Abstract
Human carcinogen 1,3-butadiene (BD) undergoes metabolic activation to 3,4-epoxy-1-butene (EB), hydroxymethylvinyl ketone (HMVK), 3,4-epoxy-1,2-butanediol (EBD) and 1,2,3,4-diepoxybutane (DEB). Among these, DEB is by far the most genotoxic metabolite and is considered the ultimate carcinogenic species of BD. We have shown previously that BD-exposed laboratory mice form 8- to 10-fold more DEB–DNA adducts than rats exposed at the same conditions, which may be responsible for the enhanced sensitivity of mice to BD-mediated cancer. In the present study, we have identified 1,4-bis-(N-acetyl-l-cystein-S-yl)butane-2,3-diol (bis-BDMA) as a novel DEB-specific urinary biomarker. Isotope dilution high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry was employed to quantify bis-BDMA and three other BD-mercapturic acids, 2-(N-acetyl-l-cystein-S-yl)-1-hydroxybut-3-ene/1-(N-acetyl-l-cystein-S-yl)-2-hydroxy-but-3-ene (MHBMA, from EB), 4-(N-acetyl-l-cystein-S-yl)-1,2-dihydroxybutane (DHBMA, from HMVK) and 4-(N-acetyl-l-cystein-S-yl)-1,2,3-trihydroxybutane (THBMA, from EBD), in urine of confirmed smokers, occupationally exposed workers and BD-exposed laboratory rats. Bis-BDMA was formed in a dose-dependent manner in urine of rats exposed to 0–200 p.p.m. BD by inhalation, although it was a minor metabolite (1%) as compared with DHBMA (47%) and THBMA (37%). In humans, DHBMA was the most abundant BD-mercapturic acid excreted (93%), followed by THBMA (5%) and MHBMA (2%), whereas no bis-BDMA was detected. These results reveal significant differences in metabolism of BD between rats and humans.
Introduction
1,3-Butadiene (BD) is a high volume industrial chemical extensively used in the production of synthetic rubber, resins and polymers (1,2). BD is also an environmental chemical present in automobile exhaust, automotive fuel, forest fires and cigarette smoke (1,2). C57BL/6 × C3H F1 mice exposed to 6.25–200 p.p.m. of BD by inhalation developed lymphocytic lymphoma and tumors of the heart, lung, forestomach, Harderian gland, preputial gland, liver, mammary gland and the ovary (3). Sprague-Dawley rats exposed to 1000–8000 p.p.m. BD exhibited neoplasms in the mammary gland, brain, Zymbal gland, uterus, pancreas, testis and thyroid gland (4). Occupational exposure of humans to BD is associated with an increased risk of leukemia, lymphatic and hematopoietic cancer (5,6). Based on the above evidence, BD has been classified as a known human carcinogen in the Twelfth Annual Report on Carcinogens published by the National Toxicology Program (2011) (1).
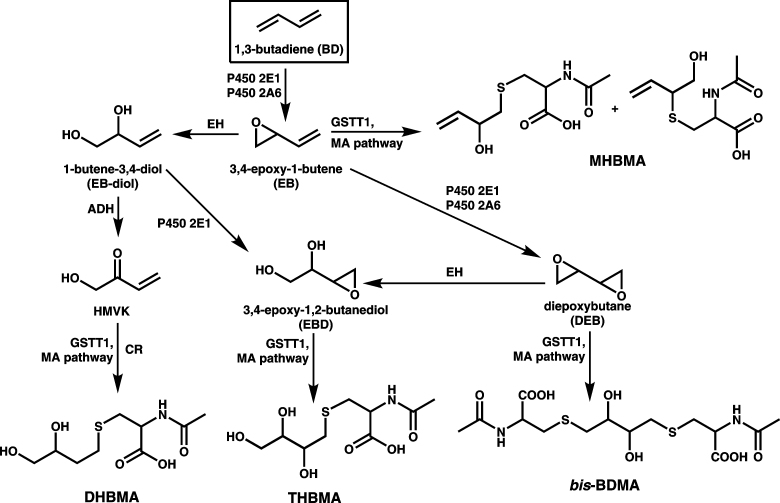
Although the exact mechanisms of BD-mediated cancer are unknown, metabolic activation of BD to DNA-reactive intermediates is required for its genotoxic and carcinogenic activity (7). BD is initially metabolized to (R,S)-3,4-epoxy-1-butene (EB), which can be further oxidized to form 1,2,3,4-diepoxybutane (DEB) or hydrolyzed to 1-butene-3,4-diol (EB-diol) (Scheme 1) (2). EB-diol can in turn undergo CYP450 2E1-mediated oxidation to 3,4-epoxy-1,2-butanediol (EBD) (2) or alcohol dehydrogenase-mediated conversion to hydroxymethylvinyl ketone (HMVK) (8) (Scheme 1). Although all BD-derived epoxides are direct mutagens (2), DEB is 30-fold more genotoxic than EB and 100-fold more mutagenic than EB-diol (9), probably due to its bifunctional nature that enables DEB to cross-link cellular biomolecules. Epoxide hydrolase-mediated hydrolysis appears to be the predominant pathway of metabolic deactivation of EB, DEB and EBD in rats and humans (2,10).
Scheme 1.
Metabolic pathways of 1,3-butadiene and the formation of urinary mercapturic acids, MHBMA, DHBMA, THBMA and bis-BDMA.
If not inactivated, BD-derived electrophilic metabolites can modify DNA nucleobases to give a wide range of adducts. EB-mediated alkylation generates N7-(2-hydroxy-3-buten-1-yl)guanine (EB-Gua I), N7-(1-hydroxy-3-buten-2-yl)guanine (EB-Gua II), N3-(2-hydroxy-3-buten-1-yl)adenine (EB-Ade I) and N3-(1-hydroxy-3-buten-2-yl) (EB-Ade II) (7). DNA alkylation by DEB initially produces 2-hydroxy-3,4-epoxybut-1-yl monoadducts (11,12), which can be hydrolyzed to the corresponding N7-(2,3,4-trihydroxy-3-buten-2-yl) lesions or can further react with another nucleophilic site of DNA to form exocyclic deoxyadenosine lesions such as 1,N 6-(1-hydroxymethyl-2-hydroxypropan-1,3-diyl)-2′-deoxyadenosine (13,14), DNA–DNA cross-links (15–18) and DNA–protein cross-links (19,20).
Previous studies in laboratory animals have revealed significant species and gender differences in susceptibility to BD, which have been attributed to differences in metabolism. Specifically, laboratory mice developed tumors at ~200-fold lower BD concentrations than rats (3,4), supposedly a result of higher BD bioactivation/detoxification ratio in the mouse (21,22). Epoxide hydrolase-mediated hydrolysis of DEB was more efficient in human liver microsomes than in rat microsomes, and negligible in mouse microsomes (22). Female mice were more susceptible to BD-mediated cancer than males exposed at the same conditions (3) and exhibited greater numbers of DNA–DNA cross-links than males (23). In contrast, hemoglobin adduct measurements in BD-exposed Czech workers suggested that females absorbed or metabolized BD to a lesser extent than males (24). If present, any interspecies and gender differences in metabolism of BD are critically important for human cancer risk assessment.
