Abstract
Background. The combined measles, mumps, and rubella (MMR) vaccine has been successfully administered for >20 years. Because of this, protection by maternal antibodies in infants born to vaccinated mothers might be negatively affected.
Methods. A large cross-sectional serologic survey was conducted in the Netherlands during 2006–2007. We compared the kinetics of antibody concentrations in children and women of childbearing age in the highly vaccinated general population with those in orthodox Protestant communities that were exposed to outbreaks.
Results. The estimated duration of protection by maternal antibodies among infants in the general population, most of whom were born to vaccinated mothers, was short: 3.3 months for measles, 2.7 months for mumps, 3.9 months for rubella, and 3.4 months for varicella. The duration of protection against measles was 2 months longer for infants born in the orthodox communities, most of whom had unvaccinated mothers. For rubella, mothers in the orthodox communities had higher concentrations of antibodies as compared to the general population.
Conclusion. Children of mothers vaccinated against measles and, possibly, rubella have lower concentrations of maternal antibodies and lose protection by maternal antibodies at an earlier age than children of mothers in communities that oppose vaccination. This increases the risk of disease transmission in highly vaccinated populations.
Keywords: MMR vaccination, waning, maternal antibodies
(See the editorial commentary by Gans and Maldonado on pages 1–3.)
In many industrialized countries, the introduction of measles, mumps, and rubella (MMR) vaccine into national immunization programs proved successful in reducing the incidence of these infectious diseases [1, 2]. Infants typically receive the first dose of vaccine around the first year of age [3]. Maternally derived antibodies provide the primary protection for infants prior to this first vaccine dose. The initial concentration of maternal antibodies in a newborn is highly correlated with the antibody concentration in their mother [4–8]. Subsequently, there is waning of the maternal antibody levels in the infant, leaving the child susceptible to infections. Optimal timing of the first dose of vaccine can contribute to keeping this period as short as possible. This is important because, among European infants aged <1 year, measles risk and severity are greater than the risk and severity among those aged ≥1 year [9]. The optimal timing of the first MMR vaccine dose depends on 2 main factors. First, the infant's immune system should be sufficiently mature to respond to the vaccine antigens. Second, levels of maternal antibodies must be low enough to ensure that they do not neutralize the live, attenuated strains in the vaccine. Insight in the kinetics and determinants of maternal antibody concentrations is therefore very important [10].
A known determinant of the maternal measles virus antibody concentration is the vaccination status of the mother. Mothers who received MMR vaccine tend to have a lower concentration of measles virus–specific antibodies than mothers who naturally acquired measles [11–13]. Infants born to measles-vaccinated mothers are hence likely to have lower levels maternal antibodies at birth and a shorter period of protection than infants of mothers who acquired measles naturally [14–16]. In countries with high MMR vaccination coverage, such as the Netherlands, most women of childbearing age are vaccinated against measles and have avoided natural infection. A lower duration of protection by maternal antibodies against measles might provide a motivation to lower the age at which the first dose of measles vaccine is administered to infants, but the degree and duration of immune response is uncertain when the vaccine is administered to infants aged <12 months. Since the measles vaccine is combined with mumps and rubella vaccines into the trivalent MMR vaccine, the question arises how the vaccination history of the mother affects the duration of protection against mumps and rubella. At present, it is not known how long infants are protected by maternal antibodies against infection or how long they remain susceptible to mumps and rubella before the first dose of vaccine is administered.
The Netherlands provides a unique setting in which to study the effects of maternal vaccination on the kinetics of maternal antibodies because, owing to orthodox Protestant beliefs, a considerable proportion of the population refuses vaccination [15]. Since many of these individuals are sociogeographically clustered in the so-called Dutch Bible belt, outbreaks of measles, mumps, and rubella are still occurring here [16–18]. The last outbreaks of measles and rubella occurred in 1999–2000 and 2004–2005, respectively. We studied the duration of protection against measles, mumps, and rubella by comparing infants in the general population, in which most mothers have been vaccinated, with infants in orthodox reformed communities, in which mothers tend to refuse vaccination [15]. We use statistical modeling to infer the kinetics of maternal antibody levels in infants and to quantify any difference in duration of immunity between infants of mothers in the general population and mothers in orthodox reformed communities. To validate our comparison between these 2 groups, we also considered varicella, against which vaccination is not included in the national immunization program.
