Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide. Methylene blue (MB) has known energy-enhancing and antioxidant properties. This study tested the hypothesis that MB treatment reduces lesion volume and behavioral deficits in a rat model of mild TBI. In a randomized double-blinded design, animals received either MB (n=5) or vehicle (n=6) after TBI. Studies were performed on 0, 1, 2, 7, and 14 days following an impact to the primary forelimb somatosensory cortex. MRI lesion was not apparent 1 h after TBI, became apparent 3 h after TBI, and peaked at 2 days for both groups. The MB-treated animals showed significantly smaller MRI lesion volume than the vehicle-treated animals at all time points studied. The MB-treated animals exhibited significantly improved scores on forelimb placement asymmetry and foot fault tests than did the vehicle-treated animals at all time points studied. Smaller numbers of dark-stained Nissl cells and Fluoro-Jade® positive cells were observed in the MB-treated group than in vehicle-treated animals 14 days post-TBI. In conclusion, MB treatment minimized lesion volume, behavioral deficits, and neuronal degeneration following mild TBI. MB is already approved by the United States Food and Drug Administration (FDA) to treat a number of indications, likely expediting future clinical trials in TBI.
Key words: : antioxidant, mitochondria, MRI, oxidative stress, vasogenic edema
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability.1 Common causes of TBI include falls, violence, injuries from wars, and vehicle and sport accidents. The initial direct mechanical damage in TBI is followed by secondary damage that includes impaired cerebral blood flow (CBF) and oxygen delivery, cerebrovascular autoregulation, and metabolic function. Ischemia-like events (such as membrane depolarization, ion dysregulation, oxidative stress, excitotoxicity, and inflammation, among others) subsequently lead to apoptotic and necrotic cell death. The multidimensional cascades of secondary brain injury in TBI offer many potential targets for therapeutic interventions.
Mitochondria are the cells' power plants that generate adenosine triphosphate (ATP), necessary for sustaining normal cellular function and repair. As the brain is dependent upon a continuous supply of energy to maintain cellular integrity, damage to the mitochondria can be detrimental. Mitochondrial dysfunction may occur within minutes of TBI, and persist for days following the injury. In addition, reactive oxygen species (ROS) – natural byproducts of oxygen metabolism – increase rapidly following TBI, and overwhelm the cells' natural antioxidant capabilities, resulting in oxidative stress. Treatments that can restore mitochondrial function have the potential to improve outcomes following TBI injury. As such, mitochondria have become an important target for neuroprotection in TBI.2,3
Methylene blue (MB) is used clinically to treat methemoglobinemia, malaria, carbon monoxide poisoning, and cyanide poisoning.4 At low doses, MB forms a reversible reduction-oxidation system with auto-oxidizing capacity.5 Low-dose MB has redox recycling properties in that it acts as an electron cycler and facilitates electron transfer in the mitochondrial electron transport chain by accepting electrons from nicotinamide adenine dinucleotide (NADH), and transferring them to cytochrome c, bypassing complex I-III.6 MB thus enhances or sustains ATP production in cells7–9 and brain oxygen consumption in vitro.10 In bypassing complex I-III to generate ATP, MB also minimizes free radical production in the mitochondrial electron transport chain, which could have positive effects under metabolic stressed conditions (i.e., brain injury) in which excessive production of free radicals may lead to cellular damage and cell death. Moreover, MB can induce long-lasting effects by modifying mitochondrial pathways.11 In vivo studies also show that MB significantly affects cerebral metabolism, hemodynamics, and evoked responses in normal rats; namely, that, MB enhances global glucose uptake, oxygen consumption, CBF12 and evoked responses.13 Recently, MB treatment has been shown to reduce behavioral impairments in animal models of Parkinson's disease14 and Alzheimer's disease,15,16 and to reduce cerebral infarct volume by histology.17,18
The goal of this study was to evaluate the therapeutic efficacy of MB in a rat model of mild TBI. We tested the hypothesis that MB treatment reduces lesion volume, neurologic deficit, and neuronal degeneration following mild TBI in rats. In a randomized, double-blinded design, animals received either MB or vehicle after TBI. MRI lesion progression, forelimb placement asymmetry, and foot fault behavioral tests were longitudinally evaluated up to 14 days post-TBI. Immunohistology (Nissl and Fluoro- Jade® B) was used to quantify neuronal degeneration.
Methods
Animal preparations
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center, San Antonio. Male Sprague–Dawley rats (250–350 g, n=12) were anesthetized initially with 5% isoflurane mixed with room air and maintained at 1.5% isoflurane throughout all surgical and imaging procedures. The animal was secured in a stereotaxic frame and a Ø5 mm craniotomy was created over the left somatosensory cortex (S1: +0.25 mm anterior and 3.5 mm lateral to bregma), exposing the dura matter. The intact dura matter was impacted using a pneumatic controlled cortical impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA) fitted with a Ø3 mm tip (5.0 m/sec, 250 μs dwell time, 1 mm depth) to mimic a mild focal TBI. The cranial opening was gently sealed with bone wax following the impact, and the scalp was sutured closed and antibiotic ointment applied. Buprenex (0.05 mg/kg) was given subcutaneously every 12 h for 3 days.
In a randomized double-blinded design, half of the animals received MB (USP Pharmaceutical grade; American regent Inc, Shirley, NY) at 1 mg/kg 1 h post-TBI and 0.5 mg/kg 3 h post-TBI via tail vein (each dose was infused over 30 min), and the other half received saline vehicle. A daily dose of 0.5–5 mg/kg MB is considered safe for humans and animals.4,11,19,20 MRI was performed on the day of the TBI procedure (1–3 h post-TBI), and 1, 2, 7, and 14 days after TBI.
