorin promoted neurite outgrowth in high-density cultures. Thus, whereas most 2H3-immunopositive neurons exhibited a flat, fibroblast-like morphology in the absence of Meteorin (Figure 4A and C) (process length, 117.2±10.2 μm (N=26)), they exhibited a large soma and long neurites in its presence (Figure 4B and D) (370.5±29.9 μm (N=22)). These neurons were also selectively stained with antibodies to calcitonin gene-related peptide (CGRP) or to Ret, but not with those to parvalbumin, indicating that they were small or intermediate neurons (Figure 4I–K). When DRG cells were cultured at a low density, however, Meteorin failed to show such neuritogenic activity. Most neurons thus remained flat and fibroblast-like in appearance even in the presence of Meteorin (Figure 4L and M) (process length: 118.3±13.8 μm (N=22) without Meteorin; 122.8±6.1 μm (N=25) with Meteorin). In contrast, in the presence of NGF, most 2H3-positive neurons exhibited a large soma and extended long neurites (Figure 4N) (419.0±40.5 μm (N=24)). Unlike NGF, Meteorin by itself was thus unable to stimulate neurons directly. These observations therefore suggested either that Meteorin promotes neurite extension indirectly by stimulating non-neuronal cells such as satellite glia, or that it acts on neurons in combination with another factor released from non-neuronal cells.
Figure 4.
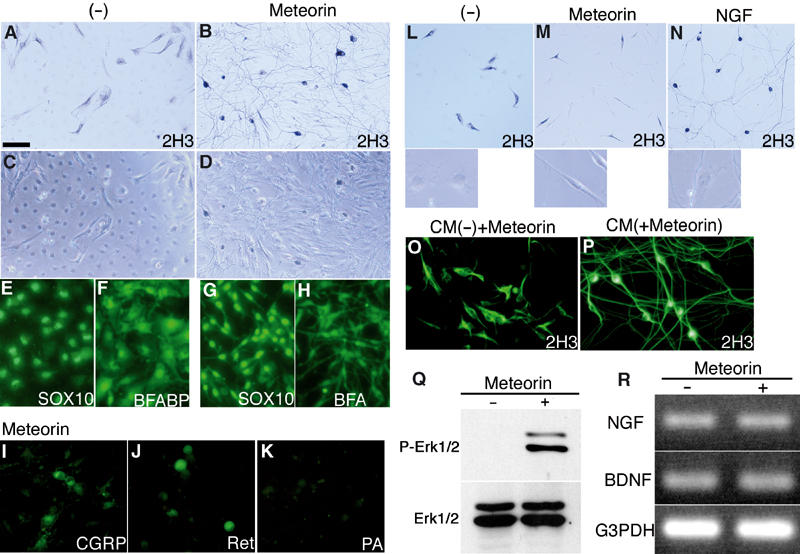
Axon-extending activity of Meteorin depends on satellite glial cells. (A–H) Dissociated cells of the E14.5 mouse DRG were cultured for 48 h at a high density in the absence (A, C, E, F) or presence (B, D, G, H) of Meteorin (80 ng/ml). They were then stained with the 2H3 antibody to identify neurons (A, B); the same fields were observed with Nomarski optics in (C) and (D), respectively. A dense axonal network and large neuronal cell bodies are apparent in the presence of Meteorin (B). Meteorin also changed the morphology of 2H3-negative, non-neuronal cells from flat and polygonal (C) to bipolar (D) (79.5±5.8% of cells (N=81–112)). Cells were alternatively stained with antibodies to SOX10 (E, G) or to BFABP (F, H). Meteorin-responsive non-neuronal cells were positive for SOX10 and BFABP, indicating that they were satellite glial cells. Scale bar (shown in (A)): 100 μm (A–D) or 60 μm (E–H). (I–K) DRG cells cultured at a high density with Meteorin were stained with antibodies to CGRP (I), to Ret (J), or to parvalbumin (K). CGRP-positive small and Ret-positive intermediate neurons, but not parvalbumin-positive large neurons, are apparent. Scale bar, 60 μm. (L–N) DRG cells were cultured for 48 h at a low density in the absence (L) or presence of either Meteorin (80 ng/ml) (M) or NGF (20 ng/ml) (N). They were then stained with the 2H3 antibody. The morphology of satellite glial cells is shown in the lower panels. 2H3-positive neurons remained flat and fibroblast-like in appearance in the absence or presence of Meteorin, but they manifested a large soma and extended long axons in the presence of NGF. In contrast, satellite glial cells adopted a bipolar morphology in response to Meteorin (81.5±3.8% of cells (N=31–47)), but remained flat and polygonal in the presence of NGF. Scale bar: 150 μm (main panels) or 40 μm (insets). (O, P) The satellite glia (∼90% pure) and neurons (>90% pure) were purified from the mouse DRG at E14.5 by sequential plating on tissue grade dishes. Satellite glia-enriched cells were conditioned for 48 h in D/F12-N2 without (O) or with (P) Meteorin (80 ng/ml). CM was prepared from each culture. Fresh Meteorin (80 ng/ml) was then added to the former CM. DRG neurons were cultured with each CM for 48 h and were stained with the 2H3 antibody. With the CM in which Meteorin was supplemented later, neurons remained flat and fibroblast-like in appearance (O). In contrast, with the CM prepared from Meteorin-treated satellite glia (P), neurons had large soma and elongated axons. Scale bar, 100 μm. (Q) Satellite glia-enriched cells were treated for 20 min with or without Meteorin. Cell lysates were prepared and were subjected to immunoblot analysis with antibodies to phosphorylated ERK1/2 (P-Erk1/2) or ERK1/2. Meteorin activated phosphorylation of ERK1/2 in satellite glia. (R) Total RNA prepared from DRG cells that had been cultured with or without Meteorin was subjected to RT–PCR analysis with primers specific for NGF, BDNF, or G3PDH (internal control) cDNAs.
