Abstract
An obstacle to development of methods to quantify β-cell turnover from pancreas tissue is the lack of conversion factors for the frequency of β-cell replication or apoptosis detected by immunohistochemistry to rates of replication or apoptosis. We addressed this obstacle in islets from 1-mo-old rats by quantifying the relationship between the rate of β-cell replication observed directly by time-lapse video microscopy (TLVM) and the frequency of β-cell replication in the same islets detected by immunohistochemistry using antibodies against Ki67 and insulin in the same islets fixed immediately after TLVM. Similarly, we quantified the rate of β-cell apoptosis by TLVM and then the frequency of apoptosis in the same islets using TdT-mediated dUTP nick-end labeling and insulin. Conversion factors were developed by regression analysis. The conversion factor from Ki67 labeling frequency (%) to actual replication rate (%events/h) is 0.025 ± 0.003 h−1. The conversion factor from TdT-mediated dUTP nick-end labeling frequency (%) to actual apoptosis rate (%events/h) is 0.41 ± 0.05 h−1. These conversion factors will permit development of models to evaluate β-cell turnover in fixed pancreas tissue.
Keywords: Ki67, TdT-mediated dUTP nick-end labeling, insulin, conversion factor
both type 1 and type 2 diabetes are characterized by a deficiency of β-cells (5, 6, 22, 30). Pancreas transplantation restores glycemic control in type 1 and type 2 diabetes (10, 26). However, given the insufficient number of pancreases available for transplantation and the risks of prolonged immunosuppression, restoration of β-cells by transplantation is not a viable option for most people with diabetes.
A potential alternative strategy is restoration of β-cells through endogenous regeneration. Induction of diabetes in young rodents by β-cell toxins or partial surgical pancreas resection has been followed by regeneration of β-cell mass and reversal of diabetes (4, 40). The origin of these β-cells has been actively debated: some have proposed duplication of existing β-cells, and others have suggested formation of new β-cells from a variety of sources (7). Although ongoing lineage studies will provide insights into the origins of β-cells in mice (3, 11, 12, 27, 37), this approach is not available in humans and does not provide guidance as to the rate of β-cell formation that might be anticipated in humans. Thus, although there is indirect evidence that there might be ongoing β-cell formation in adult humans with type 1 diabetes (22), there is no information as to rate of β-cell turnover in human pancreas. Although methods do exist for detection of β-cell replication and β-cell apoptosis, these methods cannot yet be used to compute the rate of β-cell replication or apoptosis in humans.
Incorporation of labeled nucleotides [bromodeoxyuridine (BrdU)-labeling index], which has been used to estimate the β-cell replication rate in rodents (15, 38), cannot be used in humans, since it requires administration of BrdU (a toxic and mutagenic substance) before pancreas collection. Moreover, BrdU can disturb the cell cycle, and its use cannot distinguish cell replication from DNA repair (7). Immunostaining of the nuclear protein Ki67 is an alternative method for quantification of the frequency of cell replication. Ki67 is expressed in all phases of the cell cycle, except the resting phase (G0) and for a short period at the beginning of the G1 phase (7). Ki67 is not detected during DNA repair. Therefore, although Ki67 labeling is more sensitive and specific as an estimate of the frequency of β-cell replication than use of BrdU (7), it does not provide a rate of replication.
TdT-mediated dUTP nick-end labeling (TUNEL) determined by immunohistochemistry is the most widely used approach to quantify cell death and has the advantage in this regard, in that it detects apoptosis and necrosis in tissue samples (9, 18). In common with Ki67 staining, TUNEL permits evaluation of the frequency, but not the rate, of cell death in a tissue of interest.
The goal of the present studies was to establish conversion factors that would provide 1) the rate of β-cell replication from the frequency of β-cell replication determined by Ki67 and insulin immunostaining and 2) the rate of β-cell apoptosis from the frequency of apoptosis detected by TUNEL and insulin immunostaining. These goals are important, because they must be accomplished before models intended to quantify β-cell turnover in humans can be developed.
MATERIALS AND METHODS
Study design.
Our strategy involved use of time-lapse video microscopy (TLVM) to directly quantify the rate of β-cell apoptosis or replication and then fix that tissue and employ immunohistochemistry to determine the corresponding frequency of β-cell replication or apoptosis. The requirements for this strategy are as follows.
First, we established conditions in which steady-state rates of β-cell replication or β-cell apoptosis were present for ≥24 h in pilot experiments using INS-1 cells and isolated islets. Second, we established conditions in which we could reproducibly identify β-cells during TLVM from the subsequent immunostaining of islets (for insulin). Third, we needed to observe β-cell replication and apoptosis at a variety of rates to establish a relationship between these two parameters. This was particularly challenging for β-cell replication in islets, since we discovered (as have others recently) that, even in rodent islets, β-cell replication is rare in adults. The challenge was overcome by use of islets from rats at 1 mo of age, when the postnatal expansion of β-cell numbers through β-cell replication is still occurring.