Behavioral assessments were made 1–3 days prior to TBI and again 1, 2, 7, and 14 days post-TBI prior to the MRI experiments in the same animals. Behavioral tests were not performed on the day of TBI induction, because of incomplete recovery from anesthetic. Immunohistology was done after MRI on day 14 post-TBI. The 14 day end-point was chosen based on a subset of studies in which no apparent differences in lesion volumes between 14 and 28 days post-TBI were observed.
MRI
MRI (Bruker 7-Tesla Pharmascan) was performed to longitudinally monitor lesion volume. The animal was anesthetized with 1.5% isoflurane and secured in a MRI-compatible stereotaxic holder with ear and tooth bars. A transceiver surface coil 2 cm in diameter was placed on top of the rat's head. T2-weighted images were acquired using a fast spin-echo sequence with repetition time (TR) 3 sec (90 degree flip angle), effective echo time (TE) 18, 54, 90, and 126 ms, 8 echo train length, seven 1.0 mm thick coronal images, field of view (FOV) 2.56×2.56 cm, matrix 96×96 and reconstructed to 128×128, and four transients for signal averaging.21 Images were co-registered across time points using QuickVol and MRIAnalysisPak software.22 The lesion volumes were determined as pixels that had T2 values higher than the mean plus two standard deviations of the value in the homologous contralesional region.23
Functional assessment
Sensorimotor function was assessed using the asymmetry forelimb placement (cylinder) test and foot fault test24 1–3 days prior to TBI, and again 1, 2, 7, and 14 days post-TBI. The forelimb asymmetry placement test was performed with videotaping to assess the use of forelimbs. The rat was placed in a transparent cylinder (20 cm diameter, 30 cm height) for 5 min or until 30 placements were made. A mirror was positioned under the cylinder to enable the video recorder to see directly into the cylinder. The behavior was scored by counting the number of left or right individual forelimb placements, and the number of simultaneous right and left (both) forelimb placements onto the wall of the cylinder during rearing. The forelimb asymmetry index was calculated as: number of forelimb placements for each individual limb+ ½ (number of both placements) divided by the total number of placements.
The foot fault test was performed with videotaping to assess limb misplacement during locomotion. The rat was placed on an elevated grid floor (e.g., size 45.7 cm×27.9 cm with grid openings of ∼3.96 cm2 and 2.54 cm2) for 5 min, or until 50 steps were taken with one (right hind) limb. The rat was allowed to move freely on the grid, and the total number of steps and the number of times each limb fell below the grid opening were counted. The percentage of foot faults for each limb was calculated as the number of right or left forelimb or hindlimb foot faults divided by the total number of steps taken.
Nissl staining
Nissl staining was used to measure morphological changes.25,26 Anesthetized rats were perfused with ice-cold heparinized phosphate buffered saline, followed by ice-cold 4% buffered paraformaldehyde on day 14 post-TBI. Brains were removed and fixed for 2–5 h at 4°C, and subsequently cryopreserved in 30% sucrose for 48 h. Coronal sections (25 μm thick) were cut on a cryostat and affixed to gelatin-coated slides and dried overnight at 37°C. Slides were hydrated through a series of graded alcohols to distilled water followed by 0.1% cresyl violet acetate for 7 min. Brain sections were then dehydrated, cleared in xylene and cover-slipped with mounting medium.
Images were acquired on an Olympus BX60F microscope equipped with an Olympus DP70 camera using a 100×oil objective for morphological analysis. A 10×objective was utilized to acquire images for mosaic full brain images assimilated using Microsoft ICE software. Intact neurons were defined as nonbasophilic neurons with pale nuclei and discrete nucleoli, and having an intact neuronal body. Dark stained neurons were defined as neurons with abnormal morphologies such as hyperbasophilic neurons and those with shrunken morphology. Four slices from each brain were selected for analysis by determining corresponding slices to the images acquired from T2 MRI using stereotaxic coordinates. The slices were 1 mm apart to ensure adequate sampling throughout the lesioned area. For each slice imaged, 10 regions located under the impact zone were selected for imaging by sampling beginning directly under the impact zone 3.5 mm from the median plane and 1.5 mm from the edge of the cortex. Additional images were acquired on either side of and below this area in two rows spaced 250 μm apart. Ten corresponding regions on the contralateral side were selected and imaged at 60×(175μm x 175μm) from each brain collected. The numbers of dark stained cells were counted and averaged. Results were expressed as the number of dark stained cells per field.
Fluoro-Jade B staining
Fluoro-Jade B is an anionic fluorescein derivative used to stain degenerating neurons.27,28 Sections were incubated in a solution of 1% NaOH in 80% ethanol for 5 min, followed by hydration in graded ethanols (75, 50, and 25%) to distilled water for 5 min each. The sections were then incubated for 10 min in 0.06% potassium permanganate, rinsed with distilled water, and incubated in 0.004% Fluoro-Jade B (Hist-Chem Inc, Jefferson, AR) for 20 min. Sections were rinsed with distilled water three times for 2 min each. The slides were then dried on a slide warmer for 10 min, cleared with Histo-Clear and cover-slipped with DPX (Fluka). Images were acquired on a Nikon C1si microscope using a 60×water immersion objective. Image analysis was performed similar to that done for Nissl staining.