Urinary metabolites can provide a sensitive, non-invasive measure of carcinogen metabolism to the ultimate tumorigenic species. As shown in Scheme 1, EB, HMVK and EBD are excreted in urine as 2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene/1-(N-acetyl-L-cystein-S-yl)-2-hydroxy-but-3-ene (MHBMA), 4-(N-acetyl-L-cystein-S-yl)-1,2-dihydroxybutane (DHBMA) and 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane (THBMA), respectively (25–31). By analogy, DEB is expected to be conjugated with two molecules of glutathione and be excreted in urine as the corresponding bis-mercapturic acid, 1,4-bis-(N-acetyl-l-cystein-S-yl)butane-2,3-diol (bis-BDMA, Scheme 1). However, to our knowledge, such bis-conjugates had not been previously reported. We hypothesized that bis-BDMA, if formed in vivo, can serve as a non-invasive biomarker of BD metabolism to its ultimate carcinogenic species, DEB. To test this hypothesis, we prepared synthetic bis-BDMA and developed a sensitive isotope dilution high-performance liquid chromatography (HPLC)-ESI−-MS/MS method for its analysis following in vivo formation. The new methodology was used to quantify bis-BDMA in urine of F344 rats exposed to 0–200 p.p.m. BD, workers occupationally exposed to BD, and confirmed smokers. Other BD-mercapturic acids (MHBMA, DHBMA and THBMA) were also measured to obtain a complete picture of BD metabolism. Finally, we have employed nanoHPLC-NSI+-MS/MS to detect DEB-induced DNA–DNA cross-links in liver tissues of BD-treated rats. Our results reveal important interspecies differences in the metabolic pathways of BD.
Materials and methods
Materials
N-acetyl-l-cysteine and HPLC-mass spectrometry (MS) grade formic acid were purchased from Sigma–Aldrich (St Louis, MO). HPLC-MS grade methanol and acetonitrile were obtained from Fisher Scientific (Pittsburgh, PA). All other chemicals and reagents were from Sigma–Aldrich. Isolute ENV+ 50mg/1 ml solid phase extraction (SPE) cartridges were purchased from Biotage (Charlotte, NC). R, R-Diepoxybutane was synthesized as reported previously (17). MHBMA, DHBMA, 2H6 -MHBMA, 2H7 -DHBMA and 2H3-N-acetyl-l-cysteine were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). THBMA and 2H3 -THBMA standards were available from a previous study (31). Bis-BDMA and 2H6 -bis-BDMA were synthesized in our laboratory as described below.
Synthesis of bis-BDMA and its deuterated analog (2H6-bis-BDMA)
N-acetyl-l-cysteine (280 mg, 1.70 mmol) was dissolved in 8 ml of water, and the pH of the solution was adjusted to 10 with 1N NaOH. R, R-Diepoxybutane (17) (75mg, 0.85 mmol) was added, and the reaction mixture was stirred at room temperature for 4 h. At the end of the reaction, sodium ions were removed by the addition of Bio-Rad AG 50W-X8 cation exchange resin, followed by filtration. Bis-BDMA was isolated by semi-preparative HPLC using an Agilent 1100 HPLC system interfaced to a DAD UV detector (Agilent Technologies, Santa Clara, CA). HPLC separation was achieved with a Synergi Hydro RP column (250 × 10.00mm; 4 μ) (Phenomenex, Torrance, CA) using isocratic elution with 6% acetonitrile in 0.1% trifluoroacetic acid/water. Under these conditions, bis-BDMA eluted as sharp peak at 19.6min. HPLC fractions containing bis-BDMA (19.0–20.2min) were manually collected and concentrated under vacuum to yield a white powder. 2H6-bis-BDMA was similarly synthesized starting with 2H3-N-acetyl-l-cysteine (Supplementary Scheme S1, available at Carcinogenesis Online).
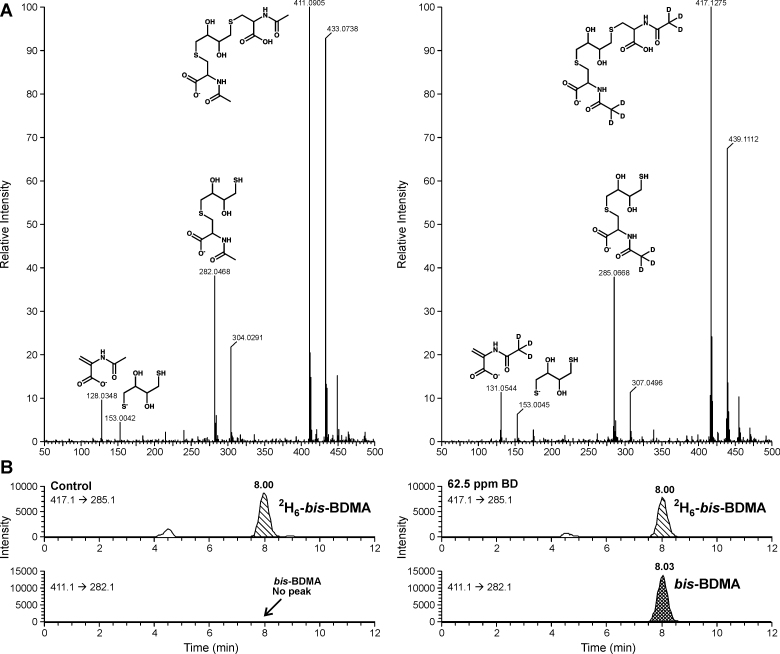
Bis-BDMA and 2H6-bis-BDMA were structurally characterized by nuclear magnetic resonance (NMR) and high resolution mass spectrometry. 1H NMR of bis-BDMA (Supplementary Figure S1, available at Carcinogenesis Online): δ 8.3 (2H, s, cys-NH), 4.4 (2H, m, αC-cys), 3.7 (2H, s, internal OH), 3.6 (2H, m, CHOH), 3.0-2.8 (4H, m, βC-cys), 2.7-2.5 (4H, m, SCH2), 1.9 (6H, s, COCH3) (Supplementary Figure S1, available at Carcinogenesis Online). High resolution mass spectrometry results (Figure 1A): bis-BDMA (ESI−-MS: 411.0905 [M-H]−, MS/MS: 282.0468, 153.0042 and 128.0348); 2H6-bis-BDMA (ESI−-MS: 417.1275 [M-H]−, MS/MS: 285.0668, 153.0045 and 131.0544). Stock solutions of bis-BDMA and 2H6-bis-BDMA were prepared in water and stored at −20°C.
Fig. 1.
Full scan accurate mass ESI−-QTOF spectrum of bis-BDMA and 2H6-bis-BDMA obtained on a Waters Acquity UPLC/Synapt G2 QTOF mass spectrometer (A) and HPLC-ESI−-MS/MS detection of bis-BDMA in urine of F344 rats exposed to 0 p.p.m. of BD and 62.5 p.p.m. of BD (B).
Animals and treatment
All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee. Male and female F344 rats (aged 27–29 days) were purchased from Charles River Breeding Laboratories (Raleigh, NC) and housed in cages with animal chow and water in temperature- and humidity-controlled rooms with a 12 h light/dark cycle (NIH Publication 86-23, 1985) at the Environmental Exposure and Inhalation Health Facility (at the University of Texas Medical Branch at Galveston). Following a 7 day acclimation period, the animals were randomly separated into air control and exposure groups. Rats were exposed whole body to nominal 62.5 or 200 p.p.m. BD in air for 2 weeks (6h/day, 5 days/week) in Hinnar exposure chambers. Rats in the third chamber received filtered air only as a control group. Stock BD (99+ %; from Air Gas Southwest, Texas City, TX) was metered using mass flow valve, diluted with filtered room air and pumped into the exposure chambers. BD concentrations in exposure chambers were determined by gas chromatography using a Shimadzu GC-17A gas chromatograph equipped with a DB-1 GC column and an FID detector. Fresh animal chow and water were provided during the exposure period. Following exposures, the leftover food and water were discarded, and the animals were returned to their appropriate housing box until the next exposure.