METHODS
Study Population
Since 1987, children in the Netherlands have been offered MMR vaccine at the ages of 14 months and 9 years, with a 3-year catch-up program for preschool-aged children during 1987–1989. This program was preceded by separate measles vaccination for 14-month-old infants, introduced in 1976, and separate rubella vaccination for 11-year-old girls, introduced in 1974. The MMR vaccine coverage in the Netherlands is >90% [19].
A large cross-sectional serologic study was conducted in the Netherlands over a 2-year period, in 2006 and 2007. Participants were recruited from a nationwide sample of the general population, using a 2-stage cluster sampling technique. First, 40 municipalities were selected at random, weighted by their population size. Second, for each of these 40 municipalities, an age-stratified sample of 380–500 individuals aged <80 years was drawn at random from the municipal population register; the realized sample size depended on the response rate among the municipalities. In total, 19 781 individuals were invited in the nationwide sample. Participants were also recruited from 8 selected municipalities in the Dutch Bible belt, where vaccination coverage is low. As in the nationwide sample, for each of these 8 municipalities, an age-stratified sample of 380–952 individuals aged <80 years was drawn at random from the municipal population register. In total, 4366 individuals were invited into the low-immunization-coverage sample. Of the 24 147 individuals invited to participate in the study, 7904 (32.7%) eventually participated by both filling out a questionnaire that included questions about religious beliefs and by donating a single blood sample. An overview of the study is presented in Supplementary Tables 1 and 2 in the Supplementary Materials. A more detailed description of the setup of this cross-sectional serologic study is available elsewhere [20].
We selected study participants who were either unvaccinated infants (ie, individuals aged <14 months and not vaccinated with the MMR vaccine) or women of childbearing age (ie, women aged 20–44 years, the age of 98.5% of mothers who gave birth in the Netherlands during 2007; available at: http://www.cbs.nl). We weighted the data of participating women such that their age distribution matched the age distribution of mothers of newborns in the Dutch population in 2007. Within this study population, we subsequently defined 2 groups: 1243 women and 434 children in the general population, including those from the orthodox Protestant communities who did not have orthodox Protestant beliefs, and 53 women and 19 children in the orthodox Protestant communities who had orthodox Protestant beliefs.
The vaccination history of the participants was checked by vaccination certificates brought by the individuals and, where possible, a copy from the regional vaccine administration offices archives. Information on the vaccination history of the mother of the participants was not available.
Serologic Analysis
Blood samples from the study participants were analyzed for levels of antibodies against measles, mumps, rubella, and varicella. Fluorescent bead–based multiplex immunoassays were performed using Luminex technology (based on complete virus particles) to determine the concentrations of immunoglobulin G against measles, mumps, rubella, and varicella [21]. The procedure was calibrated to the World Health Organization (WHO) standard cutoff criteria. For measles, the second international WHO standard was used. Diagnostic cutoffs for discriminating between susceptible and immune individuals are 0.2 IU/mL for measles [22, 23], 45 RU/mL for mumps [24], 10 IU/mL for rubella [25], and 0.26 IU/mL for varicella. Antibody levels below the lower limits of quantification (LLOQ) were assumed to be equivalent to half of the LLOQ (ie, 0.002 IU/ mL for measles, 0.2 RU/mL for mumps, 0.02 IU/mL for rubella, and 0.01 IU/mL for varicella).
Statistical Analysis
We used a statistical model [26] to capture the kinetics of both passively acquired maternal antibodies and actively produced antibodies against measles, mumps, rubella, and varicella. The parameters of interest were the average concentration of maternal antibodies at birth (m), the decay rate of maternal antibodies (d), a base concentration of antibodies for susceptible individuals (b), and a concentration of antibodies for immune individuals (m + c). We fitted this statistical model of antibody kinetics to the age-specific serologic data for each of the 2 groups, using the method of maximum likelihood. We used maximum likelihood estimates to estimate the parameters and their significance and, additionally, the age at which the antibody concentrations in infants dropped below levels that protect against infection (ie, the diagnostic cutoff). The half-life of maternal antibodies was calculated as h = log(2)/d. All statistical analyses were performed in R [27]. For more information, see the Supplementary Materials.