Statistical analysis
Unpaired t tests were used to compare T2 lesion volumes between vehicle- and MB-treated groups. Differences in the number of dark-stained Nissl neurons and Fluoro- Jade B positive cells between vehicle- and MB-treated animals were assessed using a two tailed t test. Mann–Whitney U tests were used to compare differences in asymmetrical limb use and the percentage of foot faults between vehicle-and MB-treated animals. Values are presented as mean±SEM. Statistical significance was set at p<0.05. Multiple regression analysis was performed to determine the correlation between MRI determined lesion volume and asymmetry scores, foot fault scores, and Nissl and Fluoro-Jade B staining values.
Results
MB reduced lesion volume
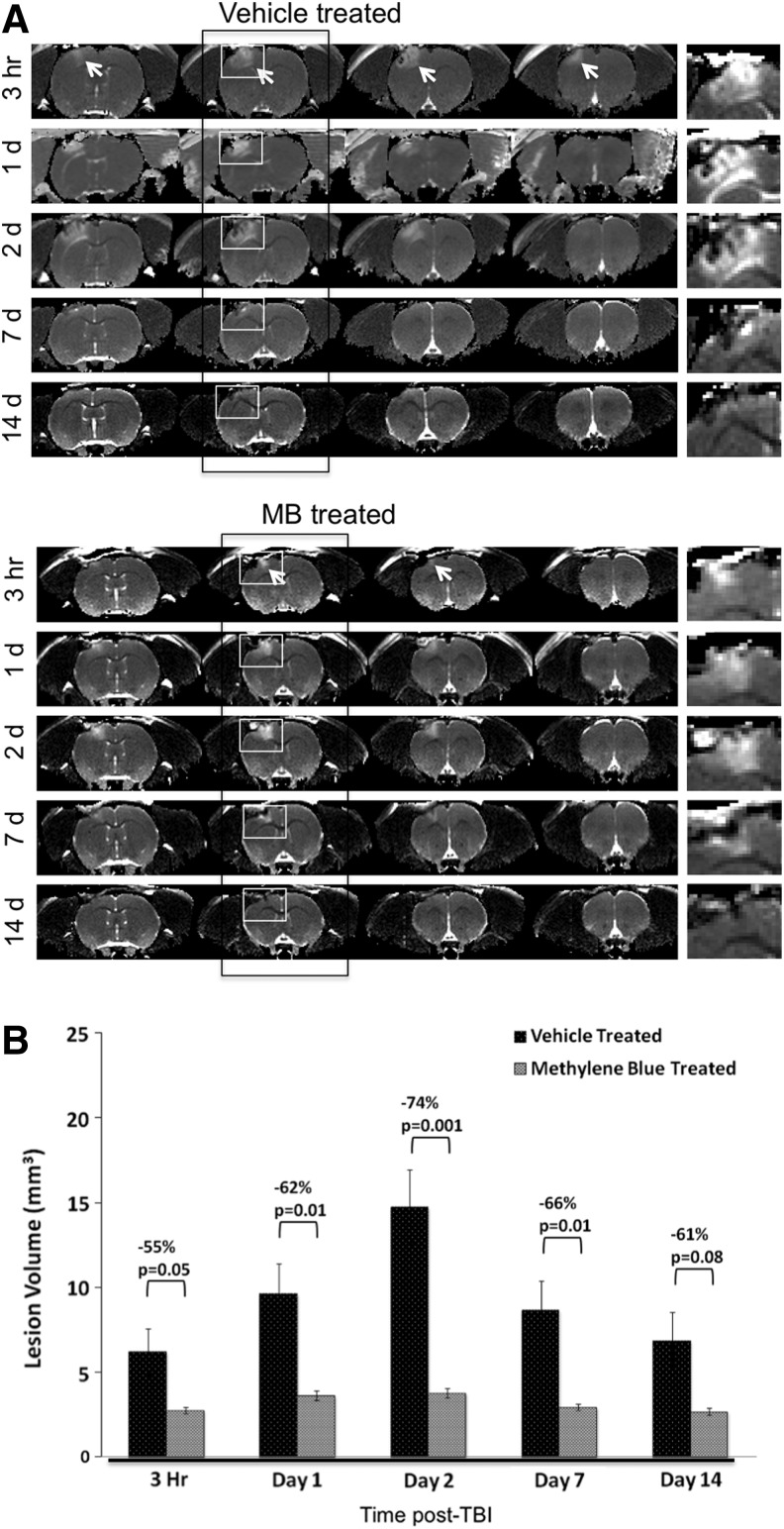
Multislice T2 maps show the temporal progression of TBI lesions in a vehicle-treated and an MB-treated animal (Fig. 1A). The hyperintense area in the S1 cortex (arrows) indicates the lesion, which has been enlarged in the panels to the right of each representative group in Figure 1A. Within the lesions, there were also hypointense spots, likely indicating signs of hemorrhage. T2 MRI at 1 h after TBI showed no apparent lesion in either the vehicle- or MB-treated group. MRI lesions were apparent 3 h after TBI in both treatment groups. In the vehicle-treated group, the lesion volume peaked at 2 days, and decreased by 14 days post-TBI (Fig. 1B). In the MB-treated group, by contrast, the lesion volumes did not change substantially with time, and were significantly smaller than those of the vehicle-treated group at all time points studied. These results indicate that MB treatment markedly reduces TBI lesion volume and vasogenic edema.
FIG. 1.