We next examined the effects of Meteorin on the non-neuronal cells of ganglia. These non-neuronal cells were positive for SOX10 and brain fatty acid-binding protein (BFABP), suggesting that they were satellite glia (Figure 4E–H). In the absence of Meteorin, these cells exhibited a flat, polygonal morphology (Figure 4C, E, F, and L). In the presence of Meteorin, however, they adopted a bipolar morphology (Figure 4D, G, H, and M). In contrast to its neuritogenic activity, this effect of Meteorin on satellite glia was independent of cell density (process length in the high-density culture: 66.0±9.5 μm (N=26) without Meteorin; 130.6±18.6 μm (N=27) with Meteorin) (process length in the low-density culture: 68.7±11.3 μm (N=27) without Meteorin; 132.5±9.9 μm (N=21) with Meteorin). Treatment of the satellite glia with Meteorin induced phosphorylation of intracellular ERK1/2 (Figure 4Q). These data thus indicate that Meteorin acts directly on satellite glial cells and induces a change in their morphology, and that neurite extension is secondarily induced by the transformed satellite glial cells.
To examine the nature of this secondary signal, satellite glial cells were cultured with or without Meteorin. Conditioned medium (CM) prepared from each culture was tested for its neuritogenic activity with DRG neurons. The latter CM failed to show the neuritogenic activity even if Meteorin was supplemented later (Figure 4O) (process length: 120.5±10.2 μm (N=24)). In contrast, the former CM induced long neurites (Figure 4P) (375.6±29.8 μm (N=22)). These observations further support that Meteorin acts on satellite glia to produce the secondary factor, which is most likely a secreted protein.
Given that Meteorin selectively stimulates small and intermediate neurons and that this effect is dependent on satellite glia, we next examined whether Meteorin upregulates the expression of NGF or BDNF genes in satellite glia. However, Meteorin did not increase the expression level of these genes in satellite glia (Figure 4R), indicating that the secondary signal produced by these cells is not one of these neurotrophins.
Meteorin transforms cerebellar astrocytes into radial glia
Given that the principal target of Meteorin appears to be glial cells and that Meteorin is expressed widely in developing neural tissues, we next examined whether Meteorin affects other types of glial cells. Culture of P2 mouse cerebellar explants in the absence of Meteorin resulted in the emergence of only a few glial fibrillary acidic protein (GFAP)-positive astrocytes with short processes from the explants (Figure 5A). The addition of Meteorin to the explants, however, induced the emergence of many GFAP-positive cells that exhibited a bipolar morphology and long processes (Figure 5B) (process length 233.2±17.2 μm (N=14)), and which were positive for RC2 (Figure 5D), a marker of radial glia, suggesting that Meteorin triggered the transformation of cerebellar astrocytes into radial glia.
Figure 5.

Meteorin transforms cerebellar astrocytes into radial glial cells. (A–F) Cerebellar explants derived from P2 mice were cultured for 48 h in the absence (A, C, E) or presence (B, D, F) of Meteorin (80 ng/ml) and then stained with antibodies to GFAP (A, B) or to RC2 (C, D) or with the 2H3 antibody (E, F). In the presence of Meteorin, elongated GFAP+, RC2+ cells emerged from the explants. Axonal extension was not affected by Meteorin. Scale bar (shown in (A)), 100 μm. (G–J) Astrocytes were purified from the mouse cerebellum at P2–P4, cultured for 48 h without (G, I) or with (H, J) Meteorin, and then stained with antibodies to GFAP (G, H) or to BLBP (I, J). Astrocytes adopted a bipolar morphology and expressed BLBP in the presence of Meteorin. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole in (I). Scale bar, 30 μm.
