Summary
Integrin α6Aβ4 is up-regulated in colorectal cancers. Knockdown of α6A in adenocarcinoma cell lines revealed a sustained reduction of cell growth both in cellulo and in xenografts as well as a repression of a number of Wnt/β-catenin pathway end points.
Abstract
The integrin α6 subunit pre-messenger RNA undergoes alternative splicing to generate two different splice variants, named α6A and α6B, having distinct cytoplasmic domains. In the human colonic gland, these splice variants display different patterns of expression suggesting specific functions for each variant. We have previously found an up-regulation of the α6β4 integrin in colon adenocarcinomas as well as an increase in the α6A/α6B ratio, but little is known about the involvement of α6Aβ4 versus α6Bβ4 in this context. The aim of this study was to elucidate the function of the α6Aβ4 integrin in human colorectal cancer (CRC) cells. Expression studies on a panel of primary CRCs confirmed that the up-regulation of the α6 subunit in CRC is a direct consequence of the increase of the α6A variant. To investigate the functional significance of an α6A up-regulation in CRC, we specifically knocked down its expression in well-established CRC cell lines using a small-hairpin RNA approach. Results showed a growth rate reduction in all α6A knockdown CRC cell lines studied. The α6A silencing was also found to be associated with a significant repression of a number of Wnt/β-catenin pathway end points. Moreover, it was accompanied by a reduction in the capacity of these cells to develop tumours in xenografts. Taken together, these results demonstrate that the α6A variant is a pro-proliferative form of the α6 integrin subunit in CRC cells and appears to mediate its effects through the Wnt/β-catenin pathway.
Introduction
The integrin superfamily is composed of the transmembrane receptors responsible for mediating epithelial-basement membrane interactions. Integrins are formed by the heterodimeric association of an α and a β subunit and, to date, 18 α and 8 β subunits have been identified, which can combine to form 24 distinct integrins (1). The existence of multiple splice variants and post-translational modification of most subunits increases the variety of integrins (2). These receptors can mediate intracellular signalling despite their lack of intrinsic kinase activity. Indeed, ligand binding (i.e. laminin, collagen and fibronectin) induces the recruitment of intracellular kinases and adaptor proteins via the cytoplasmic C-terminal domains of either integrin subunit, mediating intracellular signalling to regulate a large spectrum of cell processes including proliferation, adhesion, migration and apoptosis (3,4).
Colorectal cancer (CRC) is the second leading cause of cancer death in North America (5) and accumulating studies confirm an important role for integrin receptors in human colorectal tumourigenesis (6–9). Interestingly, the α6 integrin subunit can heterodimerize with either β1 or β4 to form the α6β1 or α6β4 integrins but in the gut epithelium as well as in CRC, the α6 integrin subunit predominantly associates with β4 (10–12). Moreover, both the α6 and β4 integrin subunits appear to be over-expressed in primary tumours of the human colon (12–14) and CRC cell lines (12,13,15), suggesting an important role for this integrin in CRC progression (7,9). Although the β4 subunit exists as five splice variants, it is the β4A variant subunit that is predominantly expressed in the gut (11). We have previously described a cytosolic variant of β4A resulting from the proteolytic cleavage of the C-terminal domain (cdt), called β4cdt−, that is non-functional for adhesion to laminin and associated with normal intestinal proliferative epithelial cells but it is the wt form, β4cdt+, that is predominantly present in CRC and in all CRC cell lines studied (11,13). To date, signalization from β4 in cancer has been well characterized. For instance, outside-in signalling leads to phosphorylation of the cytoplasmic C-terminal domain of β4, recruitment of SHC/GRB2 and/or IRS1/IRS2 and downstream activation of the MAPK/ERK and PI3K/AKT pathways (16), thus regulating major cell processes involved in tumourigenesis (16–19).
Although the majority of α6β4 functions in cancer have been attributed to the β4 subunit, recent evidence suggests that signalization events mediated by the α6 subunit can also regulate the processes involved in tumourigenesis including proliferation and metastasis (20–25). However, the α6 integrin subunit exists as two splice variants, α6A and α6B, generated by the alternative splicing of exon 25, resulting in the formation of two distinct cytoplasmic domains (26). The existence of two variants with distinct C-tails suggests that each may have a specific function in the regulation of cellular processes. In support of this, a study performed using two yeast hybrids has shown that the PDZ domain of each variant can interact with specific intracellular molecules (27,28). Furthermore, other studies have demonstrated that each variant initiates different intracellular signalling events, such as paxilin phosphorylation (29) and RAS-MEK-ERK activation (30). In human tissues, the α6A and α6B variant subunits display distinct patterns of expression (26) as for instance in the skin where α6A is exclusively associated with basal cells. Previous results from our laboratory have shown that in the normal human small intestine, the α6A variant is predominantly associated with proliferative cells in the glands, whereas the α6B variant is mainly localized in quiescent and differentiated cells in the villus epithelium (31). Although also detected in the normal colon, this pattern of expression is lost in primary tumours where α6A becomes ubiquitously expressed in all CRC cells (12), supporting the possibility that inclusion of the α6A subunit into α6β4 integrin generates a pro-proliferative integrin (7). In the context where deregulation of cell proliferation is one of the hallmarks of cancer and that the α6 subunit appears to be involved in the process (20–25), we propose that the pro-proliferative function of α6 is specifically mediated by its α6A splice variant in CRC.
In the present study, we tested this hypothesis using a knockdown approach targeting the mature α6A messenger RNA (mRNA) and found that α6A ablation significantly reduced CRC cell proliferation both in vitro and in xenografts. Furthermore, we also found that this effect was accompanied by a decline in the Wnt/β-catenin signalling pathway.
Materials and methods
Primary antibodies and materials
Primary antibodies used for the detection of the α6A and α6B variants were anti-α6A [western blot (WB): 1/500, immunofluorescence (IF): 1/100] (1A10, Millipore, Etobicoke, Ontario) and anti-α6B (WB: 1/500, IF: 1/100) (6B4, Millipore). These antibodies were originally a generous gift from Dr A.Sonnenberg (Division of Cell Biology, The Netherlands Cancer Institute, Amsterdam, The Netherlands). Other primary antibodies used were anti-integrin β4 (WB: 1/5000, IF: 1/100) (3E1, Millipore), anti-integrin α6 (IF: 1/1000) (GOH3, Millipore), anti-β-actin (WB: 1/75 000) (C4, Millipore), anti-active-β-catenin (WB: 1/2500) (8E7, Millipore) recognizing the dephosphorylated form of β-catenin on Ser37 and Thr41 (sites of GSK3β phosphorylation), anti β-catenin (WB: 1/2500) (610153, BD Biosciences, Mississauga, Ontario), anti-DVL2 (WB: 1/2500) (30D2, Cell Signaling Technology, Danvers, MA), anti-cytokeratin 18 (WB: 1/1 000 000) (CY-90, Sigma–Aldrich, Oakville, Ontario), anti-histone H1 (WB: 1/1000) (AE-4, Santa Cruz Biotechnologies, Santa Cruz, CA), anti-integrin β1 (WB: 1/1000) (Mab13, BD Biosciences), anti-GSK3β (WB: 1/5000) (27C10, Cell Signaling Technology) and anti-H3K27me3 (WB: 1/1000) (07-449, Millipore). The pharmacological inhibitor of GSK3β (SB216763, S3442, Sigma–Aldrich) was used at a final concentration of 20 µM. The protease inhibitor cocktail (P8340) was purchased from Sigma.
Cell culture and generation of CRC cells knocked down for α6A subunit expression
The CRC cell lines Caco-2/15 and T84 (polarized) as well as HT29 and DLD-1 (non-polarized) were obtained from the American Type Culture Collection (www.ATCC.org) and cultured as described (11–13). All cells were grown in an atmosphere of 95% air and 5% CO2 at 37°C. Colon cancer cells were plated at 60% confluence 24 h prior to infection with lentiviruses prepared with MISSION® shRNA (Sigma–Aldrich) plasmids for the human α6A integrin containing the shRNA sequence: 5′-CCG GCC TTT GGA CTG AAA GGA GAA ACT CGA GTT TCT CCT TTC AGT CCA AAG GTT TTT G. The negative control shGFP contained the sequence: 5′: CCG GGT GGG CAT CAA AGA CGT GTT TCT CGA GAA ACA CGT CTT TGA TGC CCA CTT TTT G and shctl: 5′: CCG GGT GGG CAT CAA AGA CGT GTT TCT CGA GAA ACA CGT CTT TGA TGC CCA CTT TTT G. At 3 days post-infection, stable cell lines were selected by adding 5–10 µg/ml puromycin to the culture medium (Qiagen, Mississauga, Ontario). Cultures were used after 14 days of selection.
