Abstract
The immune system protects the host from pathogenic microbes, but tight regulation of the evoked response is requisite to limit bystander damage. The interleukin (IL)-10 family of cytokines, composed of 9 members: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, and 3 distantly related members, IL-28A, IL-28B, and IL-29, plays a central role in this regulation. IL-10 family cytokines emerged before the adaptive immune response and elicit diverse host defense mechanisms, especially from epithelial cells during an infection. IL-10 family cytokines are also essential for maintenance and integrity of tissue epithelial layers. These cytokines promote innate immune responses from tissue epithelia that limit the damage caused by both viral and bacterial infections. They also facilitate tissue healing after infection/inflammation. In this regard, IL-10 suppresses pro-inflammatory responses, limiting tissue disruption resulting from an inflammatory response. Thus, a central functional theme of IL-10 family cytokines is their role in tissue protection. This review focuses on IL-10, the founding member of this family of cytokines, and integrates recent data on the function and regulation of IL-10 during bacterial infections. Emphasis is placed on the role of IL-10 in Pseudomonas aeruginosa keratitis and the subsequent infectious/inflammatory processes evoked.
Introduction
In general, bacterial keratitis is associated with contact lens wear, and adverse events have a significant medical and financial impact, with ∼25–30,000 cases occurring annually in the USA.1 A gram-negative bacterium, P. aeruginosa, is a common isolate among keratitis-inducing microbes.2 Among numerous virulence factors, it produces endotoxin (ie, lipopolysaccharide) that rapidly elicits an acute inflammatory response in the cornea which can contribute to eradication of the bacterium, but unless precisely regulated, destructive events such as stromal disruption, ulceration, and loss of vision can occur. The innate immune response in the cornea has received considerable attention, with experimental infection studies focusing on infiltration of leukocytes (eg, neutrophils [PMN], macrophages, dendritic, and T cells), production of pro- and anti-inflammatory cytokines,3 and the interplay of cellular apoptosis versus necrosis.4 Studies have provided consistent evidence that a key regulatory molecule associated with better disease outcome in murine models of P. aeruginosa keratitis is the anti-inflammatory cytokine IL-10.5,6
The functions of interleukin (IL)-10 identified by Mosmann in 1989,7 with a focus on infectious diseases have been recently reviewed.8,9 IL-10 is a general suppressive cytokine, it functions to inhibit pro-inflammatory responses from innate and adaptive immunity, and it prevents tissue lesions caused by exacerbated adaptive immune responses. IL-10 is, thus, often described as a central cytokine during the resolution phase of an inflammatory response. Consistent with this pattern of protection, and as discussed later, blocking the IL-10 pathway in mice causes spontaneous development of inflammatory bowel disease. However, on the other hand, evolutionarily pathogens have exploited the functions of IL-10 to repress the normal host inflammatory response during infections, thus establishing chronic infectious states. For example, increased IL-10 expression has been associated with many chronic bacterial and viral infections; in fact, some viruses can produce IL-10 to directly suppress the immune responses of the host.10 Specifically, the induction of IL-10 in dendritic cells (DCs) and macrophages represents a powerful mechanism of immune evasion used by various pathogens. Either IL-10 itself or the subsequent induction of T regulatory cells impairs pathogen control and clearance in infection models using lymphocytic choriomeningitis virus,11 Schistosoma mansoni, 12 Mycobacterium tuberculosis,13 and Candida albicans.14 Thus, if IL-10 is absent, better clearance of some pathogens with no enhanced immunopathology11,15 has been noted, but during other infections its absence can be accompanied by immunopathology that is detrimental to the host without affecting pathogen load.16–20 This suggests that the function of IL-10 is probably not compensated by other regulatory mechanisms or members of the IL-10 cytokine family, and that the role for IL-10 in limiting inflammatory responses in vivo may not exhibit redundancy.