To confirm that this transformation represented a direct effect of Meteorin on astrocytes, we isolated astrocytes from the cerebellum of mice at P2–P4 and cultured them in the absence or presence of Meteorin. In the absence of Meteorin, most GFAP+ astrocytes remained fibroblast-like in appearance (Figure 5G) (process length: 38.6±5.4 μm (N=26)). In the presence of Meteorin, however, many GFAP+ astrocytes adopted an elongated bipolar morphology (Figure 5H) (process length: 254.3±34.0 μm (N=23)). In addition, the expression of brain lipid-binding protein (BLBP), another marker for radial glia, was upregulated in the Meteorin-treated cells (Figure 5I and J) (4.5±2.3% of total cells (N=45–64) without Meteorin; 83.6±2.4% (N=41–55) with Meteorin). Cell proliferation was not affected by Meteorin (BrdU+ population: 30.8±6.7% (N=17) without Meteorin; 28.0±5.4% (N=19) with Meteorin).
These data thus suggest that Meteorin induces the conversion of astrocytes to radial glia. Given that radial glia guide the migration of cerebellar granule cells, we examined the migration of and axonal extension by granule cells in cerebellar explant cultures. Meteorin did not affect neuronal migration or axonal extension. Granule cells with 2H3-positive axons and small cell bodies emerged in a similar manner from explants cultured in the absence or presence of Meteorin (Figure 5E and F) (the length of axon: 342.8±39.5 μm (N=12) without Meteorin; 322.8±25.5 μm (N=11) with Meteorin).
Meteorin promotes astrocyte differentiation
We next examined the effects of Meteorin on the differentiation of neural progenitors. Neurospheres were prepared from the cerebral cortex at E11.5. Almost all cells of neurospheres maintained in the presence of fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF) were positive for Nestin and were thus neural, neuronal, or glial progenitors (data not shown). Withdrawal of these mitogens resulted in the appearance of neurons, astrocytes, and oligodendrocytes in the cultures. The addition of Meteorin after mitogen withdrawal induced a marked increase in the number of GFAP+ astrocytes (Figure 6A and B). This effect was specific to astrocytes and similar to that of LIF (Figure 6G). Meteorin thus did not increase the number of neurons positive for microtubule-associated protein 2 (MAP2) or of oligodendrocytes positive for the O4 antigen (Figure 6C–F). This effect was not due to an increase in cell proliferation (BrdU+ population without Meteorin 37.8±5.8% (N=17); with Meteorin 39.0±3.1% (N=19)).
Figure 6.
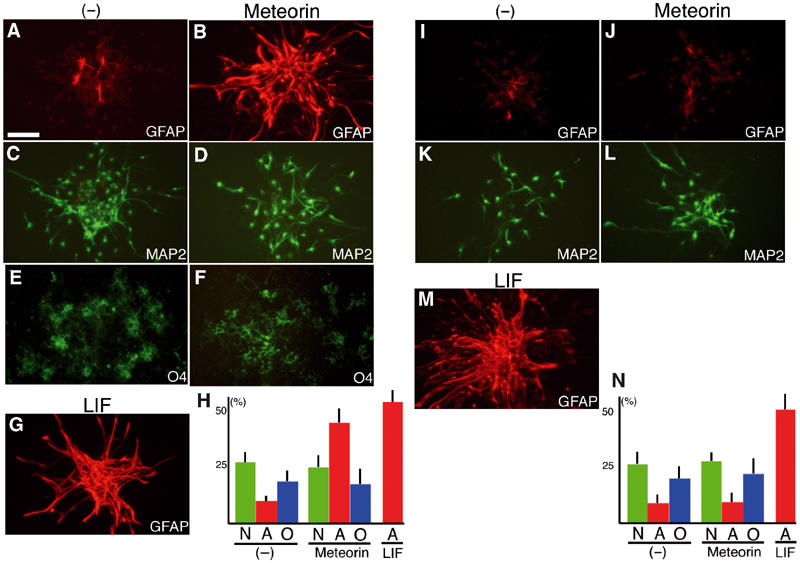
Meteorin increases the number of astrocytes during differentiation of neurosphere cells. (A–H) Neurospheres prepared from the E11.5 mouse cortex were cultured for 60 h in the absence (A, C, E) or presence of either Meteorin (80 ng/ml) (B, D, F) or LIF (80 ng/ml) (G, H). The spheres were then stained with antibodies to GFAP (A, B, G) or to MAP2 (C, D, H) or with the O4 antibody (E, F). Both Meteorin and LIF induced a marked increase in the number of GFAP+ astrocytes. Scale bar (shown in (A)), 100 μm. Proportion of each cell type is shown in (H) (three experiments (N=15–17) for each condition). N, neuron; A, astrocyte; O, oligodendrocyte. (I–N) Neurospheres were formed in culture medium containing FGF2 and EGF in the absence (I, K) or presence of either Meteorin (80 ng/ml) (J, L) or LIF (80 ng/ml) (M). The spheres were then allowed to differentiate for 60 h in the absence of these factors before staining with antibodies to the indicated proteins. The number of GFAP+ astrocytes was increased by LIF but not by Meteorin. Scale bar, 100 μm. Proportion of each cell type is shown in (N) (three experiments (N=15–17) for each condition).