Growth curve assay
Stable cell populations of T84, Caco-2/15, HT29 and DLD-1 were seeded in 6-well plates at 2×105 cells/dish in their respective media. Cell number was measured, using a Z1 Coulter Counter (Beckman, Mississauga, Ontario).
Cell proliferation assays
Proliferation assays using 5-bromo-2-deoxyuridine (BrdU) incorporation were performed according to the manufacturer’s instructions (Roche, Laval, Quebec) as described previously (12). All experiments were performed in triplicate and repeated three times.
In situ terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling (ISEL) assays
Analysis of apoptosis index was evaluated using the ApopTag® Plus Fluorescein In Situ Apoptosis Detection Kit (S7111, Millipore) and performed according to the manufacturer’s instructions. Briefly, 5×104 cells were seeded onto serum-pretreated coverslips in a 12-well plate (Falcon) and allowed to adhere for 48h under normal culture conditions and processed for in situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (ISEL) ISEL assays. The percentage of apoptotic cells was evaluated by the number of positive ISEL cells over the total number of cells stained by 4′,6-diamidino-2-phenylindole (×100). Cytochalasin D (Sigma–Aldrich) was added at 1 µM for a positive control of apoptosis.
Nuclear extracts
Nuclear extracts were prepared using cells at 80% confluence. Briefly, cells were washed three times with phosphate-buffered saline (PBS) 1x and harvested with 4ml PBS 1x. Cells were centrifuged at 3000 r.p.m. for 5 min at 4°C then rapidly resuspended in a hypotonic buffer (HB: 10 µM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1.5 µM MgCl2, 3 µM KCl, 0.5 µM dithiothreitol and 1% protease inhibitor cocktail in bidistilled H2O) and centrifuged at 3000 r.p.m. for 5 min at 4°C. Supernatants were removed and cells were resuspended in HB for 10 min at 4°C. Cells were then homogenized with a micropestle and centrifuged for 15 min at 4°C. The supernatant was removed and cells were resuspended in low salt buffer (20 µM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1.5 µM MgCl2, 6 µM KCl, 25% glycerol, 0.2 µM ethylenediaminetetraacetic acid, 5 µM dithiothreitol and 1% protease inhibitor cocktail in bidistilled H2O) followed by resuspension in a high salt buffer (20 µM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1.5 µM MgCl2, 0.4 M KCl, 25% glycerol, 0.2 µM ethylenediaminetetraacetic acid, 5 µM dithiothreitol and 1% protease inhibitor cocktail in bidistilled H2O), shaken for 30 min at 4°C and centrifuged at 14 000 r.p.m. for 30 min at 4°C. The supernatants were solubilized 1:3 in 4X concentrated Laemmli buffer prior to loading and analysis for nuclear proteins by WB.
Cellular fractionation
Subcellular fractionation was performed using the Subcellular Proteome Extraction Kit (539790, Millipore) according to the manufacturer’s instructions. Cells were allowed to grow 3 days prior to subcellular compartment extraction (cytosolic: F1; membrane: F2; nuclear: F3; cytoskeletal: F4). Enriched fractions were confirmed by WB for the detection of GSK3β (cytoplasmic soluble fraction), integrin β1 (membrane fraction), trimethylated lysine 27 on histone 3 (nuclear fraction) and keratin 18 (cytoskeletal fraction).
Western blot
WB analyses were performed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels under denaturing conditions as described previously using 50 µg of whole cell lysate per lane, except for α6A and α6B immunodetections, which were performed under non-denaturing conditions using 120 µg of whole cell lysate per lane (12,31). Primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies (anti-mouse, anti-rabbit, GE Healthcare—Amersham Bioscience, Baie d’Urfe, Quebec) and developed using the Immobilon Western® kit (Millipore).
Immunofluorescence
IF was performed as described previously (32). Briefly, 5×104 T84 cells were seeded onto serum-pretreated coverslips in a 12-well plate (Falcon), allowed to adhere for 48h under normal culture conditions and processed for IF experiments. For α6 and β4 staining, cells were fixed in 2% paraformaldehyde, non-specific sites were blocked for 1 h at room temperature with 5% Blotto–PBS (pH 7.4) and both primary and secondary antibodies were diluted in 5% Blotto–PBS (pH 7.4). For α6A and α6B staining, cells were fixed in MeOH and EtOH, respectively. Non-specific sites were blocked for 1 h at room temperature in a 2% bovine serum albumin solution in PBS (pH 7.4) and both primary and secondary antibodies were diluted in 2% bovine serum albumin–PBS (pH 7.4). Cells were treated with a 0.2% Triton X-100 solution for 5 min prior to antibody incubation. Primary antibodies were detected with Alexa Fluor 488 or 594 goat anti-mouse secondary antibodies (Invitrogen, A11017, A11032) and Alexa Fluor 488 goat anti-rat secondary antibody (Invitrogen, A11006).
Transfections and luciferase assays
TOPflash and FOPflash reporter plasmids (Millipore) were transfected into CRC cell lines with Effectene transfection reagent (Qiagen) using the manufacturer’s instructions. Firefly and renilla luciferase activities were measured using the dual luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, each cell line was plated at 5×104 cells/well in 12-well plates. Cells were co-transfected with TOPflash or FOPflash reporter plasmids and the renilla luciferase expression plasmid (Promega) and treated with dimethyl sulfoxide or SB216763 for 48 h. Data were obtained by calculating the ratio of firefly/renilla luciferase expressions for the TOPflash and FOPflash reporter plasmids. The FOPflash ratio was subtracted from the TOPflash ratio. Data represent three separate experiments performed in triplicate.
Human colorectal tissues
Samples of 97 CRC and paired normal tissues (at least 10cm from the tumour) were obtained from patients undergoing surgical resection without prior neoadjuvant therapy. Tissues were obtained after patients’ written informed consent, according to a protocol approved by the Institutional Human Subject Review Board of the Centre Hospitalier Universitaire de Sherbrooke. Diagnoses, staging and grading were performed by the pathologists of the Department of Pathology of the Centre Hospitalier Universitaire de Sherbrooke.
RNA extraction, reverse transcription–polymerase chain reaction and quantitative PCR
RNA extraction and reverse transcription were performed as described previously (33). For competitive PCR, conditions and primers used to co-amplify α6A and α6B have been described previously (31). For quantitative PCR (qPCR), primers used to amplify α6, RPLPO and B2M have been described previously (12,13,33). α6A was amplified using a PrimeTime assay (IDT, Coralville, IA) composed of primers with the sequences 5′-GATCCTTACAGCATGGTATCGG and 5′-AAGAGAGGCTTACTTCTGATGC and a double-quenched hydrolysis probe containing the 5′ fluorophore FAM and the sequence 56-FAM/TCGTACCTA/ZEN/GAGCGTTTAAAGAATCCACAC/3IABkFQ. α6B was amplified using a PrimeTime assay (IDT) composed of primers with the sequences 5′ATT CTC GCT GGG ATC TTG ATG and 5′GAT CCT TAC AGC ATG GTA TCG G and a double-quenched hydrolysis probe containing the 5′ fluorophore FAM and the sequence 56-FAM/TGG AAG TGT/ZEN/GGA TTC TTT AAA CGC TCT/3IABkFQ/. Other primers used were for LGR5—LGR5-F: 5′-TGC TCT TCA CCA ACT GCA TC and LGR5-R: 5′-CTC AGG CTC ACC AG ATC CTC; for CCD2—CCD2-F: 5′-TGG GGA AGT TGA AGT GGA AC and CCD2-R: 5′-TCA TCG ACG GTG GGT ACA T; for CCD1—CCD1-F: 5′-AAC TAC CTG GAC CGC TTC CT and CCD1-R: 5′-CCA CTT GAG CTT GTT CAC CA and for DVL2—DVL2-F: 5′-GCC TAT CCA GGT TCC TCC TC and DVL2-R: 5′-AGA GCC AGT CAA CCA CAT CC. qPCR was performed using an Mx3000P (Stratagene, Mississauga, Ontario) as described previously (33). Relative mRNA levels were established by normalization to a pool of cDNA and calculated according to the Pfaffl mathematical model (34).