IL-10-Deficient Animal Model
Experimentally, the first IL-10-deficient mouse model was reported 20 years ago, and much has been learned about IL-10 through its study.21 IL-10-deficient mice exhibit prolonged and exaggerated immune responses toward antigen. Often, this may be accompanied by unbalanced inflammation and tissue damage; and they often develop chronic enterocolitis.21,22 This pathology is lessened by maintenance of the animals under germ-free conditions, and it suggests a role for the gut microbiota in disease and, therefore, a role for IL-10 in regulating homeostasis among commensal microorganisms.23 Similarly, IL-10-deficient mice develop prolonged and exacerbated fever in response to lipopolysaccharide22 and a lethal immune response to acute infection with Toxoplasma gondii, not seen in their wild-type counterparts.17 Several studies of human autoimmune disease also provide evidence that the level of IL-10 detected in patient samples has an inverse correlate with the severity of the disease.24–28 In another disease, juvenile onset arthritis, a single-nucleotide polymorphism associated with reduced IL-10 mRNA expression correlates with a greater number of joints affected with arthritis.26 Collectively, these studies in mice and humans illustrate the importance of IL-10 in immune regulation and the impact of its dysregulation in many diverse diseases.
Sources of IL-10 and Function
IL-10 was initially described as a T helper 2 (Th2)-type cytokine, but other additional work suggested that its production was associated with regulatory T (Treg) cell responses.16,29,30 In fact, IL-10 is expressed by many cells of the adaptive immune system, including Th1, Th2, and Th17 T cell subsets, Treg cells, CD8+ T cells, and B lymphocytes (reviewed in Refs.16,19,20,30–32). It is also expressed by cells of the innate immune system, including DCs, mast cells, macrophages, natural killer (NK) cells, and PMN.16 Thus, IL-10 production appears associated with many types of immune cells of both innate and acquired responsiveness, affirming its critical role as a feedback regulator of diverse immune responses.
By acting on DCs and macrophages, IL-10 inhibits the development of Th1-type T-cell responses (reviewed in Refs.5,16); it also may lead to the suppression of Th2 cell responses (reviewed in Ref.33). In addition to an autocrine inhibitory effect of IL-10 on macrophages and DCs, and since IL-10 can be produced by various T-cell subsets (Th1, Th2, and Th17), an additional feedback loop exists to limit the innate effector functions of macrophages and DCs and their subsequent activation of T cells. However, IL-10 enhances the differentiation of IL-10-secreting Treg cells, a positive regulatory loop for its induction (reviewed in Refs.30,33).
DCs and macrophages are activated through the recognition of pathogen-derived products by pattern recognition receptors (PRRs) that signal and trigger expression of cytokines, chemokines, and other molecules.34 Both macrophages35–39 and DC38,40–45 can express IL-10 in vitro after activation of specific PRRs. In addition, DC,43,46 macrophages,47 and PMN48 can express IL-10 in vivo. Significant amounts of IL-10 are produced by macrophages and myeloid DCs after stimulation with toll like receptor (TLR) 4 and 9 ligands.38 Furthermore, activation of macrophages through TLRs results in high levels of IL-10 production, whereas myeloid DCs produce reduced amounts and plasmacytoid DCs produce little to no detectable IL-10.38 In addition, IL-10 can be induced by TLR-independent stimuli; for instance, the C-type lectins and ligation of CD40 also enhance IL-10 production by TLR-stimulated macrophages.37
Tissue Macrophages
Tissue macrophages perform a dynamic role in host defense and maintenance of tissue integrity and can be classified into at least 2 subtypes: M1 (classical) and M2 (alternative), based on their function. M1 cells are activated by lipopolysaccharide and/or interferon (IFN)-γ to elaborate pro-inflammatory cytokines and contribute to tissue inflammation.49 On the other hand, M2 cells are stimulated by Th2 cytokines IL-4 and/or IL-13 and promote, among other functions, helminthic immunity, fibrosis, allergy, and immunomodulation.50 Stimulation of macrophages with IL-4 and IL-13 leads to activation of the transcription factor STAT6, which is required for M2 polarization.51
In addition, activation of the nuclear receptors PPARγ and PPARδ is required for development of the M2 phenotypic response.52,53 M2 macrophages are characterized by an increase in arginase-1 gene expression and activity,50 which enables conversion of L-arginine to L-ornithine and promotes polyamine synthesis and tissue repair.54 The M2 phenotype is also characterized by up-regulation of C-type lectins, mannose receptor, and IL-10, all of which contribute to their immunomodulatory function.55 Importantly, distinct metabolic programs are required to support energy demands of M1 and M2 macrophages. M1 cells rely primarily on glycolytic metabolism, mediated by HIF-1α; while M2 cells utilize fatty acid oxidation mediated by PPARγ and the transcriptional coactivator, PGC-1β.51,56,57 This suggests that macrophage metabolism and inflammatory phenotype are linked, and it provides insights into possible additional regulatory control of macrophage polarization through metabolic pathways, which will be discussed later.