To enable urine collection from BD-exposed animals, groups of female and male rats (n = 4 per exposure level and sex) were randomly selected and transferred from exposure chambers into metabolic cages at the end of the 7th or the 8th day of BD exposure. Rats were held in metabolic cages, with access to food and water, until the next exposure period, when they were returned to Hinnar exposure chambers. Urine excreted by individual rats during overnight housing in metabolic cages was placed in Eppendorf tubes, snap frozen, and stored at −80°C. Water and food were provided in a separate area to prevent the introduction of drinking water into urine collection tubes.
At the end of the final exposure period, animals were euthanized via cardiac puncture. Liver and lung tissues were collected, flash frozen in liquid nitrogen, and stored at −80°C. Frozen urine and liver tissue samples were shipped on dry ice to the University of Minnesota, where they were stored at −80°C until analysis.
Human subjects
For the occupational exposure study, 72 subjects working in a BD production facility were included (Supplementary Table S1, available at Carcinogenesis Online). Of these, 40 were controls (administrative workers) and 32 were BD production workers. BD exposures were determined by personal monitoring tubes for 8h work shifts on 10 separate occasions over a 4 month interval for each study subject. The details of the occupational exposure study have been reported previously (32). The experimental protocol was approved by the Institutional Review Boards at Regional Institute of Hygiene of Central Bohemia and the University of Vermont. Archived urine samples were shipped to the University of Minnesota on dry ice and stored at −80°C.
Smoker urine samples (n = 36, overnight or 24 h urine collection, Supplementary Table S1, available at Carcinogenesis Online) were obtained from the University of Minnesota Tobacco Research Programs and the University of Hawaii Cancer Center. Smoking status was confirmed by cotinine analysis.
Quantitation of bis-BDMA
Rat or human urine samples (100 μl) were vortexed and mixed with 100 μl of 50mM ammonium formate buffer (pH 2.5) and 10 μl of formic acid. 2H6-bis-BDMA internal standard (60ng) was added, and the samples were mixed and centrifuged at 13 000 r.p.m. for 15 min. The supernatant was loaded onto Isolute ENV+ cartridges (1ml/50mg) preconditioned with methanol (3ml) and 0.3% formic acid (3ml). The cartridges were washed with 1.5 ml of 0.3% formic acid, followed by 0.75 ml of 5% methanol in 0.3% formic acid and dried under vacuum for 20min. Bis-BDMA and its internal standard were eluted with 1.2 ml of 2% formic acid in methanol. SPE eluates were concentrated under vacuum and reconstituted in 30 μl of water. Typically, 3 μl of this solution was injected onto the HPLC column for HPLC-ESI−-MS/MS analysis.
HPLC-ESI−-MS/MS analysis of bis-BDMA was conducted with an Agilent 1100 HPLC system (Agilent Technologies) coupled with a Thermo-Finnigan TSQ Quantum Discovery mass spectrometer (Thermo Scientific Corp., Waltham, MA). HPLC separations were carried out on a SIELC Primesep D column (2.1 × 100mm, 5 μm particle size) equipped with a guard column (Primesep D; 2.1 × 10mm). The column was maintained at 50°C and eluted with a gradient of water (solvent A) and 1% formic acid in 50% acetonitrile (solvent B), at a flow rate of 200 μl/min. Gradient program was as follows (time, % of solvent B): 0–8min, 40 to 48% B; 8–10min, 48 to 75% B; 10–13min, isocratic at 75% B; 13–15min, 75 to 40% B; 15–25min, final equilibration at 40% B. The HPLC eluent was directed into the MS detector during 4.5–10min of the chromatographic run.
The TSQ Vantage triple quadrupole instrument (Thermo Scientific), was operated in the negative electrospray ionization (ESI−) mode. Typical MS parameters were as follows: spray voltage, −3500V; sheath gas pressure, 50 psi; capillary temperature, 250°C; collision energy, 16; source CID, −9V; collision gas pressure, 1.0 mTorr; Q1 (full width at half maximum), 0.4; Q3 (full width at half maximum), 0.7; scan width, 0.4 m/z and scan time, 0.4 s. The MS parameters were optimized by direct infusion of authentic standards and may differ between runs. The mass spectrometer was operated in the selected reaction monitoring (SRM) mode. The SRM transitions used for quantification of bis-BDMA were: m/z 411.1 → 282.1 (bis-BDMA) and m/z 417.1 → 285.1 (2H6 -bis-BDMA internal standard). For confirmation purposes, additional SRM transitions were also monitored (m/z 411.1 → 153.1, 411.1 → 128.1 for bis-BDMA and m/z 417.1 → 153.1, 417.1 → 131.1 for 2H6 -bis-BDMA). HPLC-ESI−-MS/MS methodology was fully validated by analyzing control rat urine spiked with known amounts of bis-BDMA and 2H6 -bis-BDMA. Method limit of detection (LOD), limit of quantification, precision, accuracy, SPE recovery, and matrix effects were determined as described in the Supplementary Materials and methods, available at Carcinogenesis Online.
Quantitative analysis of MHBMA, DHBMA and THBMA
Rat or human urine samples (200 μl) were spiked with 60ng each of 2H6 -MHBMA and 2H7 -DHBMA and processed by SPE on Waters Oasis HLB cartridges. The SPE eluates were dried, reconstituted in 30 μl of 0.1% formic acid and analyzed by HPLC-ESI−-MS/MS using a Pursuit 3 Diphenyl column (2.1 × 150mm, 3 µm) eluted with a gradient of 0.1% formic acid and acetonitrile. For quantitative analysis of THBMA, urine aliquots (100 μl) were spiked with 2H3 -THBMA internal standard, and, processed by Isolute ENV+ SPE as described previously (31). The HPLC-ESI−-MS/MS conditions for analysis of THBMA were same as described above for bis-BDMA, except that the gradient program was modified as follows (time, % of solvent B): 0–6min, 15 to 21% B; 6–9min, 21 to 75% B; 9–12min, isocratic at 75% B; 12–15min, 75 to 15% B; 13–25min, finally maintained at 15% B.
Determination of bis-N7G-BD adducts in rat liver DNA
NanoHPLC-NSI+-MS/MS methodology developed in our laboratory (18) was used to quantify DEB-induced bis-N7G-BD cross-links in liver DNA of rats exposed to 0, 62.5 or 200 p.p.m. BD by inhalation. In brief, DNA was extracted using NucleoBond AXG500 anion exchange cartridges (Machery-Nagel, Düren, Germany). DNA concentrations were determined by HPLC-UV analysis of dG in enzymatic digests (18). DNA (100 μg) was spiked with 50fmol of 15N10-bis-N7G-BD (internal standard for quantitation) and subjected to neutral thermal hydrolysis at 70°C for 1h to release N7-guanine adducts as free bases. Partially depurinated DNA was removed by ultrafiltration. Bis-N7G-BD and its internal standard were enriched by offline HPLC and reconstituted in 0.01% aqueous acetic acid. Samples (8 μl) were injected onto a trap column connected to a manually packed Zorbax SB-C18 nano HPLC column (75 μm × 200mm, 5 μ), which was eluted with 0.01% acetic acid in 1:1 methanol:acetonitrile. Bis-N7G-BD cross-links were quantified by isotope dilution nanoHPLC-NSI+-MS/MS. The SRM transitions for bis-N7G-BD and 15N10-bis-N7G-BD internal standard were m/z 389.1 [M + H]+ → m/z 238.1 [M + H – Gua]+ and m/z 399.1 [15N10-M+H]+ → m/z 243.1 [M + H − [15N5]Gua]+, respectively. Accurate quantification of bis-N7G-BD was achieved by comparing the areas of nanoHPLC-NSI+-MS/MS peaks corresponding to the analyte and its internal standard using standard curves (18).
