Abstract
Upregulation of fatty acid synthase (FASN), a key enzyme of de novo lipogenesis, is associated with metastasis in colorectal cancer (CRC). However, the mechanisms of regulation are unknown. Since angiogenesis is crucial for metastasis, we investigated the role of FASN in the neovascularization of CRC. The effect of FASN on tumor vasculature was studied in orthotopic CRCs, the chick embryo chorioallantoic membrane (CAM) and Matrigel plug models using immunohistochemistry, immunofluorescent staining and confocal microscopy. Cell secretion was evaluated by ELISA and antibody arrays. Proliferation, migration and tubulogenesis of endothelial cells (ECs) were assessed in CRC–EC coculture models. In this study, we found that stable knockdown of FASN decreased microvessel density in HT29 and HCT116 orthotopic CRCs and resulted in ‘normalization’ of tumor vasculature in both orthotopic and CAM models. Furthermore, FASN regulated secretion of pro- and antiangiogenic factors, including vascular endothelial growth factor-A (VEGF-A). Mechanisms associated with the antiangiogenic activity noted with knockdown of FASN included: downregulation of VEGF189, upregulation of antiangiogenic isoform VEGF165b and a decrease in expression and activity of matrix metalloproteinase-9. Furthermore, conditioned medium from FASN knockdown CRC cells inhibited activation of vascular endothelial growth factor receptor-2 and its downstream signaling and decreased proliferation, migration and tubulogenesis of ECs as compared with control medium. Together, these results suggest that cancer cell-associated FASN regulates tumor vasculature through alteration of the profile of secreted angiogenic factors and regulation of their bioavailability. Inhibition of FASN upstream of VEGF-A and other angiogenic pathways can be a novel therapeutic strategy to prevent or inhibit metastasis in CRC.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the USA (1). Approximately 60% of patients diagnosed with CRC have locally advanced or metastatic disease, which is associated with a worse prognosis. Treatment options for advanced CRC are limited and novel therapeutic targets are important for development of therapeutic strategies that may improve survival.
Fatty acid synthase (FASN), a key enzyme of lipid biosynthesis, is significantly upregulated in many cancers, including CRC (2). Upregulation of lipogenic enzymes provides selective proliferative and survival advantages for cancer cells (3,4). The expression level of FASN is highest in metastatic tumors and correlates with poor prognosis (5,6). We recently demonstrated that short hairpin RNA-mediated inhibition of lipogenic enzymes significantly reduced expression of CD44, a transmembrane protein implicated in metastasis, and attenuated signaling downstream of the CD44/c-MET complex, significantly reducing systemic metastases in nude mice (6). In contrast, overexpression of FASN, together with androgen receptors in immortalized human prostate epithelial cells, resulted in development of invasive adenocarcinoma in nude mice; these findings demonstrate that overexpression of FASN plays an important role in neoplastic transformation of epithelial cells and development of metastasis (7).
Although a connection between activation of lipogenesis and aggressive metastatic behavior has been shown, the mechanism by which FASN regulates metastasis remains unclear. Metastasis is a complex multistep process requiring aggressive cancer cell behavior and alteration of the tumor microenvironment (TME) (8). The vascular niche is an important component of the TME (8,9), and angiogenesis plays a crucial role in tumor initiation, progression and metastasis (10). Angiogenic factors secreted by cancer cells modulate proliferation, survival, tubulogenesis and sprouting of endothelial cells (ECs) (11). Activated by cancer cells, ECs release specific endothelial-derived growth factors that might directly regulate tumor growth and contribute to establishment of the unique TME that can promote cancer proliferation, invasiveness and metastasis (12). Induction of vascular endothelial growth factor-A (VEGF-A) is a critical step in tumor angiogenesis (13). In fact, CRC is one of the most extensively studied malignancies with regard to the relationship between angiogenesis and clinical outcome, and the cumulative analysis of these studies demonstrates that expression of VEGF-A and microvessel density (MVD) predict poor prognosis in patients with CRC (14).
Despite multiple preclinical studies demonstrating the relevance of inhibition of VEGF signal transduction pathways (15), the clinical benefit of anti-VEGF therapy is limited due to acquired resistance (16). Moreover, certain tumors are relatively insensitive to VEGF inhibition (10). We demonstrate that FASN regulates secretion of multiple angiogenic factors, including VEGF-A, in CRC cells. More importantly, inhibition of FASN in CRC cells is associated with low MVD and normalization of blood vessel structure and appearance in vivo. We consistently observed a significant reduction in VEGF-A in the extracellular space within the vascularized areas of orthotopic tumors. Furthermore, stable inhibition of FASN in CRC cells results in inhibition of proliferation, migration and tubulogenesis of ECs and attenuation of vascular endothelial growth factor receptor-2 (VEGFR-2) signaling in vitro. Considering that FASN is highly expressed in CRC, our findings suggest that inhibition of FASN may be a potential therapeutic strategy for targeting angiogenesis in advanced CRC.
Materials and methods
Cell lines, small interfering RNA
CRC lines KM20, HT29, HCT116 and SW480 were authenticated November 2011, Genetica DNA Laboratories, Cincinnati, OH (6). Stable FASN knockdown CRC cell lines were established and lipid biosynthesis was assessed (6). FASN cDNA (ID6172538; Open Biosystem, Chicago, IL) was cloned into the pEGFP vector. Stable overexpression was established by transfecting SW480 cells with pEGFP-FASN vector and Gentamicin (Invitrogen, Austin, TX) selection. Clonetics™ Lung Microvascular Endothelial Cell System (CC-2527 and CC-3202; Cambrex, East Rutherford, NJ) was used.
In vivo studies
Male athymic nudenu/nu mice (Charles River Laboratories, Wilmington, MA) were housed in the Markey Cancer Center Small Animal Facility. All procedures were performed using protocols approved by the UK Animal Care and Use Committee. HCT116 and HT29 (1 × 106 cells/25 μl), either control or FASN stable knockdown, were injected into the colon wall of 8-week-old athymic nude mice (at least five mice for each group) using the Karl-Storz Coloview system as described previously (17). Prior to colonoscopy, mice were anesthetized with ketamine. The injections were videotaped and high resolution imaging of colon tumors was performed in living mice at the end of experiment, 2 weeks postinjection. The colon tumors with surrounding normal tissues were resected, fixed and analyzed using hematoxylin and eosin, immunohistochemistry (IHC) and immunofluorescence (IF) staining. For evaluation of tumor vasculature in the Matrigel plug assay, 2 × 106 cells were mixed with 500 μl of growth factor reduced Matrigel (BD, Franklin Lakes, NJ) and implanted under the skin of mice (n = 4 per group, two implants per mouse). Experiments ended on day 7. Matrigel plugs and adjacent skin were imaged and fixed. Sections of Matrigel plugs were analyzed using IHC.
Antibody array
Human Angiogenesis antibody Array 1 was purchased from RayBiotech (Norcross, GA). The experiment was carried out with HCT116 and HT29 cell lines. One milliliter of medium conditioned on control or FASN knockdown cells (6 × 105 cells per well) in the absence or presence of human lung microvascular endothelial cells (HMVEC-L) in coculture (3 × 105 in the top chamber) for 24h was analyzed according the manufacturer’s instructions. The relative levels of cytokines in the medium were determined by densitometry analysis of the intensities of signals and normalized to positive control (Alpha Innotech Imaging system and AlphaEase software).