Xenografts
Female CD1 nu/nu mice were purchased from Charles River (Wilmington, MA). All experimental protocols were approved by the Ethics Committee for Animal Experimentation of the Université de Sherbrooke. For tumour growth, a total of 2 × 106 cells suspended in 100 µl Dulbecco's modified Eagle's medium were injected into the dorsal subcutaneous tissue of 5-week-old female CD1 nu/nu mice. After injection of shα6A and shctl T84, HT29 and DLD-1 cell populations, tumour volumes were determined by external measurement [V = (d 2 × D)/2] as soon as growing tumours became palpable and were followed until killing. At the time of killing, tumours were dissected and weighed. A portion of each tumour was used for mRNA extraction and subsequent qPCR analysis. Another portion was embedded in Optimum Cutting Temperature compound (Canemco-Marivac, Lakefield, Quebec), cut into 5–10 μm sections and stained with haematoxylin and eosin for histological analysis. Slide sections were viewed using an FSX100 Bio Imaging Navigator microscope (Olympus, Center Valley, PA).
Data presentation and statistical analyses
Each experiment was repeated at least three times and representative results were shown. Student’s paired t-test and analysis of variance (ANOVA) using Bonferroni’s multiple comparison test were used to analyse the results and data were considered to be significantly relevant at P ≤ 0.05 and are presented as mean ± SEM. Statistical calculations were performed using Prism 3.0 software (GraphPad Software, San Diego, CA).
Results
Correlation between total α6 subunit and α6A variant expression in CRC
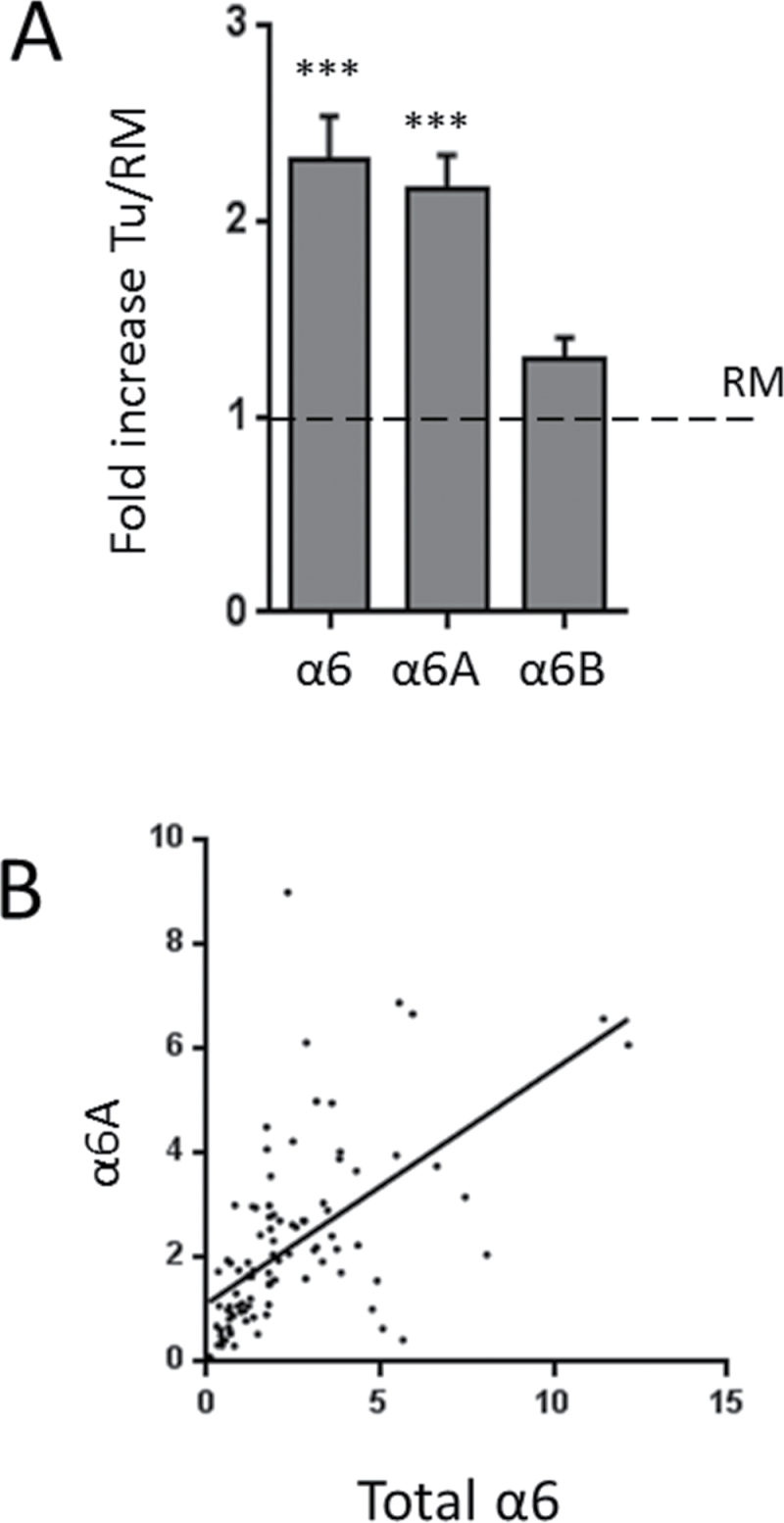
We have shown previously that α6B/α6A integrin subunit ratios were significantly reduced in a relatively small set of human CRC samples at the transcript level (12). In an attempt to extend these observations, the mRNA levels of total α6 as well as the individual α6A and α6B variants were analyzed by qPCR in 97 CRCs and their corresponding resection margins (RMs). The level of total α6 subunit mRNA was found to be increased in CRC samples compared with RMs by more than 2-fold, as well as that of α6A, whereas the level of α6B remained stable (Figure 1A). Moreover, a close correlation was observed between the levels of α6 and α6A mRNA (P ≤ 0.0001, Pearson r = 0.588) in human CRC (Figure 1B). When each sample was analyzed individually, the expression of α6A in CRC compared with corresponding RMs was found to be increased in 69 patients, similar in 16 patients and reduced in 12 patients. Taking into consideration that significant levels of α6A are expressed in the crypt of the normal colonic mucosa of the RM, these results confirm that a large proportion of CRC cells express significant levels of α6A. Furthermore, up-regulation of α6A was observed for all tumour stages or grades (Supplementary Figure 1, available at Carcinogenesis Online) although the presence of somatic mutations of adenomatous polyposis coli (APC) had no impact on α6A expression (data not shown). Taken together, these data confirm a sustained up-regulation of the α6A splice variant in human CRC.
Fig. 1.
Expression of the α6 integrin subunit in human CRCs. (A) qPCR analysis of α6, α6A and α6B transcript levels in 97 primary colorectal tumours (Tu) relative to their matched RMs. Data were normalized using B2M levels as the reference gene. ***P ≤ 0.001. (B) Graph showing the correlation between α6 and α6A transcript expression in the 97 primary colorectal tumours. Pearson r = 0.588, P ≤ 0.0001.