Results
Development of HPLC-MS/MS methodology for bis-BDMA
It has been proposed that the susceptibility of a given organism toward BD-induced cancer is determined by the extent of metabolic activation of BD to its ultimate carcinogenic metabolite, DEB (21). In support of this notion, we have previously reported that DNA of laboratory mice (more sensitive species) contained 5- to 10-fold greater numbers of DEB-specific bis-N7G-BD adducts than rats (less sensitive species) exposed at the same conditions (23). However, human tissue and blood samples are not readily available for biomarker analysis. We therefore examined the feasibility of using urinary bis-BDMA as a novel, non-invasive biomarker of BD bioactivation to DEB.
Authentic standards of bis-BDMA and its deuterated analog were prepared by reacting DEB with N-acetyl-l-cysteine and 2H3-N-acetyl-l-cysteine, respectively (Supplementary Scheme S1, available at Carcinogenesis Online). Both standards were characterized by proton NMR (Supplementary Figure S1, available at Carcinogenesis Online) and high resolution mass spectrometry (Figure 1A) and were used for the development of an isotope dilution HPLC-ESI-MS/MS method for bis-BDMA in urine. In our approach, bis-BDMA is isolated by SPE and quantified by HPLC-ESI−-MS/MS using isotope dilution with the corresponding deuterated internal standard (2H6 -bis-BDMA).
Our HPLC-ESI−-MS/MS method for bis-BDMA is based on selected reaction monitoring of MS/MS transitions corresponding to the C-S bond cleavage (m/z 411.1 → 282.1 for the analyte and m/z 417.1 → 285.1 for the deuterated internal standard, respectively, Figure 1B). Analysis of standard solutions containing fixed amounts of 2H6-bis-BDMA internal standard and increasing amounts of bis-BDMA confirmed that HPLC-ESI−-MS/MS responses were linear between 0.05–160ng of bis-BDMA (on column) (Supplementary Figure S2A, available at Carcinogenesis Online). Further method validation was conducted by spiking control rat urine (100 μl) with 2H6 -bis-BDMA (60ng) and bis-BDMA (0.5–1600ng), followed by SPE and HPLC-ESI−-MS/MS analysis. An excellent correlation was observed between the measured and the expected concentrations of bis-BDMA (Supplementary Figure S2B, available at Carcinogenesis Online) (R 2 = 0.9999). HPLC-ESI−-MS/MS LOD and limit of quantification for bis-BDMA in human urine were determined to be 1 and 5ng/ml, respectively (Supplementary Table S2, available at Carcinogenesis Online). The intraday and interday precision (% relative standard deviation) of our analytical method determined by repeated analysis of bis-BDMA (100ng) spiked into control rat urine sample (100 µl) were 0.88 and 1.17%, respectively. Comparison of HPLC-ESI−-MS/MS peak areas in the presence and in the absence of rat urine matrix (post-SPE) revealed a minimal signal suppression of the analyte by the sample matrix (<2%). Method accuracy was determined to be 96.2–100.5%. Freeze-thaw stability studies of bis-BDMA in a non-smoker urine have revealed minimal analyte losses over time. Complete HPLC-ESI−-MS/MS method validation parameters are compiled in Supplementary Table S2, available at Carcinogenesis Online.
Quantification of BD-mercapturic acids in urine of F344 rats exposed to BD by inhalation
The validated HPLC-ESI−-MS/MS method was used to quantify bis-BDMA in urine of F344 rats (n = 4 per exposure level and sex) treated with 0, 62.5 or 200 p.p.m. BD by inhalation for 2 weeks (6h/day, 5 days/week). The concentrations of other BD-mercapturic acids (MHBMA, DHBMA and THBMA, Scheme 1) were also determined in order to obtain a complete picture of BD metabolism in the rat. A concentration-dependent increase in bis-BDMA amounts was observed in groups exposed to 62.5 and 200 p.p.m. BD (right panel in Figure 1B, Figure 2A), whereas no bis-BDMA was detected in urine of control rats exposed to filtered air (left panel in Figure 1B, Figure 2A). The identity of bis-BDMA peak observed for exposed rat urine samples was further confirmed by examining two additional MS/MS transitions (Supplementary Figure S3, available at Carcinogenesis Online) and by reanalyzing the same samples on a different HPLC column (Synergi Hydro-RP, data not shown). Authentic bis-BDMA standard and the analyte detected in rat urine exhibited identical MS/MS spectra and HPLC retention times (Supplementary Figure S3, available at Carcinogenesis Online).
Fig. 2.
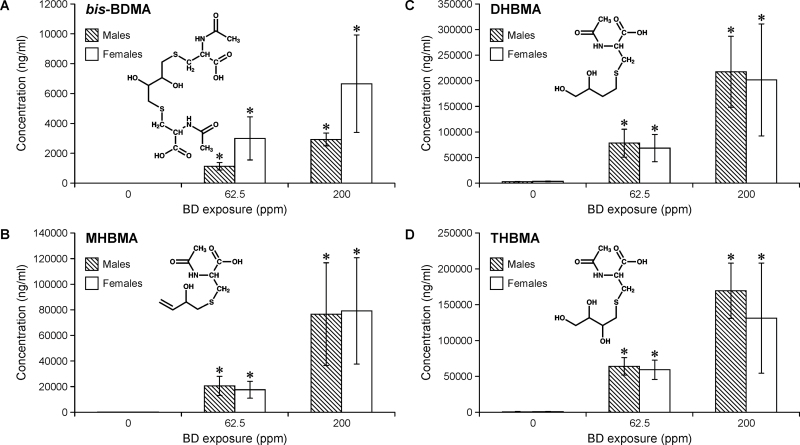
Concentrations of bis-BDMA (A), MHBMA (B), DHBMA (C) and THBMA (D) in urine of F344 rats exposed to 0, 62.5 or 200 p.p.m. BD by inhalation for 2 weeks (6h/day, 5 days/week). Error bars represent standard deviation values obtained upon analysis of quadruplicates. Statistical significance is represented for comparisons between exposed and control samples (P < 0.05). The differences between urinary BD-mercapturic acid concentrations in male and female rats were not statistically significant.
Mean concentrations of bis-BDMA in rat urine following exposure to 200 p.p.m. BD were 4.8±2.9 μg/ml, whereas the corresponding amounts of MHBMA, DHBMA and THBMA in the same samples were 77.9±37.8, 209.5±85.1 and 150.3±59.9 μg/ml, respectively (Figure 2). Urinary concentrations of BD-mercapturic acid correlated with BD exposure, with 2- to 3-fold higher amounts observed in 200 p.p.m. BD exposure group as compared with 62.5 p.p.m. exposure group (Figure 2). Urine samples from unexposed rats contained background levels of MHBMA (68.5±17.1ng/ml), DHBMA (3192.7±533.2ng/ml) and THBMA (561.7±81.7ng/ml), consistent with previous studies that have observed these metabolites in unexposed animals and human subjects due to an unidentified endogenous source (25,27,31). In contrast, no bis-BDMA was detected in unexposed animals (Figure 1B, left panel and Figure 2A).
