Abstract
Phytochemical complexity of plant foods confers health-promoting benefits including chemopreventive and anticancer effects. Isolating single constituents from complex foods may render them inactive, emphasizing the importance of preserving the natural composition of whole extracts. Recently, we demonstrated in vitro synergy among the most abundant bioactive constituents of ginger extract (GE), viz., 6-gingerol (6G), 8-gingerol (8G), 10-gingerol (10G) and 6-shogaol (6S). However, no study has yet examined the in vivo collaboration among ginger phytochemicals or evaluated the importance, if any, of the natural ‘milieu’ preserved in whole extract. Here, we comparatively evaluated in vivo efficacy of GE with an artificial quasi-mixture (Mix) formulated by combining four most active ginger constituents at concentrations equivalent to those present in whole extract. Orally fed GE showed 2.4-fold higher tumor growth-inhibitory efficacy than Mix in human prostate tumor xenografts. Pharmacokinetic evaluations and bioavailability measurements addressed the efficacy differences between GE and Mix. Plasma concentration-time profiles revealed multiple peaking phenomenon for ginger constituents when they were fed as GE as opposed to Mix, indicating enterohepatic recirculation. Bioavailability of 6G, 8G, 10G and 6S was 1.6-, 1.1-, 2.5- and 3.4-fold higher, respectively, when dosed with GE compared with Mix. In addition, gingerol glucuronides were detected in feces upon intravenous administration confirming hepatobiliary elimination. These data ascribe the superior in vivo efficacy of GE to higher area under the concentration time curves, greater residence time and enhanced bioavailability, of ginger phytochemicals, when fed as a natural extract compared with artificial Mix, emphasizing the usefulness of consuming whole foods over single agents.
Introduction
A recent paradigm shift recognizes that the anticancer benefits of fruits and vegetables are due to an additive or synergistic interplay of the complex mixture of phytochemicals present in whole foods that work through complementary and overlapping mechanisms, reinforcing interdependency among the constituent components for optimal activity (1–5). Thus, it is plausible that isolating a single compound from complex foods may not be effective even at high, relatively toxic doses, whereas combinations of lower, less-toxic doses of each compound may be effective. This might also explain why clinical trials with pure single phytochemicals such as α-tocopherol, β-carotene and vitamin C have failed in the past, reinforcing the futility of isolating a single constituent from a phytochemical mixture as it may lose its bioactivity or even cause undesirable cancer-promoting effects, as in the case of β-carotene (4,6–8). Concomitantly, it highlights the importance of the lesser known, relatively under studied, minor components of the complex mixtures of fruits and vegetables that have been mistakenly ignored in evaluating the superior bioactivity of whole extracts.
Fruits and vegetables present a treasure trove of polyphenolic compounds that upon ingestion undergo extensive metabolism during passage through the gastrointestinal system primarily to prevent unwanted accumulation and to prompt their elimination from the system (9–12). Most phytochemicals are metabolically modified within the gut before entering the blood circulation. The conjugated sugar groups, if any, are cleaved in the intestinal lumen, followed by glucuronidation, sulfation and/or methylation of the aglycones, to facilitate elimination of phytochemicals subsequent to conjugation mechanisms that enhance their polarity (10,11). However, the residence time of phytochemicals in systemic circulation is responsible for their beneficial activity. Recently, identification of a vast array of phenolic metabolites in circulation has offered useful insights and allowed us to realize and explore the bioavailability, especially of poorly absorbable compounds, such as the phenolics (12,13).
An extensively consumed spice worldwide, ginger (Zingiber officinale Roscoe) is an excellent source of several bioactive phytochemicals. A variety of active components have been identified in the oleoresin, the non-volatile pungent fraction of ginger, mainly including gingerols and shogaols (14–17). The other active ginger constituents include 6-paradol, 6- and 10-dehydrogingerdione, 6- and 10-gingerdione, 4-, 6-, 8- and 10-gingerdiol, 6-methylgingerdiol, zingerone, 6-hydroxyshogaol, 6-, 8- and 10-dehydroshogaol, and diarylheptanoids (14,15,17,18). Nevertheless, gingerols and shogaols are most abundant in ginger compared with any of the other constituents. Extensive literature has successfully shown that 6-gingerol (6G), 8-gingerol (8G), 10-gingerol (10G) and 6-shogaol (6S) are the main bioactive components of ginger (14,15,19–31). Although several reports exist on the antioxidative, anti-inflammatory and antitumor properties of ginger, only marginal benefits of ginger constituents as single agents are yet known from clinical trials (28,29). A careful examination of several studies reveals that the effective in vitro doses of these ginger phytochemicals are much higher than their achievable plasma concentrations, which impose serious limitations on their potential efficacy and utility as chemopreventive supplements in humans (19–21,24,25,27,30,31).
Recently, we reported the tumor growth-inhibitory efficacy of ginger extract (GE) in prostate cancer models (32). In another follow-up study, we demonstrated that the abundantly present most active ginger phenolics namely 6G, 8G, 10G and 6S exhibit synergistic inter-relationships to exert maximum anti-proliferative efficacy (33). Whether or not this in vitro synergistic collaboration among the most active ginger phenolics holds up in in vivo to elicit much higher therapeutic efficacy than the observed tumor inhibition efficacy of GE is yet an uncharted territory. Clearly, it is comprehensible that in vitro methods fail to account for in vivo complexities such as absorption of ginger phenolics from gastrointestinal tract, their metabolic biotransformation, systemic bioavailability and clearance and thus preclude a logical explanation of in vivo therapeutic efficacy.
Here, we demonstrate the remarkably superior efficacy of GE compared with an artificial quasi-mixture (Mix) produced by combining the most active individual phytochemicals in the same ratios as they appear in the natural setting. To take this multiseries ginger study to the next level, we asked whether these active ginger phenolics solely collaborate among themselves or recruit other ginger phenolics in their complex in vivo agenda to ultimately elicit optimal therapeutic activity. Given the significance of metabolism and bioavailability to dictate the in vivo biological effects of phytochemicals, we addressed the discrepancy in the efficacy outcomes of GE and Mix using pharmacokinetic (PK) approaches. We monitored absorption and bioavailability of 6G, 8G, 10G and 6S upon oral feeding of GE compared with the Mix to underscore the presence of beneficial in vivo interactions among GE biophenolics that are largely absent in the Mix. In addition, we developed a sensitive, accurate and robust liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the quantitation of 6-, 8- and 10-gingerols and 6S to study their collaborative interactions when present in their natural milieu and the consequential pharmacological significance.
Materials and methods
Cell culture, chemicals and reagents
GE was a gift from Sabinsa Corporation. Acetonitrile (ACN) and methanol were obtained from Fisher Scientific (Pittsburgh, PA). The silica used for classical chromatography was from EMD Biosciences (Billerica, MA). Thin-layer chromatography (TLC) plates were from EMD Chemicals (Billerica, MA). PC-3, prostate cancer cells, were cultured in RPMI-1640 media supplemented with 10% heat-inactivated fetal bovine serum and 5% penicillin–streptomycin. Luciferase-expressing PC-3 cells (PC-3-luc) were from PerkinElmer (Hopkinton, MA) and were maintained in modified Eagle’s medium with 10% fetal bovine serum, Hyclone (Pittsburgh, PA). The 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide dye (thiazolyl blue tetrazolium bromide, 98% TLC), carboxy methyl cellulose and β-glucuronidase were from Sigma (St Louis, MO). The active ginger constituents, 6G, 8G, 10G and 6S, were extracted from GE and was characterized for >99% purity.