T2 MRI. (A) Representative T2 maps are shown for vehicle- and methylene blue (MB)-treated animals at 3 h, and 1, 2, 7, and 14 days post-traumatic brain injury (TBI). The arrows indicate hyperintense lesions in the ipsilesional cortex. The white boxes in the second panel indicate the enlarged image of the lesion area demonstrated to the right of each panel to further demonstrate the evolution of the lesion with time. (B) Bar graph shows the progression of lesion volumes in MB-treated animals compared with vehicle-treated animals (mean±SEM, n=6 per group,*p<0.05).
MB improved behavioral outcomes
Sensorimotor function was assessed using the forelimb asymmetry (cylinder) and foot fault tests. Before TBI, the mean forelimb asymmetry scores were not significantly different between the vehicle- and MB-treated groups (49±2% vs. 52±2%, p>0.05; Fig. 2A) indicating symmetrical use of the two forelimbs. In the vehicle-treated group, asymmetry scores worsened on days 1 and 2 after TBI, indicating increased utilization of the left (unaffected) forelimb in the vehicle-treated animals. The asymmetry scores returned toward pre-TBI values on days 7 and 14. In the MB-treated group, by contrast, forelimb asymmetry scores did not change substantially with time, and remained close to pre-TBI values across all time points studied.
FIG. 2.
Line graphs of the (A) cylinder and (B) foot fault tests for the vehicle- and methylene blue (MB)-treated animals at pre-traumatic brain injury (TBI), and at 1, 2, 7, and 14 days post-TBI (mean±SEM, n=6 each group, *p<0.05, **p<0.01 between vehicle- and MB-treated groups).
The percentages of foot faults were not statistically different between the vehicle- and MB-treated groups prior to TBI induction (5.13±0.64 vs. 6.57±1.04%, p>0.05; Fig. 2B). In the vehicle-treated group, foot fault scores worsened in the right forelimb dramatically on day 1, persisted on days 2 and 7 after TBI, and improved slightly on day 14. In the MB-treated group, by contrast, right foot faults were only slightly elevated on day 1 post-TBI but did not reach the severity observed in the vehicle group. Moreover, by day 2, the right foot fault scores returned close to pre-TBI values, and persisted through days 7 and 14. There were significantly lower numbers of foot faults in the MB-treated group than in the vehicle-treated group on days 1, 2, and 7 post TBI (p=0.043, 0.018, and 0.0058, respectively) but not on day 14. Together, these data indicated that MB markedly reduced sensorimotor deficits following TBI. These data also suggest that there were functional compensations in both groups, and that the extent of functional compensation differed between groups.
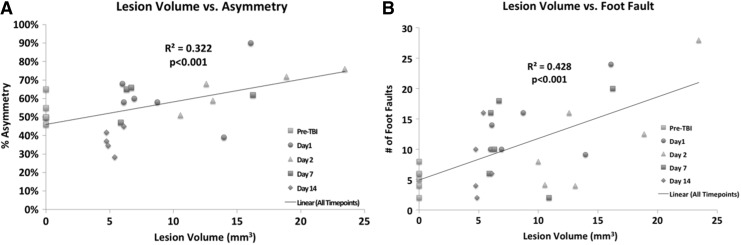
Correlation analysis between T2 MRI lesion volume and forelimb asymmetry scores for all time points yielded an R2 value of 0.32 (p=0.002) (Fig. 3A). Correlation analysis between MRI-determined lesion volume and foot fault scores for all time points yielded an R2 value of 0.43 (p=0.0002) (Fig. 3B). Vasogenic edema and T2 MRI lesion volume were in general agreement with behavioral scores.
FIG. 3.
Multiple regression correlation plots are demonstrated for T2 MRI lesion volume versus (A) asymmetry and (B) foot fault, with different symbols indicating data from different days post TBI.
MB reduced Nissl-positive cells
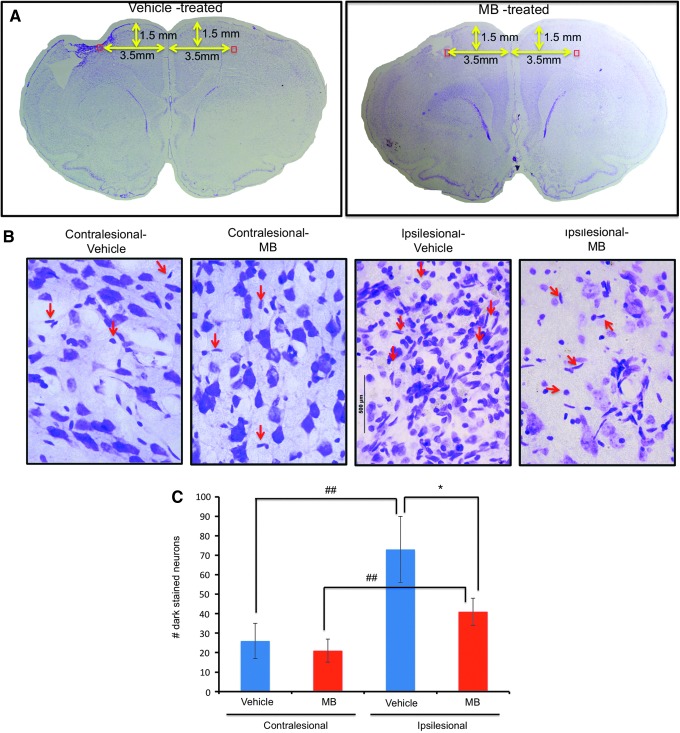
Nissl staining of degenerating neurons was analyzed in the primary somatosensory cortex below the impact zone for both experimental groups on day 14 after TBI. Mosaic images (Fig. 4A) and magnified images (Fig. 4B) demonstrated the extent of the injury and altered morphological characteristics in the ipsilesional cortex. In the vehicle-treated group, the number of Nissl-positive cells in the ipsilesional S1 cortex was 73±17 cells/field and that in the contralesional S1 cortex was 26±9 cells/field (n=6, p<0.05). In the MB-treated group, the number of Nissl-positive cells in the ipsilesional S1 cortex was 41±7 cells/field and that in the ipsilesional S1 cortex was 21±6 cells/field (n=6, p<0.05). The numbers of Nissl-positive cells in the ipsilesional S1 cortex were significantly different between the vehicle- and MB-treated groups (p<0.05). The numbers of positive Nissl cells in the contralesional S1 cortex were not significantly different between the vehicle- and MB-treated groups (p>0.05).