Specific α6A knockdown in CRC cells
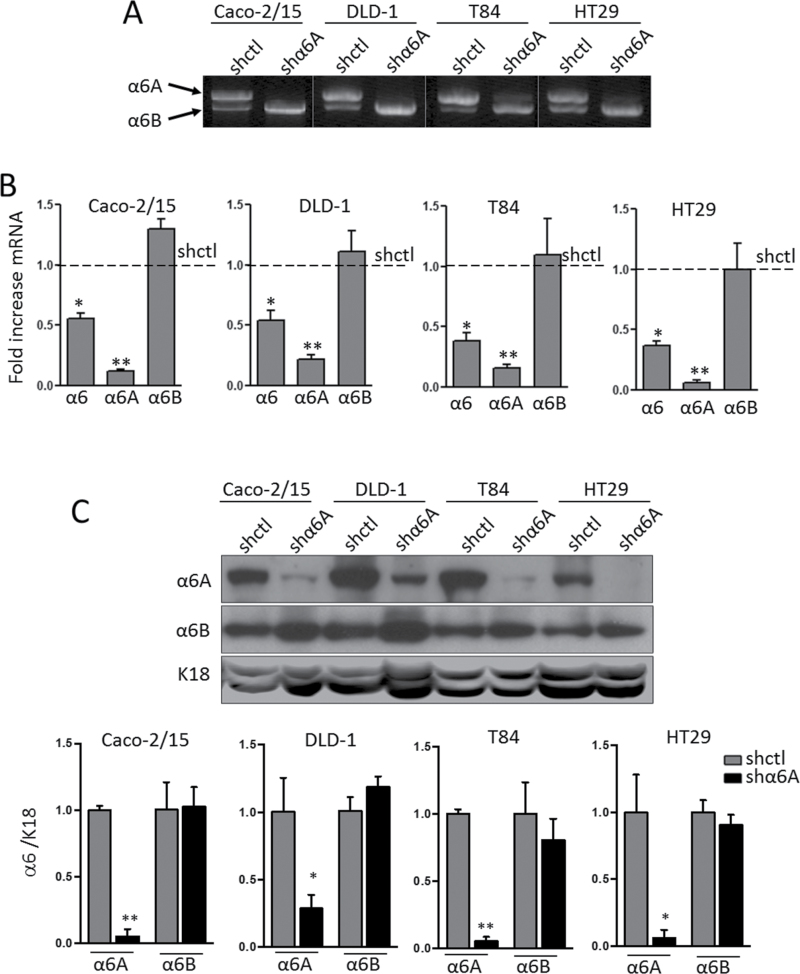
To investigate the involvement of the α6A variant in CRC cell behaviour, four well-characterized CRC cell lines—Caco-2/15, DLD-1, T84 and HT29 cells—were infected with an shRNA targeting the α6A variant. The α6A shRNA was designed in order to recognize a unique sequence of the short exon 25 (119pb) specific to the α6A mRNA transcript. Specificity of knockdown of α6A was first confirmed by competitive PCR using primers that amplify both variants of α6. The results revealed a decrease in α6A variant mRNA expression in shα6A versus shctl-treated CRC cells in all four cell lines tested (Figure 2A). Further analysis by qPCR revealed that knocking down α6A led to a decrease of ~50% of total α6 mRNA levels (Figure 2B). Analysis of splice variants showed an 80–90% decrease in α6A transcript levels in shα6A cells compared with controls, without affecting mRNA levels of α6B (Figure 2B). WB analysis of α6A and α6B performed to extend our observations at the protein level confirmed the specific abolition of the α6A variant, without significantly affecting α6B in all CRC cell lines (Figure 2C).
Fig. 2.
Knockdown of the α6A subunit in human CRC cells. (A) Representative gel showing the results of a competitive reverse transcription–polymerase chain reaction for the detection of α6A and α6B transcripts in stably expressing shctl and shα6A Caco-2/15, DLD-1, T84 and HT29 cells. (B) qPCR using probes specific for total α6, α6A and α6B confirming the specificity of abolition of α6A variant expression by shα6A relative to shctrl; *P ≤ 0.05, **P ≤ 0.01, ANOVA, n = 3. (C) Representative WB for detection of α6A and α6B subunits in shctl- and shα6A-infected Caco-2/15, DLD-1, T84 and HT29 cells. Keratin 18 (K18) was used as loading control. Densiometric analysis of α6A and α6B protein levels in shctl- versus shα6A-infected cell populations; *P ≤ 0.05, **P ≤ 0.01, t-test, n = 3.
α6A knockdown does not affect the intracellular localization of α6B
In order to verify the localization of α6B in shα6A cells, we first used IF staining on T84 cells, which display hemidesmosomes. Co-staining using a rat anti-α6 antibody and a mouse anti-β4 antibody showed that the α6 integrin subunits co-localize with the β4 subunit in both shctl (Supplementary Figure 2A–C, available at Carcinogenesis Online) and shα6A cells (Supplementary Figure 2D–F, available at Carcinogenesis Online) with a typical punctuated hemidesmosome-like staining pattern. As expected, the relative intensity of staining for both integrin subunits was lower in shα6A cells. Furthermore, using α6 variant-specific antibodies, a significant reduction of the α6A staining observed in shctl cells (Supplementary Figure 2G, available at Carcinogenesis Online) was noted in shα6A cells (Supplementary Figure 2H, available at Carcinogenesis Online), whereas the α6B staining remained comparable between shctl and shα6A cells (Supplementary Figure 2I and J, available at Carcinogenesis Online). These results suggested that abolition of α6A does not alter the distribution of α6B. In order to confirm this observation, cell subfractionation was performed on shctl and shα6A DLD-1 cells, which do not display organized hemidesmosomes. WB analysis confirmed that the membrane localization of α6B was not altered by the ablation of α6A expression (Supplementary Figure 3, F2, available at Carcinogenesis Online). As expected, a decrease in the β4 integrin was observed relative to the β1 integrin. Taken together, these two sets of observations indicate that α6A knockdown has no significant effect on α6B expression and localization.
α6A regulates cell proliferation
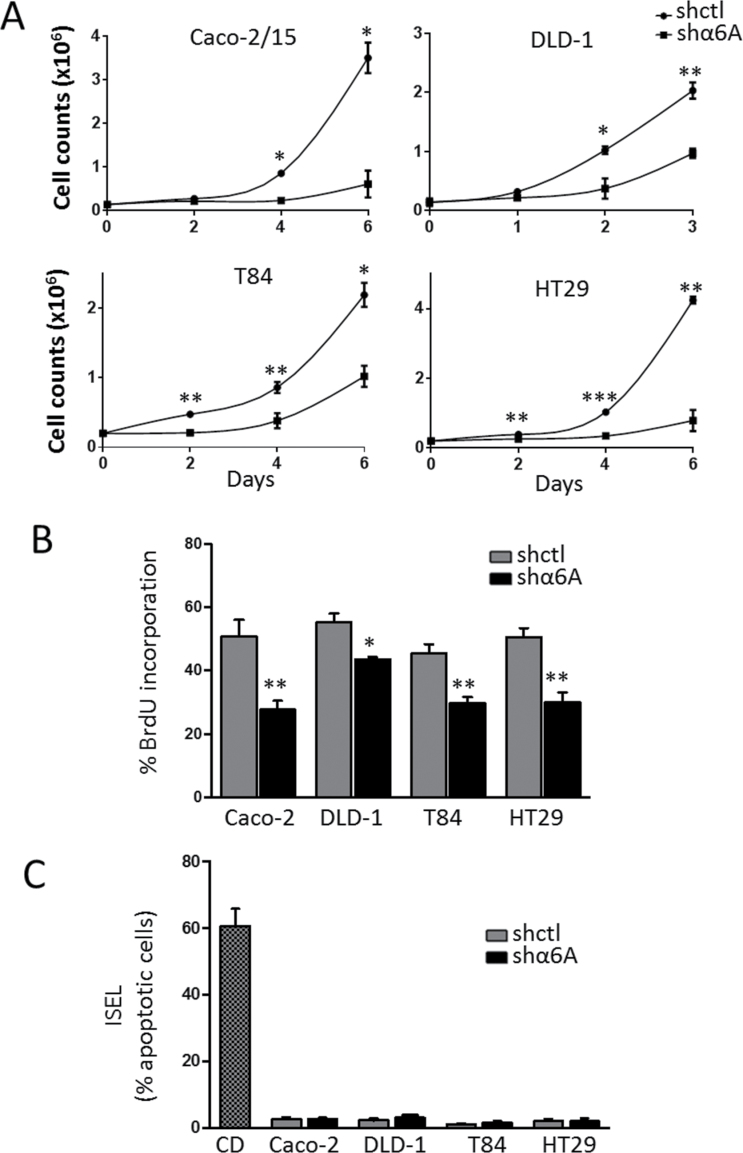
As the α6 integrin subunit was reported to be involved in cell proliferation (20,21), we further investigated if this function could be attributed to the α6A splice variant. Therefore, the involvement of the α6A variant in cancer cell growth was first assessed by establishing a growth curve using CRC cell populations knocked down for α6A, but expressing α6B, in vitro. As shown in Figure 3A, a significant reduction in cell number was observed throughout the culture beginning as early as 2 days post-seeding for T84, HT29 and DLD-1 shα6A cells in comparison with shctl cells and at 4 days for Caco-2/15 shα6A cells. Overall, abolition of the α6A subunit led to a significant and sustained reduction of the growth rate in all CRC cells tested.
Fig. 3.
Knocked down α6A splice variant decreases cell proliferation. (A) Cell counts over a 3–6 day period after the seeding of Caco-2/15, DLD-1, T84 and HT29 stably expressing shα6A or shctl. Cells were counted at the indicated times. (B) BrdU labelling assay in Caco-2/15, DLD-1, T84 and HT29 shα6A and shctl cells at 2 days post-seeding. (C) ISEL assay in Caco-2/15, DLD-1, T84 and HT29 shα6A and shctl cells at 2 days post-seeding. Cytochalasin D (CD)-treated cells were used as positive control for apoptosis. Statistical analysis between shctrl and shα6A: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, t-test, n = 3.