Having established these conditions, we studied juvenile rat islets under steady-state conditions of β-cell replication and β-cell apoptosis and then immunostained these same cells with Ki67 and insulin or TUNEL and insulin, respectively. From the resulting merged images, we established the relationship between the steady-state rate of β-cell replication (by TLVM) and frequency of insulin- and Ki67-positive cells by immunofluorescence. Similarly, we established the relationship between the rate of β-cell apoptosis (by TLVM) and the frequency of TUNEL-positive β-cells by immunofluorescence. The frequency of TUNEL-positive cells in isolated islets (0.20 ± 0.06%; Table 2) was low compared with the frequency of Ki67-positive cells (5.9 ± 1.4%; Table 1). Therefore, we also used a cytotoxin-treated β-cell line to increase the rate of apoptosis and provide a wider range of apoptosis rates to more reliably establish a relationship between the frequency of TUNEL-positive cells and the observed rate of β-cell apoptosis by TLVM.
Table 2.
Summary of rat islet immunostaining and TLVM: apoptosis
| Immunostaining |
TLVM |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cell Number | Ins(+) Cells, % | Ins(+)-TUNEL(+) Cells/Ins(+) Cells, % | Ins(+)-TUNEL(+) Cells/TUNEL(+) Cells, % | Total Cell Number | Islet Cell Apoptosis Rate, %/h | Adjusted β-Cell Apoptosis Rate, %/h | ||||||
| Expt 6 | 3,374 | 74 | 0.16 | 80 | 532 | 0.070 | 0.079 | |||||
| Expt 7 | 5,415 | 69 | 0.40 | 94 | 1,458 | 0.107 | 0.149 | |||||
| Expt 8 | 4,748 | 67 | 0.25 | 67 | 1,555 | 0.060 | 0.060 | |||||
| Expt 9 | 4,357 | 72 | 0.13 | 67 | 1,923 | 0.075 | 0.068 | |||||
| Expt 10 | 3,898 | 78 | 0.07 | 50 | 1,722 | 0.040 | 0.028 | |||||
| Total | 21,792 | 7,190 | ||||||||||
| Mean | 4,358 | 72 | 0.20 | 72 | 1,438 | 0.070 | 0.077 | |||||
| SE | 350 | 2 | 0.06 | 7 | 240 | 0.011 | 0.020 | |||||
TUNEL, TdT-mediated dUTP nick-end labeling.
Table 1.
Summary of rat islet immunostaining and TLVM: replication
| Immunostaining |
TLVM |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cell Number | Ins(+) Cells, % | Ins(+)-Ki67(+) Cells/Ins(+) Cells, % | Ins(+)-Ki67(+) Cells/Ki67(+) Cells, % | Total Cell Number | Islet Cell Replication Rate, %/h | Adjusted β-Cell Replication Rate, %/h | ||||||
| Expt 1 | 5,398 | 62 | 10.7 | 90 | 1,609 | 0.15 | 0.21 | |||||
| Expt 2 | 4,457 | 92 | 4.2 | 98 | 1,281 | 0.11 | 0.12 | |||||
| Expt 3 | 5,065 | 89 | 3.2 | 95 | 2,840 | 0.09 | 0.09 | |||||
| Expt 4 | 5,621 | 80 | 7.2 | 97 | 2,213 | 0.22 | 0.26 | |||||
| Expt 5 | 4,643 | 93 | 4.1 | 98 | 2,115 | 0.08 | 0.08 | |||||
| Total | 25,184 | 10,058 | ||||||||||
| Mean | 5,037 | 83 | 5.9 | 96 | 2,012 | 0.13 | 0.15 | |||||
| SE | 220 | 6 | 1.4 | 2 | 268 | 0.03 | 0.04 | |||||
TLVM, time-lapse video microscopy; Ins, insulin; Expt, experiment.
Rat islet isolation and culture.