M1 Versus M2 Program Induction and Keratitis
In addition, gene expression profiling of macrophages has shown that Gram-negative bacteria induce transcriptional activation of a common host response which induces genes in macrophages expressing an M1 program.58 M1 polarized cells, prototypical in Th1 responder strains of mice, such as C57BL/659 (B6), are characterized by the production of IL-12, tumor necrosis factor (TNF)-α, MIP-2, and high levels of nitric oxide synthase 2 (NOS2).60,61 Excessive or prolonged M1 polarization often leads to tissue injury and contributes to pathogenesis. In contrast, Th2 responder mice (eg, BALB/c) have a higher population of alternatively activated macrophages designated M2 cells, which produce anti-inflammatory mediators such as IL-10 and IL-1ra.61 These cells up-regulate arginase 1 production,59 and they are critical to the resolution of disease. A subset of the latter cells can be induced by agonists of TLRs, including lipopolysaccharide,58 suggesting that negative regulation of TLR signaling may be critical to avoid a detrimental inflammatory response.62 In this regard, the soluble TLRs (sTLR2 and sTLR4) act as decoy receptors by binding to their ligands and competitively blocking signaling via TLR2 and TLR463; whereas the IL-1 receptor-related protein ST2 negatively regulates TLR signaling by sequestering the recruitment of adaptor molecules such as MyD88 and TIRAP.64
On comparing the response to experimental Pseudomonas aeruginosa challenge in B6 and BALB/c mice, the ligand of ST2, IL-33 was examined at the mRNA and protein levels. Both groups of mice constitutively expressed the ligand in the normal cornea; after infection, elevated levels were detected in BALB/c over B6 mice. To test the significance of this, B6 mice were treated with recombinant mouse (rm) IL-33. These mice showed less severe disease than phosphate-buffered saline (PBS) controls, and they also exhibited decreased bacterial load, PMN infiltrate, and corneal mRNA levels for IL-1β, MIP-2, and TNF-α. Th2-type cytokines (IL-4, -5, and -10) were significantly up-regulated, and protein levels for the pro-inflammatory cytokine, TNF- α, and the anti-inflammatory cytokine, IL-10 confirmed the mRNA data. To further investigate the role of IL-33 and, in turn, IL-10 in corneal inflammation, IL-33 was overexpressed in macrophage-like, RAW264.7 cells. Overexpression significantly increased IL-10 (and IL-5), while it decreased IFN-γ and other pro-inflammatory cytokines. The role of the macrophage was further tested in infected rmIL-33 compared with PBS-injected mice. Immunostaining showed that rmIL-33 injection shifted macrophage polarization from the production of NOS2 to arginase. Furthermore, peritoneally elicited cells (B6 mice) treated with lipopolysaccharide and rmIL-33 exhibited elevated ST2 levels and a shift from IL-12 to IL-10 mRNA production. These data provide evidence that IL-33 promotes a Th2-type immune response and reduces inflammation by polarizing the macrophage production of anti-inflammatory mediators, namely, IL-10 and revealed the importance of this cytokine in ameliorating disease and participating in healing in the cornea.