Quantitative real-time PCR
Total RNA was isolated from cultured cells using RNeasy kit (Qiagen) according to the manufacturer’s instructions. Complementary DNA was synthesized using 1 μg of total RNA and a high capacity cDNA reverse transcription kit (Applied Biosystems, Austin, TX). Quantitative real-time PCR (qRT–PCR) was carried out using a TaqMan Gene Expression Master Mix (#4369016) according to the manufacturer’s protocol and TaqMan probes for human total VEGF-A (ID Hs00900055_m1) and human GAPDH (# 4333764F; Applied Biosystems). Following specific primers and probes with FAM at the 5′-end and TAMRA at the 3′-end for human VEGF189 (NM_003376) and VEGFA165b (NM_001033756) were synthesized (Applied Biosystems) and used for evaluation of respective isoforms expression: VEGF189-F, 5′-TGTGA ATGCAGACCAAAGA AAGA-3′; VEGF189-R, 5′-CGTTTTTGCCCCTTTCCC-3′; VEGF189 Probe, AGAGCAAG ACAAGAAAAAAAATCAGTTC; VEGFA165b-F, 5′-CAAGA AAATCCCTGTGGGCC-3′; VEGFA165b-R, 5′-TGAGAGA TCTGCAAG TA CGTTCG-3′; VEGFA165b Probe 5′-TGCTCAGAGCGGAGAAAGCATTTGTT TG-3′.
Conditioned medium
Equal numbers of CRC cells were plated in 100mm dishes or 6-well plates and allowed to attach. Cells were cultured with cell type-specific serum-free medium [for enzyme-linked immunosorbent assay (ELISA), proliferation and activation of HMVEC-L experiments] or with 1% fetal bovine serum (for tube formation assay) for 24 h. Prior to use, medium was centrifuged at 1000 r.p.m. for 3min to remove any cell contaminants.
ELISA, proliferation, migration and tube formation assays
Secretion of VEGF-A was determined using Quantikine Human VEGF immunoassay, DVE00 (R&D Systems, Minneapolis, MN) and RayBio Human VEGF ELISA, ELH-VEGF001 (Norcross, GA) according to the manufacturer’s instructions and normalized to the number of cells.
HMVEC-L proliferation was measured in 96-well plates. A total of 2000 cells were plated per well, cultured for 24 h and then starved overnight. Conditioned medium was added for 48 h (replenished at 24 h) and cellular proliferation was measured using CellTiter 96@ Aqueous One Solution Proliferation Assay, #G3580 (Promega, Madison, WI).
HMVEC-L migration was assessed using the Boyden chamber assay. Briefly, 2 × 104 CRC cells were plated in the lower chamber. After attachment, medium was changed to 1% fetal bovine serum medium and conditioned on cells for 24h. The inserts were coated with collagen, blocked with 1% of bovine serum albumin and 5 × 104 HMVEC-L cells were added. After 24 h HMVEC-L migration was quantified as described previously (6).
Tube formation was evaluated in 48-well plates. 6 × 104 HMVEC-L cells in 500 μl of conditioned medium were added on top of polymerized growth factor-reduced Matrigel, 120 μl per well, #354230 (BD) for 8h. Tubules were labeled with Calcein AM (2.5 μg/ml) for better visualization and the length of tubules was quantified using NIC-Elements BR 3.10 Ink software.
The chick chorioallantoic membrane assay
Fertilized white leghorn eggs (Charles River, North Franklin, CT) were incubated at 37.6°C and 70–80% humidity. Chick Embryo Ex-Ovo Model: After 3 days of incubation, the eggs were cleaned with 70% EtOH, opened with a Dremel tool and the egg’s contents were emptied into a sterile weigh boat. The embryo is returned to the incubator for 7 days. On day 10, 2.5 × 105 HCT116 cells, either nontargeting control (NTC) or FASN knockdown, in 10 μl of Matrigel are applied on the chorioallantoic membrane (CAM). On day 14, the green fluorescent protein-labeled cancer cells disseminated from the primary tumor were detected and quantified blindly for NTC and FASNsh tumors. The rhodamine-conjugated lens culinaris agglutinin (LCA) (Vector Laboratories, Burlingame, CA) was injected into the blood stream of the embryo to improve visualization of the blood vessels and detection of cancer cells. Blood vessel density surrounding the tumor was analyzed by direct blind counting of blood vessels per field.
Statistical analysis
Descriptive statistics and bar graphs were presented to summarize several endpoints, including qRT–PCR of VEGF (VEGF-A, VEGF189, VEGF165b) levels, ELISA, microvessel analysis parameters, migration, etc. Comparisons of NTC versus FASNsh groups were performed using two-sample t-test or analysis of variance. Contrast statements were generated from the analysis of variance model to perform pairwise comparisons within each cell line so adjustments in P values due to multiple testing were not employed. Statistical analyses were performed using SAS 9.3.
Results
Stable knockdown of FASN inhibits neovascularization of orthotopic human colon tumors
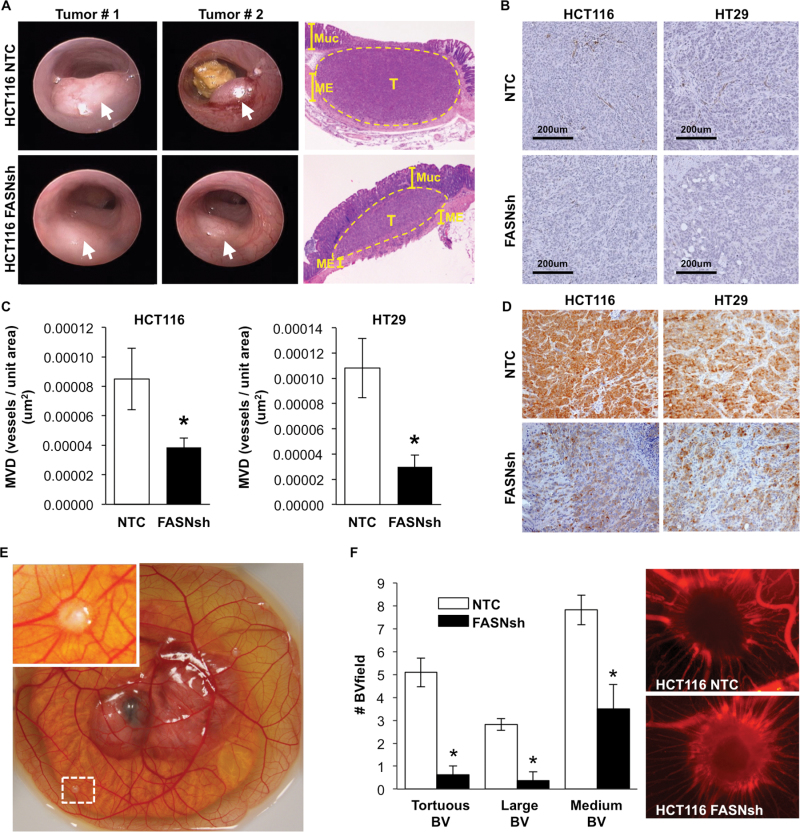
To elucidate whether expression of FASN has a role in the regulation of tumor vasculature, HCT116 and HT29 tumors, NTC and FASN knockdown, were implanted into the colonic submucosa of athymic mice using a murine colonoscope (17). Images taken during colonoscopy demonstrated that all cell lines formed well-defined tumors at 2 weeks. Hematoxylin and eosin staining of representative tumors and normal adjacent colon confirmed that most tumors were localized to the submucosa and grew through the lining and into the wall of the colon (Figure 1A). Tumor vasculature in control and FASN knockdown HCT116 and HT29 tumors was evaluated using IHC staining for CD31, an EC marker (Figure 1B). Digital images of the slides were analyzed using Microvessel Analysis algorithm (Aperio ScanScope XT scanner and software). MVD of the tumors with stable knockdown of FASN was significantly lower as compared with NTC tumors in both HCT116 and HT29 orthotopic models (Figure 1C and D). The diameter of blood vessels was also significantly decreased by inhibition of FASN in both HT29 and HCT116 tumors (Supplementary Table 1, available at Carcinogenesis Online). We next determined whether FASN expression in CRC cells affects formation of tumor vasculature utilizing the CAM and Matrigel plug models. The ‘shell-less’ CAM model was established as described by Deryugina et al. (18) Tumor cells were placed on the CAM on day 10 and the surrounding tumor vasculature was analyzed on day 14 (Figure 1E). Inhibition of FASN in HCT116 tumors established on the CAM resulted in a significant decrease in the number of torturous, large and medium blood vessels in surrounding tumor areas (Figure 1F). Consistent with these findings, analysis of dissemination of cancer cells from the primary tumor demonstrated the complete attenuation of dissemination of cancer cells in FASN knockdown HCT116 tumors as compared with control tumors (Supplementary Figure 1A and B, available at Carcinogenesis Online). We also tested the effect of FASN knockdown on tumor vasculature using the Matrigel plug assay. KM20 cells (2 × 106) were mixed with Matrigel (500 μl) and injected under the skin of athymic nude mice, and allowed to grow for 1 week. The skin vasculature of all animals bearing KM20 NTC plugs was poorly defined, very fragile and leaky upon any intervention; in contrast, we observed well-defined vasculature in skin adjacent to the FASN knockdown KM20 plugs (Supplementary Figure 2A and B, available at Carcinogenesis Online). Even though inhibition of FASN led to a slight decrease in MVD as compared with control, it was not statistically significant in this model (Supplementary Figure 2C, available at Carcinogenesis Online).