All animal studies were approved by the University of California Los Angeles, Animal Research Committee. Pancreatic islets were isolated from 1-mo-old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). The rats were euthanized by inhalation of isoflurane (Abbott Laboratories, North Chicago, IL), the bile duct was cannulated, and Hanks' working buffer with liberase (0.23 mg/ml, catalog no. 1815032; Roche Diagnostics, Mannheim, Germany) and DNase (0.1 mg/ml, ∼2,000 U/mg; catalog no. 104159, Roche Diagnostics) was directly infused into the pancreas (∼2 ml) in situ through the bile duct to uniformly distend the pancreas. The pancreas was resected, fat was trimmed from the pancreas, and the whole organ was placed in a 20-ml vial containing 5 ml of the same buffer and digested to permit separation of islets from exocrine tissue in a 37°C shaker for 18 min. The pancreas tissue digest was dispersed by vigorous shaking and washed four times in Hanks' buffer and subjected to Histopaque (catalog no. 10771; Sigma, St. Louis, MO) gradient separation. After centrifugation, the medium-Histopaque interface was collected and washed, and islets were hand-picked under microscopic guidance to exclude exocrine debris. Then 30–50 islets were cultured in a glass-bottomed 35-mm FluoroDish (World Precision Instruments, Sarasota, FL) with RPMI 1640 medium containing 11 mM glucose supplemented with 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin for ∼4 days to recover from the isolation procedure. To ensure that the cells would not form multilayer clusters, we coated the slides or dishes for TLVM with a human bladder carcinoma cell line (HTB-9) matrix, as described previously (24, 31). The clock time used in the experiments on these islets during TLVM represents the hours after the cell medium was changed and the islets were placed into the chamber of the microscope for TLVM, with time 0 representing the time at which the medium was changed. Different islets were used for the TLVM studies for β-cell replication (experiments 1–5; Table 1) and apoptosis (experiments 6–10; Table 2).
INS-1 cell culture and treatment.
INS-1 rat insulinoma cells (kindly provided by Dr. Christopher Rhodes) were grown in RPMI 1640 medium supplemented with 10% FBS, 50 μM β--mercaptoethanol, 10 mM HEPES, 1 mM sodium pyruvate, 2 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin and cultured at 37°C in humidified air containing 5% CO2. INS-1 cells were plated onto four-well glass chamber slides (Nalge Nunc International, Naperville, IL) at a density of 3 × 104 cells/well and grown to 50% confluency before assay. Just before TLVM experiments were begun, tissue culture medium was changed to fresh medium with or without cytokine mix, and the slide was transferred to the stage of a time-lapse video microscope (model DMIRE2, Leica Microsystems, Wetzlar, Germany). To ensure all INS-1 cells in monolayer, we coated the slides with an HTB-9 matrix.
Rat recombinant TNFα, IL-1β-, and IFNγ (catalog nos. T 5944, I 2393, and I 3275, respectively, Sigma) were diluted in distilled water or 0.1% BSA-PBS solution, and 10 ng/ml TNFα, 1 ng/ml IL-1β-, and 10 ng/ml IFNγ were mixed as a standard dose (100%) of cytokine mix. Fifty, 100, and 200% cytokine mix were used in the study.
TLVM.
TLVM was carried out using an inverted microscope equipped with phase contrast and a temperature-controlled and motorized stage (model DMIRE2, Leica Microsystems). The chamber on the stage was maintained at 37°C in 95% air-5% CO2 saturated with water, similar to a CO2 incubator. The bright-field images of INS-1 cells were acquired using a charge-coupled device camera (Hamamatsu Photonics, Hamamatsu, Japan) at ×200 magnification and Volocity (Improvision, Lexington, MA) software and collected at 10-min intervals for 48 h with six fields per treatment, which contained 737 ± 27 (SE) cells in each experiment (total 11,790 cells). The bright-field images of rat islet cells were also acquired at ×300 magnification and collected at 10-min intervals for 16 h with 10–18 fields per dish (see Tables 1 and 2 for cell numbers).
Analysis of TLVM images.
Time-lapse images were merged into a movie and then evaluated for identification of each replication or apoptosis event. The total number of islet cells in each field was counted at the beginning of the study, and then the change in the total number of β-cells during the movie was accounted for by the number of replication events subtracted by the number of apoptosis events. Replication was judged to have occurred when a single islet cell divided into two daughter cells (Fig. 1, A and B). Apoptosis was judged to have occurred when an islet cell rounded up, the nucleus condensed and subsequently fragmented, and the cell cytoplasm disintegrated via cytoplasmic blebs into apoptotic bodies (Fig. 1, C and D). Thus the rate of replication and apoptosis was calculated as the number of events per total number of cells per hour × 100 (%events/h). Because, in preliminary studies, most cells that spread from the islet in culture were subsequently shown to be fibroblast-like and insulin negative, we analyzed only the cells within the islet that spread in the monolayer. Although most of the islet cells within these fields subsequently proved to be β-cells, a small proportion were not. To address this issue, we determined the frequency of replication and apoptosis in β- and non-β-cells within these fields of islet cells. Given these data (Tables 1 and 2), we adjusted the event rates (replication and apoptosis) observed in these islet fields by TLVM by the ratio of β-cells to non-β-cells and the ratio of the frequency of events in β-cells vs. non-β-cells determined by immunostaining in each experiment. Since >70% of the islet cells in the selected fields were β-cells, this adjustment had only a modest effect on the event rates (Tables 1 and 2).