This increase in the number of astrocytes might have been due either to an instructive effect of Meteorin on neural progenitors and glial progenitors, or proliferation-promoting effect on cells that had already committed to the astrocyte lineage such as astrocyte precursor cells (APCs) and astrocytes. To distinguish between these two possibilities, we allowed neurospheres to first form in the presence of FGF, EGF, and either Meteorin or LIF, and then to differentiate in the absence of these various factors. Meteorin did not increase the number of GFAP+ astrocytes under these conditions (Figure 6I and J). The proportions of MAP2+ neurons and O4+ oligodendrocytes were also unaffected by Meteorin (Figure 6K and L; data not shown). In contrast to Meteorin, LIF induced a large increase in the number of GFAP+ astrocytes (Figure 6M). These observations thus suggest that Meteorin acts on cells that have committed to the astrocyte lineage, most likely APCs.
Discussion
Action of Meteorin on the glial cell lineage
The three major cell types of the CNS—neurons, astrocytes, and oligodendrocytes—are generated sequentially from neural progenitors in a spatially and temporally restricted manner. Neurons are the first of these three cell types to be generated, arising from neuronal progenitors early in development. Both astrocytes and oligodendrocytes are generated later from glial progenitors (Qian et al, 2000). Our observations now indicate that Meteorin is likely expressed in neural progenitors, glial progenitors, and cells of the astrocyte lineage (Figure 7A). For example, in the developing mouse spinal cord, the pattern of migration of Meteorin-expressing cells from the ventricular zone resembles that of GLAST-expressing cells and apparently differs from that of Olig2-expressing oligodendrocyte precursor cells (OPCs) and oligodendrocytes (Supplementary Figure 1) (Zhou et al, 2000). In the developing ganglionic eminences, PDGFRa- or Olig2-expressing cells that are located far from the ventricular zone do not express Meteorin (Supplementary Figure 2A–H). In the prospective amygdala (Nery et al, 2001), plp-expressing cells are negative for Meteorin expression (Supplementary Figure 2I and J). These observations suggest that, although glial progenitors express Meteorin, they lose Meteorin expression after commitment to the oligodendrocyte lineage.
Figure 7.
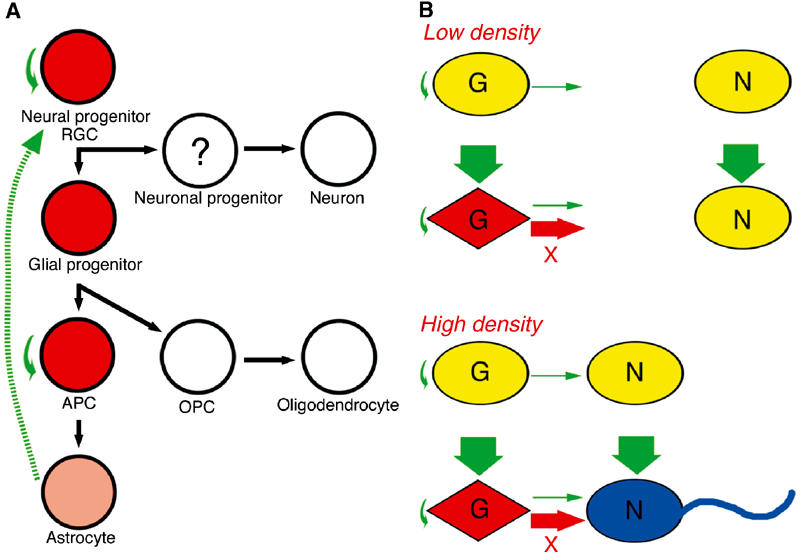
Model for the expression and action of Meteorin during neurogenesis. (A) Meteorin expression. Cells that express Meteorin are shown in red or pink (corresponding to relatively high and low expression levels, respectively); those that do not express Meteorin are shown in white. Meteorin is expressed in the astroglial lineage, most prominently in immature cells. Solid green arrows indicate putative physiological actions of Meteorin; the broken green arrow indicates in vitro activity of Meteorin in cerebellar astrocyte cultures. RGC, radial glial cell; APC, astrocyte precusor cell; OPC, oligodendrocyte precusor cell. (B) Induction of axonal extension in DRG cell cultures by Meteorin in a cell density-dependent manner. Satellite glial cells (G) that are activated by Meteorin produce an unknown factor (X) that is able to stimulate neurons (N) only at a high cell density. See text for further details.