Isolation of active constituents from GE
The methanolic extract of ~20g ginger powder (obtained from Sabinsa) was adsorbed on silica gel 60–120 mesh size and purified using 50 × 6cm intradermally column packed with stationary phase. Gradient elution steps were performed from 100% hexane to 100% ethyl acetate to separate 6G, 8G, 10G and 6S by column chromatography. Different fractions were eluted and collected and monitored by TLC using p-anisaldehyde stain. All the fractions were then further purified by high-performance LC using semi-preparative reversed phase column with mobile phase consisting ACN in water in linear gradient from 45% to 100% at a flow rate of 1 ml/min with a run time of 55min. MS was employed to analyze the purified 6G, 8G, 10G and 6S.
About 100mg of the remaining fraction of GE containing trace amounts of 6S after isolating 6G, 8G, 10G and 6S was loaded onto preparative TLC plate and was developed in a glass chamber consisting a binary mobile phase of ethyl acetate and hexane (2:8). Pure 6S was used as a standard to separate and remove the 6S from this fraction. The rest of the fraction was scraped, dissolved in methanol, filtered and concentrated for testing. High-performance LC analysis of this fraction confirmed the absence of 6G, 8G, 10G and 6S. This fraction devoid of the active ginger phytochemicals, known as GE-Mix, was employed in future studies.
Formulation recipe of GE, Mix and GE-Mix for in vivo PK studies
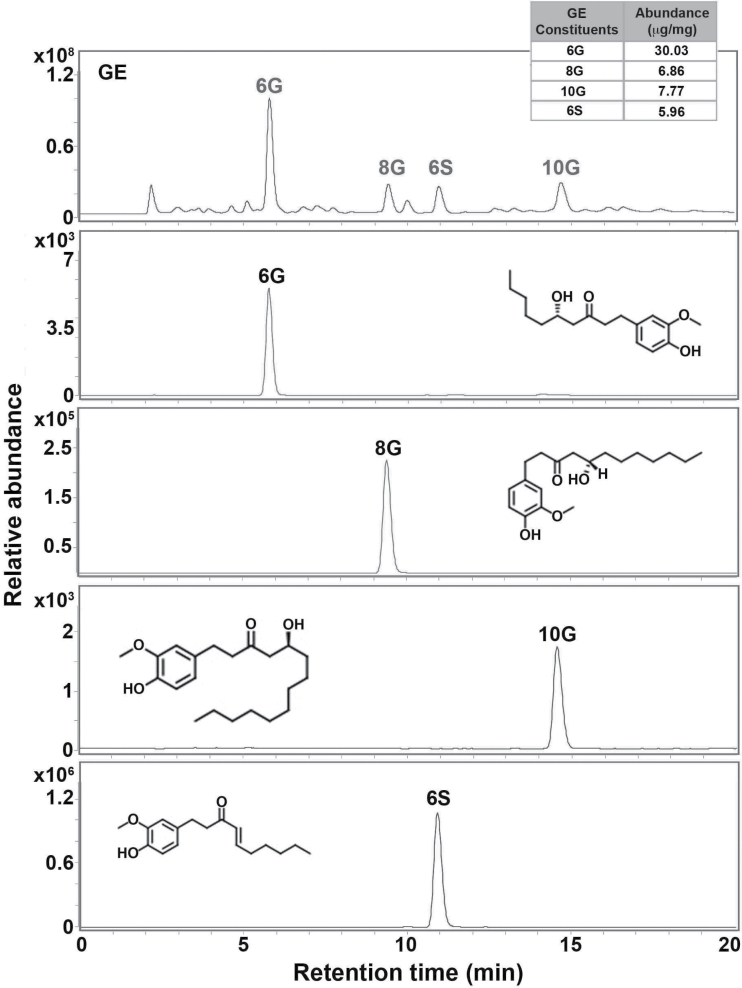
Required amount of GE, Mix and GE-Mix were weighed and appropriate volume of ethanol (10%) and polyethylene glycol 300 (30%) were added, vortex mixed and sonicated for 5min. The volume was made up with 0.25% Tween-80 in 0.5% carboxy methyl cellulose in Milli-Q® water quantity sufficient for Mix, pure standards of 6G, 8G, 6S and 10G were mixed in the same proportion as in GE (Figure 1).
Fig. 1.
Quantitation of gingerols: 6G, 8G, 10G and 6S were quantified employing LC-MS/MS with electrospray ionization in positive-ion mode. The presence of 6G (MRM: 277.2/177.2, RT: 5.8min), 8G (MRM: 305.2/137.2, RT: 9.4min), 10G (MRM: 333.2/137.2, RT: 14.6min) and 6S (MRM: 277.2/137.1, RT: 10.9min) were confirmed using analytical standards and quantified using calibration curve for each individual component (shown in the inset).
Choice of in vivo dose for efficacy and PK studies
The choice of 250mg/kg dose is based on the human PK studies performed by Zick et al. (28,29), where free forms of ginger biophenolics were detected in the human blood plasma upon oral administration of 2g GE. Upon normalizing for a 75kg body wt, this 2g dose is equivalent to 25mg/kg. Given the faster metabolic rate in mice compared with humans and the allometric scaling factor of 10 (34), a dose of 250mg/kg body wt was chosen for mouse studies. Furthermore, the total amount of the active ginger biophenolics (6G, 8G, 10G and 6S) present in GE used in this study was similar to that used in the clinical studies (5%, i.e. ~13mg in 250mg of GE). Because dose toxicology studies are often conducted for 7, 14 or 28 days (35), we chose to observe the tumor growth inhibition upon oral administration of GE/Mix/GE-Mix over a period of 28 days.
In vivo tumor growth and bioluminescent imaging
PC-3-luc cells (1 × 106) were subcutaneously injected on either flank of 6-week-old male BALB/c nude mice (Harlan Laboratories, Indianapolis, IN). When tumors were palpable, mice were randomly divided into four groups of five mice each. Control group received vehicle and the treatment groups received 250mg/kg GE, Mix and GE-Mix by oral gavage daily for 28 days. Tumor growth was monitored by measuring the luciferase activity in live mice by bioluminescent imaging in real time using the IVIS in vivo imaging system (PerkinElmer) with the Live Imaging software. Briefly, mice anesthetized with isoflurane were intraperitoneally injected 30mg/ml luciferin and imaged with a charge-coupled device camera. An integration of 20 s with four binnings of 100 pixels was used for image acquisition. The relative photon counts at the tumor sites of the mice from vehicle or GE, Mix and GE-Mix treated groups were quantitated twice a week along with tumor volume measurements for 4 weeks. Body weights were recorded twice a week to evaluate the general health and well being of animals during treatment. Mice in treatment groups exhibited normal weight gain with no signs of discomfort. All animals in control group were euthanized after fourth week due to tumor overburden in compliance. All animal experiments were performed in compliance with institutional animal care and use committee guidelines.
PK studies of GE versus Mix
PK studies were performed in male C57BL6J mice following a single oral administration of GE and Mix at 250mg/kg and intravenous (IV) dose administration at 1mg/kg of pure 6G, 8G, 10G and 6S individually. All animals were acclimatized for 3 days before dosing in the experimental area. Mice were fasted for 3h before dose administration and food was provided 3h postdose. Water was provided ad libitum through the study period. Animals were marked and housed (three per cage) in polypropylene cages and maintained in controlled environmental conditions with 12h light and dark cycles. The temperature and humidity of the room were maintained between 22±3°C and 30–70%, respectively, and ~10–15 fresh air change cycles per hour. A sparse sampling design was used to collect blood samples from animals at 5min, 10min, 15min, 30min, 1h, 2h, 4h, 6h, 8h, 12h and 24h into K2EDTA-coated tubes. Plasma was harvested from blood by centrifugation of samples at 8000g for 10min. Feces samples were collected from mice treated with IV dose of individual gingerols at 0–2, 2–4, 4–6, 6–8, 8–12 and 12–24h intervals. All samples were stored below −60°C until bioanalysis.