Fig. 1.
Increased expression of FASN is associated with increased microvessel density in orthotopic colon tumors. (A) Representative images of mouse colon tumors (arrows; 2 weeks) established using HCT116 NTC and FASNsh cells and hematoxylin and eosin staining of tumors and surrounding colon tissues (Muc–mucosa, T–tumor, ME–muscularis externa). (B) IHC staining of CRCs for CD31. (C) MVD analysis using Microvessel analysis algorithm (Aperio ScanScope XT) in eight HCT116 NTC colon tumors (19 sections) and nine HCT116 FASNsh tumors (20 sections), *P < 0.05, and three HT29 NTC colon tumors (nine sections) and three HT29 FASNsh (nine sections), *P < 0.01. (D) Expression of FASN in NTC and FASNsh HCT116 and HT29 orthotopic colon tumors assessed by IHC staining; ×20 magnification. (E) Representative image of the chicken embryo (day 14) with established HCT116 tumors on the CAM. (F) The effect of FASN knockdown on tumor surrounding vasculature in the CAM model. Quantification of tortuous, large (≥25 μm) and medium (10–25 μm) blood vessels surrounding tumors and the representative images of tumors and surrounding vasculature labeled with rhodamine-conjugated lens culinaris agglutinin.
Taken together, these data suggest a potential involvement of tumor-specific expression of FASN in regulation of tumor vasculature.
FASN regulates expression and secretion of angiogenic factors in CRC
The induction of tumor vasculature or the ‘angiogenic switch’ depends on the balance between pro- and antiangiogenic molecules (19). To test whether FASN regulates tumor vasculature through altered secretion of angiogenic factors by CRC cells, conditioned media from control and FASN knockdown CRC cells was analyzed using Human Angiogenesis Antibody Array 1. To account for the effect of a cross-talk between CRC and ECs, we also analyzed medium from NTC and FASNsh cells cocultured with HMVEC-L cells. As shown in Supplementary Table 2, available at Carcinogenesis Online, FASN regulates secretion of several pro- and antiangiogenic factors in CRC cells. In fact, knockdown of FASN in HT29 cells resulted in a decrease in secretion of several proangiogenic factors such as transforming growth factor beta (active form), the growth-regulated protein family, thrombopoietin, platelet-derived growth factor and VEGF-A (array detects VEGF-A165 and VEGF-A121). In HCT116 cells, secretion of growth-regulated protein family members, angiogenin and interleukin 6 (proangiogenic factors) was significantly downregulated with knockdown of FASN; secretion of tissue inhibitor of metalloproteinase-1 (TIMP-1) and tissue inhibitor of metalloproteinase-2 (TIMP-2) (antiangiogenic factors) was significantly upregulated. We also observed that the profile of secreted factors by CRC cells is different in the presence or absence of HMVEC-L in coculture, suggesting the importance of evaluation of CRC cell secretion in EC-CRC coculture model.
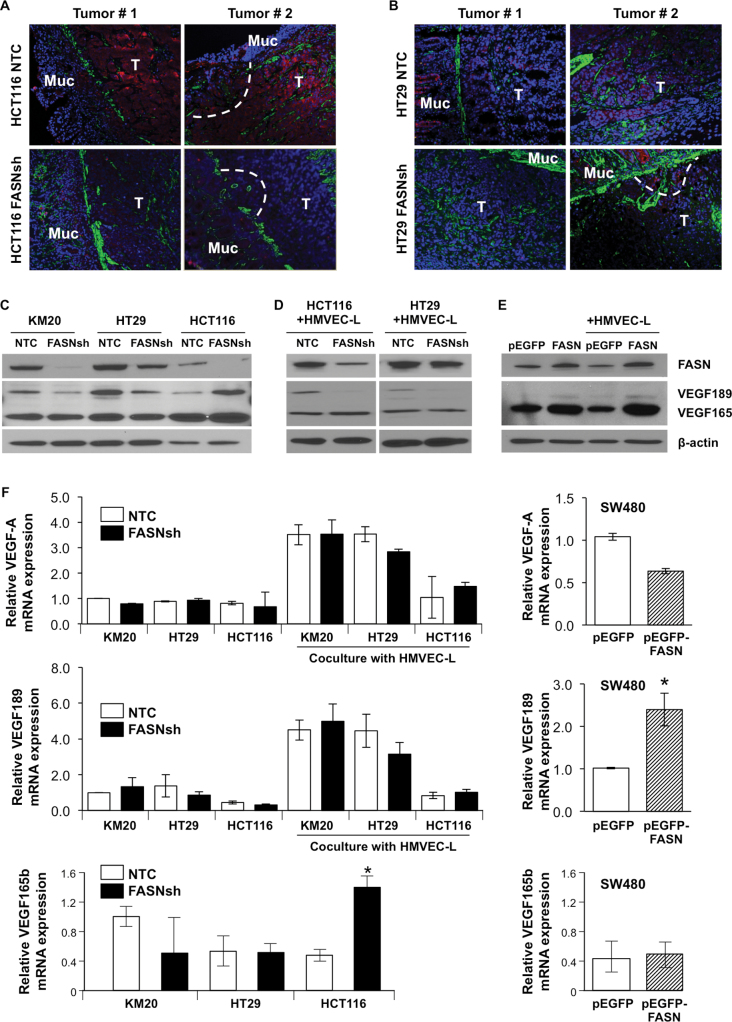
VEGF-A is a major cytokine that induces activation of ECs to promote formation of new blood vessels (13) and often represents a crucial rate-limiting step in angiogenesis (20). VEGF-A is subjected to multilevel regulation including the pattern of expression of VEGF-A isoforms, processing these isoforms in a cell, their secretion and, more importantly, their bioavailability in the extracellular environment (21).Therefore, we first tested whether FASN regulates expression of VEGF-A in HCT116 and HT29 orthotopic colon tumors. Inhibition of FASN resulted in a significant decrease in the level of VEGF-A in both models (Figure 2A and B).