In the astrocyte lineage, Meteorin expression appears to be restricted to relatively immature cell populations. Meteorin expression is gradually lost in GLAST-expressing astrocytes located in the postnatal cerebral parenchyma (Figure 2K and L), and is not detected in two major types of astrocytes in the adult cerebrum, fibrous astrocytes and protoplasmic astrocytes (Miller and Raff, 1984) (Supplementary Figure 3G). In the developing cerebellum, Meteorin is expressed in the VZ and GLAST-positive migrating glial precursors. Among three subclasses of astrocytes in the adult cerebellar cortex, bushy protoplasmic astrocytes, smooth protoplasmic astrocytes, and Bergmann glia (Palay and Chan-Palay, 1974), Meteorin expression is restricted to Bergmann glia (Figure 2M and N) (Supplementary Figure 3H and I). Expression of Meteorin in Bergmann glia may be regulated by neurons that interact with Bergmann glia. For instance, dendritic spines of Purkinje cells were completely enwrapped by Bergmann glial cells (Spacek, 1985). Recently, modified AMPA receptors on Bergmann glia were shown to affect glial ensheathing of the dendrite spines, and synaptic trasmission of these synapses (Iino et al, 2001). In addition to Purkinje cells, Bergmann glial cells are known to interact mutually with migrating granule cells (Hatten, 1999). Furthermore, several neuron-derived factors, such as granule cell-derived Neuregulin or Purkinje cell-derived Sonic hedgehog (Rio et al, 1997; Dahmane and Ruiz-i-Altaba, 1999), are known to transform astrocytes into radial glia.
What is the function of Meteorin in neural development? Our data obtained from several culture systems suggest that Meteorin acts on cells from which it is secreted in an autocrine manner and changes their state of differentiation. Various factors, including the cytokines LIF and CNTF, are known to promote astrogenesis. The binding of these cytokines to their receptors results in activation of the JAK-STAT signaling pathway (Johe et al, 1996; Bonni et al, 1997; Nakashima et al, 1999). The Notch-Delta signaling system also contributes to astrocyte generation (Tanigaki et al, 2001). Both these groups of factors promote astrogenesis instructively. In the neurosphere differentiation assay, Meteorin promoted astrocyte differentiation in a selective manner, with the emergence of MAP2+ neurons and O4+ immature oligodendrocytes being unaffected. Furthermore, stimulation of astrocyte differentiation by Meteorin, in contrast to the action of LIF, was not detected in the presence of FGF2. These observations indicate that, unlike instructive factors such as LIF and CNTF, Meteorin promotes astrogenesis by acting on cells that have already committed to the astrocyte lineage. Given that Meteorin is expressed only at a low level in mature astrocytes, the principal target of Meteorin may be APCs.
Although Meteorin may play a role in astrocyte differentiation, Meteorin is already expressed in neural progenitors during active neurogenesis. Precocious astrogenesis is suppressed during this early period of neurogenesis by at least two distinct mechanisms. One such mechanism is the expression of neurogenic basic helix–loop–helix (bHLH) transcription factors such as neurogenin1 and -2 and Mash1 (Nieto et al, 2001; Sun et al, 2001). These transcription factors either competitively bind CBP/p300 and prevent its association with STAT3 or prevent cytokine-mediated STAT3 phosphorylation. Activation of the GFAP promoter by the canonical Notch signaling pathway is also suppressed at this time (Ge et al, 2002). The second mechanism for suppression of precocious astrogenesis is methylation of glia-specific genes (Takizawa et al, 2001). Thus, despite its expression in neural progenitors, the promotion of astrogenesis by Meteorin might be prevented by these mechanisms.