To confirm that the decrease in cell number was the result of a specific reduction in cell proliferation, all cell lines were subjected to BrdU incorporation and ISEL assays. A significant reduction in cells entering S-phase was revealed by BrdU incorporation for the four shα6A cell lines relative to their corresponding shctl cells (Figure 3B), while at the same time, ISEL experiments showed that the apoptotic index was negligible in all colorectal cell lines (Figure 3C). These results confirm the pro-proliferative function of the α6A variant on human CRC cells.
α6A variant knockdown reduces tumour growth in xenografts
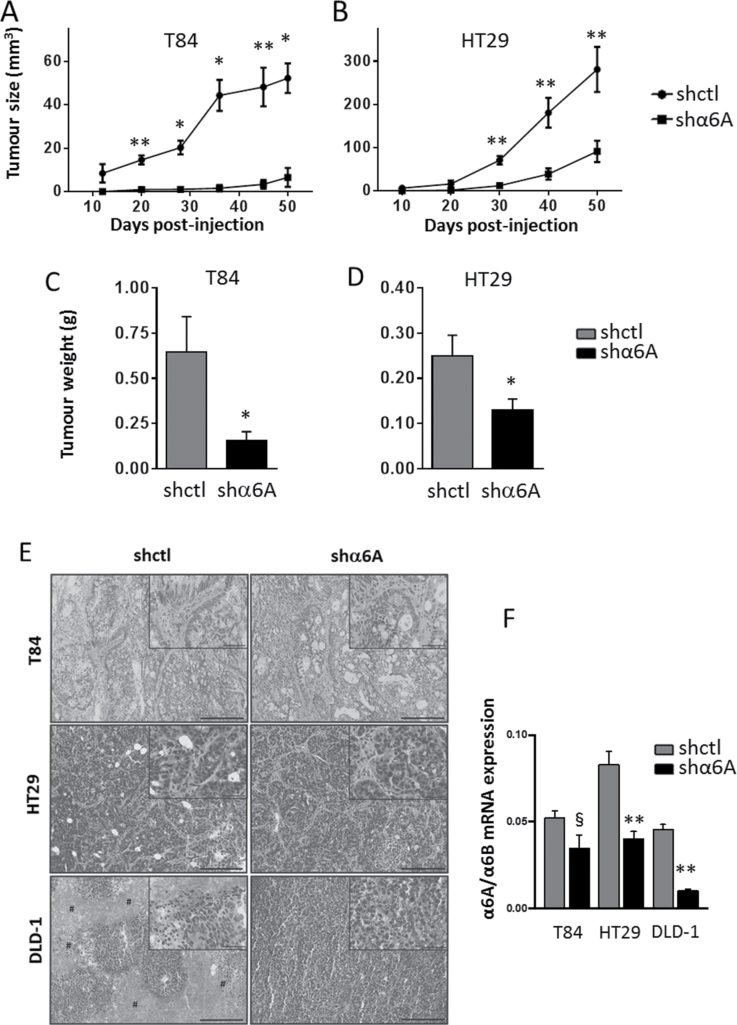
The capacity of α6A knockdown cells to form tumours in vivo was next evaluated by subcutaneous injection of nude mice with T84, HT29 and DLD-1 cells. Caco-2/15 cells were not included in this assay because of the long latency period required to observe tumour formation in nude mice with this cell line. Interestingly, we found that the latency period for the detection of palpable tumours was significantly delayed for T84/shα6A cells compared with T84/shctl (36 days versus 12 days) (Figure 4A), whereas this was not so for HT29 and DLD-1 cells. However, abolition of α6A in T84 and HT29 strongly diminished their growth capacity as tumours in nude mice (Figure 4A and B), resulting in a significant reduction of the tumour weight at the time of the killing (Figure 4C and D). The decrease in proliferation rate observed in DLD-1 shα6A cells in vitro was not transposed into a significant reduction in tumour growth and weight (data not shown). However, histological haematoxylin and eosin analysis showed that DLD-1 shctl xenograft tumours displayed large necrosis/oedema regions, a feature not observed in DLD-1 shα6A xenograft tumours (Figure 4E). This observation could explain the lack of difference in tumour size development observed, despite the decrease in proliferation in DLD-1 shα6A cells. On the other hand, no histological difference was observed between shctl and shα6A xenograft tumours from T84 and HT29 cells (Figure 4E). As shown in Figure 4F, qPCR analysis of α6A and α6B in tumours confirmed that the α6A knockdown is retained in HT29 and DLD-1 and tends to be retained in T84 xenogafts even after 50 days (P < 0.01 for HT29 and DLD-1; P < 0.08 for T84). Taken together, these results demonstrate that the α6A variant can regulate tumour growth in at least a subset of CRC cell lines in xenografts, confirming the pro-proliferative effect of the α6A variant.
Fig. 4.
Knockdown of α6A variant in human CRC cells inhibits their growth in xenografts. (A and B) Tumour growth (mm3) following subcutaneous injection of 2×106 T84 and HT29 stably expressing shα6A and shctl cells into nude mice. Tumour volumes were determined by external measurement [V = (d 2 × D)/2]. (C and D) Weight (g) of tumours from T84 and HT29 shctl and shα6A cells at the time of killing. Statistical analysis between shctrl and shα6A: *P ≤ 0.05, **P ≤ 0.01, t-test, n = 4. (E) Representative haematoxylin and eosin staining images for T84, HT29 and DLD-1 shctl and shα6A xenograft tumours. Scale bars = 200 μm for main panels and 50 μm for inserts. Hash symbols denote necrosis/oedema regions. (F) qPCR analyses for the expression of α6A and α6B transcript levels in xenograft tumours from T84, HT29 and DLD-1 shctl and shα6A cells. Data are expressed by α6A normalized to α6B levels. **P ≤ 0.01, § P = 0.0783, t-test, n = 4.
α6A knockdown regulates the Wnt/β-catenin pathway
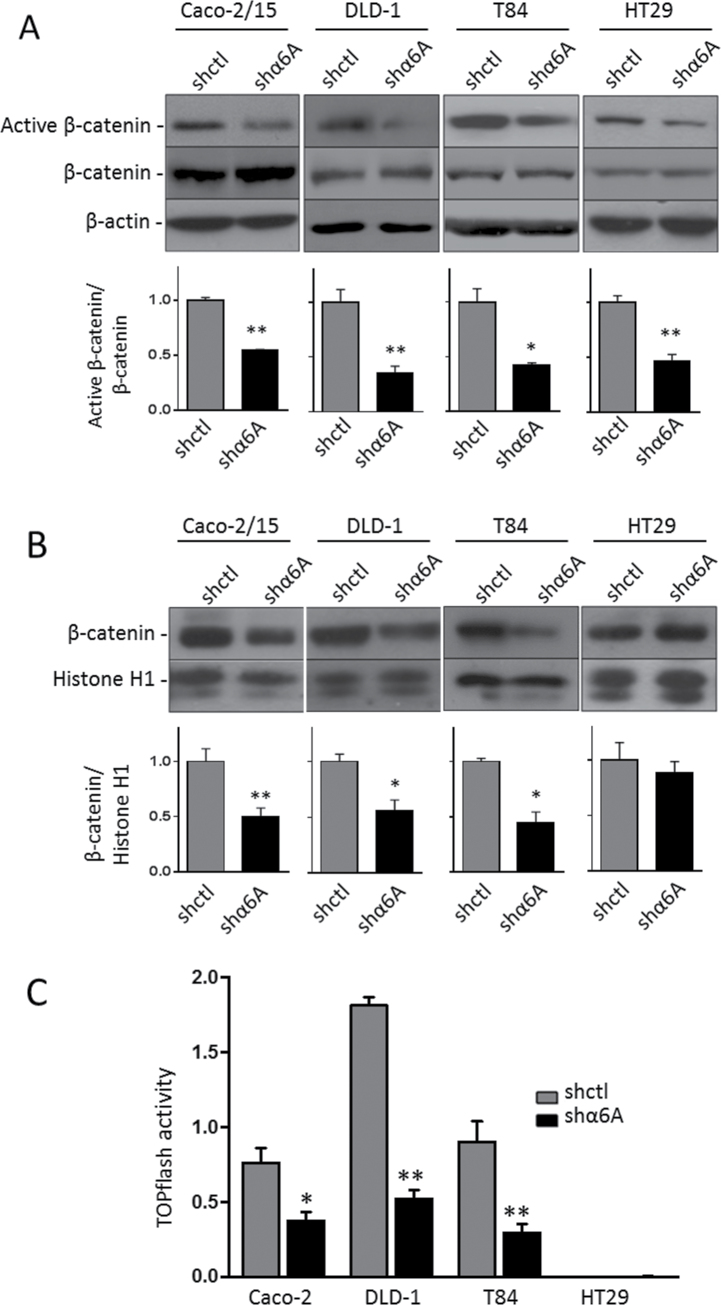
The Wnt/β-catenin pathway is one of the most important regulators of cell proliferation in various systems and is often strongly deregulated in human CRC (35,36), thus suggesting a possible link between α6A expression and Wnt/β-catenin activity in CRC cells. Wnt/β-catenin activity was first evaluated by determining the level of GSK3β phosphorylation of β-catenin at ser37/thr41 using an active β-catenin antibody. WB analyses of whole cell lysates showed a statistically significant decrease in the levels of active β-catenin in all shα6A cell lines relative to total β-catenin compared with shctl cells (Figure 5A). This overall decrease was also reflected in WB analyses of nuclear extracts, where β-catenin was reduced in α6A knockdown Caco-2/15, DLD-1 and T84 (Figure 5B). These results suggest that in these CRC cells, depletion of the α6A integrin variant interferes with the Wnt/β-catenin pathway by enhancing the phosphorylation of β-catenin by GSK3β, targeting it to proteasome degradation and consequently reducing its accumulation in the nucleus.