FIG. 4.
Nissl staining 14 days post-traumatic brain injury (TBI). (A) Mosaic images of the vehicle and methylene blue (MB)-treated animals. The red box indicates the location of the zoom-in images. (B) Magnified images of a vehicle- and an MB-treated TBI animal obtained using a 60×objective. The red arrows indicate typical dark-stained neurons. (C) Bar graph of the number of dark-stained Nissl bodies for vehicle- and MB-treated animals (mean±SEM, n=6 each group, *p<0.01, ##p<0.01). Color image is available online at www.liebertpub.com/neu
MB reduced Fluoro-Jade B positive cells
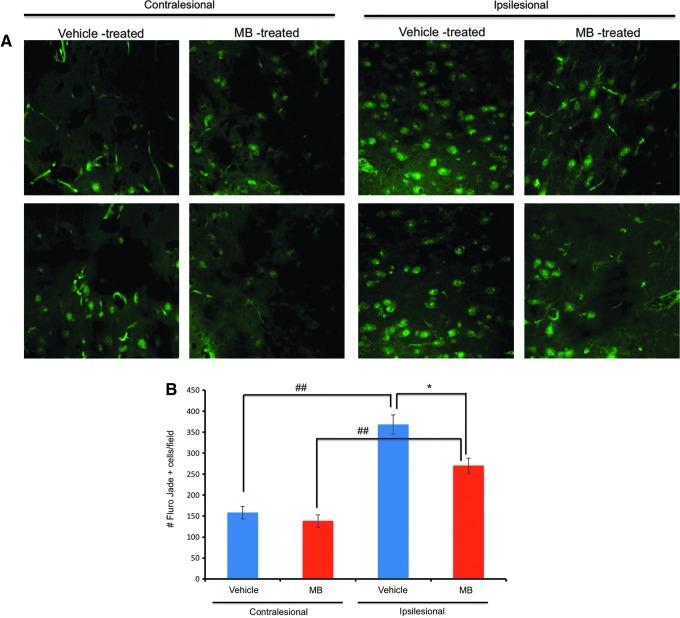
Fluoro-Jade B staining for degenerating neurons was performed 14 days after TBI. Figure 5A demonstrates representative images from the contralesional and ipsilesional cortex from vehicle- and MB-treated animals. In the vehicle-treated group, the number of Fluoro-Jade B positive cells (white arrows) in the ipsilesional S1 cortex was 368±23 cells/field, and that in the contralesional S1 cortex was 158±4.8 cells/field (n=6, p<0.05) (Fig. 5B). In the MB-treated group, the number of Fluoro- Jade B positive cells in the ipsilesional S1 cortex was 270±18 cells/field, and that in the contralesional S1 cortex was 149±15 cells/field (n=6, p<0.05). The numbers of Fluoro-Jade B positive cells in the ipsilesional S1 cortex were significantly different between the vehicle- and MB-treated groups (p<0.05). The numbers of Fluoro-Jade B positive cells in the contralesional S1 cortex were not significantly different between the vehicle- and MB-treated groups (p>0.05). The results from the Nissl and Fluoro-Jade B experiments indicated that MB reduced neurodegeneration following TBI.
FIG. 5.
Fluoro-Jade® B staining 14 days post-traumatic brain injury (TBI). (A) Representative images of vehicle- and methylene blue (MB)-treated animals at 60×magnification. (B) Bar graph of the number of Fluoro-Jade B positive cells per field for vehicle- and MB-treated animals (mean±SEM, n=6 each group, *p<0.01, ##p<0.05). Color image is available online at www.liebertpub.com/neu
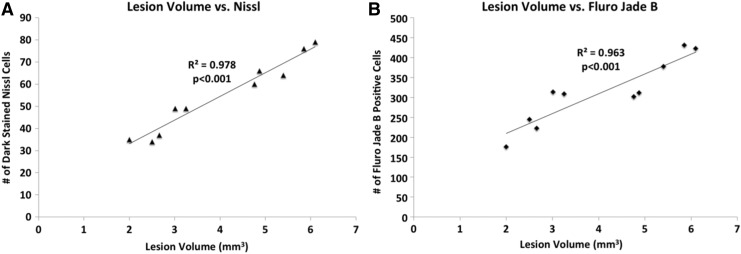
Correlation analysis between T2 MRI lesion volume and Nissl values at 14 days revealed significant correlation with an R2 value of 0.978 (p=0.000005) (Fig. 6A). Correlation analysis between T2 MRI lesion volume and Fluoro-Jade B values at 14 days revealed significant correlation, with an R2 value of 0.963 (p=0.008) (Fig. 6B). Vasogenic edema and T2 MRI lesion volume were in general agreement with markers of neurodegeneration.
FIG. 6.