Enzymatic hydrolysis of gingerol conjugates
To confirm the presence of glucuronide conjugates, plasma (200 µl) and feces samples (homogenized with buffer 1:3, 200 μl) were treated with β-glucuronidase (50 μl, 500 units) and incubated at 37°C for 1h.
Plasma protein binding assay
Binding potential of 6G, 8G, 10 G and 6S in GE and Mix was assessed using ultra-centrifugation technique. The study was conducted at 0.3, 1 and 3mg/ml in triplicate. Appropriate volumes of GE or Mix stock solution (10 µl) were spiked into plasma (990 µl) to attain the final concentrations. The microfuge tube was mixed by inversion and incubated at 37°C for 30min. An aliquot (50 μl) of the incubated samples was collected in microfuge tube. The remaining samples were subjected to ultra-centrifugation at 40 000g for 1h and aliquots of supernatant (50 μl) were carefully collected into microfuge tubes.
Bioanalysis
All in vitro and in vivo samples were processed by protein precipitation method. An aliquot (50 µl) of sample was added with 20 µl internal standard (capsaicin), 180 µl ACN and vortex mixed for 3min. The tubes were centrifuged at 8000g for 10min and an aliquot of supernatant was transferred into auto-sampler vials for analysis.
The stock solutions of 6G, 8G, 10G, 6S and capsaicin (internal standard) were prepared in ACN:water (95:5) at 1mg/ml. A calibration curve range of 0.002–2 µg/ml was employed for the quantification of analytes and internal standard concentration was 100ng/ml for each analysis. The calibration curve consisted of blank, blank with internal standard and six non-zero calibration standards. The calibration standards were within ±15% of the nominal concentration and lower limit of quantification was within ±20% of nominal.
All samples were analyzed using LC-MS/MS method (Agilent 6410 series). A positive ionization mode with multiple reaction monitoring (MRM, m/z Q1/Q3) of 6G (m/z 277.2/177.2, retention time [RT] 5.8min), 8G (m/z 305.2/137.2, RT 9.4min), 10G (m/z 333.2/137.2, RT 14.6min), 6S (m/z 277.2/137.1, RT 10.9 min) and IS (m/z 306.2/137.1, RT 6.8min) was employed. The ion spray voltage was set at 3000V, ionization temperature was set as 200°C and drying gas flow rate was 10 l/min. Data acquisition and quantitation were performed using Mass Hunter software (Agilent Technologies, Wilmington, DE). Separation was achieved using HP1100 series LC (Agilent Technologies) equipped with a photodiode array detector, using an Agilent Zorbax reversed-phase (SB-C18, 3.0 × 250mm, 5.0 μm) column. A gradient method was employed to separate the individual GE components using mobile phase A (0.1% formic acid in water) and mobile phase B (ACN). The gradient elution method with 60% B at 0min, 90% B at 20min, held for 10min, back to 60% B at 40min with a flow rate of 0.4 ml/min. An injection volume of 10 μl was used for analysis.
PK analysis
PK parameters were calculated from the concentration-time data using the non-compartmental analysis tool of validated WinNonlin® software (version 5.2, Pharsight, St Louis, MO). The area under the concentration time curve (AUClast and AUCinf) was calculated by the linear trapezoidal rule. Following oral administration, peak concentration (C max) and time for the peak concentration (imax) were the observed values. The clearance and volume of distribution (V ss) were estimated following IV dose administration. The elimination rate constant value (k) was obtained by linear regression of the log-linear terminal phase of the concentration-time profile using at least three declining concentrations in terminal phase with a correlation coefficient of >0.8. The terminal half-life value (T 1/2) was calculated using the equation ln2/k. Oral bioavailability was calculated by taking the ratio of dose normalized AUClast following oral administration to IV administration.
Statistical analysis
All the values are expressed as mean ± standard deviation (SD) where applicable. The Student’s t-test was used to determine the differences among various treatments, with P-value of <0.05 (α = 0.05) considered statistically significant. Furthermore, a two-way analysis of variance was performed to evaluate the differences between vehicle- and GE-/Mix-fed groups for in vivo efficacy studies and P-values were obtained from two-sided tests for statistical significance.
Results
To discern the importance of the natural ‘milieu’ of GE and in vivo interactions between the most active ginger phytochemicals, we artificially created a Mix of the active GE constituents, 6G, 8G, 10G and 6S, hereafter called as Mix. Essentially, the first step was to isolate, purify and spectrally characterize the aforementioned GE biophenolics using column chromatography (Supplementary Figure 1, available at Carcinogenesis Online). Further, to formulate the Mix, the purified ginger phytochemicals (6G, 8G, 10G and 6S) were then mixed exactly in the same ratio and concentrations at which they appear in the GE. The quantitation of individual ginger phytochemicals in GE was performed using LC-MS/MS, which indicated that 1mg of GE consists of 30.03 μg of 6G, 6.86 μg of 8G, 7.77 μg of 10G and 5.96 μg of 6S (Figure 1).
Having formulated a Mix with matched concentrations of the most abundant and active ginger phytochemicals, we asked if oral feeding with Mix recapitulated or surpassed GE’s remarkable in vivo efficacy. To this end, we performed an in vivo experiment to evaluate tumor inhibition by Mix compared with GE in a real-time non-invasive bioluminescent prostate cancer model.
GE is superior to Mix in inhibiting prostate tumor growth
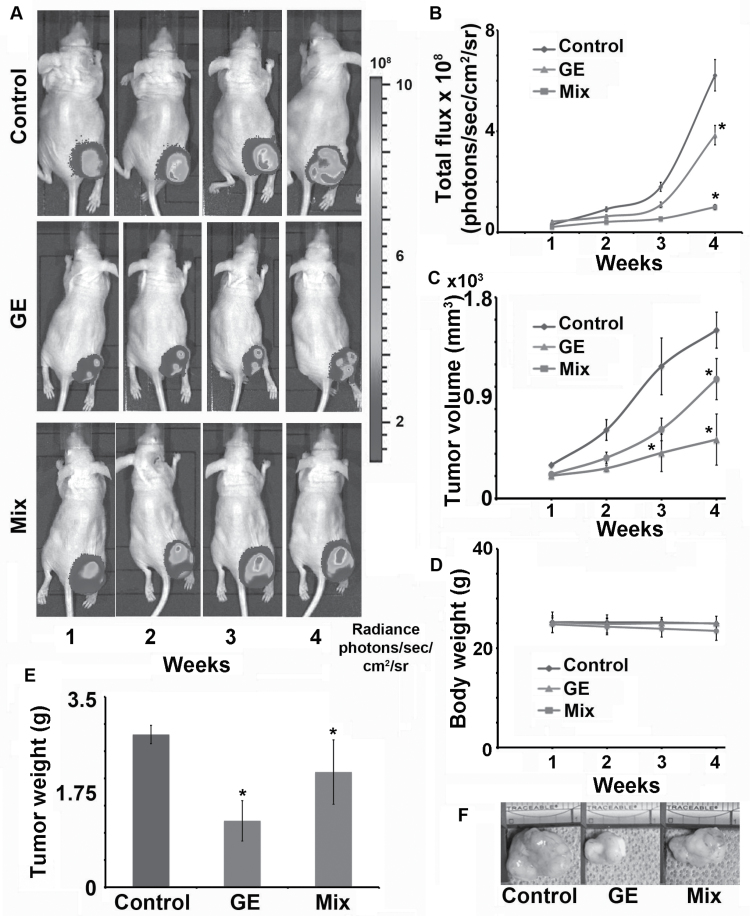
We compared the in vivo tumor growth-inhibiting efficacy of GE and Mix in human prostate cancer xenografts implanted subcutaneously in the right flanks of athymic nude mice. A human prostate cancer PC-3 cell line stably expressing luciferase enzyme (PC-3-luc) was employed, which facilitates real-time non-invasive monitoring of prostate cancer growth. Animals were randomized and divided into three groups of five mice each for the study. The study included one vehicle-fed control group and two treatment groups fed daily with 250mg/kg of GE and Mix via oral gavage for 4 weeks. Our results indicated that both treatment groups exhibited a time-dependent inhibition of tumor growth (Figure 2A–C), compared with vehicle-treated controls. Quantitative comparison of the relative total photon flux that reflected tumor volume revealed that GE caused ~68% inhibition of tumor growth compared with Mix, which was only ~28% as measured at the end of week 4 (P < 0.05; Figure 2B, C and E).