Fig. 1.
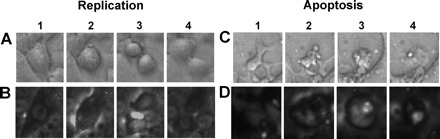
Detection of replication and apoptosis during time-lapse video microscopy (TLVM). A and B: sequential images of an INS-1 cell and a rat islet cell, respectively, undergoing replication. C and D: sequential images of an INS-1 cell and a rat islet cell, respectively, undergoing apoptosis. Replication was judged to have occurred when 1 cell divided into 2 daughter cells (1–4). Apoptosis was judged to have occurred when a cell rounded up (2 and 3), the nucleus condensed and subsequently fragmented, and the cell cytoplasm disintegrated via cytoplasmic blebs into apoptotic bodies (4).
Ki67 staining and detection.
After completion of the TLVM studies, culture chambers or dishes were immediately washed in PBS and then fixed in 4% paraformaldehyde for 30 min. For immunostaining, sections were washed in TBS, permeabilized using PBS-0.2% Triton X-100, and blocked with blocking buffer, and the slides were incubated with mouse anti-Ki67 antibody (1:50 dilution; catalog no. M7248, Dako, Carpenteria, CA) overnight and then with donkey-derived secondary antibodies conjugated to Cy3 (Jackson Immuno Research Laboratories, West Grove, PA). The slides were immunostained with guinea pig anti-insulin (1:100 dilution; Zymed Laboratories, South San Francisco, CA) for 2 h and then with donkey-derived secondary antibodies conjugated to FITC (Jackson ImmunoResearch Laboratories). All slides were mounted with 4′,6-diamidino-2-phenylindole-containing mounting medium (Vector Laboratories, Burlingame, CA). Fluorescent slides were viewed using a Leica DM6000 microscope (Leica Microsystems), and images were acquired using Openlab (Improvision) software. Replication was analyzed by counting Ki67-positive β-cells in each islet, which contained 5,037 ± 220 islet cells in each experiment (total 25,184 cells), and expressed as frequency (percentage of β-cells; Table 1).
DNA staining with TUNEL.
After completion of the time-lapse studies, culture chambers or dishes were immediately washed in PBS and then fixed in 4% paraformaldehyde for 30 min. For immunostaining, sections were washed in TBS, permeabilized in PBS-0.2% Triton X-100, and subjected to TUNEL staining by use of an in situ cell death detection kit [12156792910 TMR Red (for isolated islet cells) and 11684809910 AP (for INS-1 cells), Roche Diagnostics]. For islet cells, this reaction was blocked with blocking buffer, and the slides were immunostained with guinea pig anti-insulin (1:100 dilution; Zymed Laboratories) for 2 h and then with donkey-derived secondary antibodies conjugated to FITC (Jackson ImmunoResearch Laboratories). All slides were mounted with 4′,6-diamidino-2-phenylindole-containing medium. Fluorescent slides were viewed using a Leica DM6000 microscope, and images were acquired using Openlab software. Apoptosis was analyzed by counting TUNEL-positive β-cells in each islet, which contained 4,358 ± 350 islet cells and 2,213 ± 218 INS-1 cells in each experiment (total 21,792 islet cells and 35,411 INS-1 cells), and expressed as frequency (percentage of β-cells; Table 2).
Data analysis.
Values are means ± SE, except as otherwise noted. Since standard errors for frequency and event rate of INS-1 data represent the errors of measurement related to immunofluorescence and TLVM, respectively (i.e., each datum results from the mean of 4 replicates), general laws of error were experimentally established for the two techniques.
For immunofluorescence, the resulting law of error is
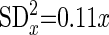 |
(1) |
where SDx is standard deviation of frequency measurements, which is well known as Poisson's law, and x is frequency measurement.
For TLVM, the resulting law of error is
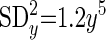 |
(2) |
where SDy is standard deviation of event rate and y is event rate. These laws were applied to data obtained from studies in isolated islets.
To determine the conversion factors from frequency to event rate, a weighted total least-squares (TLS) algorithm for fitting a straight line (21) was implemented by MATLAB (Mathworks, Natick, MA); this algorithm takes into account the measurement errors affecting frequency and event rate. The intercept was set to zero, because if a 0% frequency is measured after TLVM, that event rate is 0%/h.
RESULTS
β-Cell replication.