Multiple regression correlation plots are demonstrated for T2 MRI lesion volume versus (A) Nissl staining and (B) Fluoro-Jade® B staining analysis at day 14 post-traumatic brain injury (TBI).
Discussion
This study demonstrated a neuroprotective effect of MB in a rat model of mild TBI using a double-blinded randomized design. MB minimized lesion volume and decreased functional deficits compared with vehicle-treated animals. Immunohistological staining for neuronal degeneration corroborated MRI lesion volume and behavioral data. MB neuroprotection against mild TBI in our study is consistent with previous MB studies that reported reduced behavioral deficits in animal models of Parkinson's disease,11 Alzheimer's disease,14–16 and cerebral infarct volume,17,18 supporting the notion that MB's energy-enhancing and antioxidant properties have therapeutic effects in a number of neurological injuries and disorders.
TBI-induced edema peaked at day 2 and resolved substantially by days 7 and 14. The reduction in edema by day 14 is in general agreement with improvement in the behavioral scores. Lesion volume and behavioral scores also generally correlated well with the improvement by MB treatment. However, despite the presence of lesions at day 7 and 14, forepaw asymmetry scores returned to normal for both groups. These results suggest that there is likely functional compensation (or reorganization). By contrast, the forelimb foot fault scores remained significantly abnormal on day 7 and returned closer to normal on day 14. These differences in our studies suggest that the foot fault test is more sensitive to milder injury, especially in the initial days following injury. The two behavioral tests showed different but complementary sensitivities to injury in this animal TBI model.
In vivo studies have shown that MB readily crosses the blood–brain barrier. MB concentration in the brain is 10–20 times higher than that in the circulation after intravenous injection in rats.29 No negative side effects of MB in animals or humans at low doses of MB (0.5–5 mg/kg) have been reported,4,11 but adverse effects (e.g., methemoglobinemia) have been reported at high MB doses (>10mg/kg)11 because MB interferes with electron transfer function in the mitochondria. The dosage utilized in this study was based on published results that daily low-dose MB is safe in humans and rats.4,11,19,20
A single MB dose of 1 mg/kg affects cerebral metabolism, hemodynamics, and evoked responses in normal rats. MB enhances global glucose uptake, oxygen consumption, and CBF in normal rats.12 In addition, MB markedly potentiates forelimb evoked blood oxygenation level-dependent (BOLD), CBF and cerebral metabolic rate of oxygen (CMRO2) changes focally in the forelimb somatosensory cortices,13 suggesting that MB has effects localized to regions with enhanced activity or metabolic stress. Moreover, during mild hypoxia,13 MB-treated rats are better able to sustain global glucose uptake, oxygen consumption, and CBF than are vehicle-treated rats.12 MB-treated animals have also shown larger functional MRI (fMRI) responses and O2 consumption changes than have vehicle-treated rats.13 These findings further support the notion that MB is an energy enhancer in vivo under metabolically stressful conditions. The ability to sustain some level of ATP production after TBI likely accounted, in part, for the observed reduction in vasogenic edema, lesion volume, neuronal cell death, and behavioral deficits.
A likely mechanism of neuroprotection is that MB acts as an energy enhancer, sustaining some level of ATP production after TBI. Other studies have targeted the mitochondria to sustain energy production and mitochondrial integrity following TBI. For example, 2-methylthioadenosine diphosphate trisodium salt, which targets the mitochondria through the P2Y1 receptor, stimulates energy production through an IP3 mediated pathway, was recently reported to decrease edema formation and neuronal loss in a closed-skull model of TBI in mice.26 In addition to stimulation of ATP production through the mitochondrial electron transport chain, the preservation of mitochondrial integrity has also been targeted using hyperoxia treatment. In a fluid percussion injury model, hyperoxia treatment significantly improved mitochondrial metabolic activity, and contributed to increased B-cell lymphoma 2 (Bcl-2) expression and increased oxygen availability.30,31 Loss of mitochondrial integrity can also affect the mitochondrial permeability transition pore, leading to increased release of pro-apoptotic factors following injury. Cyclosporine A binds to cyclophilin D, and results in stabilization of the mitochondrial permeability transition pore, reduced axonal damage, and lesion volume following TBI.32–34 Phase II clinical trials have demonstrated that cyclosporin A improves cerebral perfusion pressure and cerebral metabolism, and cyclosporin A has moved into Phase III clinical trials.35–37 Taken together, these data provide evidence that improving mitochondrial integrity and production of ATP results in improved outcome following TBI.
In addition to being an energy enhancer, MB also has antioxidant properties. Therefore, MB also likely reduces oxidative damage from excessive oxygen free radicals following TBI. MB has been shown to decrease ROS production in ischemia/reperfusion injury38 and neuron cell death induced by oxidative stress.39 In addition, MB inhibits rotenone-induced lipid peroxidation,8 and decreases oxidative damage following ischemic reperfusion injury.40,41 Many studies have targeted reduction in the production of ROS from the mitochondria following brain injury.42,43 Superoxide dismutases (SOD), which are considered one of the primary antioxidant defenses in cells, have been shown to maintain cytosolic calcium levels and reduce superoxide.44 The free radical scavenger, α-tocopherol, has also been shown to exert neuroprotective effects in experimental models of TBI by decreasing lipid peroxidation, neuronal necrosis, and reactive gliosis, and by reducing edema formation.45,46 MB's antioxidant property likely contributed to the observed reduction in vasogenic edema, lesion volume, neuronal cell death, and behavioral deficit in our study.