Fig. 2.
Oral administration of GE exhibited enhanced inhibition of human prostate tumor xenograft growth in nude mice compared with Mix. Male nude mice were subcutaneously injected with 106 PC-3-luc cells. (A) Representative bioluminescent images of one animal per group indicating progression of tumor growth over 4 weeks. (B) Graphical representation of quantitative radiance measured as the number of photons leaving a square centimeter of tissue and radiating into a solid angle of 1 steradian (photons/sec/cm2/sr) from control vehicle, GE- and Mix-fed mice for 4 weeks. (C) Tumor growth monitored (by vernier calipers) and presented as tumor volume in cubic millimeter, over a period of 4 weeks. (D) Graphical representation of body weight of vehicle, GE- and Mix-treated mice. (E) Graphical representation of tumor weight. (F) Representative photographic images of excised tumors [*P < 0.05 (two-way analysis of variance), as compared with controls; B, C and E]. Error bars refer to ±SD. Statistical significance in percent tumor growth between control and treatment groups was achieved after week 3.
Plasma concentration-time profile of GE shows multiple peaking phenomenon
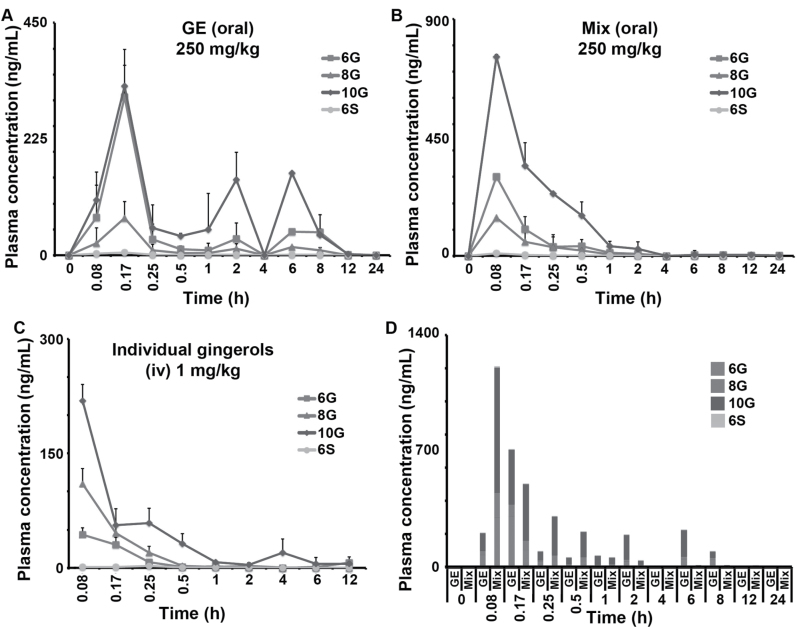
Intriguingly, despite identical concentrations of the four major active biophenolics in both GE and Mix, we found a remarkable difference (40%) in the inhibitory efficacy of GE and Mix. To gain insights into this discrepancy, we evaluated the levels of these four ginger biophenols in the plasma following oral administration of GE and Mix (250mg/kg) in C57/BL6J mice (three mice per group). All the plasma samples were then evaluated for the presence of 6G, 8G, 10G and 6S followed by their quantitation using LC-MS/MS (Figure 3A–C). To assess the bioavailability of these components, 6G, 8G, 10G and 6S were dosed individually at an IV dose of 1mg/kg.
Fig. 3.
GE feeding results in EHR causing multiple peaking phenomenon. Plasma concentration-time profiles of gingerols following oral administration of 250mg/kg (A) GE, (B) Mix and following IV administration of 1mg/kg, (C) individual gingerols and (D) comparison of plasma concentration-time profiles of gingerols achieved at each time point upon feeding GE and Mix. Error bars refer to + SD.
The PK parameters of individual GE biophenolics following IV and oral administration were calculated using the plasma concentration time profiles (Tables I and II). Following IV administration, the clearance of all gingerols was more than normal liver blood flow in mice (90ml/min/kg). The volume of distribution was 20-fold higher than normal body water (0.7 l/kg) suggesting extensive distribution of these components. The half-life of gingerols varied between 0.8 and 6h. Following oral administration, all gingerols showed Cmax at 10min with GE and 5min with Mix (Figure 3A and B, Table II).
Table I.
PK parameters of gingerols following IV bolus administration of active individual ginger phytochemicals in C57BL/6J mice (dose: 1mg/kg)
| Analyte | C 0 (ng/ml) | AUClast (ng*h/ml) | Clearance (ml/min/kg) | V SS (l/kg) | MRT (h) | T 1/2 (h) |
|---|---|---|---|---|---|---|
| 6G | 62.20 | 26.66 | 468.01 | 281.68 | 6.43 | 6.06 |
| 8G | 264.91 | 33.64 | 493.96 | 13.31 | 0.43 | 0.79 |
| 10G | 847.47 | 168.59 | 91.60 | 21.93 | 2.98 | 3.28 |
| 6S | 0.96 | 1.15 | 14044.40 | 946.28 | 0.93 | 1.36 |
C 0, back extrapolated concentration; MRT: mean residence time.
Table II.
PK parameters of gingerols following oral gavage administration of GE and Mix in C57BL/6J mice (dose GE: 250mg/kg; Mix: prorated to amount present in GE)
| Analyte | T max (h) | C max (ng/ml) | MRT (h) | AUClast (ng*h/ml), PO | Bioavailability, F | Fold increase in F | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GE | Mix | GE | Mix | GE | Mix | GE | Mix | GE | Mix | ||
| 6G | 0.17 | 0.08 | 305.9±92.45 | 300.69±2.95 | 3.95 | 3.34 | 219.04 | 137.97 | 110 | 69 | 1.6 |
| 8G | 0.17 | 0.08 | 71.74±32.61 | 144.71±0.49 | 2.81 | 1.45 | 48.65 | 44.49 | 84 | 77 | 1.1 |
| 10G | 0.17 | 0.08 | 327.26±40.12 | 756.62±7.62 | 4.57 | 1.34 | 957.34 | 281.56 | 385 | 113 | 3.4 |
| 6S | 0.17 | 0.08 | 5.68±0.18 | 10.57±4.72 | 4.63 | 2.10 | 7.37 | 2.97 | 330 | 133 | 2.5 |
F, bioavailability—calculated using dose normalized AUClast; MRT: mean residence time; PO, single oral dose.