Fig. 2.
FASN regulates expression of VEGF-A. IF staining of (A) HCT116 NTC and HCT116 FASNsh, and (B) HT29 NTC and HT29 FASNsh orthotopic tumors for VEGF-A (red), smooth muscle actin (green) and 4′,6-diamidino-2-phenylindole (blue). T–tumor, Muc–mucosa, dashed line-local invasion. (C) Immunoblot analysis for VEGF-A in CRC cell lines. (D) Expression of VEGF-A in HCT116 and HT29 in the presence of HMVEC-L in coculture for 24h. (E) Immunoblot analysis for VEGF-A in SW480 cells with overexpression of FASN in the presence or absence of HMVEC-L. (F) qRT–PCR analysis of mRNA for VEGF-A, VEGF189 and VEGF165b in CRC cells with altered expression of FASN. mRNA expression was calculated using the ΔΔCt method areas (means ± SD of triplicate determinations, *P < 0.05 versus control).
To further investigate the effect of FASN on VEGF-A signaling, we assessed expression of VEGF-A messenger RNA (mRNA) in three different CRC cell lines (KM20, HT29 and HCT116) with stable knockdown of FASN. Knockdown of FASN in these cell lines is associated with attenuation of de novo palmitate synthesis as determined by stable isotope labeling (6). Consistent with previously published data (22), we detected expression of four different isoforms of VEGF-A (VEGF121, VEGF145, VEGF165 and VEGF189) in CRC cells (Supplementary Figure 3, available at Carcinogenesis Online). However, analysis of total cell lysates using an antibody which immunoreacts with all isoforms of VEGF-A demonstrated that VEGF165 and VEGF189 are the primary isoforms translated into protein in the CRC cell lines tested (Figure 2C). Knockdown of FASN did not affect expression of VEGF165 in KM20 and HT29 cell lines, but increased its expression in the HCT116 cell line in the absence or presence of HMVEC-L in coculture (Figure 2C and D). Expression of VEGF189 was decreased following knockdown of FASN in KM20 and HT29 cell lines (in the absence or presence of HMVEC-L) and in the HCT116 cell line (in the presence of HMVEC-L) (Figure 2C and D). To test whether overexpression of FASN affected expression of VEGF-A, SW480 cells were stably transfected with pEGFP-control or pEGFP-FASN plasmids. Overexpression of FASN resulted in an increase in expression of VEGF-A in SW480 cells in the presence or absence of HMVEC-L (Figure 2E).
To elucidate whether FASN regulates expression of VEGF-A isoforms at the mRNA level, expression of total VEGF-A, VEGF189 and VEGF165b mRNA was measured using qRT–PCR in control and FASN knockdown cell lines in the presence or absence of HMVEC-L. No significant difference was observed in VEGF-A and VEGF189 mRNA expression among the cell lines tested (Figure 2F). In contrast to the VEGF165, VEGF165b isoform is known to elicit an antiangiogenic effect and is downregulated in CRC (23). Knockdown of FASN in CRC cells did not affect the level of VEGF165b mRNA in KM20 and HT29 cell lines (Figure 2F), but upregulated its expression in HCT116 cells, which is consistent with an increase in protein expression of this isoform shown in Figure 2C and D. Overexpression of FASN in SW480 cells led to a 2-fold increase in the level of VEGF-A189 mRNA expression and a decrease in the total expression of VEGF-A isoforms (Figure 2F).
Taken together these findings suggest that the expression of FASN in CRC cells regulates secretion of angiogenic factors including VEGF-A. Furthermore, high expression of FASN is associated with an increased expression of VEGF-A in vivo and an increased expression of VEGF189, an isoform associated with an aggressive phenotype and metastasis in CRC in vitro (24).
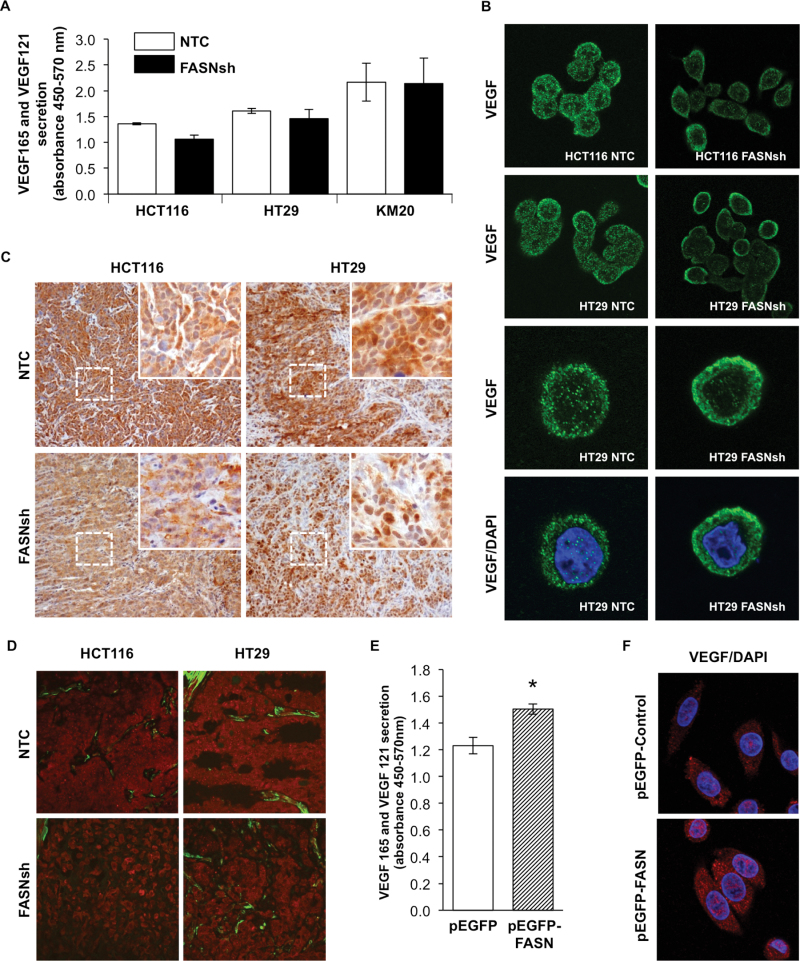
FASN controls bioavailability of VEGF-A in CRC
The ability of VEGF-A to induce angiogenesis depends on the presence of active isoforms within the TME (25). To test whether changes in expression of VEGF-A mediated by FASN correspond to changes in secretion of VEGF-A, we assessed secretion of VEGF-A by ELISA. Even though we observed a slight decrease in secretion of VEGF121 and VEGF165, when FASN was knockdown in HCT116 and HT29 cell lines, these changes were not statistically significant (Figure 3A). It can be explained by the fact that VEGF-A ELISA only detects freely released VEGF121 and VEGF165 isoforms; it cannot detect VEGF189, an isoform that is tightly bound to extracellular matrix (ECM) or sequestered in cell membranes (26), or significant fraction of VEGF165 can remain bound to the cell surface or the ECM (27). Additionally, this assay does not distinguish between VEGF165 and VEGF165b isoforms.