Macrophage Depletion in Keratitis
Of course, one cannot exclude the importance of the PMN in pseudomonas keratitis,5 but evidence of a central role for macrophages continues to build, and was shown in earlier studies that tested the role of this cell in susceptible (cornea perforates), B6 versus resistant (cornea heals), BALB/c mice. This was achieved by macrophage depletion using subconjunctival injections of clodronate-containing liposomes before corneal infection. Both groups of mice treated with clodronate liposomes compared with PBS liposomes exhibited more severe disease. In B6 mice, the cornea perforated more rapidly and the eye became extremely shrunken, and in BALB/c mice, worsened disease was noted, as evidenced by perforation of the cornea rather than the expected healing response. Use of a myeloperoxidase assay as an indicator of PMN number detected significantly more PMN in the cornea of both groups of mice treated with clodronate liposomes versus PBS liposomes. In independent experiments, enzyme-linked immunosorbent assay (ELISA) analysis showed that protein levels for IL-1β, MIP-2, and MIP-1α, all of which were able to regulate PMN chemotaxis, were also elevated in both groups of clodronate-liposome-treated mice. Bacterial load in B6 mice treated with clodronate liposomes was unchanged at 3 days and was higher in control-treated mice at 5 days postinfection (p.i.); whereas in BALB/c mice, bacterial load was significantly elevated in the cornea of mice treated with clodronate liposomes at both 3 and 5 days p.i. mRNA expression levels for pro- (IFN-γ and TNF-α) and anti- (eg, IL-10) inflammatory cytokines were also determined in BALB/c mice treated with clodronate liposomes versus control-treated mice. Expression levels for IFN- γ were significantly elevated in mice treated with clodronate liposomes at 3 and 5 days p.i., while IL-10 levels (mRNA and protein) were reduced in the absence of the macrophage. These data strongly suggest and provide evidence that macrophages not only control resistance to P. aeruginosa corneal infection through regulation of PMN number, bacterial killing, and balancing pro- and anti-inflammatory cytokine levels, but also regulate induction of IL-10, which appears requisite to corneal healing.
Neuropeptide Substance P and Spantide
In humans, keratitis caused by P. aeruginosa develops rapidly and may lead to corneal perforation, with a higher incidence of disease associated with extended-wear contact lens use.65,66 Experimental work has shown that Th1 responder mouse strains (eg, C57BL/6) are suscepible (cornea perforates), whereas Th2 strains (eg, BALB/c) are resistant (ie, the cornea heals)67 after bacterial infection. More recently,68 we have shown an interrelationship between the neuropeptide substance P (SP) and production of IFN-γ in the infected BALB/c cornea. Evidence has shown that in the infected cornea, NK cells are the source of IFN-γ, express the NK-1R, and participate in the regulation of PMN infiltration. Evidence has also shown that SP regulates the production of IFN-γ indirectly through the regulation of IL-18 and directly through the interaction with the NK-1R on NK cells. Collectively, the data demonstrate a unique link between neuropeptide regulation of the innate immune response in resistant mice and protection against P. aeruginosa–induced corneal perforation. Alternately, evidence also indicates that SP plays an important role in augmenting inflammatory responses principally by regulating the function of cells such as DCs and macrophages, via the NK-1R.69,70 SP, a product of both nerves and leukocytes, is present in many areas of the central and peripheral nervous system. In this regard, the cornea is one of the most densely innervated tissues in the body and is richly supplied by both sensory and autonomic