Furthermore, a comparison of mean plasma concentration-time profile of 6G, 8G, 10G and 6S as shown in Figure 3D, revealed that these components, when fed as a Mix without the ‘natural milieu’ are eliminated at a faster rate compared with GE. Upon oral feeding, 10G achieved a higher Cmax (756.62±7.62ng/ml) in case of Mix compared with GE (327.26±40.12ng/ml) and was statistically significant (P < 0.001). Similarly, 8G also showed statistically significant (P = 0.02) difference in Cmax between Mix (144.71±0.49ng/ml) and GE (71.74±32.61ng/ml). However, 6G and 6S showed similar Cmax when fed as GE or Mix (P > 0.05; Figure 3, Table II). Interestingly, the exposure (AUClast) of 6G, 10G and 6S was 2- to 3-fold higher when GE was fed orally compared with Mix except for 8G, which showed a similar profile for both. No statistical analysis was performed on AUClast as it was a sparse sampling design with composite profile. Surprisingly, a second and third Cmax peak was observed for all ginger phytochemicals in case of GE at 2 and 6h, a phenomenon usually observed in case of enterohepatic recirculation (EHR) of molecules from the intestine back to systemic circulation (36). A similar profile was observed in case of IV administration of pure standards (Figure 2C). Surprisingly, this multiple peaking phenomenon was not observed in case of Mix, thus setting grounds for further investigation of GE’s peculiar PK profile.
EHR of GE biophenolics
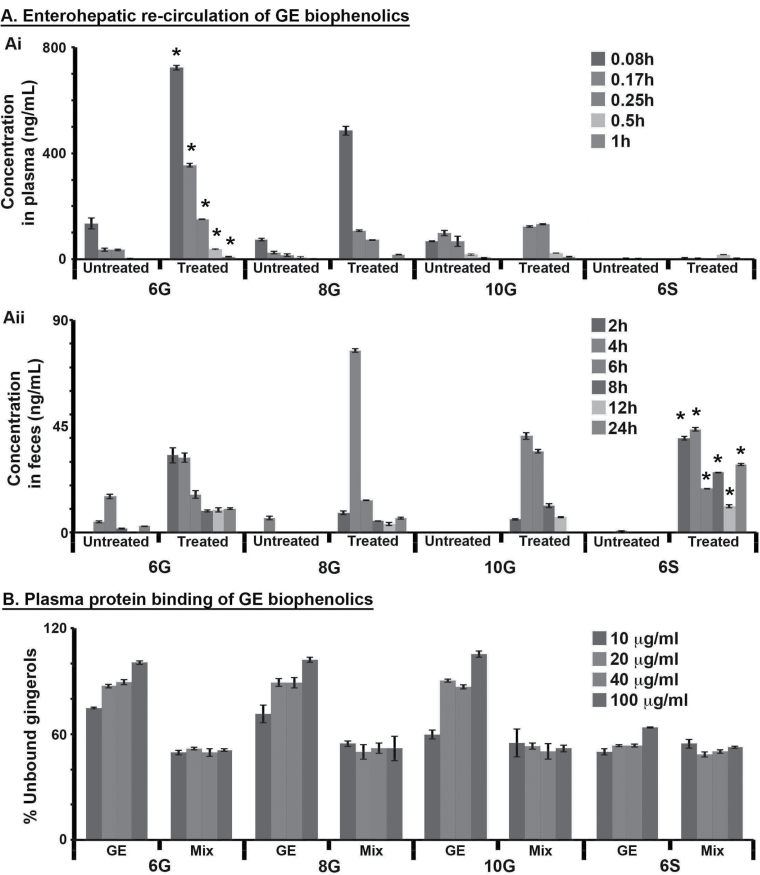
Following intake of drugs or natural products, the disposition of these molecules occurs by several pathways, of which biotransformation is the most notable. Also, the drug or natural product metabolites may even be excreted into the bile or to general circulation. Multiple peaks were observed in case of 6G, 8G and 10G upon GE feeding at 2 and 6h following an initial Cmax at 5min. This multiple peaking phenomenon is perhaps the consequence of EHR of GE phenolics, where their glucuronidated forms that are ready to be eliminated via feces are broken down by the glucuronidase enzymes in the intestine to be released as free forms of gingerols, which are then available for reabsorption. To confirm this, plasma and feces samples obtained after IV administration of pure GE biophenolics were subjected to β-glucuronidase hydrolysis, an enzyme responsible for de-conjugation of glucuronide metabolites in the intestine. The hydrolyzed samples were then analyzed and quantitated for 6G, 8G, 10G and 6S using high-performance LC-MS. Their amounts (ng/ml) in enzyme-treated and untreated samples were then compared (Figure 4Ai and Aii). The presence of significant amounts of 6G and 8G in enzyme-treated plasma samples at 5, 10 and 15min (Figure 4Ai) indicates these gingerols undergo conjugation in the intestine/liver to enable elimination. On the other hand, 10G and 6S were present in very low amounts in their glucuronidated forms.
Fig. 4.
β-Glucuronidase activity assay in plasma and feces obtained from mice IV injected with individual active ginger constituents. (Ai) Comparison of plasma concentration-time profile of 6G, 8G, 6S and 10G in untreated and β-glucuronidase-treated plasma samples following IV dose administration of individual ginger phytochemicals. The values of 6G, 8G, 10G and 6S in the enzyme-treated plasma samples were compared with the untreated samples using independent sample t-test. There was no significant difference in values of 8G, 10G and 6S (P > 0.005) but were different for 6G (*P < 0.005). (Aii) Comparison of concentration-time profiles of 6G, 8G, 6S and 10G in untreated and β-glucuronidase-treated feces samples following IV dose administration. The values of 6G, 8G, 10G and 6S in treated feces samples were compared with untreated samples using independent sample t-test. There was no significant difference in values of 6G, 8G and 10G (P > 0.005) but were different for 6S (*P < 0.001). (B) Plasma protein binding of gingerols in GE versus Mix. Percentage of unbound gingerols in GE is more than in Mix when compared at different concentrations. Error bars refer to ±SD.
Although the plasma concentration profile of ginger conjugates indicate their rapid elimination from the system, the data from feces samples subjected to β-glucuronidase hydrolysis (Figure 4Aii) corroborated that all the four components are conjugated and eliminated through bile. In the treated feces samples, the free forms of 6G, 8G, 10G and 6S upon quantitation were found to exist in very high amounts. This observation clearly indicates that upon GE feeding, even though the ginger biophenolics are conjugated as early as 5min and for a possible elimination (Figure 4Ai), they can undergo systemic circulation for prolonged time (Figures 2C and 4Aii), thus resulting in higher exposure and greater residence times in the system. It is thus clearly evident from the above observations that GE biophenolics undergo EHR (Figure 2C). The PK profiles of GE and Mix containing the same amounts of 6G, 8G, 10G and 6S (Figure 2A and B) clearly indicate that in their natural setting, these GE phenolics undergo EHR, whereas in a Mix, they get eliminated within 2h. Thus, we next asked as to why these components exhibit different behavior when present in different matrices.