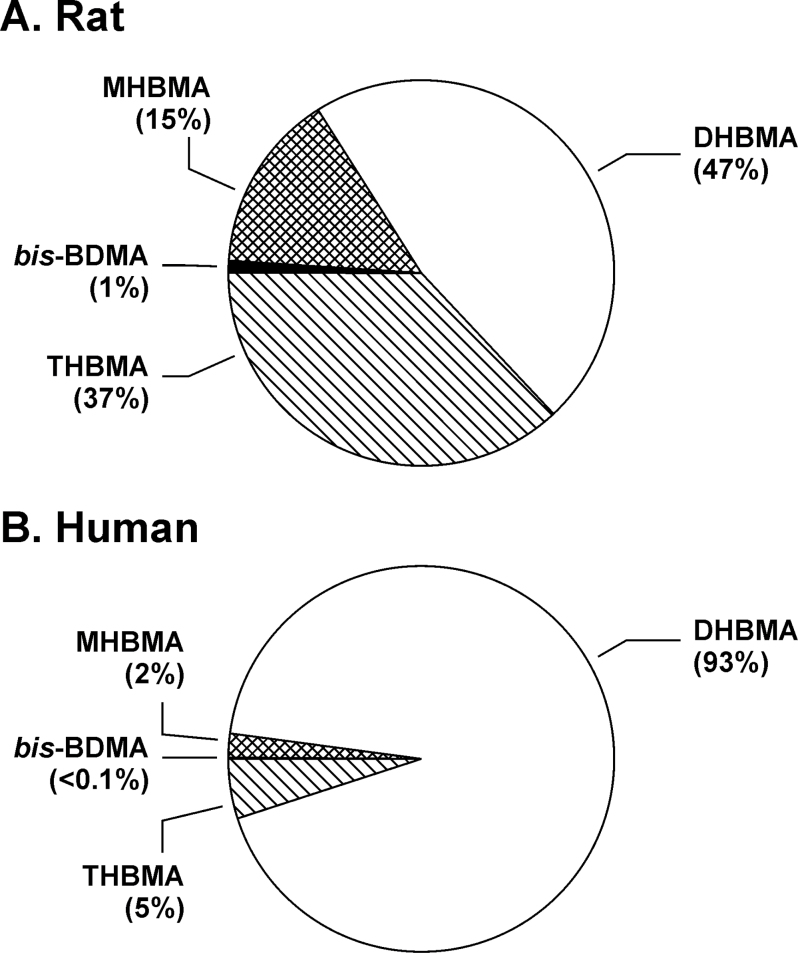
Urinary concentrations of bis-BDMA were 2- to 3-fold higher in female rats as compared with the male animals exposed under the same conditions, although the difference was not statistically significant (Figure 2A, P = 0.06). In contrast, female rats excreted slightly lower levels of MHBMA, DHBMA and THBMA as compared with males (P > 0.3, Figure 2C and D). Although these results are suggestive of gender differences in BD metabolism, further studies with greater numbers of animals are needed to confirm these initial observations. The relative molar concentrations of BD-mercapturic acids in urine of BD-exposed rats were in the order: DHBMA (47%) > THBMA (37%) > MHBMA (15%) > bis-BDMA (1%) (Figure 3A). These results are consistent with previous studies that examined BD-induced hemoglobin adducts in blood of rats exposed by inhalation (33). Additionally, the relative contribution of bis-BDMA was higher in females (1.6–2%) as compared with males (0.6–0.7%) (Supplementary Table S3, available at Carcinogenesis Online).
Fig. 3.
Relative contributions of individual mercapturic acids to total amounts of BD-mercapturic acids in urine of F344 rats (n = 16) exposed to 62.5 or 200 p.p.m. BD (A) and smokers (n = 36) (B).
Quantification of BD-mercapturic acids in urine of smokers and occupationally exposed workers
To examine the possibility of bis-BDMA formation in humans, two populations chronically exposed to BD were examined: smokers and occupationally exposed individuals (Supplementary Table S1, available at Carcinogenesis Online). Workers at a BD polymer production plant (n = 32) were exposed to 0.1–2 p.p.m. BD, whereas the corresponding controls (office workers, n = 40) were exposed to <0.02 p.p.m. BD (Supplementary Table S1, available at Carcinogenesis Online). Current smokers (n = 36, 20±7 cigarettes per day) were exposed to BD due to its presence in cigarette smoke (~46 μg/cigarette in mainstream smoke) (34). For comparison, the other three urinary BD-mercapturic acids (MHBMA, DHBMA, THBMA) were also analyzed.
Mean urinary concentrations of MHBMA, DHBMA and THBMA in exposed workers were 82±138, 3094±4162 and 157±158ng/mg creatinine, respectively. In smokers, the corresponding concentrations were 11±12, 631±452 and 31±20ng/mg creatinine, respectively. DHBMA was the major BD metabolite excreted in human urine (93%), followed by THBMA (5%) and MHBMA (2%) (Figure 3B). In contrast, the concentrations of bis-BDMA in both groups were below the LOD of our method (1ng/ml) (Supplementary Figure S4A, available at Carcinogenesis Online). Bis-BDMA was not detectable even when a more sensitive capillary HPLC-ESI-MS/MS method was employed (LOD, 0.1ng/ml), (Supplementary Figure S4B, available at Carcinogenesis Online) or when larger urine volumes were analyzed (up to 1ml, results not shown). These results are consistent with previous studies of BD metabolism in liver microsomes and BD–hemoglobin adduct measurements, which suggested that BD to DEB conversion is less efficient in humans than in laboratory rats and mice (7,22,35).
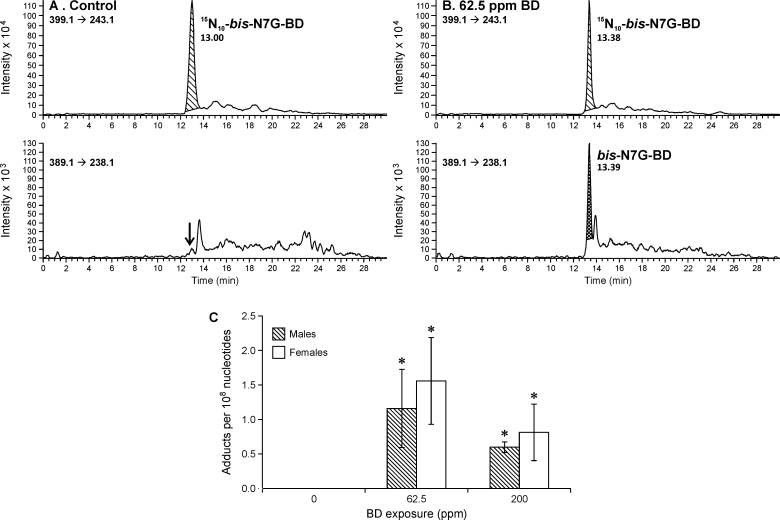
Dose–response studies of DEB-derived bis-N7G-BD adducts in rat liver DNA
In order to examine the correlation between urinary BD-mercapturic acids and BD–DNA adduct concentrations in tissues, DEB-specific bis-N7G-BD adducts were quantified in liver DNA of laboratory rats exposed to BD by inhalation. NanoHPLC-nanoNSI+-MS/MS methodology recently reported by our group (18) was employed in these studies. As expected, no bis-N7G-BD was detected in control animals (Figure 4A), whereas BD-exposed animals contained significant amounts of DEB-induced bis-N7G-BD cross-links (Figure 4B). Interestingly, dose–response relationships for bis-N7G-BD adducts in rat liver were not linear, with higher adduct numbers observed following exposure to 62.5 p.p.m. BD as compared with 200 p.p.m. BD treatment (Figure 4C). As was the case with bis-BDMA, female rats produced higher numbers of bis-N7G-BD adducts (1.6 adducts/108 nucleotides) as compared with males (1.2 adducts/108 nucleotides), although this difference was not statistically significant due to the small group size (Figure 4).