nerve fibers.71 In past studies, the distribution of neuropeptides, including SP, was elegantly shown in the human cornea,71 but limited information was available for the mouse cornea, before or after infection. In past studies, we examined the distribution of SP in the mouse cornea and a disparate distribution of the neuropeptide in susceptible (more) B6 versus resistant (less) BALB/c mice was documented by enzyme immunoassay and staining. Due to the pro-inflammatory functions of SP and since there was increased amounts of the neuropeptide in the B6 cornea, blocking SP binding to the neurokinin 1 receptor with spantide I was done. This prevented P. aeruginosa–induced corneal perforation in susceptible B6 mice. This study also tested the effect of SP injection on the resistance response (cornea heals) of BALB/c mice.72 The day before infection, mice were injected intraperitoneally with SP or PBS. Disease was graded by clinical score, slit lamp, plate count, real-time RT-PCR and ELISA assays, and PMN) were quantitated using a myeloperoxidase assay. Mice injected with SP exhibited worsened disease on days 1–7 after infection compared with controls. SP injection resulted in elevated PMN levels and viable bacterial counts in the cornea at 3 and 5 days after the infection. mRNA expression for NF-κB and type 1 cytokines (eg, IFN-γ), as well as for TNF-α, MIP-2, IL-18, IL-6, and IL-1β, was significantly elevated; whereas cytokines IL-10 (and TGF-β) were significantly reduced. Differences in mRNA expression were selectively confirmed at the protein level by ELISA for NF-κB, IL-1β, and IL-10. These data provide evidence that the neuropeptide SP is a potent neuro-immunoregulator which promotes susceptibility in the resistant BALB/c mouse by overcoming the anti-inflammatory effects of IL-10.
SP and Growth Factors
In addition, SP regulation of growth factors was examined after infection,73 as others have reported that they are essential in tissue repair and have healing properties when administered exogenously in noninfectious wound or trauma models.74,75 This study73 revealed that the SP injection had a localized effect and increased growth factors such as hepatocyte growth factor (HGF) in both the normal and infected cornea. Unfortunately, this effect was overwhelmed by a pro-inflammatory cytokine response, leading to increased stromal destruction, higher bacterial plate counts, and a decrease in M2 arginase-producing cells (critical to disease resolution through production of anti-inflammatory mediators such as IL-10). These data, based on experimental modeling, also led us to conclude that treatment with SP to hasten wound closure was contraindicated clinically in the cornea in the presence of a bacterial infection.75
Growth Factors and IL-10 Regulation
Our interest in growth factors and their possible participation in regulation of IL-10 continued to increase, based on the fact that metabolic status may also participate in and shift/regulate the host response to various diseases. In this regard, the mechanistic target of rapamycin (mTOR) is a critical nutrient/energy sensor that functions to couple nutrient availability to the regulation of downstream metabolic processes, including glycolysis, protein synthesis, and lipogenesis.76,77 mTOR exists in a rapamycin-sensitive complex called mTORC1 that is negatively regulated by the tuberous sclerosis complex which is composed of TSC1 and TSC2.78 Genetic loss of either of these leads to constitutive mTORC1 activation.79 It is also significant that recent studies have provided evidence that mTOR controls many aspects of T-cell biology, including differentiation, activation, and cell quiescence.80 A role for mTOR also has been demonstrated in macrophage polarization using Tsc1Δ/Δ macrophages that have a marked defect in M2 