Plasma protein binding of gingerols when present in GE versus Mix
Binding of a drug to plasma proteins plays a major role in influencing efficacy, drug distribution and toxicity. Extensive literature underscores that only unbound drugs circulating in the blood have better access to target tissues. To discern the differences in plasma protein binding of ginger phytochemicals present in GE and Mix, we conducted a plasma protein binding study. We found that the binding of 6G, 8G and 10G to plasma proteins was lesser in GE compared with Mix. With an increase in concentration from 10 to 100 µg/ml, the free fraction increased except for 6S, which showed similar binding profile in both GE and Mix (Figure 4B). To summarize, GE phenolics when present in GE are primarily present in free form (more unbound) thus facilitating their free transportation to the target tissues, which thus contributes toward the better efficacy of GE compared with Mix.
Other less abundant GE phenolics collaborate with main GE constituents to enable delivery of optimal in vivo benefits
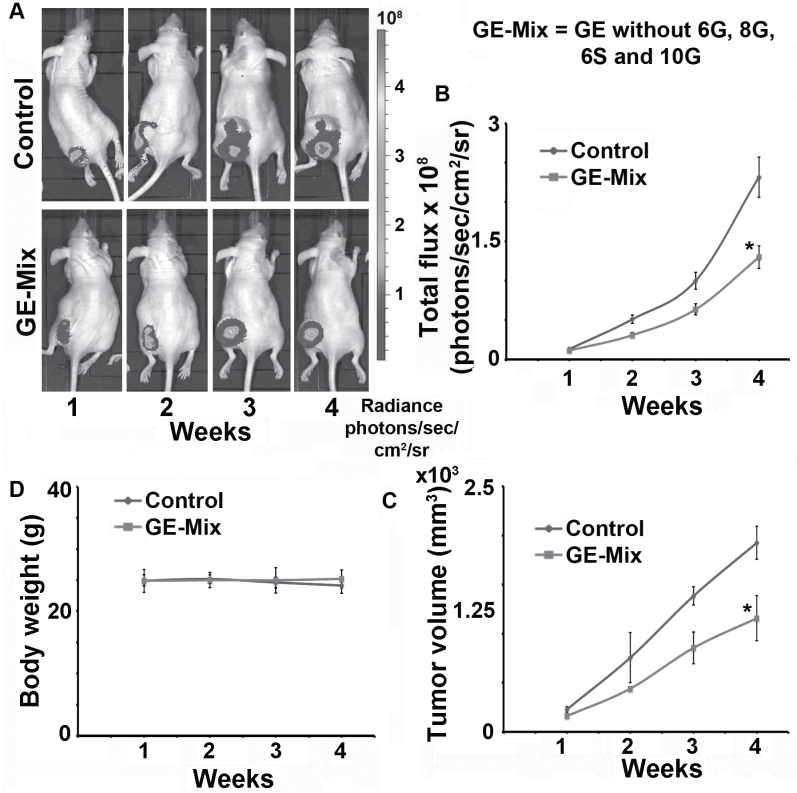
Given that 6G, 8G, 10G and 6S in GE are present in their natural plant matrix along with other ginger phytochemicals like 6-paradol, 8-, 10- shogaol, zingerone, zerumbone and so on (14–18,37), we cannot exclude the contributions from these minor components. To rule out their input, we prepared another fraction lacking in 6G, 8G, 10G and 6S, which we referred to as GE-Mix (aka GE minus Mix). Essentially, it was obtained by combining all the ginger subfractions collected after isolating 6G, 8G, 10G and 6S from GE. We tested the in vivo efficacy of GE-Mix to account for the activity of the residual GE components (Figure 5). Intriguingly, GE-Mix fed animals showed ~35% inhibition in tumor growth compared with vehicle-fed controls indicating that several other ginger phytochemicals beyond the most abundant ones too can exert anticancer activity, possibly through additive/synergistic interactions.
Fig. 5.
Oral administration of GE-Mix also exhibited inhibition of human prostate tumor xenograft growth in nude mice. Male nude mice were subcutaneously injected with 106 PC-3-luc cells. (A) Representative bioluminescent images of one animal per group indicating progression of tumor growth over 4 weeks. (B) Graphical representation of quantitative radiance measured as the number of photons leaving a square centimeter of tissue and radiating into a solid angle of 1 steradian (photons/s/cm2/sr) from vehicle and GE-Mix fed mice for 4 weeks. (C) Tumor growth monitored (by vernier calipers) and presented as tumor volume in cubic millimeter, over a period of 4 weeks. (D) Graphical representation of body weight of vehicle control and GE-Mix treated mice [*P < 0.05 (two-way analysis of variance), as compared with controls; B and C]. Error bars refer to ±SD. Statistical significance in percent tumor growth between control and treatment groups was achieved after week 3.
Discussion
Over the past several years, the debate on the use of whole foods versus single agents to achieve optimal health benefits has spurred numerous studies that have consistently proven that consumption of whole food extracts underlies improved therapeutic efficacy over single-isolated constituents. Primarily, this has been speculatively attributed to the presence of additive/synergistic interactions among the phytochemicals in the former along with other factors including solubility, physiochemical characteristics and PKs of the compounds (1–5,38,39). Nonetheless, attempts to improve the efficacy of whole extracts by isolating the most active fraction(s) or single agents alone are also being intensely investigated. Given the perplexing phytocomplexity of plant extracts, several questions arise. Is it even possible to selectively isolate only the components responsible for efficacy, while ignoring the insignificant partners? Would the resultant ‘most active’ fraction(s) show significant improvement, while working via the same pathways as the parent extract? Would the ‘single-isolated component’ or ‘most active fraction’ be absorbed by the body in the same way as the whole extract? These are some of the stimulating questions that led us to formulate this study, wherein we provide compelling in vivo evidence that ginger biophenolics collaboratively interact with each other to deliver maximum health benefits. This study also shows for the first time that upon oral feeding, gingerols present in GE (6G, 8G and 10G) undergo EHR to enhance their bioavailability and reabsorption into the systemic circulation, thus imparting maximum tumor growth inhibiting efficacy.
Given the existence of additive and/or synergistic interactions among the active ginger constituents, 6G, 8G, 10G and 6S in vitro (33), an obvious next step was to investigate if these in vitro interactions hold up in an in vivo situation. Our data demonstrate existence of in vivo interactions among GE phytochemicals as evidenced by the tumor growth-inhibiting efficacy of GE (~68%) compared with that of Mix (~28%). The superior efficacy of GE by 40% unmasked other partners that contributes toward GE’s remarkable activity in addition to 6G, 8G, 10G and 6S (Figure 2A and B). Further credence to this notion accumulated when we ‘created’ a subfraction of GE called GE-Mix that lacked the majorly well-known four bioactive components and tested for its in vivo anticancer efficacy. Surprisingly, GE-Mix showed ~35% inhibition of tumor growth (Figure 5) indicating that the less known partners other than 6G, 8G, 10G and 6S also possess significant anticancer potential.
Despite the increasing popularity of whole food extracts, their PK and absorption, distribution, metabolism and excretion data are scant or lacking. Many components of most food extracts undergo phase I and/or phase II metabolism in vivo, with cytochrome P450s and uridine diphosphate glucuronosyltransferases majorly dictating overall bioavailability of the active components in the human body upon ingestion (9,10,12,13). To the best of our knowledge, no studies have yet compared the PK and bioavailability of the active components when fed as single agents or fed in their natural extract setting. Indeed, the pharmacological activity is gained only when the ‘active agents’ or ‘active metabolites’ attain as well as sustain appropriate levels so that they can be optimally distributed to the tumor tissue for therapeutic action. In our context, we found no studies that have compared the bioavailability of ginger phytochemicals when they are fed as an artificially formulated mixture of single agents (at concentrations identical to those present in the natural extract) to the whole extract (phytochemicals present in their natural milieu). Keeping in mind the collaborative interactions among GE phytochemicals, we conducted PK studies to recognize any differences in the bioavailability of 6G, 8G, 10G and 6S when they are consumed as GE or Mix.