RESULTS
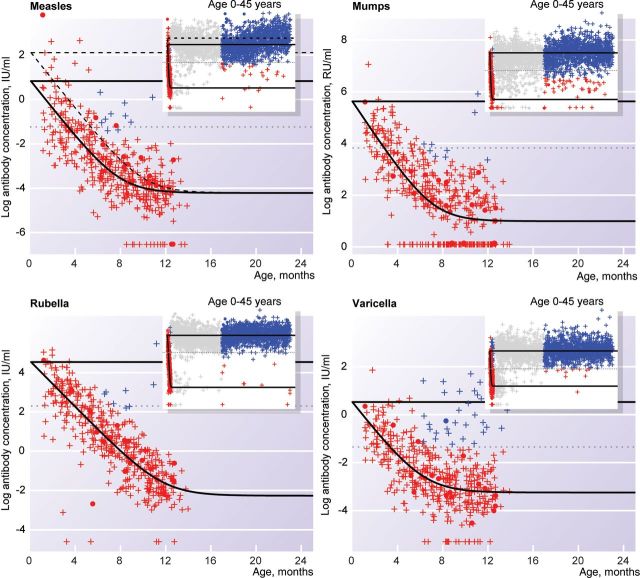
For measles, women of childbearing age in the general population were more likely to be vaccinated against measles than those in the orthodox Protestant communities (51.2% vs 12.6%; Table 1). Women of childbearing age and infants in the general population had a lower measles antibody concentration than infants and women of childbearing age in the orthodox Protestant communities (P < .0001). The concentration of maternal antibodies at birth was 4.13-fold lower in the general population as compared to the orthodox Protestant communities (Figure 1 and Supplementary Table 4). This corresponds to a 3.6-fold lower antibody concentration. The decay rate for maternal measles antibodies was 7.77 per year (Supplementary Table 4). This implies that, each month, the concentration of maternal antibodies halves and, therefore, that a 3.6-fold lower level of maternal antibodies at birth decreases the period of protective by almost 2 months (Figure 1). The duration of protection against measles was 3.3 months for newborns in the general population and 5.3 months for newborns in the orthodox Protestant communities.
Table 1.
Vaccination Coverage for Measles, Mumps, and Rubella Among Women of Childbearing Age Participating in the Dutch National Seroprevalence Study (Pienter 2), the Netherlands, 2006–2007
| General Population |
Orthodox Protestant Community |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine Coverage, % |
Vaccine Coverage,% |
|||||||
| Age | No. | Measles | Mumps | Rubella | No. | Measles | Mumps | Rubella |
| 20–24 y | 270 | 85.6 | 82.2 | 83.5 | 17 | 29.4 | 23.5 | 23.5 |
| 25–29 y | 270 | 74.4 | 55.2 | 73.4 | 16 | 31.3 | 25.0 | 37.5 |
| 30–34 y | 241 | 48.5 | 1.2 | 67.6 | 8 | 0 | 0 | 0 |
| 35–39 y | 247 | 11.3 | 0.4 | 45.4 | 6 | 0 | 0 | 16.7 |
| 40–44 y | 215 | 0 | 0 | 32.1 | 6 | 0 | 0 | 0 |
| Weighted average | 51.2 | 25.3 | 65.6 | 12.6 | 10.1 | 17.2 | ||
Figure 1.
Immunoglobulin G concentrations, by age, against measles, mumps, rubella, and varicella among individuals aged 0–24 months. Red markers represent individuals with maternal antibodies who did not seroconvert, and blue markers represent individuals who seroconverted. Dots represent individuals in the orthodox Protestant community, and pluses represent individuals in the general population. Grey horizontal lines indicate the diagnostic cutoffs below which individuals are susceptible to infection. Black lines represent the fitted model for antibody kinetics; with the dashed line indicating age-specific antibody level for orthodox Protestant individuals, and the solid lines indicating the age-speciic antibody level for individuals from the general population, respectively. The inset shows a wider age range; the grey dots are female participants who are not included in the analysis.
For mumps, we found a much smaller difference in vaccination coverage among women of childbearing age between the general population and the orthodox Protestant communities (25.3% vs 10.1%; Table 1). There were no statistically significant differences in antibody levels between the 2 groups. The decay rate for maternal mumps antibodies was 8.01 per year (Supplementary Table 4). This corresponds to a half-life of about 1 month. The duration of protection against mumps was 2.7 months for newborns in the general population and those in the orthodox Protestant communities.
For rubella, we found a large difference in vaccination coverage among women of childbearing age (65.6% in the general population vs 17.2% in the orthodox Protestant communities). Most of these vaccinated women received a single dose of vaccine at the age of 11 years and had possibly acquired natural infection earlier in life. Women of childbearing age in the general population had lower rubella antibody concentrations than those in the orthodox Protestant communities, with a difference of 55.19-fold (95% confidence interval, −6.22 to 116.60; P = .0296, by the likelihood ratio test). This corresponds to a 1.1-fold lower antibody concentration. The overall difference in antibody level between the 2 study groups was borderline nonsignificant (P = .0952). The decay rate for maternal rubella antibodies was 7.01 per year (Supplementary Table 4); this corresponds to a half-life of about 1 month. The duration of protection against rubella was 3.9 months for newborns in the general population and those in the orthodox Protestant communities. Projection of the existing 1.1-fold difference between women of childbearing age onto a difference in duration of protection among infants revealed that infants in the general population would be protected for 0.8 months less than infants in the orthodox Protestant communities.