Fig. 5.
Regulation of the Wnt/β-catenin pathway by the α6A variant subunit. Representative WB and graph of the densiometric analysis for the detection of active β-catenin and total β-catenin in the whole cell extract (A) and β-catenin in nuclear extracts (B). β-Actin served as loading control in cell extracts and histone H1 for nuclear extracts. Statistical analysis between shctrl and shα6A: *P ≤ 0.05, **P ≤ 0.01, t-test, n = 3. (C) TOPflash assay of the response of β-catenin/TCF4 promotor activity in the α6A variant knocked down cell lines and their corresponding shctrl. Results showed the net luciferase/renilla ratio (Topflash − FOPflash). Statistical analysis between shctrl and shα6A: *P ≤ 0.05, **P ≤ 0.01, t-test, n = 3.
To evaluate the functional significance of reduced β-catenin levels, the activity of the Wnt/β-catenin pathway was further analyzed by the luciferase assay using a responsive β-catenin/TCF4/LEF reporter plasmid (TOPflash). As shown in Figure 5C, a sharp decrease in TOPflash activity was observed in Caco-2/15, DLD-1 and T84 shα6A cells compared with shctl. In our hands, HT29 cells displayed a below-detection level of TOPflash activity compared with the other cell lines.
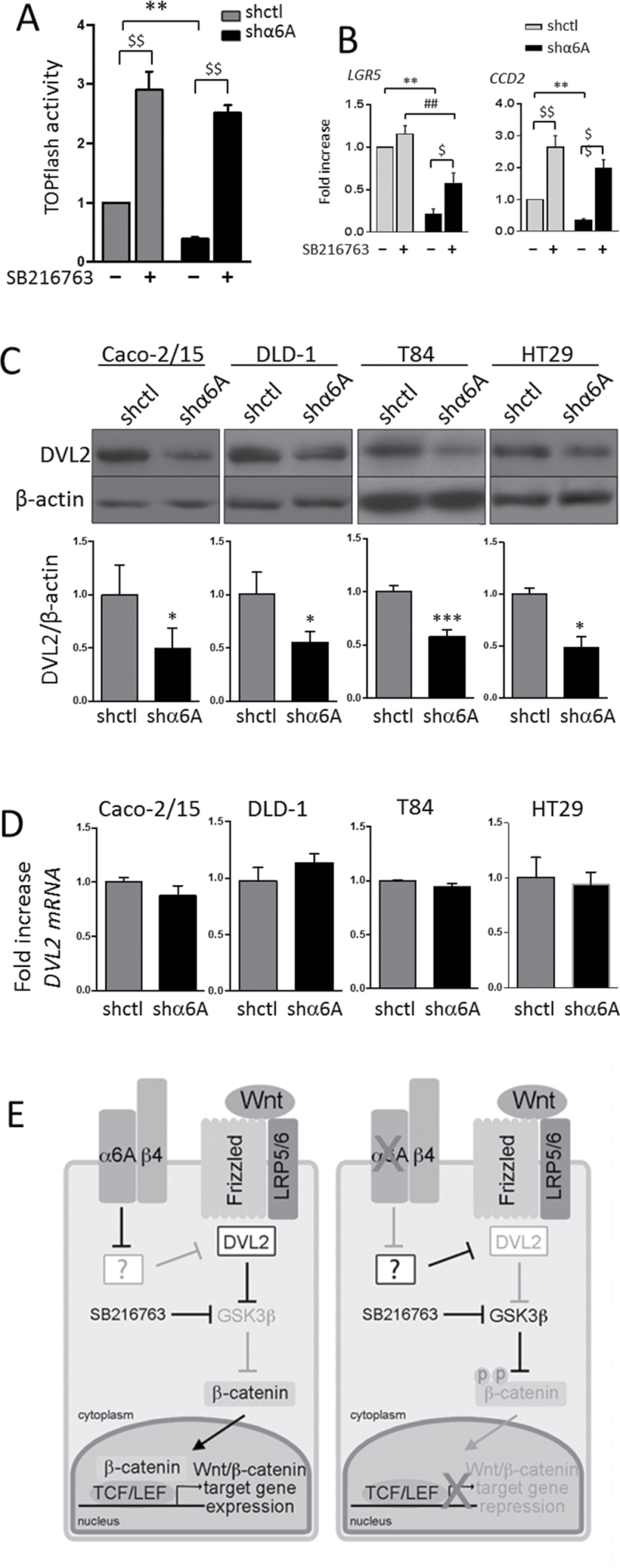
Inhibition of GSK3β rescues Wnt/β-catenin pathway
To further investigate the possible contribution of GSK3β to α6A-mediated β-catenin activation, we evaluated the effect of GSK3β inhibition on the rescue of responsive β-catenin/TCF4/LEF reporter plasmid activity and target gene expression in α6A knocked down T84 cells. As shown in Figure 6, we found that the pharmacological inhibition of GSK3β with SB21673 led to a significant stimulation of TOPflash activity in both shctl and shα6A cells (Figure 6A). Moreover, GSK3β inhibition stimulated TOPflash activity to the same level in both shctl and shα6A cells. To further extend these observations, the effect of α6A knockdown and GSK3β inhibition on Wnt/β-catenin target gene expression was analyzed by qPCR. We chose to investigate LGR5, CCD1 and CCD2, three well-documented target genes of the Wnt/β-catenin pathway in the intestine (37,38). First, shα6A cells were found to display a significant reduction of mRNA levels for LGR5 and CCD2 relative to shctl cells (Figure 6B), confirming the inhibition of Wnt/β-catenin transcriptional activity on these two target genes. GSK3β inhibition in shα6A cells resulted in a significant stimulation of LGR5 and CCD2 mRNA expression (Figure 6B). When both shctl and shα6A cells treated with the GSK3β inhibitor were compared, LGR5 mRNA expression was still significantly lower in shα6A cells than in shctl cells, whereas CCD2 mRNA expression was similar (Figure 6B), suggesting at least a partial rescue. No significant change in CCD1 mRNA expression was observed under these conditions (data not shown). These results suggest a role for the α6A integrin subunit in the control of Wnt/β-catenin activity and some of its target genes through GSK3β, which in turn may regulate CRC proliferation.
Fig. 6.