From pilot studies, we noted that rat islets flattened out over ∼4 days in culture on a bladder cancer cell line matrix, so the edges of the islet were a monolayer that could readily be evaluated by TLVM for replication (or apoptosis) events (Figs. 1 and 2A). We also noted in pilot studies that β-cell replication was very rare in adult (≥10-wk-old) rat islets but was much more readily identified in islets from juvenile (4-wk-old) rats. The rat islets were removed from conventional cell culture chambers after 4 days in culture following isolation and transferred to the chamber of the microscope for TLVM, with medium change at the time of transfer. The clock time shown in Fig. 2 corresponds to hours after islets were transferred to the TLVM chamber and medium change. In the juvenile rat islets thus studied, the β-cell replication rate varied from 0.08 to 0.26 (0.15 ± 0.04% events/h; Table 1). The β-cell replication rate was at a steady state during TLVM and before fixation of islets for immunostaining (Fig. 2B). After the TLVM studies, we evaluated the β-cell replication frequency in the same islets by Ki67 and insulin coimmunofluorescence. As expected, the majority of islet cells were insulin positive (83 ± 6%; Table 1 and Fig. 2C). In contrast, cells spreading out of the islet (mostly with fibroblast-like properties) were insulin negative. Within the juvenile rodent islets, the frequency of Ki67-positive cells was greater in insulin-positive than insulin-negative cells (5.9 ± 1.4% vs. 1.3 ± 0.2%; Table 1).
Fig. 2.

A: representative images of a rat islet cell during TLVM. Original magnification ×300. First and last images of a rat islet cell studied by TLVM for 16 h show little overall change in cell number or islet architecture. B: rate of β-cell replication and apoptosis of rat islet cells during 16 h of TLVM (×20 objective). Values are means ± SE. C and D: immunostaining of rat islets for Ki67 and TdT-mediated dUTP nick-end labeling (TUNEL), respectively, after TLVM. Green, insulin; red, Ki67 or TUNEL; blue, nuclear 4′,6-diamidino-2-phenylindole (DAPI). Most islet cells were insulin positive; cells that spread out from islets were most often insulin negative. Ki67-positive cells were frequently seen, and most Ki67-positive cells were insulin positive (arrowheads in C). TUNEL-positive β-cells were more rare (arrowhead in D).
The relationship between rate of β-cell replication (TLVM) and frequency of β-cell replication (Ki67) was linear (r2 = 0.87, P < 0.01; Fig. 3A) and could be described as follows
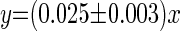 |
(3) |
where y is replication rate (%events/h) and x is Ki67-positive frequency (%). From Eq. 3, if the frequency of Ki67-positive β-cells were 1%, we would predict the corresponding rate of β-cell mitosis to be 0.025%events/h. These data imply that an average β-cell undergoing cell replication is Ki67 positive for ∼40 h, comparable to the duration observed for other cell types (16, 17, 19, 32, 34, 39, 41).
Fig. 3.

A: correlation between rate of β-cell replication observed by TLVM and frequency of Ki67-positive β-cells determined by immunostaining in rat islets (n = 5). B: correlation between rate of β-cell apoptosis and frequency of TUNEL-positive β-cells in rat islets (n = 5). Values are means ± SD. SD for each value was determined by resulting law of error based on INS-1 experiment (see Eqs. 1 and 2).
β-Cell apoptosis.
β-cell apoptosis was evaluated in INS-1 cells and isolated rat islets. In juvenile rat islets, the rate of β-cell apoptosis observed by TLVM varied from 0.028 to 0.149%events/h (0.077 ± 0.020%events/h; Table 2). The rate of β-cell apoptosis increased after cell culture medium change (time 0) but was then relatively stable during TLVM and before fixation of islets for immunostaining (Fig. 2B). During the last 8 h of TLVM, the rate of β-cell apoptosis was at steady state and similar to the corresponding rate of β-cell replication. However, despite this comparable rate of β-cell replication and apoptosis, the frequency of TUNEL-positive β-cells (0.20 ± 0.06%) was much lower than the frequency of Ki67-positive β-cells (5.9 ± 1.4%; Table 2 and Fig. 2D).
The relationship between the rate of β-cell apoptosis (TLVM) and the frequency of β-cell apoptosis (TUNEL) in juvenile rodent islets was linear (r2 = 0.77, P < 0.01; Fig. 3B) and described as follows
 |
(4) |
where y is apoptosis rate (%events/h) and x is TUNEL frequency (%).
These findings were confirmed in INS-1 cells. In INS-1 cells exposed to 0–200% cytokine mix, the rate of apoptosis increased in a dose-dependent manner (Fig. 4). Consistent with prior reports that cells exposed to proapoptotic signals preferentially undergo apoptosis, rather than mitosis (13, 24), the rate of replication declined in a dose-dependent manner with increasing dose of cytokine mix. The rates of apoptosis and replication remained at steady state from 16 to 48 h (Fig. 4, F and G).