The PK profiling of the four GE components upon oral administration of 250mg/kg of GE or Mix revealed that there were multiple Cmax peaks observed in case of GE, compared with a single peak in Mix (Figure 3A and B). This multiple peaking phenomenon, associated with recirculation of compounds from intestine to systemic circulation after getting eliminated through bile, was not observed when pure GE phytochemicals were orally gavaged as a Mix (Figure 3B). Furthermore, the plasma concentrations of the four GE components were short-lived, when present in Mix (Figure 3D). The β-glucuronidase hydrolysis of both plasma and feces samples obtained post-IV administration of pure ginger biophenolics (Figure 4Ai and Aii) confirmed that the gingerols re-enter the liver via hepatic portal vein from the intestine for reabsorption into the systemic circulation. Gingerols when fed as GE mimicked this phenomenon and exhibited multiple Cmax values, whereas Mix was eliminated from the body within 2h of feeding. In addition, a higher percentage of unbound gingerols were observed in GE compared with Mix (Figure 4B), which may aid in the enhanced availability of ginger phenolics at the target sites in the former setting. Further, the comparison of in vitro inhibitory concentrations of 6G, 8G, 10G and 6S and their respective in vivo C max values achieved in the blood plasma following the oral administration of 250 mg/kg GE reveal that the plasma levels of ginger phenolics are significantly lower than the effective in vitro concentrations (Supplementary Table 1, available at Carcinogenesis Online). Although we do not expect these single-dose levels to correlate with in vitro efficacy, our observations clearly indicate that GE phytochemicals exert maximum therapeutic properties when present in their natural setting of a complex plant matrix along with other indispensable partners.
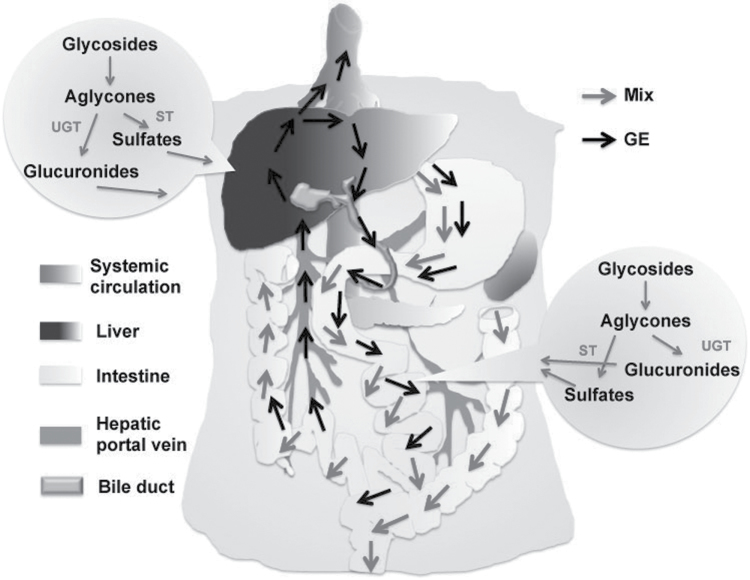
However, the intriguing question to ponder is how EHR of 6G, 8G and 10G is facilitated in case of oral GE feeding and why is it not the same with Mix? A major challenge associated with consumption of xenobiotics that undergo phase II metabolism like anthocyanins, flavanoids, polyphenols and so on is their lack of in vivo bioavailability. The therapeutic benefits of polyphenols are often the result of coupled metabolic activities of efflux/uptake transporters and conjugating enzymes, which play a major role in drug metabolism, elimination and detoxification (40–43). Conjugating enzymes like uridine diphosphate glucuronosyltransferases and sulfotransferases conjugate phenolic compounds to produce hydrophilic metabolites (44–46), which by the action of chemical pumps, also known as efflux transporters, can be diffused out of the cells. Gingerols undergo conjugation in various organs including small intestine, liver and kidneys to avoid their toxic accumulation and hence get eliminated from the system. However, sometimes, these pumps function as gatekeepers of excess metabolism and thus enable enterohepatic recycling of phenolics resulting in their reabsorption and improved half-lives. This phenomenon is better explained via the ‘revolving door’ theory (9,47), which emphasizes that effective elimination of hydrophilic phase II metabolites of xenobiotics depend on chemical pumps. When their rate of conjugation exceeds that of elimination, these metabolites tend to accumulate inside the cell, which may lead to toxicity. During such adverse conditions, a reverse reaction, where the de-conjugating enzymes like glucuronidases present in various organs including liver and intestine, release the free forms of these metabolites into the system, can be favorable (as illustrated in Figure 6). In such cases, the reabsorption of active constituents in lower amounts may actually prove to be beneficial than become toxic due to the build up (47).
Fig. 6.
GE is more efficacious than the Mix of its active constituents. After oral dose administration of Mix to mice, gingerols were eliminated at a faster rate compared with when GE was dosed. After administering GE, the exposure of gingerols was more due to EHR of gingerols, thus conferring GE improved efficacy in inhibiting prostate tumor growth compared with Mix.
Considering the perks of the revolving door mechanism in the cells of gastrointestinal system, we thus strongly believe that the reabsorption of gingerols after oral feeding of GE could be responsible for its superior efficacy over Mix (Figure 6). It is reasonable to speculate that EHR as seen in case of whole extract (GE) could likely be occurring due to the fact that a wide variety of molecules (from GE) undergo conjugation at a given time resulting in a possible elimination lag of the ‘bioactive’ components compared with Mix, which is only made up of the four bioactive phenolics. It is also likely that the whole extract offers a competition due to the varying binding affinities of different molecules for uridine diphosphate glucuronosyltransferases, and the most active molecules are retained in their unconjugated state at the expense of the ‘less active’ components, which get eliminated. This would perhaps explain enhanced transport of free active gingerols to the tumor tissues. This intricate ‘compensatory’ or ‘buffering’ mechanism offered by the complex phytochemical network is lacking for the gingerols in Mix, and perhaps may result in their faster elimination, which might underlie the sharp attenuation of efficacy. Indeed, mechanistic insights on the different phase II enzyme systems that occupy ‘center stage’ in the elimination kinetics of ginger phytochemicals will empower us with new knowledge and may result in a conceptual advancement to develop logically driven rational chemoprevention.
In conclusion, our study emphasizes the existence of a complex collaborative interplay among GE phytochemicals to confer maximum therapeutic benefits due to its favorable absorption kinetics and bioavailability. Our observations of possible EHR of gingerols, when delivered in their natural matrix, are compelling and provide impetus to investigate and design futuristic combinations/dietary supplements for prostate cancer management.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute at the National Institutes of Health (R00CA131489 and R01CA169127 to R.A.); American Cancer Society (121728-RSG-12-004-01-CNE).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- 6G
6-gingerol
- 6S
6-shogaol
- 8G
8-gingerol
- 10G
10-gingerol
- ACN
acetonitrile
- AUC
area under the concentration time curve
- GE
ginger extract
- EHR
enterohepatic recirculation
- IV
intravenous
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- MRM
multiple reaction monitoring
- PK
pharmacokinetic
- RT
retention time
- SD
standard deviation
- TLC
thin-layer chromatography.