Regulation of the Wnt/β-catenin pathway. (A and B) Inhibition of GSK3β rescues Wnt/β-catenin activity. (A) Response of β-catenin/TCF4 promotor activity in α6A knocked down T84 cells and controls ± SB216763. Statistical analysis between shctrl and shα6A: **P ≤ 0.001; statistical analysis between − versus + SB216763: $$ P ≤ 0.001; ANOVA, n = 3. (B) qPCR analysis for the expression of LGR5 and CCD2 transcript levels in shctl and shα6A cells. T84 cells were treated for 24h ± SB216763 prior to analysis. Data were normalized to RPLPO as reference gene. Statistical analysis between untreated shctrl and shα6A: **P ≤ 0.001; statistical analysis between SB216763-treated shctrl and shα6A: ## P ≤ 0.001; statistical analysis between − versus + SB216763: $ P ≤ 0.05, $$ P ≤ 0.001; ANOVA, n = 3. (C and D) Knockdown of α6A reduces DVL2 protein levels in the four cell lines tested. (C) Representative WB and graph of the densiometric analysis of the detection of DVL2 protein levels in shctl and shα6A cells. Statistical analysis between untreated shctrl and shα6A: *P ≤ 0.05, ***P ≤ 0.0001, t-test, n = 3. (D) qPCR analysis for the expression of DVL2 transcript levels in shctl and shα6A cells. Data were normalized to RPLPO levels. (E) Working model for the involvement of the α6Aβ4 integrin in the regulation of the Wnt/β-catenin pathway. (Left) The α6Aβ4 integrin is over-expressed in CRC. When present, α6A regulates positively DVL2 at the protein level. DVL2, which inhibits GSK3β-mediated β-catenin phosphorylation, enhances β-catenin stability and translocation into the nucleus for the activation of the transcription of specific target genes involved in cell proliferation. (Right) Knockdown of the α6A subunit in CRC cells results in a decrease in DVL2 levels, thus allowing β-catenin phosphorylation by GSK3β. β-Catenin being targeted to degradation is no longer translocated to the nucleus resulting in repression of the transcription of Wnt/β-catenin-specific target genes. In this context, pharmacological inhibition of GSK3β with SB216763 restores Wnt/β-catenin pathway activity by bypassing the regulation of DVL2 by the α6A integrin subunit. The ‘?’ box denotes a still unknown mechanism by which α6A could be involved in the repression of key proteins regulating DVL2 degradation. Pathways/molecules activated are in black, whereas those inhibited are in grey.
Down-regulation of DVL2
The promoting effect of α6A on the expression of the active/nuclear form of β-catenin and the fact that inhibition of GSK3β could rescue the activity of the Wnt/β-catenin pathway suggest that α6A could interfere with a signalling event upstream to GSK3β. Thus, we investigated the possibility that α6A could act through the main GSK3β regulator, DVL2 (39). Interestingly, WB analyses of whole cell lysates revealed that knockdown of α6A led to a significant decrease in the protein levels of DVL2 in all four cell lines tested (Figure 6C) although its transcript levels were not affected (Figure 6D), suggesting that integrin α6Aβ4 regulates the Wnt/β-catenin pathway through DVL2.
Discussion
Over the past several years, there has been an increase in evidence showing that integrin receptors can have important functions in various steps of cancer progression, as main modulators of cell survival, invasion, migration and proliferation (6–9,19,24). Moreover, in cancer, although integrins can be mutated, they are more often found to be regulated at the level of expression and constitutively activated in lipid rafts (1,4,6) as observed for the α6β4 integrin in CRC (12). Up-regulation of the α6 integrin subunit is not restricted to CRC, as other cancer types such as breast cancer and glioblastoma have been shown to express high levels of α6, which correlates with poor survival (21,25). However, the integrin α6 subunit is expressed in the form of α6A and α6B variants in most tissues (26) although each variant may mediate distinct cell functions (27–31). In the human colon, the α6B variant has been detected in the quiescent cells of the surface epithelium, whereas the α6A variant has been found to be associated with the proliferative cells in the normal crypts and in colorectal primary tumours (12). In this study, we found that the overall increase of α6 subunit found in CRC occurs almost exclusively under its α6A splice variant form. More importantly, we also identified specific functions of the α6A variant as a pro-proliferative component for CRC cells and a regulator of the Wnt/β-catenin pathway.
Alternative splicing is a process by which exons are excluded/included into the mRNA, which allows for a considerable increase in protein diversity. Numerous studies have shown that alternative splicing is involved in the generation of pro-tumoural variants in cancer (40). Herein, we demonstrated that the inclusion of exon 25 during α6 integrin pre-mRNA processing is favoured in CRC and leads to the formation of a pro-proliferative variant of the α6 subunit, α6A. Interestingly, this integrin seems to have a common effect on CRC cells, since all four tested CRC cell lines in which α6A was knocked down showed a reduction in in vitro cell proliferation and a decrease in S-phase entry. A reduction in tumour growth in xenografts was also observed. Interestingly, the reduction of cell proliferation and/or tumour growth of mammary carcinoma cells, MCF7 breast cancer cells, glioblastoma cells and human liposarcoma cells has been reported previously following the knockdown of α6 integrin subunit expression (20,21,23,41). However, this is the first time that such pro-proliferative and pro-tumoural growth mediated by the α6 integrin subunit can be specifically attributed to one of its variants, α6A.
How the α6A(β4) integrin specifically regulates the Wnt/β-catenin pathway to modulate cell proliferation remains to be determined. Integrins interact with scaffold and kinase proteins to generate intracellular signalling events. Since α6A and α6B differ only by their cytoplasmic domains, the pro-proliferative function of the α6A variant could be attributed to the recruitment of and/or interaction with specific cytoplasmic partners. For instance, it has been reported that α6A can interact with MSS4 and α6B with BIN1 via their GFFKR motif (28). MSS4 has been found to act as a regulator of the stress response and apoptosis (42), whereas BIN1 has been characterized as a tumour suppressor through its strong ability to inhibit c-MYC transcriptional activity (43). On the other hand, GIPC, a glut1-binding protein, has been found to bind to the type I PDZ domains of both α6A and α6B, the interaction being stronger on α6A (27). Incidentally, an small interfering RNA-targeting GIPC has been found to inhibit pancreatic cancer growth in an orthotopic mouse model (44).
The Wnt pathway has been recognized as the dominant force behind the proliferative activity of the intestinal epithelium both in its physiological state and in CRC (45), thus suggesting a possible relation between the pro-proliferative α6A(β4) integrin and the activation of the Wnt/β-catenin pathway in both normal colonic crypts and in CRC. In normal crypt cells, the Wnt/β-catenin pathway is mainly modulated by the Wnt ligands of the stem cell niche (45). In CRCs, Wnt activation mainly occurs through mutation of the APC gene (35,46), which regulates β-catenin phosphorylation, thus favouring the accumulation of β-catenin in the nucleus, which after binding with T-cell factor results in the transcriptional activation of pro-proliferative target genes (47). However, it has been established that a number of mutations allow the retention of APC function and regulation of the activity of β-catenin in CRC cells despite mutation in APC (35,46,48). In agreement with these findings, herein using CRC cell lines that harbour various APC mutations (46), we demonstrated that abolition of α6A expression has a significant impact on a series of end points used to monitor the Wnt/β-catenin pathway. Indeed, based on the observations that down-regulation of α6A levels results in a reduction of the relative amounts of both active (evaluated as the non-GSK3β phosphorylated form) and nuclear β-catenin, whereas the pharmacological inhibition of GSK3β restored the activity of the Wnt/β-catenin pathway as evaluated by TOPflash assays and the modulation of specific target genes including LGR5 and CCD2, we suggest that α6A interferes with the activity of GSK3β. Interestingly, the expression of DVL2, which is recruited to the Wnt receptor complex upon ligand activation and acts on the activation of Wnt/β-catenin signalling by preventing constitutive proteolytic destruction of β-catenin (39), was significantly diminished at the protein level in α6A knocked down CRC cells, suggesting that the α6Aβ4 integrin acts upstream from GSK3β. The mechanism still remains to be elucidated, but it is noteworthy that selective degradation pathways involving autophagy and prickle-1-dependent proteasomes have been reported previously for DVL. Indeed, during starvation-induced autophagy, the E3 ubiquitin ligase Von Hippel–Lindau protein tumour suppressor (pVHL) was shown to mediate DVL ubiquitination and its recognition by p62/SQSTM1, targeting it for selective autophagy (49). On the other hand, prickle-1, a planar cell polarity protein, can interact directly with DVL to mediate its ubiquitination and subsequent proteasome degradation via its destruction box (D-box) motif (50). Thus, via their regulatory functions on DVL degradation, pVHL and prickle-1 could negatively regulate Wnt/β-catenin activity.
In conclusion, this study identified for the first time a specific cell function for the α6A splice variant. Our results suggest that α6A(β4) regulates cell proliferation and the Wnt/β-catenin pathway through DVL2/GSK3β (Figure 6E). However, the α6A cytoplasmic-associated protein responsible for this mechanism remains to be elucidated, as well as the molecular mechanisms responsible for the increase of α6A in CRC cells.