Fig. 3.
FASN regulates the bioavailability of VEGF-A. (A) Secretion of VEGF121 and VEGF165 by CRC cell lines measured by ELISA. (B) Localization of VEGF-A (green) in HCT116 and HT29 cells with knockdown of FASN assessed by confocal microscopy. ×40 and ×60 (×4 zoom) magnification. (C) IHC staining of orthotopic HCT116 and HT29 colon tumor sections for VEGF-A. ×20 (insets ×180) magnification. (D) Localization of VEGF-A assessed in tumor sections by confocal microscopy. VEGF-A (red), smooth muscle actin (green), ×60 magnification. (E) The effect of FASN overexpression on secretion of VEGF121 and VEGF165 by SW480 cells measured by ELISA. (F) Expression of VEGF-A in SW480 cells with overexpression of FASN, ×40 magnification.
To further assess the role of FASN in regulation of VEGF-A in CRC cells, we used microscopy. Confocal imaging of cells revealed an apparent increase in localization of VEGF-A around the plasma membrane in HCT116 and HT29 FASN knockdown cells (Figure 3B), suggesting that inhibition of FASN may affect release of VEGF-A from the cell. Three-dimensional confocal imaging of the cells was performed to confirm these observations (data not shown).
To test whether knockdown of FASN affects localization of VEGF-A in vivo, we analyzed expression of VEGF-A in orthotopic colon tumors. Consistent with our in vitro data, IHC staining of HCT116 and HT29 tumor sections for VEGF-A demonstrated that FASN knockdown resulted in a decrease in the expression of VEGF-A, an increase in VEGF-A localized to the plasma membrane and a decrease in its presence in ECM (Figure 3C). A similar pattern of VEGF-A distribution was detected in the orthotopic tumor sections analyzed by confocal microscopy (Figure 3D). In contrast to FASN knockdown CRC cells, overexpression of FASN in the SW480 cell line resulted in a significant increase in secretion of VEGF121 and VEGF165 as determined by ELISA (Figure 3E); however, we did not observe any changes in the localization of VEGF-A within the cell (Figure 3F). Together, these findings suggest that FASN expression regulates localization within a cell and secretion of VEGF-A in CRC.
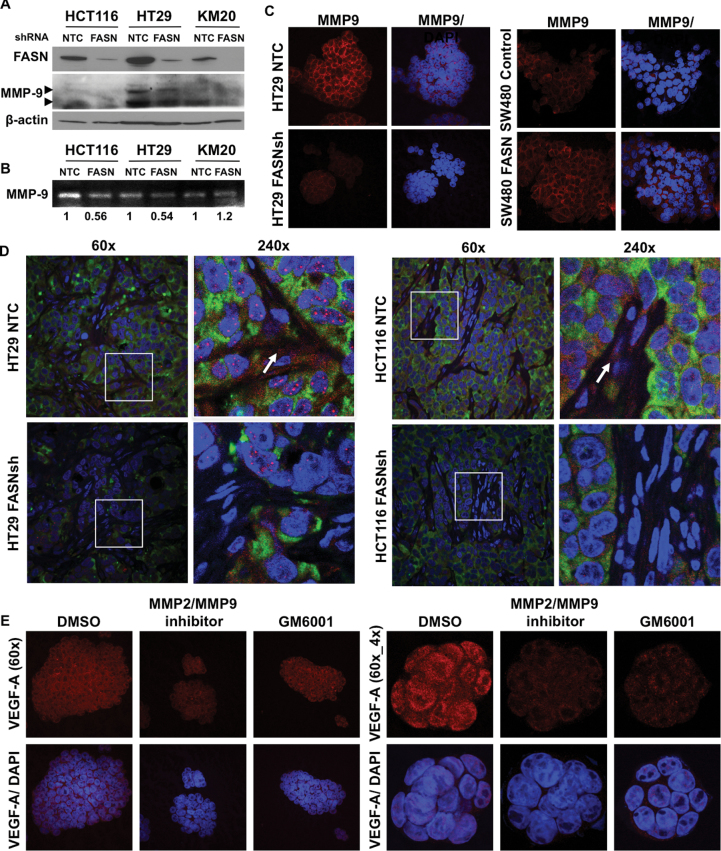
FASN regulates bioavailability of VEGF-A through a decrease in expression and activity of MMP9
Upon secretion, VEGF-A becomes bound to the ECM and the state of free versus bound VEGF-A dictates its effect on a vascular network (21). Matrix metalloproteinases (MMPs) have been implicated in regulation of the VEGF-A bioavailability from extracellular stores or from cells directly (21). MMP-9 is particularly important in the release of bioactive VEGF-A isoforms from cells and ECMs (25,28). Furthermore, the level of MMP-9 correlates with preoperative levels of circulating VEGF and poor outcomes in CRC patients (25). Immunoblot analysis of CRC cell lines with stable knockdown of FASN demonstrated a significant decrease in the level of MMP-9 when FASN was inhibited (Figure 4A). Zymography confirmed a significant inhibition of MMP-9 activity in medium from HCT116 and HT29 cells with FASN knockdown (Figure 4B). Consistent with this data, IF staining demonstrated a significantly lower level of expression of MMP-9 in FASN knockdown HT29 versus control cells (Figure 4C). In contrast, overexpression of FASN led to a significant upregulation of MMP-9 in SW480 cells (Figure 4C).
Fig. 4.
FASN regulates bioavailability of VEGF-A via alteration of activity of MMPs. (A) Immunoblot for MMP-9 in CRC cell lines with stable knockdown of FASN (top arrow-proenzyme, bottom arrow-activated enzyme). (B) Zymography and densitometry analysis for MMP9 in CRC cell lines with stable knockdown of FASN. (C) Expression of MMP-9 in CRC cell line assessed by confocal microscopy, ×60 magnification. (D) IF staining of HCT116 and HT29, NTC and FASNsh, orthotopic tumors for VEGF-A (red), MMP-9 (green) and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Arrows point at VEGF-A associated with ECM. (E) Expression of VEGF-A in HT29 cells treated with MMPs inhibitors for 24h.
Consistent with this data, IF staining of orthotopic HCT116 and HT29 tumors demonstrated that a decrease in expression of MMP-9 in FASN knockdown tumors is associated with a significant decrease of VEGF-A in the ECM, and this difference was particularly apparent in highly vascularized areas (Figure 4D). Interestingly, the Angiogenesis Antibody Array (Supplementary Table 2, available at Carcinogenesis Online) and the MMP Antibody Array (data not shown) demonstrated that secretion of TIMP-1, TIMP-2 and TIMP-4, a family of proteins that inhibits a wide range of MMPs, is upregulated with knockdown of FASN in HCT116 cells, suggesting that the activity of MMPs, other than MMP9, may be affected by altered lipogenesis. To confirm that the effect is mediated by MMPs, we treated HT29 cells with the MMP2/9 inhibitor (20 μM) and GM6001, pan MMP inhibitor, (50 μM) for 24 h. Treatment of HT29 cells with both inhibitors decreased the expression of VEGF-A and its localization to the plasma membrane (Figure 4E) in a manner similar to that seen in CRC cells with stable knockdown of FASN. The MMP2/MMP9 inhibitor had a more prominent effect on VEGF-A, suggesting that these enzymes may play a predominant role in the regulation of VEGF-A bioavailability. These findings suggest that FASN regulates the bioavailability of VEGF-A isoforms, at least in part, by an upregulation of expression and enzymatic activity of MMPs, in particular MMP-9.