For varicella, no participants were vaccinated. The level of varicella antibodies did not differ between the 2 study groups. The decay rate for maternal varicella antibodies was 7.36 per year (Supplementary Table 4). The duration of protection against varicella was 3.4 months for newborns.
We did not find differences between the maternal antibody level in infants at birth and those of women at childbearing age for any of the infections studied. The decay rate of antibodies in newborns did not differ between infants in the general population and those in the orthodox Protestant community (Supplementary Table 4).
DISCUSSION
In our analysis of maternal antibodies against measles, mumps, rubella, and varicella in a cross-sectional serologic study of the Dutch population, we found that the average age at which infants lose protection lies well before 14 months, when the first dose of MMR vaccine is administered. We compared individuals sampled from the general population with a group of individuals randomly selected from orthodox Protestant communities (the Dutch Bible belt), where the vaccination coverage among mothers is much lower than that in the general population. This comparison suggests that, as vaccination coverage among mothers increases, the level of maternal antibodies at birth among infants and the duration of the protection afforded by maternal antibodies among newborns decrease. The most likely explanation for this is that MMR vaccine induces lower antibody levels than natural infection with measles, mumps, and rubella and that antibody levels of vaccinated cohorts are no longer boosted by exposure to wild-type infection.
For measles, in which the vaccination coverage among mothers differed by almost 40% between groups, we found that the period of maternal antibody protection among infants in the general population was 2 months shorter than that among infants from orthodox Protestant communities. For rubella, in which the vaccination coverage among mothers differed by almost 50% but the difference in exposure to natural infection must have been much less, we found no significant difference in the duration of protection by maternal antibodies. However, if the significant difference between the adult women is carried onward to the infants, we see that infants in the general population were protected for 0.8 month less by maternal antibodies than infants in orthodox Protestant communities. For both mumps, in which the vaccination coverage among mothers differed by only 15% between groups, and varicella, we found no statistically significant difference in the duration of protection by maternal antibodies.
We assessed the duration of protection by maternal antibodies, using a cross-sectional study. The overall response was 33.5% (33% for the national sample and 35% for the low-immunization-coverage sample), which nowadays is relatively high for cross-sectional studies. Because of the large number of participants, we do not expect a self-selecting bias. Additionally, the advantage of this study design is that we have a large number of individuals who are representative of a large population. A possible disadvantage is the risk of finding associations between infants and women of childbearing age that do not exist between pairs of infants and mothers when using a longitudinal design. We address the potential for such spurious associations in each step of our argument. First, we observed little difference between antibody levels among seroconverted women of childbearing age and the initial levels among newborns within each of the 2 defined groups. This suggests that the level of antibodies is passed on from mother to child. This is in line with previous studies that showed that levels of maternal antibodies among children at birth were highly associated with the antibody levels of their mothers [11, 28]. Some previous studies reported an increase in antibody levels in infants at birth, compared with levels in their mothers; our study suggests this increase must be small, but this does not provide statistical evidence for the absence of such a difference. Second, we observed no difference in the decay rate of maternal antibodies between the 2 groups. This suggests that the decline in levels of maternal antibodies with age is an autonomous process that is similar for maternal antibodies. Third, we observed that antibody concentrations among women from the general population were lower than those among women from the orthodox Protestant communities. Other studies of maternal antibody levels confirm that such qualitative findings could be due to differences in vaccination status [11, 13]. These results, when taken together, provide evidence for a shorter protection period among infants in the general population than among those in the orthodox Protestant communities.
The 2 groups in our study differ markedly in vaccination coverage among women of childbearing age. To test for other factors that could affect our comparison, such as family size, we also compared the concentration of antibodies against varicella between the 2 groups. Vaccination against varicella has not been introduced in the Netherlands, and the vaccination coverage for varicella is 0% in both groups. Because of this, any difference with respect to varicella will suggest an intrinsic relevant difference between the groups that is due to a factor other than vaccination. In this sense, the analysis for varicella serves as a negative control [29]. We observed no differences between the groups with respect to varicella, and therefore we expect no major role for other factors that could affect our comparison. This provides a firm basis for attributing the observed difference in maternal antibody levels in newborn children to the vaccination history of their mother.