Fig. 4.
Representative traces for nanoHPLC-NSI+-MS/MS analysis of bis-N7G-BD adduct in liver DNA of F344 rats exposed to 0 p.p.m. of BD (A) and 62.5 p.p.m. of BD (B). Concentrations of bis-N7G-BD adducts in liver tissues of F344 rats (C) exposed to 0, 62.5 or 200 p.p.m. BD by inhalation for 2 weeks (6h/day, 5 days/week). Error bars represent standard deviation values obtained upon analysis of quadruplicate samples. Statistical significance is represented for comparisons between exposed and control samples (P < 0.05). The differences between bis-N7G-BD adduct concentrations in liver DNA of male and female rats were not statistically significant (P > 0.2).
Discussion
A potent human and animal carcinogen, BD is metabolically activated to four electrophilic species: EB, HMVK, EBD and DEB (Scheme 1) (2,8). Among these, DEB displays the greatest genotoxicity and is considered the ultimate carcinogenic metabolite of BD (2). DEB is 50-fold more effective at inducing sister chromatid exchanges and chromosomal aberrations in human lymphocytes than EB (36,37) and is two orders of magnitude more mutagenic than EB and EBD in TK6 lymphoblasts (9). More efficient DEB formation from BD in laboratory mice is thought to be responsible for their increased sensitivity to BD-induced cancer as compared with laboratory rats (23,38). Previous studies of DEB formation in humans are limited, although experiments with human liver microsomes suggest that any DEB produced in humans is rapidly detoxified via epoxide hydrolase-catalyzed hydrolysis (22).
Urinary mercapturic acids are commonly used as biomarkers of exposure to carcinogens and as a measure of their metabolic activation to DNA-reactive intermediates (39). In the present work, bis-BDMA was employed as a novel urinary biomarker for DEB formation from BD. To our knowledge, bis-BDMA has not been previously reported in vivo, although Boogaard et al. (40) detected the formation of a conjugation product of DEB with two molecules of glutathione following in vitro incubation of DEB with radiolabeled glutathione in cytosolic tissue fractions. Furthermore, analogous bis-glutathione conjugates have also been reported for hexachloro-1,3-butadiene (41), vinyledene chloride (42) and 1,2-dibromoethane (43). Such conjugates are formed via consecutive reactions of the two electrophilic groups with two molecules of glutathione. Although the first reaction is catalyzed by glutathione-S-transferases, the second conjugation step may be either spontaneous or enzymatic (43). The resulting bis-glutathion-S-yl conjugates can undergo further processing by glutamyltransferases, cysteinyl glycinases and acetylases in the kidney via the mercapturic acid pathway and are excreted in urine as the corresponding bis-N-acetylcysteine (mercapturic acid) conjugates. Interestingly, Cho and Guengerich have shown that glutathione–DEB monoconjugates are formed in vivo and are more mutagenic than DEB itself (44,45).
The availability of specific urinary biomarkers for DEB, EB, HMVK and EBD (bis-BDMA, MHBMA, DHBMA and THBMA, respectively, Scheme 1), has enabled us to evaluate the formation of all four electrophilic metabolites of BD in vivo (BD-exposed rats and humans exposed to BD in the workplace or via smoking). Although MHBMA, DHBMA and THBMA were observed in urine of both species, bis-BDMA was detected in BD-treated rats, but not in BD-exposed humans (Figure 3). This can be explained by more efficient bioactivation of BD to DEB or less efficient detoxification of DEB in the rat as compared with humans (22).
The relative molar concentrations of MHBMA, DHBMA and THBMA differed significantly between the two species. In humans, DHBMA accounted for 93% of total urinary BD-mercapturic acids (Figure 3B), whereas the other three BD-mercapturic acids were much less abundant, with THBMA at 5%, MHBMA at 2% and bis-BDMA at <0.1% (Figure 3B). In contrast, in the rat, DHBMA accounted for only 47% of metabolites, closely followed by THBMA (37%). In the latter species, MHBMA comprised for 15% and bis-BDMA for 1% of BD-mercapturic acids excreted (Figure 3A). Our results are in agreement with previous investigations of BD metabolism in rats and humans. For example, the concentrations of BD metabolites in blood of rats exposed to 62.5 or 200 p.p.m. BD followed the order: EB-diol (precursor of DHBMA) > EBD (precursor of THBMA) > EB (precursor of MHBMA) > DEB (precursor of bis-BDMA) (46). Furthermore, the relative abundance of the corresponding hemoglobin adducts in blood of BD-exposed rats was THB-Val (from EBD) > HB-Val (from EB) > Pyr-Val (from DEB) (7).
Background amounts of MHBMA (68.5±17.1ng/ml), DHBMA (3192.7±533.2ng/ml) and THBMA (561.7±81.7ng/ml) were observed in urine of control animals exposed to filtered air only. This finding is consistent with a previous report by McDonald et al. (25) who detected MHBMA and DHBMA in urine of control F344 rats (10 and 1500ng/ml, respectively). Georgieva et al. (33) also observed significant background levels of the corresponding BD-hemoglobin adducts (HB-Val and THB-Val) in unexposed rats (47), suggesting that there is a significant endogenous source of these metabolites in mammals. In our recent paper (31), we observed detectable amounts of THBMA in urine of non-smokers (16.3ng/ml), although smokers excreted higher amounts of this metabolite (30.7ng/ml). In contrast, DEB-specific biomarkers bis-N7G-BD (Figure 4) and Pyr-Val were not detected in control animals (33,47).
Although higher concentrations of DEB-derived bis-BDMA and bis-N7G-BD DNA–DNA cross-links were observed in female rats as compared with males (Figures 2A and 4C), these gender differences were not statistically significant. This is consistent with our earlier study, which found that bis-N7G-BD adducts were more abundant in female rats than in male rats following exposure to 625 p.p.m. BD (23). Furthermore, Boysen et al. (48) reported that the concentrations of DEB-specific hemoglobin adducts (pyr-Val) were 2- to 4-fold higher in female rats as compared with males following exposure to 1000 p.p.m. BD, although no gender differences were observed following 200 p.p.m. BD exposure (23,33). However, we also observed that the relative formation of bis-BDMA was slightly higher in female rats as compared with male rats (Supplementary Table S3, available at Carcinogenesis Online), in support of possible gender differences in BD metabolism. Increased formation of DEB in females may be responsible for their enhanced susceptibility toward BD-induced cancer as revealed in animal inhalation studies (3).