Functional properties of ECs are regulated by the level of FASN expression in CRC cells
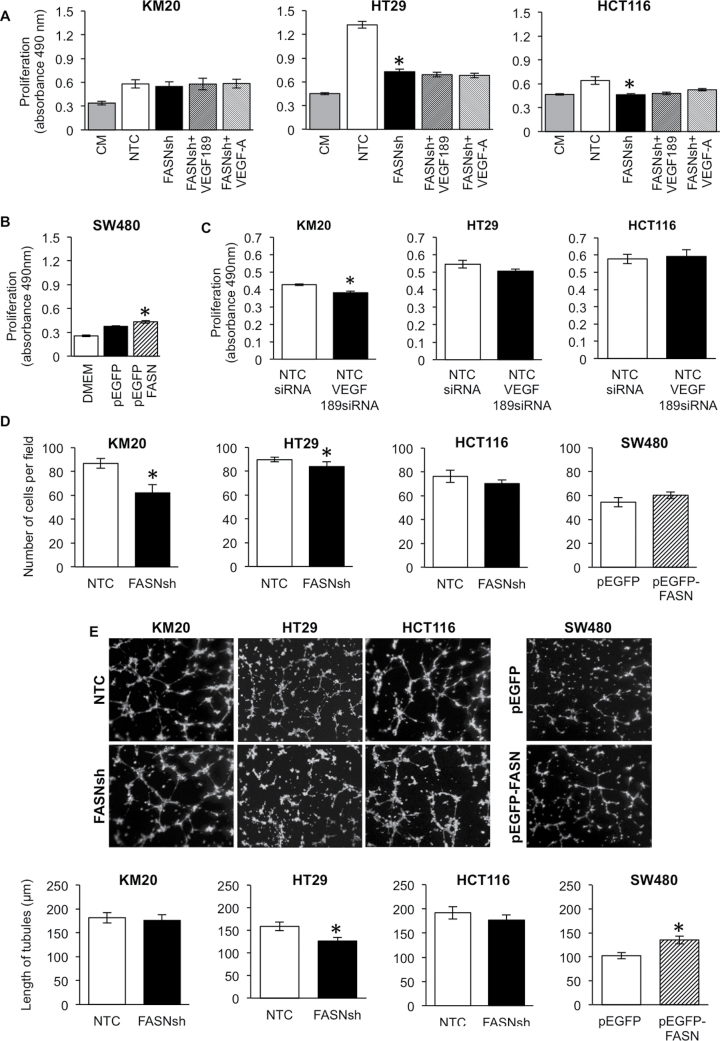
The angiogenic factors secreted by cancer cells regulate proliferation, survival, tubulogenesis and migration of ECs (10,12). To elucidate whether tumor-specific expression of FASN affects the functional properties of ECs, we assessed the effect of conditioned media from CRC cells with an altered expression of FASN on the proliferation of HMVEC-L cells. Conditioned media from FASN knockdown HCT116 and HT29, but not KM20 cells, significantly inhibited proliferation of HMVEC-L cells compared with control medium (Figure 5A). Addition of recombinant VEGF-A (10ng/ml) or VEGF189 (4ng/ml) to conditioned medium from FASN knockdown cells did not significantly affect proliferation, suggesting that upregulation of VEGF-A alone is not sufficient to rescue the phenotype induced by FASN knockdown, and that other angiogenic factors likely play an important role in the regulation of EC proliferation (Figure 5A). In contrast, conditioned media from SW480 cells overexpressing FASN significantly increased proliferation of HMVEC-L versus control (Figure 5B). Furthermore, knockdown of VEGF189 using VEGF189 small interfering RNA, in KM20, but not HCT116 or HT29, inhibited proliferation of HMVEC-L cells, supporting our previous data and suggesting that factors other than VEGF189 regulate proliferation of HMVEC-L (Figure 5C). Supplementary Figure 4, available at Carcinogenesis Online shows the efficiency of VEGF189 knockdown.
Fig. 5.
Cancer cell-associated FASN regulates functional properties of ECs. (A and B) The effect of FASN expression in CRC cells on proliferation of HMVEC-L measured by MTS assay. ECs were cultured on control or conditioned medium for 48h. (C) The effect of VEGF189 knockdown in CRC cells on proliferation of HMVEC-L. (D) Migration of HMVEC-L cells toward CRC cells with altered expression of FASN for 20h. (E) The effect of FASN expression in CRC cells on HMVEC-L tubulogenesis. The length of tubules was quantified after 8h of exposure to conditioned medium (Nikon software). *P < 0.05 versus control.
Attracted by proangiogenic factors, ECs become motile and invasive (10). To test whether expression of FASN in CRC cells affects migration of ECs, HMVEC-L cells were seeded onto the top chamber of a Transwell and allowed to migrate toward either control or FASN knockdown CRC cells. A significant decrease in migration of HMVEC-L was observed when the cells migrated toward FASN knockdown KM20 and HT29 cells (Figure 5D). Migration of HMVEC-L was not significantly affected by alteration of FASN expression of HCT116 and SW480 cells.
Tubulogenesis of ECs is a hallmark of vessel formation (10). Exposure of HMVEC-L to conditioned media from the HT29 cell line with knockdown of FASN significantly decreased the ability of ECs to form tubules as compared with medium from control cells (Figure 5E). In contrast, conditioned media from SW480 cells overexpressing FASN significantly increased tubulogenesis (Figure 5E). Taken together, these findings suggest that the functional properties of ECs depend on the level of FASN expression in CRC cells.
Activation of VEGFR-2 on the surface of HMVEC-L and its downstream signaling depends on the level of FASN expression in CRC cells
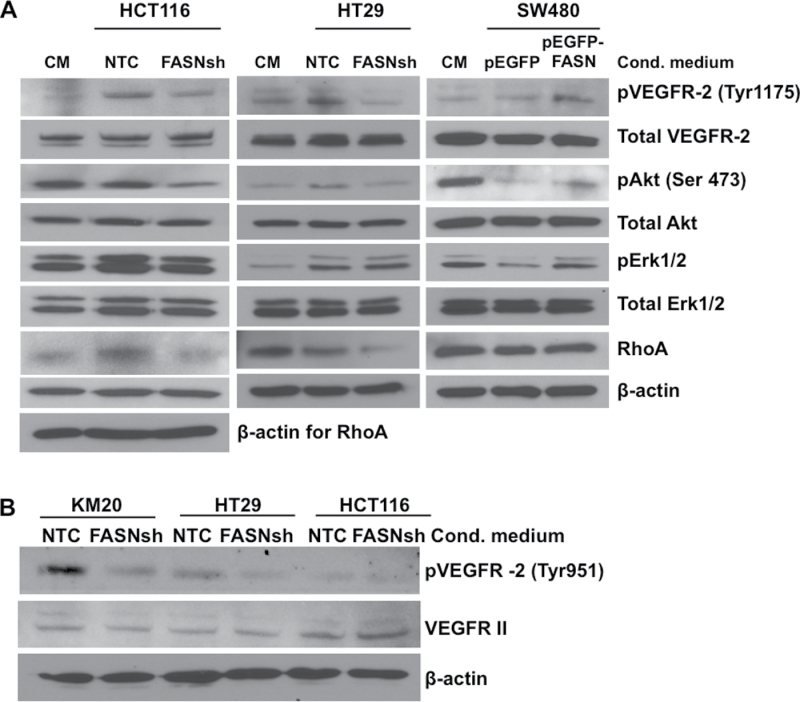
VEGFR-2 is a major mediator of the mitogenic, angiogenic and permeability-enhancing effects of VEGF-A in ECs (13). Notably, blockade of VEGF-A production or VEGFR-2 activation results in suppression of vascular growth and a concomitant reduction in tumor mass and metastasis (21). Since our data indicated that FASN may regulate secretion and bioavailability of VEGF-A, we investigated whether conditioned media from CRC cells with an altered expression of FASN had a different effect on the expression and activation of VEGFR-2 versus control medium. Flow cytometry analysis of VEGFR-2 on the surface of HMVEC-L cells demonstrated exposure of HMVEC-L cells for 6 or 24 h to conditioned media from control or FASN knockdown CRC cells does not affect VEGFR-2 presence on the cell surface (Supplementary Figure 5, available at Carcinogenesis Online). Activation of VEGFR-2 resulted in phosphorylation of numerous residues within the cytoplasmic domain (15). Activation of VEGFR-2 on Tyr1175 and Tyr951 was significantly lower when HMVEC-L cells were stimulated with conditioned media from FASN knockdown HCT116 and HT29 cells compared with conditioned media from control cells (Figure 6A and B). We also observed a concurrent decrease in pAkt (Tyr473) in HT29 cells and pAkt (Tyr473) and pErk1/2 in HCT116 cells, the major mediators of signaling downstream of VEGFR-2 (12). In contrast, conditioned media from SW480 cells overexpressing FASN enhanced activation of VEGFR-2 and its downstream targets as compared with control medium (Figure 6A). We also noted that expression of RhoA, a protein important in EC migration, survival and cell permeability (29), is regulated in HMVEC-L cells by the level of FASN expression in CRC cells (Figure 6A).