Our observations suggest that mass vaccination with MMR shortens, in due time, the duration of protection by maternal antibodies against measles, mumps, and rubella. Our study was conducted 20 years after introduction of the MMR vaccine, in 1987, when about 25% of women of childbearing age were vaccinated with MMR vaccine when they were young. This proportion of women of childbearing age who have been vaccinated with MMR will increase rapidly in the coming years because the vaccination coverage of each age cohort is >90%. We expect that this will further shorten the duration of protection against measles and rubella by maternal antibodies in infants and that a decreasing duration of protection against mumps by maternal antibodies will become more detectable among infants in the near future.
The average age at which a child loses the protection of its maternal antibodies and becomes susceptible to measles, mumps, and rubella lies well before the age of first MMR vaccination. It is extremely important to protect this large number of susceptible children, who have a high probability of a severe outcome when infected. An obvious solution is to lower the age at which the first dose of MMR is administered, but this could lower the vaccine efficacy because immunization at a younger age is hampered by different factors, such as the immaturity of the immune response [3, 30]. An alternative solution is to temporarily lower the age at which the first dose of MMR vaccine is administered to one when the risk of exposure to measles is high. In the Netherlands, MMR vaccination is currently recommended to all children aged 6–14 months of age who are at increased risk of acquiring measles during travel abroad to regions where measles is endemic. This intervention will probably also be implemented within the Netherlands should a measles outbreak occur there. Furthermore, as the effects of MMR vaccination on maternal protection become more pronounced over the next decade, the current MMR vaccination schedule will need to be adapted to continue to provide optimal protection to infants and their mothers.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The funding institution had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Financial support. This work was funded by the Ministry of Health, Welfare, and Sport, the Netherlands.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Davidkin I, Kontio M, Paunio M, Peltola H. MMR vaccination and disease elimination: the Finnish experience. Expert Rev Vaccines. 2010;9:1045–53. doi: 10.1586/erv.10.99. [DOI] [PubMed] [Google Scholar]
- 2.Castillo-Solorzano C, Marsigli C, Danovaro-Holliday MC, Ruiz-Matus C, Tambini G, Andrus JK. Measles and rubella elimination initiatives in the Americas: lessons learned and best practices. J Infect Dis. 2011;204(Suppl 1):S279–83. doi: 10.1093/infdis/jir216. [DOI] [PubMed] [Google Scholar]
- 3.Redd SC, King GE, Heath JL, Forghani B, Bellini WJ, Markowitz LE. Comparison of vaccination with measles-mumps-rubella vaccine at 9, 12, and 15 months of age. J Infect Dis. 2004;189(Suppl 1):S116–22. doi: 10.1086/378691. [DOI] [PubMed] [Google Scholar]
- 4.de Voer RM, van der Klis FR, Nooitgedagt JE, et al. Seroprevalence and placental transportation of maternal antibodies specific for Neisseria meningitidis serogroup C, Haemophilus influenzae type B, diphtheria, tetanus, and pertussis. Clin Infect Dis. 2009;49:58–64. doi: 10.1086/599347. [DOI] [PubMed] [Google Scholar]
- 5.Kebede S, Nokes DJ, Cutts FT, Nigatu W, Sanderson F, Beyene H. Maternal rubella-specific antibody prevalence in Ethiopian infants. Trans R Soc Trop Med Hyg. 2000;94:333–40. doi: 10.1016/s0035-9203(00)90347-x. [DOI] [PubMed] [Google Scholar]
- 6.Oyedele OO, Odemuyiwa SO, Ammerlaan W, Muller CP, Adu FD. Passive immunity to measles in the breastmilk and cord blood of some Nigerian subjects. J Trop Pediatr. 2005;51:45–8. doi: 10.1093/tropej/fmh073. [DOI] [PubMed] [Google Scholar]