Fig. 4.

A–D: representative images of INS-1 cells during TLVM (×20 objective). Number of nontreated control cells increased ∼150% during TLVM for 48 h (A and B). Net number of cells treated with 200% cytokine mix was not increased, and many dead or dying cells were seen after 48 h (C and D). E–G: cell number, replication rate, and apoptosis rate during TLVM experiment in INS-1 cells. Replication and apoptosis rates were at steady state for the last 32 h of TLVM (F and G). Values are means ± SE (n = 4 in each group).
When the same slides were subsequently stained for TUNEL, the frequency of β-cell apoptosis also increased in proportion to the applied dose of cytokine mix (Fig. 5, A and B). There was a linear relationship (r2 = 0.95, P < 0.01; Fig. 5C) between the rate of apoptosis (TLVM) and the frequency of β-cell apoptosis (TUNEL) in INS-1 cells during the last 16 h of incubation. Moreover, the relationship between rate of β-cell apoptosis (TLVM) and frequency of TUNEL-positive β-cells [y = (0.38 ± 0.05)x] was comparable to that observed in primary rat islets (Eq. 4).
Fig. 5.
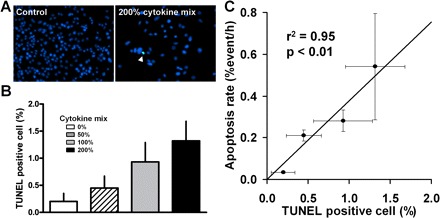
A: immunostaining of INS-1 cells after TLVM (×20 objective). Green, TUNEL; blue, nuclear DAPI. Number of cells decreased and apoptosis increased (arrowhead) in cells treated with 200% cytokine mix. B: frequency of TUNEL-positive INS-1 cells. C: correlation between rate of apoptosis observed by TLVM and frequency of TUNEL-positive cells determined by immunostaining in INS-1 cells. Values are means ± SE (n = 4 in each group).
From Eq. 4, if the frequency of TUNEL-positive β-cells is 1%, we would predict that the corresponding rate of β-cell apoptosis is 0.4%events/h. This relationship implies that, on average, a β-cell undergoing apoptosis is TUNEL positive for ∼2.5 h, a finding that is consistent with published data from other cell types (1, 2, 8, 14, 17, 28, 29).
DISCUSSION
In this study, we used TLVM and immunohistochemistry to develop factors to permit conversion of frequency to rate of β-cell replication and apoptosis. The conversion factors from the frequency of labeling (Ki67 and TUNEL) to the actual event rate were 0.025 ± 0.003 and 0.41 ± 0.05 h−1 for β-cell replication and β-cell apoptosis, respectively.
To determine the conversion factors, we first derived, from replicate data, models to establish errors affecting estimates of frequency and event rate. Then, with use of the TLS algorithm, both errors were simultaneously taken into account in linear regression, and the conversion factors were estimated as the slope of the line (Fig. 3, A and B). The conventional approach for error analysis, which considers errors affecting only one variable (i.e., frequency or event rate) leads to biased estimates. For example, in replication data, the conversion factor is 0.025 h−1 with the TLS algorithm, 0.028 h−1 when only errors in frequency are considered and event rate is assumed error free, and 0.023 h−1 in the reciprocative situation.
The resulting conversion factors are consistent with the available data in other cell types as well as predictions of duration of the cell cycle and apoptosis in β-cells. The conversion factors imply that β-cells undergoing replication or apoptosis are positive for Ki67 or TUNEL for ∼40 or ∼2.5 h, respectively. Although the duration of the process of programmed cell death by apoptosis can be 12–24 h from initiation to cell disintegration, the duration of the final execution phase corresponding to nuclear condensation by light microscopy is several hours on the basis of morphological observation of the epithelial lining of the small intestine in vivo (1, 29). TUNEL detects only this final execution phase of apoptosis (nuclear fragmentation) and is consistent with our calculated period of 2.5 h.
On a technical note, it is important to emphasize that, in this study and in those in which we have reported the frequency of apoptosis in tissues, we consider a cell to be TUNEL positive only if the nucleus is clearly positive for TUNEL staining and the cell is still distinguishable as a cell. When the rate of apoptosis is high, it is not unusual to observe TUNEL-positive nuclear debris that is no longer within the confines of a cell. This debris presumably remains for variable periods after cell death, depending on the time taken for it to be cleared by macrophages. If this TUNEL-positive debris is included in counts of apoptosis, the conversion factors provided here would not be valid, and the calculated rates of apoptosis would be greatly exaggerated.