Fig. 6.
High expression of FASN in CRC cells correlates with activation of VEGFR-2 and its downstream signaling in HMVEC-L. (A) Immunoblot for pVEGFR-2 (Tyr1175), pAkt (Ser473) and pErk1/2 in HMVEC-L cells stimulated for 15min with medium conditioned on CRC cells with altered expression of FASN. Serum-free medium was used as a control. (B) Immunoblot for pVEGFR-2 (Tyr951) and total VEGFR-2 in HMVEC-L cells stimulated for 24h with medium conditioned on CRC cells with FASN knockdown.
Discussion
Angiogenesis is an essential component of metastasis; therefore, we focused this study on elucidation of the role of FASN in regulation of tumor vasculature. We demonstrate that knockdown of FASN is associated with a low MVD and a decrease in VEGF-A bioavailability, a predominant stimulator of angiogenesis (30). Considering that increased MVD and high expression of VEGF-A predict poor prognoses in patients with CRC (14), our data suggest that FASN could be a potential antiangiogenic target for advanced CRC.
We demonstrated that stable knockdown of FASN in human CRC cell lines regulates activation of ECs through differential expression of VEGF-A isoforms. A similar phenomena was observed in melanoma and oral squamous carcinoma cell lines, when treated with pharmacological inhibitors of FASN cerulenin and orlistat (31). VEGF121, VEGF165 and VEGF189 are the VEGF-A isoforms predominantly detected in colorectal tumor tissues (24). Consistent with this study, we identified VEGF121, VEGF145, VEGF165 and VEGF189 mRNA in tested CRC cell lines. Since immunoblot analysis showed that VEGF165 and VEGF189 are the primary isoforms detected at the protein level, we focused our study on these two isoforms. Evaluation of clinical samples demonstrated that expression of VEGF165 has significant correlation with a smaller tumor size, whereas VEGF189 has significant correlation with advanced stages and poor prognosis in CRC (24,32). Our data demonstrates that inhibition of FASN expression is associated with a decrease in expression of VEGF189 protein, suggesting that the beneficial effect of FASN inhibition on tumor vasculature may be mediated by this isoform. The fact that knockdown of FASN did not affect expression of VEGF189 mRNA suggests that FASN may regulate the translation or protein stability of this isoform. In contrast, overexpression of FASN in SW480 cells induces expression of VEGF-A isoforms at both the mRNA and proteins level. Even though we did not see any changes in expression of VEGF165 and VEGF165b in HT29 and KM20 cell lines, we saw a significant increase in VEGF165 protein in the HCT116 cell line. This increase can be explained by a dramatic upregulation of VEGF165b isoform detected by qRT–PCR.
Our observation that knockdown of FASN promotes association of VEGF-A with the plasma membrane and decreases an abundance of VEGF-A within ECM in vitro and in vivo suggests that FASN may affect the release of VEGF-A from a cell. VEGF-A bioavailability is regulated by proteolytic release or cleavage by various proteases (25). MMPs have been implicated in liberation of VEGF-A from cells and extracellular stores, thus, increasing its bioavailability and inducing angiogenic switch (21,28). Since inhibition of FASN attenuates expression of CD44 (6), which is necessary for activation of MMPs including MMP-9 (33), VEGF-A processing by proteases may be inhibited, and that would result in a decrease in VEGF-A expression and secretion, and may promote its accumulation at the plasma membrane. Furthermore, our results demonstrate that an increase in FASN is associated with increased expression of MMP-9 and bioavailability of VEGF-A in vitro and in vivo. These results are consistent with the study showing that production of MMP-9 increases bioavailability of VEGF-A and other ECM-sequestered proangiogenic factors in the mouse model (34). Consistently, our results from the angiogenesis and MMP antibody arrays show that knockdown of FASN enhances secretion of TIMP-1, TIMP-2 and TIMP-4 by CRC cells. As an alternative mechanism, we do not exclude the possibility that changes in the lipid content due to inhibition of FASN might affect the structural properties of the plasma membrane and interfere with cell secretion. Although additional investigations are necessary, these findings suggest that inhibition of FASN may affect multiple factors that are critical in regulation of tumor vasculature and, consequently, metastasis.
Our results show that stable knockdown of FASN in CRC cells affects proliferation, migration and tubulogeneisis of HMVEC-L. Different responses of ECs to condition medium from various CRC cell lines most likely depend on genetic background and mutational status of these cells. We were not able to evaluate the effect of overexpression of FASN in the cell lines which were used for knockdown due to a high level of FASN expression in these cells (6) and possible induction of toxicity by extensive de novo production of lipids when we introduce additional FASN into cells. Interestingly, even though we could not achieve a significant overexpression of FASN, we observed an increase in VEGF189 when HCT116 cells were sorted for expression of pEGFP-FASN (Supplementary Figure 6, available at Carcinogenesis Online). Overexpression of FASN in SW480, a cell line which expresses a low level of FASN compared with other CRC cell lines, predominantly affected tubulogenesis by increasing the length of capillary structures formed on Matrigel. The observed activation of VEGFR-2 points to the importance of VEGF-A in regulation of functional properties of ECs in our model; however, the fact that supplementation of medium with VEGF189 or VEGF-A could not rescue the proliferation of ECs suggests that other factors play a role in activation of ECs and alteration of their functional properties.