Meteorin expression during neurogenesis may reflect another function of the encoded protein such as maintenance of the undifferentiated state. Such dual functions have been described for Notch signaling. Notch signaling thus promotes the generation of astrocytes at the gliogenic stage, but it maintains the proliferation of neural progenitors and inhibits neuronal differentiation at earlier (neurogenic) times. Activated Notch also promotes radial glial identity (Gaiano et al, 2000). Radial glia have recently been shown to be neural progenitors that are able to generate neurons (Malatesta et al, 2000; Alvarez-Buylla et al, 2001; Hartfuss et al, 2001). Like Notch, Meteorin is expressed in the early neuroepithelium and in radial glial cells, as well as acts on purified cerebellar astrocytes to promote radial glial formation. The expression pattern and biological activity of Meteorin are consistent with the idea that Meteorin produced by the radial glia act in an autocrine manner to maintain these progenitor cells.
Meteorin promotes axonal extension in the presence of glia
Meteorin induces axonal outgrowth of sensory neurons. Our data suggest that the satellite glia are the primary target of Meteorin action and axonal extension is a secondary effect of such glial stimulation. According to our model (Figure 7B), the concentration of endogenous Meteorin is too low to activate the satellite glia in culture. These cells are activated, however, by exogenous Meteorin and generate a secondary signal. This secondary signal is unable to stimulate neurons at low cell density, probably because it fails to achieve a sufficient concentration. It is able to induce axonal elongation at high cell density, however.
What might this secondary signal be? In both DRG explant and dissociated cell cultures, Meteorin appeared to specifically affect CGRP-positive small and Ret-positive intermediate neurons. Neurotrophins such as NGF and BDNF are therefore potential candidates for the signal. However, the abundance of the mRNAs for NGF or BDNF was not affected by exogenous Meteorin in DRG cell cultures, making this possibility unlikely. Alternatively, the secondary signal may be a secreted protein that (1) elicits axonal extension by itself, (2) acts synergistically with Meteorin, or (3) increases the responsiveness of neurons to NGF or BDNF.
Why does Meteorin activate neurons indirectly through surrounding glia instead of directly? One advantage might be the adaptation of axonal extension to the surrounding environment. Neurons must know the timing and destinations of axonal extension from their surroundings. Schwann cells and their precursors serve as an important source of signals required for nerve development (Jessen and Mirsky, 1999). The indirect action of Meteorin that links glial stimulation and axonal extension might thus provide important information for neurons on their surroundings.
Another advantage is that by acting through glia more diversity in signaling is achievable than acting on neurons directly. Diversity can be generated by changing the level and timing of stimulation. For example, isoforms of transforming growth factor-β, like Meteorin, have no neurotrophic activity alone on purified neurons, but do exhibit such activity in mixed culture systems. They act on neurons in an autocrine–paracrine manner and synergize with other factors such as CNTF, neurotrophins, and GDNF (Krieglstein and Unsicker, 1996; Krieglstein et al, 1998). Diversity might also be generated by the spatial distribution of glial cells. The same signal may thus induce different biological effects in neurons depending on which part of the cell receives the signal (Heerssen and Segal, 2002). For example, in developing retinal ganglion cells, BDNF reduces arborization when applied to dendritic terminals, but increases it when applied to axon terminals (Lom and Cohen-Cory, 1999). Activation by Meteorin of satellite glia in contact with neuronal cell bodies and of Schwann cells in contact with axons might thus elicit different biological effects within individual neurons.
Several factors act in various combinations to elicit different effects on neurons. For example, hepatocyte growth factor promotes axonal extension in combination with NGF, but not in combination with BDNF or NT3 (Maina et al, 1997). Transforming growth factor-β3 promotes the survival of DRG neurons in combination with NT3, but not with NGF (Krieglstein and Unsicker, 1996). Further diversity in the effects of Meteorin may thus also be generated by its acting in concert with other factors.
Materials and methods
Isolation of Meteorin
cDNA libraries were constructed from P19 cells that had been cultured for 2 days in the absence or presence of 1 μM RA. Several cDNA clones corresponding to genes whose expression was increased by RA treatment were isolated by a sensitive subtraction procedure (Saijoh et al, 1996). One of these clones was derived from Meteorin. A full-length Meteorin cDNA was obtained by 5′ RACE with an E11 mouse Marathon Ready cDNA library (Clontech).