The relatively short duration of the TUNEL-positive final commitment phase of apoptosis implies that the frequency of TUNEL-positive cells in most tissues is low. Even a relatively modest increase in frequency of TUNEL-positive cells can correspond to a very significant increase in cell death through apoptosis. In addition, the relatively low frequency of apoptosis determined by TUNEL implies that it is important to evaluate a large cell sample size to minimize sampling and counting errors, since these will be multiplied by the conversion factor. Also, since TUNEL is also positive in necrotic tissue, it is particularly important to obtain tissue that is rapidly fixed after removal. In occasional reports of frequency of apoptosis as high as 3%, the explanation would imply an explosive loss of tissue or, perhaps more likely, the presence of postmortem autolysis.
Although a β-cell is TUNEL positive for only a short time when undergoing apoptosis, we report that β-cells undergoing replication are Ki67 positive for ∼40 h. This indicates that if 100% of β-cells are positive for Ki67, the doubling rate will be ∼40 h. Interestingly, this time period is similar to the doubling time of INS-1 cells, in which 100% of cells are in the active cell cycle (G1 to M phase). Moreover, Russ et al. (33) reported that ∼30% of human islet cells cultured in vitro with doubling time of 7 days (∼150 h) were positive for Ki67, which is consistent with the present conversion factor. Swenne (35, 36) reported that the cell cycle length of fetal islet cells is 14.9 h. Our estimated cell cycle length of juvenile β-cells (i.e., time during which they are Ki67 positive) was ∼40 h. This discrepancy is likely due to different techniques. Swenne used [3H]thymidine to label DNA synthesis after synchronization of fetal islets by hydroxyurea to estimate cell cycle length. Since [3H]thymidine incorporation occurs in DNA synthesis during the cell cycle and also with DNA repair, to the extent that the latter is present, cell replication is overestimated and computed cell length is underestimated. Using time-lapse microscopy, we previously reported that the cell cycle length of rat insulinoma cells is ∼30 h (24, 31). In theory, in contrast to the use of TUNEL to detect apoptosis, use of Ki67 to detect replication does not require evaluation of such a large number of cells, given the relatively high frequency that would be detected during active β-cell replication.
This is most readily illustrated by considering an islet in which the numbers of β-cells being formed exclusively through replication of existing β-cells is equal to the number of β-cells undergoing apoptosis. In this circumstance, the frequency of Ki67-positive β-cells would be expected to be ∼20 times the frequency of TUNEL-positive β-cells. An important implication of this notion is that comparable precision and accuracy for estimation of the rate of β-cell apoptosis and replication would require evaluation of ∼20-fold more cells for insulin and TUNEL staining than for insulin and Ki67 staining.
The intended purpose of the present studies was to develop conversion factors in the human pancreas. A limitation in this respect is that the studies were performed in rat, rather than human, islets. Although we performed some pilot studies with human islets, they were unsuitable for the present purpose for several reasons. 1) β-Cell replication was almost never detected in human islets, even in culture on a cell matrix. Although β-cell replication is detectable in human infants, donor islets were available only from adults. 2) In contrast to rodent islets, which are obtained immediately after death of a previously healthy animal, human islets for research are obtained from pancreases procured from terminally ill brain-dead donors after removal of multiple organs (heart, lungs, liver, and kidneys) for transplantation. Moreover, the isolated islets are then typically shipped overnight before use. Perhaps not surprisingly, these human donor islets typically have high rates of β-cell apoptosis and often a relatively small proportion of β-cells vs. non-β-cells. Therefore, we concluded from pilot studies that human islets were not suitable for the replication or apoptosis TLVM studies planned here. Moreover, we chose to use juvenile rat islets after we established that the rate of β-cell replication from islets obtained during this period of postnatal expansion was sufficiently high to be readily detected, in contrast to islets from adult rats. It is of interest that the frequency of replication by Ki67 and apoptosis by TUNEL staining in these islets closely approximates that in human pancreas obtained during postnatal expansion (20, 23) as well as rodent pancreas procured at the same age (25, 27). Although we corroborated the findings for apoptosis in a cell line (INS-1 cells) and islets, obviously, the cell line cannot be used to establish a range of frequency of β-cell replication, since, by definition, all the cells in the cell line are in the cell cycle.
In summary, we established the conversion factors from frequency of Ki67 and TUNEL labeling (%) to actual event rate (%events/h) in β-cells. Since Ki67 and TUNEL labeling are widely used to assess β-cell replication and apoptosis in various species, including humans, we believe that these conversion factors will be helpful in development of models to quantify β-cell turnover in health and diabetes.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-077967, Juvenile Diabetes Research Foundation Grant 7-2005-1152, the Larry L. Hillblom Foundation, and the Manpei Suzuki Diabetes Foundation.