References
- 1. HemaIswarya S., et al. (2006). Potential synergism of natural products in the treatment of cancer. Phytother. Res., 20, 239–249 [DOI] [PubMed] [Google Scholar]
- 2. Jacobs D.R., Jr, et al. (2009) Food synergy: an operational concept for understanding nutrition. Am. J. Clin. Nutr., 89, 1543S–1548S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu R.H. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr., 78, 517S–520S [DOI] [PubMed] [Google Scholar]
- 4. Liu R.H. (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr., 134, 3479S–3485S [DOI] [PubMed] [Google Scholar]
- 5. Wagner H. (2011). Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia, 82, 34–37 [DOI] [PubMed] [Google Scholar]
- 6. Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. (1994). The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med., 330, 1029–1035 [DOI] [PubMed] [Google Scholar]
- 7. Greenberg E.R., et al. (1994). A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N. Engl. J. Med., 331, 141–147 [DOI] [PubMed] [Google Scholar]
- 8. Tsao A.S., et al. (2004). Chemoprevention of cancer. CA. Cancer J. Clin., 54, 150–180 [DOI] [PubMed] [Google Scholar]
- 9. Manach C., et al. (2004). Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr., 79, 727–747 [DOI] [PubMed] [Google Scholar]
- 10. Manach C., et al. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr., 81, 230S–242S [DOI] [PubMed] [Google Scholar]
- 11. Williamson G., et al. (2005). Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr., 81, 243S–255S [DOI] [PubMed] [Google Scholar]
- 12. Silberberg M., et al. (2006). The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur. J. Nutr., 45, 88–96 [DOI] [PubMed] [Google Scholar]
- 13. Calani L., et al. (2012). Colonic metabolism of polyphenols from coffee, green tea, and hazelnut skins. J. Clin. Gastroenterol., 46, S95–S99 [DOI] [PubMed] [Google Scholar]
- 14. Govindarajan V.S. (1982). Ginger–chemistry, technology, and quality evaluation: part 1. Crit. Rev. Food Sci. Nutr., 17, 1–96 [DOI] [PubMed] [Google Scholar]
- 15. Govindarajan V.S. (1982). Ginger-chemistry, technology, and quality evaluation: part 2. Crit. Rev. Food Sci. Nutr., 17, 189–258 [DOI] [PubMed] [Google Scholar]
- 16. Tao Y., et al. (2009). Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem., 57, 10014–10021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vasala P.A. (2004). Ginger. In Peter K.V. (ed.), Handbook of Herbs and Spices. Vol. I Woodhead, Cambridge, England: vol. I. [Google Scholar]
- 18. Yoshikawa M., et al. (1993). [Qualitative and quantitative analysis of bioactive principles in Zingiberis Rhizoma by means of high performance liquid chromatography and gas liquid chromatography. On the evaluation of Zingiberis Rhizoma and chemical change of constituents during Zingiberis Rhizoma processing]. Yakugaku Zasshi, 113, 307–315 [DOI] [PubMed] [Google Scholar]
- 19. Jeong C.H., et al. (2009). [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res., 69, 5584–5591 [DOI] [PubMed] [Google Scholar]
- 20. Kim S.O., et al. (2005). [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene, 24, 2558–2567 [DOI] [PubMed] [Google Scholar]
- 21. Lee H.S., et al. (2008). [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem., 19, 313–319 [DOI] [PubMed] [Google Scholar]
- 22. Kundu J.K., et al. (2009). Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr., 61, 182–192 [DOI] [PubMed] [Google Scholar]
- 23. Lumb A.B. (1994). Effect of dried ginger on human platelet function. Thromb. Haemost., 71, 110–111 [PubMed] [Google Scholar]
- 24. Park K.K., et al. (1998). Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett., 129, 139–144 [DOI] [PubMed] [Google Scholar]
- 25. Park Y.J., et al. (2006). [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med. J., 47, 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shukla Y., et al. (2007). Cancer preventive properties of ginger: a brief review. Food Chem. Toxicol., 45, 683–690 [DOI] [PubMed] [Google Scholar]
- 27. Wang C.C., et al. (2003). Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic HL-60 cells. In Vivo, 17, 641–645 [PubMed] [Google Scholar]
- 28. Zick S.M., et al. (2008). Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev., 17, 1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zick S.M., et al. (2010). Quantitation of 6-, 8- and 10-gingerols and 6-shogaol inhumanplasma by high-performanceliquidchromatography withelectrochemicaldetection. Int. J. Biomed. Sci., 6, 233–240 [PMC free article] [PubMed] [Google Scholar]
- 30. Chen C.Y., et al. (2009). Effect of [10]-gingerol on [ca2+]i and cell death in human colorectal cancer cells. Molecules, 14, 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dugasani S., et al. (2010). Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol., 127, 515–520 [DOI] [PubMed] [Google Scholar]
- 32. Karna P., et al. (2012) Benefits of whole ginger extract in prostate cancer. Br. J. Nutr., 107, 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brahmbhatt M., et al. (2013). Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer, 65, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reagan-Shaw S., et al. (2008). Dose translation from animal to human studies revisited. FASEB J., 22, 659–661 [DOI] [PubMed] [Google Scholar]
- 35. Gad S.C.(ed.) (2007). Themouse. In Animal Models in Toxicology. CRC Press, Taylor & FrancisGroup, Boca Raton, FL, pp. 19–146 [Google Scholar]
- 36. Roberts M.S., et al. (2002). Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin. Pharmacokinet., 41, 751–790 [DOI] [PubMed] [Google Scholar]
- 37. Baliga M.S., et al. (2011). Update on the chemopreventive effects of ginger and its phytochemicals. Crit. Rev. Food Sci. Nutr., 51, 499–523 [DOI] [PubMed] [Google Scholar]
- 38. Jacobs D.R., Jr, et al. (2007) Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev., 65, 439–450 [DOI] [PubMed] [Google Scholar]
- 39. Ulbricht C.E., et al. (2010). Phytochemicals in the oncology setting. Curr. Treat. Options Oncol., 11, 95–106 [DOI] [PubMed] [Google Scholar]
- 40. Wells P.G., et al. (2004). Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos., 32, 281–290 [DOI] [PubMed] [Google Scholar]
- 41. Jana S., et al. (2009). Role of phase II drug metabolizing enzymes in cancer chemoprevention. Curr. Drug Metab., 10, 595–616 [DOI] [PubMed] [Google Scholar]
- 42. Bravo L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev., 56, 317–333 [DOI] [PubMed] [Google Scholar]
- 43. Olson J.A., et al. (1992) Enhancement of biological activity by conjugation reactions. J. Nutr., 122, 615–624 [DOI] [PubMed] [Google Scholar]
- 44. McCarver D.G., et al. (2002). The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J. Pharmacol. Exp. Ther., 300, 361–366 [DOI] [PubMed] [Google Scholar]
- 45. Gregory P.A., et al. (2004). Regulation of UDP glucuronosyltransferases in the gastrointestinal tract. Toxicol. Appl. Pharmacol., 199, 354–363 [DOI] [PubMed] [Google Scholar]
- 46. Ritter J.K. (2000). Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem. Biol. Interact., 129, 171–193 [DOI] [PubMed] [Google Scholar]
- 47. Liu Z., et al. (2007). Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol., 3, 389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]