Purification of Meteorin
For the preparation of polyclonal antibodies to Meteorin, rabbits were injected with one of two chemically synthesized oligopeptides, Meteorin-Npep (SWRGSGLTQEPGS) and Meteorin-Cpep (PLFKEGSSEGQGRAS). Antibodies were affinity-purified on an antigen column and used for immunoblot analysis. An expression vector (pEF-BOS-Meteorin-His-IRES-Puro) that encodes COOH-terminally His6-tagged mouse Meteorin (Meteorin-His6) was constructed and introduced by transfection into CHO-K1 cells (RIKEN cell bank). The stable clone (clone 78) that expressed Meteorin at the highest level was cultured for 3 days in OPTI-MEM (Gibco), after which the recombinant Meteorin-His6 protein was purified from the CM with Talon metal affinity resin (Clontech). The purified protein was desalted and concentrated with a centrifugal filter device (Millipore), supplemented with BSA(1 mg/ml), and stored at −80°C until use.
Cell culture
For DRG explant culture, E12.5–14.5 mouse DRGs were embedded in a collagen gel, and incubated for 24 h in D/F-N2 (DMEM–F12 (1:1) plus N2 supplement (Gibco)) with or without purified Meteorin-His6, mouse NGF (Becton Dickinson), human BDNF (Peprotech), or human NT3 (Peprotech). The density of axons was quantified as a pixel density, which was estimated from the digitalized pictures by using NIH-Image J program. For dissociation culture, DRG cells were incubated for 48 h in D/F-N2 at a density of 1 × 105 cells per well (high cell density) or 5 × 103 cells per well (low cell density) in a p-ornithine/laminin-coated 48-well plate. For cerebellar explant culture, pieces of the external granule cell layer from P2–4 mouse cerebellum were incubated for 48 h in D/F-N2 with or without Meteorin-His6. Astrocytes were isolated from the cerebellum by density gradient centrifugation with Percoll and sequential plating as described previously (Hatten, 1985). For the neurosphere assay, E11.5 mouse cortex was dissociated and cultured in DMEM-F12 supplemented with human EGF (20 ng/ml) (Roche), human FGF2 (20 ng/ml) (Roche), 0.6% glucose, 2 mM glutamine, 3 mM NaHCO3, 5 mM HEPES, insulin (25 μg/ml), transferrin (100 μg/ml), 20 nM progesterone, 60 μM putrescine, and 30 μM selenium chloride (Sigma). Neurosphere differentiation was induced in medium without EGF and FGF2 in the wells of a p-ornithine-coated 48-well plate. Meteorin-His6 or mouse LIF (AMRAD) was included in the medium during sphere formation (for 72 h) or during differentiation (for 60 h).
In situ hybridization, Northern blot, and RT–PCR analyses
Nonradioactive in situ hybridization was performed as previously described (Nishino et al, 1999). Probes included those specific for mouse Meteorin, rat Protein zero (kindly provided by M Wegner; Peirano et al, 2000)), mouse GLAST (kindly provided by M Watanabe; Shibata et al, 1997), mouse Olig2, mouse PDGFRa, and mouse Plp (kindly provided by H Takebayashi and K Ikenaka) mRNAs. For Northern blot analysis, poly(A) RNA was prepared from adult mouse organs and was probed consecutively with 32P-labeled fragments of Meteorin and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNAs. RT–PCR analysis was performed with a Cell-to-cDNA kit (Ambion) and the following primers: NGF-S (5′-TCAGCATTCCCTTGACACAGC-3′); NGF-AS (5′-TCCAGTGTTTGGAGTCGATGC-3′); BDNF-S (5′-GAGCTGAGCGTGTGTGACAG-3′); BDNF-AS (5′-GGTCAGTGTACATACACAGG-3′); G3PDH-S (5′-GGCCGGTGCTGAGTATGTCG-3′); and G3PDH-AS (5′-GCACGTCAGATCCACGACGG-3′). PCR was performed with Ampli-Taq Gold (Takara) according to the following protocol: 95°C for 9 min; 28 (NGF, BDNF) or 24 (G3PDH) cycles of 95°C for 20 s, 55°C for 30 s, and 72°C for 1 min; and 72°C for 10 min.