- 7.Sauerbrei A, Groh A, Bischoff A, Prager J, Wutzler P. Antibodies against vaccine-preventable diseases in pregnant women and their offspring in the eastern part of Germany. Med Microbiol Immunol, 2002;190:167–72. doi: 10.1007/s00430-001-0100-3. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg JP, Westerbeek EA, van der Klis FR, Berbers GA, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87:67–72. doi: 10.1016/j.earlhumdev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Muscat M, Bang H, Wohlfahrt J, Glismann S Mølbak K; EUVAC.NET Group. Measles in Europe: an epidemiological assessment. Lancet. 2009;373:383–9. doi: 10.1016/S0140-6736(08)61849-8. [DOI] [PubMed] [Google Scholar]
- 10.Mulholland EK, Griffiths UK, Biellik R. Measles in the 21st century. N Engl J Med. 2012;366:1755–7. doi: 10.1056/NEJMp1202396. [DOI] [PubMed] [Google Scholar]
- 11.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado YA, Lawrence EC, DeHovitz R, Hartzell H, Albrecht P. Early loss of passive measles antibody in infants of mothers with vaccine-induced immunity. Pediatrics. 1995;96:447–50. [PubMed] [Google Scholar]
- 13.Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25:6296–304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 14.van den Hof S, Berbers GA, de Melker HE, Conyn-van Spaendonck MA. Sero-epidemiology of measles antibodies in the Netherlands, a cross-sectional study in a national sample and in communities with low vaccine coverage. Vaccine. 1999;18:931–40. doi: 10.1016/s0264-410x(99)00348-5. [DOI] [PubMed] [Google Scholar]
- 15.Ruijs WL, Hautvast JL, van der Velden K, de Vos S, Knippenberg H, Hulscher ME. Religious subgroups influencing vaccination coverage in the Dutch Bible belt: an ecological study. BMC Public Health. 2011;11:102. doi: 10.1186/1471-2458-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Hof S, Conyn-van Spaendonck MA, van Steenbergen JE. Measles epidemic in the Netherlands, 1999–2000. J Infect Dis. 2002;186:1483–6. doi: 10.1086/344894. [DOI] [PubMed] [Google Scholar]
- 17.Hahné S, Macey J, van Binnendijk R, et al. Rubella outbreak in the Netherlands, 2004–2005: high burden of congenital infection and spread to Canada. Pediatr Infect Dis J. 2009;28:795–800. doi: 10.1097/INF.0b013e3181a3e2d5. [DOI] [PubMed] [Google Scholar]
- 18.Karagiannis I, van Lier A, van Binnendijk R, et al. Mumps in a community with low vaccination coverage in the Netherlands. Euro Surveill. 2008;13 pii: 18901. [PubMed] [Google Scholar]
- 19.van Lier EA, Oomen PJ, Giesbers H, Drijfhout IH, de Hoogh PAAM, Melker HE. Bilthoven, the Netherlands: RIVM; 2011. Immunization coverage National Immunization Programme in the Netherlands: Year of report 2011. [Google Scholar]
- 20.van der Klis FR, Mollema L, Berbers GA, de Melker HE, Coutinho RA. Second national serum bank for population-based seroprevalence studies in the Netherlands. Neth J Med. 2009;67:301–8. [PubMed] [Google Scholar]
- 21.Smits GP, van Gageldonk PG, Schouls LM, van der Klis FR, Berbers GA. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin Vaccine Immunol. 2012;19:396–400. doi: 10.1128/CVI.05537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 23.Christenson B, Bottiger M. Methods for screening the naturally acquired and vaccine-induced immunity to the measles virus. Biologicals. 1990;18:207–11. doi: 10.1016/1045-1056(90)90008-n. [DOI] [PubMed] [Google Scholar]
- 24.Harmsen T, Jongerius MC, van der Zwan CW, Plantinga AD, Kraaijeveld CA, Berbers GA. Comparison of a neutralization enzyme immunoassay and an enzyme-linked immunosorbent assay for evaluation of immune status of children vaccinated for mumps. J Clin Microbiol. 1992;30:2139–44. doi: 10.1128/jcm.30.8.2139-2144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol. 1996;106:170–4. doi: 10.1093/ajcp/106.2.170. [DOI] [PubMed] [Google Scholar]
- 26.van den Hof S, Wallinga J, Widdowson MA, Conyn-van Spaendonck MA. Protecting the vaccinating population in the face of a measles epidemic: assessing the impact of adjusted vaccination schedules. Epidemiol Infect. 2002;128:47–57. doi: 10.1017/s0950268801006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org . [Google Scholar]
- 28.Leuridan E, Hens N, Hutse V, Aerts M, Van Damme P. Kinetics of maternal antibodies against rubella and varicella in infants. Vaccine. 2011;29:2222–6. doi: 10.1016/j.vaccine.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–8. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morein B, Abusugra I, Blomqvist G. Immunity in neonates. Vet Immunol Immunopathol. 2002;87:207–13. doi: 10.1016/s0165-2427(02)00078-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.