Due to a strong correlation between distant metastasis in advanced CRC and angiogenesis (14), tumor vasculature is one of the primary targets for CRC therapy. Despite recent clinical data demonstrating a benefit of anti-VEGF therapy, progression of the disease eventually occurs in many patients suggesting an upregulation of the compensatory angiogenic pathways (27). Moreover, recent studies raise the argument that antiangiogenic treatment may trigger more invasive and metastatic tumors (10,35) and suggest that sustained ‘normalization’ of abnormal tumor vasculature, instead of destruction of tumor vasculature, may be a better approach in the treatment of metastasis (34). Using the orthotopic, CAM and Matrigel plug models, we show that inhibition of FASN improves vessel structure and appearance and is associated with a complete attenuation of dissemination of CRC cells from primary tumors, thus requiring further investigation of FASN as a target for angiogenesis and metastasis.
In summary, the current study, for the first time, provides evidence that inhibition of de novo lipogenesis, by targeting FASN in CRC cells, may balance the profile of secreted anti- and proangiogenic factors including VEGF-A isoforms and thus, inhibit tumor neovascularization, ‘normalize’ tumor surrounding vasculature and, consequently, prevent metastasis in CRC. To support these data, we show that activation and functional properties of ECs are regulated by the level of FASN expression in CRC cells. Furthermore, we show that activity of MMPs, in particular MMP-9, is important in regulating the expression and bioavailability of VEGF-A by FASN.
The fact that FASN appears to be upstream of multiple angiogenic pathways, makes FASN an attractive antiangiogenic target warranting further investigation. Our results strongly suggest that targeting FASN may provide a long-term benefit in normalizing tumor vasculature, improving drug delivery and prolonging survival of patients with advanced CRC. Furthermore, the fact that FASN is secreted by cancer cells (Supplementary Figure 7, available at Carcinogenesis Online) and elevated FASN levels have been detected in the blood of patients with breast, prostate, colon and ovarian cancers compared with normal subjects suggesting that FASN may be used not only as a therapeutic target, but also as a potential diagnostic and prognostic biomarker (36).
Supplementary material
Supplementary Tables 1 and 2 and Figures 1–7 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (P20 CA150343 to B.M.E., T32 CA165990 to B.M.E. and K.L.O., RO1 CA133429 to T.G., RO1 CA109136 to K.L.O.); the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK048498 to B.M.E.).
Supplementary Material
Acknowledgements
The authors thank D.Gilbreath and C.Anthony for help with manuscript preparation, G.Bauman for assistance with fluorescence-activated cell sorting and G.Epperly for assistance with confocal microscopy and Aperio ScanScope XT scanner and software. Author contributions: Y.Y.Z.: study concept and design, data acquisition, statistical analysis; V.A.E.: data acquisition; P.R.: study concept and design, data acquisition; W.C.M.: data acquisition; J.T.K.: data acquisition; J.V.: data acquisition; T.G.: data acquisition; K.L.O.: study concept and design; J.M.N.: data acquisition; E.Y.L.: critical revision for content; H.L.W.: statistical analysis; B.M.E.: study concept and design, study supervision.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CAM
chorioallantoic membrane
- CRC
colorectal cancer
- EC
endothelial cells
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- FASN
fatty acid synthase
- HMVEC-L
human lung microvascular endothelial cells
- IHC
immunohistochemistry
- MMPs
matrix metalloproteinases
- mRNA
messenger RNA
- MVD
microvessel density
- qRT–PCR
quantitative real-time PCR
- TME
tumor microenvironment
- VEGF-A
vascular endothelial growth factor-A
- VEGFR-2
vascular endothelial growth factor receptor-2.
References
- 1. Jemal A., et al. (2011). Global cancer statistics. Cancer J. Clin., 61, 69–90 [DOI] [PubMed] [Google Scholar]
- 2. Kuhajda F.P. (2006). Fatty acid synthase and cancer: new application of an old pathway. Cancer Res., 66, 5977–5980 [DOI] [PubMed] [Google Scholar]
- 3. Zhan Y., et al. (2008). Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin. Cancer Res., 14, 5735–5742 [DOI] [PubMed] [Google Scholar]
- 4. Uddin S., et al. (2009). High prevalence of fatty acid synthase expression in colorectal cancers in Middle Eastern patients and its potential role as a therapeutic target. Am. J. Gastroenterol., 104, 1790–1801 [DOI] [PubMed] [Google Scholar]
- 5. Camassei F.D., et al. (2003). Expression of the lipogenic enzyme fatty acid synthase (FAS) in retinoblastoma and its correlation with tumor aggressiveness. Invest. Ophthalmol. Vis. Sci., 44, 2399–2403 [DOI] [PubMed] [Google Scholar]
- 6. Zaytseva Y.Y., et al. (2012). Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res., 72, 1504–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Migita T., et al. (2009). Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J. Natl Cancer Inst., 101, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joyce J.A., et al. (2009). Microenvironmental regulation of metastasis. Nat. Rev. Cancer, 9, 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dvorak H.F., et al. (2011). Tumor microenvironment and progression. J. Surg. Oncol., 103, 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potente M., et al. (2011). Basic and therapeutic aspects of angiogenesis. Cell, 146, 873–887 [DOI] [PubMed] [Google Scholar]
- 11. Folkman J., et al. (1987). Angiogenic factors. Science, 235, 442–447 [DOI] [PubMed] [Google Scholar]
- 12. Butler J.M., et al. (2010). Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer, 10, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrara N., et al. (2003). The biology of VEGF and its receptors. Nat. Med., 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 14. Des Guetz G., et al. (2006). Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br. J. Cancer, 94, 1823–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olsson A.K., et al. (2006). VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol., 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 16. Crawford Y., et al. (2009). VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res., 335, 261–269 [DOI] [PubMed] [Google Scholar]
- 17. Zigmond E., et al. (2011). Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One, 6, e28858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deryugina E.I., et al. (2008). Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem. Cell Biol., 130, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folkman J., et al. (1989). Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature, 339, 58–61 [DOI] [PubMed] [Google Scholar]
- 20. Nowak D.G., et al. (2008). Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J. Cell Sci., 121(Pt 20), 3487–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S., et al. (2005). Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol., 169, 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uthoff S.M., et al. (2002). VEGF isoforms and mutations in human colorectal cancer. Int. J. Cancer, 101, 32–36 [DOI] [PubMed] [Google Scholar]
- 23. Díaz R., et al. (2008). p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int. J. Cancer, 123, 1060–1067 [DOI] [PubMed] [Google Scholar]
- 24. Cressey R., et al. (2005). Alteration of protein expression pattern of vascular endothelial growth factor (VEGF) from soluble to cell-associated isoform during tumourigenesis. BMC Cancer, 5, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hawinkels L.J., et al. (2008). VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur. J. Cancer, 44, 1904–1913 [DOI] [PubMed] [Google Scholar]
- 26. Park J.E., et al. (1993). The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell, 4, 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrara N. (2002). VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer, 2, 795–803 [DOI] [PubMed] [Google Scholar]
- 28. Belotti D., et al. (2003). Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res., 63, 5224–5229 [PubMed] [Google Scholar]
- 29. Bryan B.A., et al. (2010). RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J., 24, 3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrara N. (2002). Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol., 29 (6 suppl. 16), 10–14 [DOI] [PubMed] [Google Scholar]
- 31. Seguin F., et al. (2012). The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br. J. Cancer, 107, 977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tokunaga T., et al. (1998). Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br. J. Cancer, 77, 998–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zöller M. (2011). CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer, 11, 254–267 [DOI] [PubMed] [Google Scholar]
- 34. Hanahan D., et al. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21, 309–322 [DOI] [PubMed] [Google Scholar]
- 35. Ebos J.M., et al. (2011). Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol., 8, 210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu H., et al. (2010). Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker. Int. J. Biochem. Mol. Biol., 1, 69–89 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.