Immunological analysis
Immunological analysis was performed with the following primary antibodies: phospho-p44/42 MAPK (Cell Signaling Tech. 1:1000), p44/42 MAPK (Cell Signaling Tech. 1:1000), 2H3 (DSHB; 1:1000 dilution), SOX10 (Chemicon; 1:100), BFABP (kindly provided by T Muller (Kurtz et al, 1994); 1:1000), CGRP (Sigma; 1:2000), Ret (MBL; 1:400), parvalbumin (Sigma; 1:400), GFAP (DAKO; 1:600), RC2 (DSHB; 1:1000), BLBP (kindly provided by N Heintz (Feng et al, 1994); 1:1000), MAP2 (Sigma; 1:400), and O4 (Chemicon; 1:100). Immune complexes were detected with fluorescein isothiocyanate- or Cy3-conjugated antibodies to mouse or rabbit immunoglobulin G or to mouse immunoglobulin M (DAKO; 1:500), or with a peroxidase-based Vectastain kit (Vector).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Acknowledgments
We thank S Ohishi, K Mochida, M Nishijima, and Y Ikawa for techninal assistance, as well as T Muller, N Heintz, M Wegner, M Watanabe, K Ikenaka, and H Takebayashi for reagents. This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan, and by CREST.
References
- Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2: 287–293 [DOI] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME (1997) Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278: 477–483 [DOI] [PubMed] [Google Scholar]
- Campbell K, Gotz M (2002) Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci 25: 235–238 [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz-i-Altaba A (1999) Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126: 3089–3100 [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N (1994) Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12: 895–908 [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G (2000) Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26: 395–404 [DOI] [PubMed] [Google Scholar]
- Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G, Weinmaster G, Sun YE (2002) Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res 69: 848–860 [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M (2001) Characterization of CNS precursor subtypes and radial glia. Dev Biol 229: 15–30 [DOI] [PubMed] [Google Scholar]
- Hatten ME (1985) Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol 100: 384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME (1999) Central nervous system neuronal migration. Annu Rev Neurosci 22: 511–539 [DOI] [PubMed] [Google Scholar]
- Heerssen HM, Segal RA (2002) Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci 25: 160–165 [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S (2001) Glia–synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292: 926–929 [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R (1999) Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci 22: 402–410 [DOI] [PubMed] [Google Scholar]
- Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD (1996) Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev 10: 3129–3140 [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Henheik P, Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K (1998) Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J Neurosci 18: 9822–9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Unsicker K (1996) Distinct modulatory actions of TGF-beta and LIF on neurotrophin-mediated survival of developing sensory neurons. Neurochem Res 21: 843–850 [DOI] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T (1994) The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120: 2637–2649 [DOI] [PubMed] [Google Scholar]
- Lom B, Cohen-Cory S (1999) Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci 19: 9928–9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R (1997) Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev 11: 3341–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127: 5253–5263 [DOI] [PubMed] [Google Scholar]
- Miller RH, Raff MC (1984) Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 4: 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Takizawa T, Ochiai W, Yanagisawa M, Hisatsune T, Nakafuku M, Miyazono K, Kishimoto T, Kageyama R, Taga T (2001) BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci USA 98: 5868–5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T (1999) Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284: 479–482 [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G (2001) Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128: 527–540 [DOI] [PubMed] [Google Scholar]
- Nieto M, Schuurmans C, Britz O, Guillemot F (2001) Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron 29: 401–413 [DOI] [PubMed] [Google Scholar]
- Nishino J, Mochida K, Ohfuji Y, Shimazaki T, Meno C, Ohishi S, Matsuda Y, Fujii H, Saijoh Y, Hamada H (1999) GFR alpha3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron 23: 725–736 [DOI] [PubMed] [Google Scholar]
- Palay S, Chan-Palay V (1974) Cerebellar Cortex. New York: Springer Verlag [Google Scholar]
- Peirano RI, Goerich DE, Riethmacher D, Wegner M (2000) Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol 20: 3198–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S (2000) Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28: 69–80 [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Dutton R, Tan SS, Gearing DP, Bartlett PF, Murphy M (1996) Leukaemia inhibitory factor or related factors promote the differentiation of neuronal and astrocytic precursors within the developing murine spinal cord. Eur J Neurosci 8: 291–299 [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G (1997) Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron 19: 39–50 [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Fujii H, Meno C, Sato M, Hirota Y, Nagamatsu S, Ikeda M, Hamada H (1996) Identification of putative downstream genes of Oct-3, a pluripotent cell-specific transcription factor. Genes Cells 1: 239–252 [DOI] [PubMed] [Google Scholar]
- Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y (1997) Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci 17: 9212–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J (1985) Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol 171: 245–252 [DOI] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH (1996) Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383: 624–627 [DOI] [PubMed] [Google Scholar]
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME (2001) Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104: 365–376 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Palmer TD, Takahashi J, Gage FH (1998) Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol Cell Neurosci 12: 340–348 [DOI] [PubMed] [Google Scholar]
- Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell 1: 749–758 [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T (2001) Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron 29: 45–55 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ (2000) Identification of a novel family of oligodendrocyte lineage-specific basic helix–loop–helix transcription factors. Neuron 25: 331–343 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3