Supplementary material
Supplementary Figures 1–3 can be found at http://carcin.oxford journals.org/
Funding
Canadian Institutes of Health Research (MOP-97836 to J.-F.B., CTP-82942 to CRC biobank).
Supplementary Material
Acknowledgements
We thank E.Herring for reviewing the manuscript and technical support, E.Tremblay for virus production and G.Bernarchez for the preparation of the colorectal tissue samples and cDNA. J.-F.B. is the recipient of a Canadian Research Chair in Intestinal Physiopathology. J.C.C. is a scholar of the Fonds de la Recherche du Québec - Santé (FRQS). J.-F.B. and J.C.C. are members of the FRQS-funded Centre de Recherche Clinique Étienne-Le Bel of the Centre Hospitalier Universitaire de Sherbrooke.
Conflict of Interest Statement: J.-F.G., N.B. and J.-F.B. are inventors of a patented technology related to integrin alpha 6. The other authors disclose no conflicts.
Glossary
Abbreviations:
- APC
adenomatous polyposis coli
- BrdU
5-bromo-2-deoxyuridine
- CRC
colorectal cancer
- IF
immunofluorescence
- ISEL
in situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- qPCR
quantitative PCR
- RM
resection margin
- WB
western blot.
References
- 1. Margadant C., et al. (2011). Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol., 23, 607–614 [DOI] [PubMed] [Google Scholar]
- 2. de Melker A.A., et al. (1999). Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays, 21, 499–509 [DOI] [PubMed] [Google Scholar]
- 3. Hynes R.O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell, 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 4. Giancotti F.G. (2003). A structural view of integrin activation and signaling. Dev. Cell, 4, 149–151 [DOI] [PubMed] [Google Scholar]
- 5. Siegel R., et al. (2012). Cancer statistics, 2012. CA Cancer J. Clin., 62, 10–29 [DOI] [PubMed] [Google Scholar]
- 6. Guo W., et al. (2004). Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol., 5, 816–826 [DOI] [PubMed] [Google Scholar]
- 7. Beaulieu J.F. (2010) Integrin α6β4 in colorectal cancer. World J. Gastrointest. Pathophysiol., 1, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S., et al. (2009). Integrin-linked kinase: a multi-functional regulator modulating extracellular pressure-stimulated cancer cell adhesion through focal adhesion kinase and AKT. Cell. Oncol., 31, 273–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercurio A.M., et al. (2001). Towards a mechanistic understanding of tumor invasion—lessons from the alpha6beta 4 integrin. Semin. Cancer Biol., 11, 129–141 [DOI] [PubMed] [Google Scholar]
- 10. Lee E.C., et al. (1992). The integrin alpha 6 beta 4 is a laminin receptor. J. Cell Biol., 117, 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basora N., et al. (1999). Expression of functionally distinct variants of the beta(4)A integrin subunit in relation to the differentiation state in human intestinal cells. J. Biol. Chem., 274, 29819–29825 [DOI] [PubMed] [Google Scholar]
- 12. Dydensborg A.B., et al. (2009). Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer, 9, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni H., et al. (2005). Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene, 24, 6820–6829 [DOI] [PubMed] [Google Scholar]
- 14. Falcioni R., et al. (1994). Integrin beta-4 expression in colorectal-cancer. Int. J. Oncol., 5, 573–578 [DOI] [PubMed] [Google Scholar]
- 15. Chao C., et al. (1996). A function for the integrin alpha6beta4 in the invasive properties of colorectal carcinoma cells. Cancer Res., 56, 4811–4819 [PubMed] [Google Scholar]
- 16. Shaw L.M. (2001). Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol. Cell. Biol., 21, 5082–5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mercurio A.M., et al. (2004). Autocrine signaling in carcinoma: VEGF and the alpha6beta4 integrin. Semin. Cancer Biol., 14, 115–122 [DOI] [PubMed] [Google Scholar]
- 18. Dans M., et al. (2001). Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem., 276, 1494–1502 [DOI] [PubMed] [Google Scholar]
- 19. Chen M., et al. (2009). Integrin alpha6beta4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J. Biol. Chem., 284, 1484–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y., et al. (2011). Integrin subunits alpha5 and alpha6 regulate cell cycle by modulating the chk1 and Rb/E2F pathways to affect breast cancer metastasis. Mol. Cancer, 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lathia J.D., et al. (2010). Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell, 6, 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marchiò S., et al. (2012). A complex of α6 integrin and E-cadherin drives liver metastasis of colorectal cancer cells through hepatic angiopoietin-like 6. EMBO Mol. Med., 4, 1156–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L., et al. (2011). Integrin α6(high) cell population functions as an initiator in tumorigenesis and relapse of human liposarcoma. Mol. Cancer Ther., 10, 2276–2286 [DOI] [PubMed] [Google Scholar]
- 24. Desgrosellier J.S., et al. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer, 10, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedrichs K., et al. (1995). High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res., 55, 901–906 [PubMed] [Google Scholar]
- 26. Hogervorst F., et al. (1993). Biochemical characterization and tissue distribution of the A and B variants of the integrin alpha 6 subunit. J. Cell Biol., 121, 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tani T.T., et al. (2001). PDZ interaction sites in integrin alpha subunits. T14853, TIP/GIPC binds to a type I recognition sequence in alpha 6A/alpha 5 and a novel sequence in alpha 6B. J. Biol. Chem., 276, 36535–36542 [DOI] [PubMed] [Google Scholar]
- 28. Wixler V., et al. (1999). Identification of novel interaction partners for the conserved membrane proximal region of alpha-integrin cytoplasmic domains. FEBS Lett., 445, 351–355 [DOI] [PubMed] [Google Scholar]
- 29. Shaw L.M., et al. (1995). The alpha 6A beta 1 and alpha 6B beta 1 integrin variants signal differences in the tyrosine phosphorylation of paxillin and other proteins. J. Biol. Chem., 270, 23648–23652 [DOI] [PubMed] [Google Scholar]
- 30. Wei J., et al. (1998). Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the alpha6 integrin subunit. J. Biol. Chem., 273, 5903–5907 [DOI] [PubMed] [Google Scholar]
- 31. Dydensborg A.B., et al. (2009). Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem. Cell Biol., 131, 531–536 [DOI] [PubMed] [Google Scholar]
- 32. Groulx J.F., et al. (2012). Autophagy is active in normal colon mucosa. Autophagy, 8, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dydensborg A.B., et al. (2006). Normalizing genes for quantitative RT-PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am. J. Physiol. Gastrointest. Liver Physiol., 290, G1067–G1074 [DOI] [PubMed] [Google Scholar]
- 34. Pfaffl M.W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res., 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Segditsas S., et al. (2006). Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene, 25, 7531–7537 [DOI] [PubMed] [Google Scholar]
- 36. Van der Flier L.G., et al. (2007). The intestinal Wnt/TCF signature. Gastroenterology, 132, 628–632 [DOI] [PubMed] [Google Scholar]
- 37. Barker N., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 38. Huang W., et al. (2007). GSK3 beta mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene, 26, 2471–2482 [DOI] [PubMed] [Google Scholar]
- 39. Gao C., et al. (2010). Dishevelled: the hub of Wnt signaling. Cell. Signal., 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 40. Miura K., et al. (2012). Splice isoforms as therapeutic targets for colorectal cancer. Carcinogenesis, 33, 2311–2319 [DOI] [PubMed] [Google Scholar]
- 41. Cariati M., et al. (2008). Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int. J. Cancer, 122, 298–304 [DOI] [PubMed] [Google Scholar]
- 42. Walter B.M., et al. (2012). Mss4 protein is a regulator of stress response and apoptosis. Cell Death Dis., 3, e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakamuro D., et al. (1996). BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat. Genet., 14, 69–77 [DOI] [PubMed] [Google Scholar]
- 44. Muders M.H., et al. (2009). Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth. Clin. Cancer Res., 15, 4095–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Flier L.G., et al. (2009). Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol., 71, 241–260 [DOI] [PubMed] [Google Scholar]
- 46. Rowan A.J., et al. (2000). APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits”. Proc. Natl Acad. Sci. USA, 97, 3352–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Korinek V., et al. (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science, 275, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 48. Yang J., et al. (2006). Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem., 281, 17751–17757 [DOI] [PubMed] [Google Scholar]
- 49. Gao C., et al. (2010). Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol., 12, 781–790 [DOI] [PubMed] [Google Scholar]
- 50. Chan D.W., et al. (2006). Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology, 131, 1218–1227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.