To evaluate the correlation between urinary metabolites of DEB and the amounts of diepoxide bound to genomic DNA, DEB-specific DNA–DNA cross-links (bis-N7G-BD) were quantified in liver DNA of the same BD-exposed rats (Figure 4). Unlike linear dose–response relationships observed for bis-BDMA, bis-N7G-BD concentrations followed a more complex dose–response relationship, with the highest adduct numbers observed at a medium exposure levels (62.5 p.p.m. BD, see Figure 4C). It has been previously hypothesized that the metabolic activation of EB to DEB in the rat is saturated at high BD concentrations (>62.5 p.p.m.) (23,49). Our data for urinary bis-BDMA in the same animals contradict this hypothesis, since urinary bis-BDMA concentrations continue to increase upon inhalation exposure to 62.5 and 200 p.p.m. BD (Figure 2A). Alternatively, our observation of decreased levels of bis-N7G-BD adducts in the high exposure group may be explained by BD-mediated epigenetic changes (50), which can lead to the induction of DNA damage response, cell cycle arrest, senescence and cell death (51,52). These possibilities warrant further investigation.
In summary, the current study for the first time establishes bis-BDMA as a novel urinary biomarker of metabolic bioactivation of BD to DEB. Unlike other BD-mercapturic acids, bis-BDMA is not present in urine of unexposed animals, suggesting that it is a specific biomarker of exposure to BD. Furthermore, bis-BDMA was detected in rats exposed to BD by inhalation, but not in occupationally exposed workers or smokers. Therefore, humans appear to be less efficient than rats in respect to metabolizing BD to DEB, revealing possible interspecies differences in metabolism of BD that may be highly relevant to human risk assessment.
Supplementary material
Supplementary Tables S1–S3, Scheme S1 and Figures S1–S4 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (CA-138338). Animal exposures were covered by a grant from the National Institute of Environmental Health Sciences (ES012689-05, sub-award 5-51624). The mass spectrometry analyses were performed at the Analytical Biochemistry Facility Core of the University of Minnesota Masonic Cancer Center, which is supported in part by grant CA-77598 from the U.S. National Cancer Institute. S.K. was partially supported by a doctoral dissertation fellowship from the University of Minnesota Graduate School.
Supplementary Material
Acknowledgements
We are thankful to B.Matter and J.Dalluge (University of Minnesota) for their assistance with the mass spectrometry analyses. We are also grateful to R.Carlson for preparing the graphics for this paper. We acknowledge that urine samples from BD production workers were obtained from an archive of available biological specimens established by R.Albertini (and maintained by the American Chemistry Council). Smoker urine samples were generously provided by Prof. L.Le Marchand (University of Hawaii) and Prof. I.Stepanov (University of Minnesota Cancer Center).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- BD
1,3-butadiene
- bis-BDMA
1,4-bis-(N-acetyl-l-cystein-S-yl)butane-2,3-diol
- DEB
1,2,3,4-diepoxybutane
- DHBMA
4-(N-acetyl-l-cystein-S-yl)-1,2-dihydroxybutane
- EB
3,4-epoxy-1-butene
- EB-diol
1-butene-3,4-diol
- EBD
3,4-epoxy-1,2-butanediol
- ESI
electrospray ionization
- HMVK
hydroxymethylvinyl ketone
- HPLC
high-performance liquid chromatography
- LOD
limit of detection
- MHBMA
2-(N-acetyl-l-cystein-S-yl)-1-hydroxybut-3-ene/1-(N-acetyl-l-cystein-S-yl)-2-hydroxy-but-3-ene
- MS
mass spectrometry
- SPE
solid phase extraction
- SRM
selected reaction monitoring
- THBMA
4-(N-acetyl-l-cystein-S-yl)-1,2,3-trihydroxybutane.
References
- 1. National Toxicology Program and Department of Health and Human Services. (2011). 1,3-Butadiene. Report on Carcinogens, Twelfth Edition. National Toxicology Program, Research Triangle Park, NC [Google Scholar]
- 2. Himmelstein M.W., et al. (1997). Toxicology and epidemiology of 1,3-butadiene. Crit. Rev. Toxicol., 27, 1–108 [DOI] [PubMed] [Google Scholar]
- 3. Melnick R.L., et al. (1990). Carcinogenicity of 1,3-butadiene in C57BL/6 x C3H F1 mice at low exposure concentrations. Cancer Res., 50, 6592–6599 [PubMed] [Google Scholar]
- 4. Owen P.E., et al. (1987). Inhalation toxicity studies with 1,3-butadiene. 3. Two year toxicity/carcinogenicity study in rats. Am. Ind. Hyg. Assoc. J., 48, 407–413 [DOI] [PubMed] [Google Scholar]
- 5. Delzell E., et al. (1996). A follow-up study of synthetic rubber workers. Toxicology, 113, 182–189 [DOI] [PubMed] [Google Scholar]
- 6. Cheng H., et al. (2007). 1,3-Butadiene and leukemia among synthetic rubber industry workers: exposure-response relationships. Chem. Biol. Interact., 166, 15–24 [DOI] [PubMed] [Google Scholar]
- 7. Swenberg J.A., et al. (2011). 1,3-Butadiene: biomarkers and application to risk assessment. Chem. Biol. Interact., 192, 150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sprague C.L., et al. (2004). Mercapturic acid urinary metabolites of 3-butene-1,2-diol as in vivo evidence for the formation of hydroxymethylvinyl ketone in mice and rats. Chem. Res. Toxicol., 17, 819–826 [DOI] [PubMed] [Google Scholar]
- 9. Cochrane J.E., et al. (1994). Mutagenicity of butadiene and its epoxide metabolites: I. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis, 15, 713–717 [DOI] [PubMed] [Google Scholar]
- 10. Krause R.J., et al. (1997). Epoxide hydrolase-dependent metabolism of butadiene monoxide to 3-butene-1,2-diol in mouse, rat, and human liver. Drug Metab. Dispos., 25, 1013–1015 [PubMed] [Google Scholar]
- 11. Tretyakova N., et al. (1997). Adenine adducts with diepoxybutane: isolation and analysis in exposed calf thymus DNA. Chem. Res. Toxicol., 10, 1171–1179 [DOI] [PubMed] [Google Scholar]
- 12. Antsypovich S., et al. (2007). Site specific N6-(2-hydroxy-3,4-epoxybut-1-yl)adenine oligodeoxynucleotide adducts of 1,2,3,4-diepoxybutane: synthesis and stability at physiological pH. Chem. Res. Toxicol., 20, 641–649 [DOI] [PubMed] [Google Scholar]
- 13. Seneviratne U., et al. (2010) Exocyclic deoxyadenosine adducts of 1,2,3,4-diepoxybutane: synthesis, structural elucidation, and mechanistic studies. Chem. Res. Toxicol., 23, 118–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goggin M., et al. (2011). Persistence and repair of bifunctional DNA adducts in tissues of laboratory animals exposed to 1,3-butadiene by inhalation. Chem. Res. Toxicol., 24, 809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goggin M., et al. (2007). HPLC-ESI+-MS/MS analysis of N7-guanine-N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene. Chem. Res. Toxicol., 20, 839–847 [DOI] [PubMed] [Google Scholar]
- 16. Park S., et al. (2004). Guanine-adenine DNA cross-linking by 1,2,3,4-diepoxybutane: potential basis for biological activity. Chem. Res. Toxicol., 17, 1638–1651 [DOI] [PubMed] [Google Scholar]
- 17. Park S., et al. (2005). Interstrand and intrastrand DNA-DNA cross-linking by 1,2,3,4-diepoxybutane: role of stereochemistry. J. Am. Chem. Soc., 127, 14355–14365 [DOI] [PubMed] [Google Scholar]