Conclusions
The present study provides evidence that MB – a unique energy-enhancer and antioxidant – is neuroprotective against mild TBI as measured by the reduction in lesion volume, behavioral deficit, and neurodegeneration. These findings suggest that targeting mitochondrial function via sustaining energy production and reducing free oxygen radicals could be a promising treatment strategy for mild TBI. Future studies will optimize multiple MB dosing and timing regimens to improve efficacy, impact other brain regions such as those involved in memory and cognition, explore milder TBI along with more sensitive behavioral tests, determine the mechanistic temporal profiles, and use additional MRI measures such as CBF, diffusion tensor, neurosvascular coupling, evoked responses, and blood–brain permeability. Combination therapy targeting multiple mechanisms will also be explored to further minimize damage and enhance recovery. Because MB is already a drug approved by the United States Food and Drug Administration (FDA), with an excellent safety profile at low doses, TBI clinical trials in humans utilizing MB can be readily explored.
Acknowledgments
The authors thank Timothy Schallert and Theresa Jones of the University of Texas (UT) at Austin for their assistance in the setup of the behavioral assays utilized in this study. Images were generated in the Core Optical Imaging Facility, which is supported by UTHSCSA, NIH-NCI P30 CA 54174 (CTRC at UTHSCSA) and NIH-NIA P01 AG19316.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nortje J., and Menon D.K. (2004). Traumatic brain injury: physiology, mechanisms, and outcome. Curr. Opin. Neurol. 17, 711–718 [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi R.K., and Beal M.F. (2008). Mitochondrial approaches for neuroprotection. Ann. N. Y. Acad. Sci 1147, 395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L., Morselli E., Kepp O., and Kroemer G. (2009). Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim. Biophys. Acta 1787, 402–413 [DOI] [PubMed] [Google Scholar]
- 4.Scheindlin S. (2008). Something old… something blue. Mol. Interv. 8, 268–273 [DOI] [PubMed] [Google Scholar]
- 5.Bruchey A.K., and Gonzalez–Lima F. (2008). Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am. J. Pharmacol. Toxicol. 3, 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifton J., 2nd, and Leikin J.B. (2003). Methylene blue. Am. J. Ther. 10, 289–291 [DOI] [PubMed] [Google Scholar]
- 7.Scott A., and Hunter F.E., Jr. (1966). Support of thyroxine-induced swelling of liver mitochondria by generation of high energy intermediates at any one of three sites in electron transport. J. Biol. Chem. 241, 1060–1066 [PubMed] [Google Scholar]
- 8.Zhang X., Rojas J.C., and Gonzalez–Lima F. (2006). Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox. Res. 9, 47–57 [DOI] [PubMed] [Google Scholar]
- 9.Lindahl P.E., and Oberg K.E. (1961). The effect of rotenone on respiration and its point of attack. Exp. Cell. Res. 23, 228–237 [DOI] [PubMed] [Google Scholar]
- 10.Riha P.D., Bruchey A.K., Echevarria D.J., and Gonzalez–Lima F. (2005). Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur. J. Pharmacol. 511, 151–158 [DOI] [PubMed] [Google Scholar]
- 11.Rojas J.C., Bruchey A.K., and Gonzalez–Lima F. (2012). Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog. Neurobiol. 96, 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin A.L., Poteet E., Du F., Gourav R.C., Liu R., Wen Y., Bresnen A., Huang S., Fox P.T., Yang S.H., and Duong T.Q. (2012). Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One 7, e46585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S., Du F., Shih Y.Y., Shen Q., Gonzalez–Lima F., and Duong T.Q. (2013). Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. Neuroimage 72C, 237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas J.C., Bruchey A.K., and Gonzalez–Lima F. (2012). Meurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog. Neurobiol. 96, 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo A.J., Sheth K.N., Kimberly W.T., Chaudhry Z.A., Elm J.J., Jacobson S., Davis S.M., Dommam G.A., Albers G.W., Stern B.J., and Gonzalez R.G. (2012). Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J. Stroke Cerebrovasc. Dis. 22, 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina D.X., Caccamo A., and Oddo S. (2011) Methylene Blue reduces aB levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 21, 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Y., Li W., Poteet E.C., Xie L., Tan C., Yan L.J., Ju X., Liu R., Qian H., Marvin M.A., Goldberg M.S., She H., Mao Z., Simpkins J.W., and Yang S.-H. (2011) Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J. Biol. Chem. 286, 16504–16515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Q., Du F., Huang S., Rodriguez P., Watts L.T., and Duong T.Q. (2013, in revison). Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS One 8, e79833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oz M., Lorke D.E., and Petroianu G.A.(2009). Methylene blue and Alzheimer's disease. Biochem. Pharmacol. 78, 927–932 [DOI] [PubMed] [Google Scholar]