Acknowledgments
We thank Ryan Galasso and Heather Gerber for technical assistance and our colleagues in the Larry Hillblom Islet Research Center at UCLA for excellent suggestions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Rubeai M, Fussenegger M (Editors). Cell Engineering. Apoptosis. Dordrecht: Kluwer Academic Publishers, 2004, vol. 4.
- 2.Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest 94: 2105–2116, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol 5: e163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 37: 232–236, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 50: 2323–2331, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 3: 758–768, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Coles HS, Burne JF, Raff MC. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 118: 777–784, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Collins RJ, Harmon BV, Gobe GC, Kerr JF. Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. Int J Radiat Biol 61: 451–453, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Dean PG, Kudva YC, Stegall MD. Long-term benefits of pancreas transplantation. Curr Opin Organ Transplant 13: 85–90, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117: 971–977, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44: 2115–2133, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol 7: 663–698, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44: 249–256, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Foa P, Maiolo AT, Lombardi L, Toivonen H, Rytomaa T, Polli EE. Growth pattern of the human promyelocytic leukaemia cell line HL60. Cell Tissue Kinetics 15: 399–404, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Forrester HB, Albright N, Ling CC, Dewey WC. Computerized video time-lapse analysis of apoptosis of REC:Myc cells X-irradiated in different phases of the cell cycle. Radiat Res 154: 625–639, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 71: 219–225, 1994 [PubMed] [Google Scholar]
- 19.Kalashnik L, Bridgeman CJ, King AR, Francis SE, Mikhalovsky S, Wallis C, Denyer SP, Crossman D, Faragher RG. A cell kinetic analysis of human umbilical vein endothelial cells. Mech Ageing Dev 120: 23–32, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49: 1325–1333, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Krystek M, Anton M. A weighted total least-squares algorithm for fitting a straight line. Meas Sci Technol 18: 3438–3442, 2007 [Google Scholar]
- 22.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48: 2221–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57: 1584–1594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier JJ, Ritzel RA, Maedler K, Gurlo T, Butler PC. Increased vulnerability of newly forming beta cells to cytokine-induced cell death. Diabetologia 49: 83–89, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes 49: 1341–1346, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Nath DS, Gruessner AC, Kandaswamy R, Gruessner RW, Sutherland DE, Humar A. Outcomes of pancreas transplants for patients with type 2 diabetes mellitus. Clin Transplant 19: 792–797, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117: 2553–2561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry VH, Henderson Z, Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol 219: 356–368, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Potten C, Wilson J. Apoptosis: The Life and Death of Cells. New York: Cambridge University Press, 2004
- 30.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29: 717–718, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Ritzel RA, Butler PC. Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes 52: 1701–1708, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Ronot X, Hecquet C, Jaffray P, Guiguet M, Adolphe M, Fontagne J, Lechat P. Proliferation kinetics of rabbit articular chondrocytes in primary culture and at the first passage. Cell Tissue Kinetics 16: 531–537, 1983 [PubMed] [Google Scholar]
- 33.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes 57: 1575–1583, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Sutherland RL, Hall RE, Taylor IW. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res 43: 3998–4006, 1983 [PubMed] [Google Scholar]
- 35.Swenne I. Effects of aging on the regenerative capacity of the pancreatic B-cell of the rat. Diabetes 32: 14–19, 1983 [DOI] [PubMed] [Google Scholar]
- 36.Swenne I. The role of glucose in the in vitro regulation of cell cycle kinetics and proliferation of fetal pancreatic β-cells. Diabetes 31: 754–760, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817–826, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Topp BG, Atkinson LL, Finegood DT. β-Cell function and β-cell mass during the development of diabetes in fa/fa rats. Am J Physiol Endocrinol Metab 293: E1730–E1735, 2007 [DOI] [PubMed] [Google Scholar]
- 39.van Furth R, Elzenga-Claasen I, van Schadewijk-Nieuwstad M, Diesselhoff-den Dulk MM, Toivonen H, Rytomaa T. Cell kinetic analysis of a murine macrophage cell line. Eur J Cell Biol 44: 93–96, 1987 [PubMed] [Google Scholar]
- 40.Wang RN, Bouwens L, Kloppel G. Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia 37: 1088–1096, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Woo KB, Funkhouser WK, Sullivan C, Alabaster O. Analysis of the proliferation kinetics of Burkitt's lymphoma cells. Cell Tissue Kinetics 13: 591–604, 1980 [DOI] [PubMed] [Google Scholar]