- 18. Sangaraju D., et al. (2012). NanoHPLC-nanoESI(+)+-MS/MS quantitation of bis-N7-guanine DNA-DNA cross-links in tissues of B6C3F1 mice exposed to subppm levels of 1,3-butadiene. Anal. Chem., 84, 1732–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loeber R., et al. (2006). Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane. Chem. Res. Toxicol., 19, 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michaelson-Richie E.D., et al. (2010). DNA-protein cross-linking by 1,2,3,4-diepoxybutane. J. Proteome Res., 9, 4356–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henderson R.F., et al. (1996). Metabolism of 1,3-butadiene: species differences. Toxicology, 113, 17–22 [DOI] [PubMed] [Google Scholar]
- 22. Krause R.J., et al. (1997). Oxidation of butadiene monoxide to meso- and (+/-)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch. Biochem. Biophys., 337, 176–184 [DOI] [PubMed] [Google Scholar]
- 23. Goggin M., et al. (2009). Molecular dosimetry of 1,2,3,4-diepoxybutane-induced DNA-DNA cross-links in B6C3F1 mice and F344 rats exposed to 1,3-butadiene by inhalation. Cancer Res., 69, 2479–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vacek P.M., et al. (2010). Hemoglobin adducts in 1,3-butadiene exposed Czech workers: female-male comparisons. Chem. Biol. Interact., 188, 668–676 [DOI] [PubMed] [Google Scholar]
- 25. McDonald J.D., et al. (2004). Analysis of butadiene urinary metabolites by liquid chromatography-triple quadrupole mass spectrometry. J. Anal. Toxicol., 28, 168–173 [DOI] [PubMed] [Google Scholar]
- 26. Albertini R.J., et al. (2001). Biomarkers for assessing occupational exposures to 1,3-butadiene. Chem. Biol. Interact., 135-136, 429–453 [DOI] [PubMed] [Google Scholar]
- 27. Urban M., et al. (2003). Determination of the major mercapturic acids of 1,3-butadiene in human and rat urine using liquid chromatography with tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci., 796, 131–140 [DOI] [PubMed] [Google Scholar]
- 28. Carmella S.G., et al. (2009). Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem. Res. Toxicol., 22, 734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckert E., et al. (2010). Determination of six hydroxyalkyl mercapturic acids in human urine using hydrophilic interaction liquid chromatography with tandem mass spectrometry (HILIC-ESI-MS/MS). J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci., 878, 2506–2514 [DOI] [PubMed] [Google Scholar]
- 30. Roethig H.J., et al. (2009). Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob. Res., 11, 1216–1225 [DOI] [PubMed] [Google Scholar]
- 31. Kotapati S., et al. (2011). Quantitative analysis of trihydroxybutyl mercapturic acid, a urinary metabolite of 1,3-butadiene, in humans. Chem. Res. Toxicol., 24, 1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albertini R.J., et al. (2007). Molecular epidemiological studies in 1,3-butadiene exposed Czech workers: female-male comparisons. Chem. Biol. Interact., 166, 63–77 [DOI] [PubMed] [Google Scholar]
- 33. Georgieva N.I., et al. (2010). Exposure-response of 1,2:3,4-diepoxybutane-specific N-terminal valine adducts in mice and rats after inhalation exposure to 1,3-butadiene. Toxicol. Sci., 115, 322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brunnemann K.D., et al. (1990). Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis, 11, 1863–1868 [DOI] [PubMed] [Google Scholar]
- 35. Boysen G., et al. (2012). Formation of 1,2:3,4-diepoxybutane-specific hemoglobin adducts in 1,3-butadiene exposed workers. Toxicol. Sci., 125, 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sasiadek M., et al. (1991). 1,3-Butadiene and its epoxides induce sister-chromatid exchanges in human lymphocytes in vitro . Mutat. Res., 261, 117–121 [DOI] [PubMed] [Google Scholar]
- 37. Kligerman A.D., et al. (1999). Comparison of cytogenetic effects of 3,4-epoxy-1-butene and 1,2:3, 4-diepoxybutane in mouse, rat and human lymphocytes following in vitro G0 exposures. Mutat. Res., 439, 13–23 [DOI] [PubMed] [Google Scholar]
- 38. Swenberg J.A., et al. (2007). Future directions in butadiene risk assessment and the role of cross-species internal dosimetry. Chem. Biol. Interact., 166, 78–83 [DOI] [PubMed] [Google Scholar]
- 39. Hecht S.S. (2002). Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis, 23, 907–922 [DOI] [PubMed] [Google Scholar]
- 40. Boogaard P.J., et al. (1996). Glutathione conjugation of 1,2:3,4-diepoxybutane in human liver and rat and mouse liver and lung in vitro . Toxicol. Appl. Pharmacol., 136, 307–316 [DOI] [PubMed] [Google Scholar]
- 41. Dekant W., et al. (1988). Enzymatic conjugation of hexachloro-1,3-butadiene with glutathione. Formation of 1-(glutathion-S-yl)-1,2,3,4,4-pentachlorobuta-1,3-diene and 1,4-bis(glutathion-S-yl)-1,2,3,4-tetrachlorobuta-1,3-diene. Drug Metab. Dispos., 16, 701–706 [PubMed] [Google Scholar]
- 42. Liebler D.C., et al. (1985). Formation of glutathione conjugates by reactive metabolites of vinylidene chloride in microsomes and isolated hepatocytes. Cancer Res., 45, 186–193 [PubMed] [Google Scholar]
- 43. Wheeler J.B., et al. (2001). Conjugation of haloalkanes by bacterial and mammalian glutathione transferases: mono- and vicinal dihaloethanes. Chem. Res. Toxicol., 14, 1107–1117 [DOI] [PubMed] [Google Scholar]
- 44. Cho S.H., et al. (2010). Mutagenicity of a glutathione conjugate of butadiene diepoxide. Chem. Res. Toxicol., 23, 1544–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cho S.H., et al. (2012). Conjugation of butadiene diepoxide with glutathione yields DNA adducts in vitro and in vivo . Chem. Res. Toxicol., 25, 706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Filser J.G., et al. (2007). Metabolism of 1,3-butadiene to toxicologically relevant metabolites in single-exposed mice and rats. Chem. Biol. Interact., 166, 93–103 [DOI] [PubMed] [Google Scholar]
- 47. Boysen G., et al. (2007). N-terminal globin adducts as biomarkers for formation of butadiene derived epoxides. Chem. Biol. Interact., 166, 84–92 [DOI] [PubMed] [Google Scholar]
- 48. Boysen G., et al. (2004). Analysis of diepoxide-specific cyclic N-terminal globin adducts in mice and rats after inhalation exposure to 1,3-butadiene. Cancer Res., 64, 8517–8520 [DOI] [PubMed] [Google Scholar]
- 49. Thornton-Manning J.R., et al. (1998). Disposition of butadiene epoxides in Sprague-Dawley rats following exposures to 8000 ppm 1,3-butadiene: comparisons with tissue epoxide concentrations following low-level exposures. Toxicol. Sci., 41, 167–173 [DOI] [PubMed] [Google Scholar]
- 50. Koturbash I., et al. (2011). Epigenetic alterations in liver of C57BL/6J mice after short-term inhalational exposure to 1,3-butadiene. Environ. Health Perspect., 119, 635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kültz D. (2003). Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J. Exp. Biol., 206(Pt 18), 3119–3124 [DOI] [PubMed] [Google Scholar]
- 52. Kültz D. (2005). Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol., 67, 225–257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.