- 20.O'Leary J.C., 3rd, Li Q., Marinec P., Blair L.J., Congdon E.E., Johnson A. G., Jinwal U. K., Koren J., 3rd, Jones J. R., Kraft C., Peters M., Abisambra J. F., Duff K. E., Weeber E. J., Gestwicki J. E., and Dickey C. A. (2010). Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol. Neurodegener. 5, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Q., Meng X., Fisher M., Sotak C.H., and Duong T.Q. (2003). Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J. Cereb. Blood Flow Metab. 23, 1479–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Q., Ren H., Cheng H., Fisher M., and Duong T.Q. (2005). Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J. Cereb. Blood Flow Metab. 25, 1265–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng X., Fisher M., Shen Q., Sotak C.H., and Duong T.Q. (2004). Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann. Neurol. 55, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez T.D., and Schallert T. (1988). Seizures and recovery from experimental brain damage. Exp. Neurol. 102, 318–324 [DOI] [PubMed] [Google Scholar]
- 25.Deitch A.D., and Moses M.J. (1957). The Nissl substance of living and fixed spinal ganglion cells. II. An ultraviolet absorption study. J. Biophys. Biochem. Cytol. 3, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts L., Sprague S., Zheng W., Galrling R., Jiminez D., Digicaylioglu M., and Lechleiter J.D. (2013). Purinergic 2Y1 receptor stimulation decreases cerebral edema and reactive gliosis in a traumatic brain injury model. J. Neurotrauma 30, 55–66 [DOI] [PubMed] [Google Scholar]
- 27.Schmued L.C., and Hopkins K.J. (2000). Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol. Pathol. 28, 91–99 [DOI] [PubMed] [Google Scholar]
- 28.Schmued L.C., and Hopkins K.J. (2000). Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 874, 123–130 [DOI] [PubMed] [Google Scholar]
- 29.O'Leary J.L., Petty J., Harris A.B., and Inukai J. (1968). Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Technol. 43, 197–201 [DOI] [PubMed] [Google Scholar]
- 30.Azbill R.D., Mu X., Bruce–Keller A.J., Mattson M.P., and Springer J.E. (1997). Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 765, 283–290 [DOI] [PubMed] [Google Scholar]
- 31.Palzur E., Zaaroor M., Vlodavsky E., Milman F., and Soustiel J.F. (2008). Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 1221, 126–133 [DOI] [PubMed] [Google Scholar]
- 32.Okonkwo D.O., and Povlishock J.T. (1999). An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 19, 443–451 [DOI] [PubMed] [Google Scholar]
- 33.Sullivan P.G., Rabchevsky A.G., Hicks R.R., Gibson T.R., Fletcher–Turner A., and Scheff S.W. (2000). Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 101, 289–295 [DOI] [PubMed] [Google Scholar]
- 34.Sullivan P.G., Thompson M., and Scheff S.W. (2000). Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161, 631–637 [DOI] [PubMed] [Google Scholar]
- 35.Empey P.E., McNamara P.J., Young B., Rosbolt M.B., and Hatton J. (2006). Cyclosporin A disposition following acute traumatic brain injury. J. Neurotrauma 23, 109–116 [DOI] [PubMed] [Google Scholar]
- 36.Mazzeo A.T., Kunene N.K., Gilman C.B., Hamm R.J., Hafez N., and Bullock M.R. (2006). Severe human traumatic brain injury, but not cyclosporin a treatment, depresses activated T lymphocytes early after injury. J. Neurotrauma 23, 962–975 [DOI] [PubMed] [Google Scholar]
- 37.Merenda A., and Bullock R. (2006). Clinical treatments for mitochondrial dysfunctions after brain injury. Curr. Opin. Crit. Care 12, 90–96 [DOI] [PubMed] [Google Scholar]
- 38.Salaris S.C., Babbs C.F., and Voorhees W.D., 3rd (1991). Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. potential new drug for the attenuation of ischemia/reperfusion injury. Biochem. Pharmacol. 42, 499–506 [DOI] [PubMed] [Google Scholar]
- 39.Poteet E., Winters A., Yan L.J., Shufelt K., Green K.N., Simpkins J.W., Wen Y., and Yang H. (2012). Neuroprotective actions of methylene blue and its derivatives. PLoS One 7, e48279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miclescu A., Sharma H.S., Martijn C., and Wiklund L. (2010). Methylene blue protects the cortical blood–brain barrier against ischemia/reperfusion-induced disruptions. Crit. Care Med. 38, 2199–2206 [DOI] [PubMed] [Google Scholar]
- 41.Bardakci H., Kaplan S., Karadeniz U., Ozer C, Bardakci Y, Ozogul C., Birincioglu C.L., and Cobanoglu A. (2006). Methylene blue decreases ischemia-reperfusion (I/R)-induced spinal cord injury: an in vivo study in an I/R rabbit model. Eur. Surg. Res. 38, 482–488 [DOI] [PubMed] [Google Scholar]
- 42.McIntosh T.K. (1993). Novel pharmacologic therapies in the treatment of experimental traumatic brain injury: a review. J. Neurotrauma 10, 215–261 [DOI] [PubMed] [Google Scholar]
- 43.Hensley K., Carney J.M., Stewart C.A., Tabatabaie T., Pye Q., and Floyd R.A. (1997). Nitrone-based free radical traps as neuroprotective agents in cerebral ischaemia and other pathologies. Int. Rev. Neurobiol. 40, 299–317 [DOI] [PubMed] [Google Scholar]
- 44.Vink R., Faden A.I., and McIntosh T.K. (1988). Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J. Neurotrauma 5, 315–330 [DOI] [PubMed] [Google Scholar]
- 45.Clifton G.L., Lyeth B.G., Jenkins L.W., Taft W.C., DeLorenzo R.J., and Hayes R.L. (1989). Effect of D, alpha-tocopheryl succinate and polyethylene glycol on performance tests after fluid percussion brain injury. J. Neurotrauma 6, 71–81 [DOI] [PubMed] [Google Scholar]
- 46.Halks–Miller M., Henderson M., and Eng L.F. (1986). Alpha tocopherol decreases lipid peroxidation, neuronal necrosis, and reactive gliosis in reaggregate cultures of fetal rat brain. J. Neuropathol. Exp. Neurol. 45, 471–484 [DOI] [PubMed] [Google Scholar]