polarization in response to IL-4, while the inflammatory response to lipopolysaccharide is enhanced. Aberrant polarization is due, at least in part, to mTORC1-mediated attenuation of Akt activity, which renders Tsc1Δ/Δ macrophages resistant to the immunomodulatory effects of Akt downstream of IL-4 and lipopolysccharide signaling. Other studies have shown that the mTOR complex regulates IL-10/IL-12 expression and that mTOR inhibition by rapamycin down-regulates IL-10 and up-regulates production of IL-12.81 mTOR remains of interest to IL-10 regulation, as it is upstream of the cytokine81–83 and is a well established target of a diverse array of microbes, growth factors, hormones, and amino acids that elicit a host innate immune response. mTOR also mediates cell growth and proliferation, ribosome biogenesis, and cytoskeletal organization.84,85 Contained within 2 functional complexes, mTORC1 and mTORC2, it is active when complexed with Raptor, mLST8, and PRAS40 (forming mTORC1), or when complexed with Rictor, mLST8, and Sin1 (forming mTORC2).84,85 mTORC1 alone is sensitive to inhibition by the macrolide antibiotic rapamycin that blocks the formation of the complex described earlier. Clinically, rapamycin has been used as an immunosuppressant in allogeneic transplantation. However, although used successfully in clinical practice for immunosuppression after kidney transplants,86 other evidence suggests that it has inflammatory side effects, which include fever, anemia, and glomerulonephritis.87 Moreover, inhibition of mTOR in mice can enhance lipopolysaccharide-induced shock that correlates with elevated levels of pro-inflammatory cytokines such as IL-12p40.88 Similarly, other work showed that inhibition of mTOR diminished IL-10 levels but elevated IL-12p40 (and IL-23) in vitro, and this was found to be protective in vivo in experimental Listeria monocytogenes infection.81 In contrast, it has been shown that in corneal P. aeruginosa infection, IL-10 is required for resistance in the BALB/c mouse and that when macrophages (a source of the cytokine) are depleted (as described above), levels of IL-10 are decreased with concurrent elevation of IFN-γ, resulting in an overall worsening of disease.5
Rapamycin Inhibition of mTOR in Bacterial Keratitis
In the P. aeruginosa infected cornea, inhibition of mTOR by rapamycin treatment increased disease in the cornea of resistant BALB/c mice through down-regulation of the anti-inflammatory cytokine IL-10 and up-regulation of pro-inflammatory IL-12p40 and IL-23. Furthermore, the decreased levels of IL-10 led to dysregulation of several downstream effectors (MMP-9, SOCS3, STAT-3, and iNOS) and elements of the TLR pathway (TLR4, TLR5, IRAK-1, IL-1R1, NFκBIL-1, Cebpb, and IFN-γ). Moreover, we demonstrated that loss of mTOR signaling generates an antiapoptotic corneal environment in which damaged and dying cells could contribute to further tissue destruction. Rapamycin treatment also increased PMN number but reduced their capacity for intracellular killing. In a rescue experiment, that is, the injection of rIL-10 along with rapamycin versus rapamycin treatment alone resulted in reduced PMN number in the cornea, strengthening the case for IL-10 as critical to disease resolution.89
Rapamycin Treatment and Growth Factors
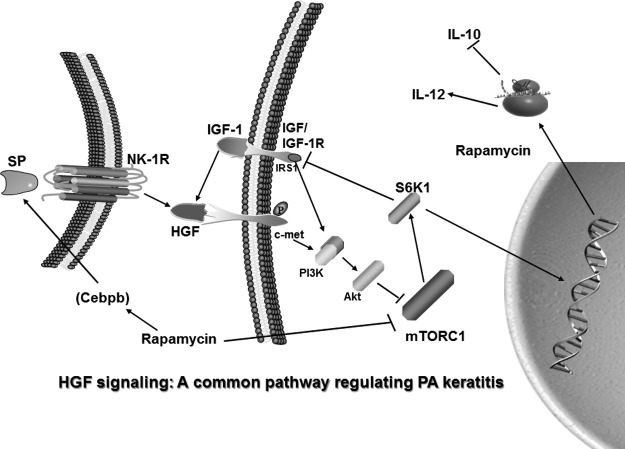
As an extension of this work, we tested the effects of rapamycin inhibition of mTOR on expression levels of growth factors, their receptors, and signaling molecules in the P. aeruginosa-infected cornea.90 Rapamycin disparately regulated infected corneal mRNA levels of EGF/EGFR and FGF-7/FGFR-2, but HGF/c-met mRNA levels increased. ELISA confirmed elevated HGF protein. Rapamycin did not change PI3KCα or Akt signaling molecule expression, down-regulated S6K1, but up-regulated IGF-1R mRNA levels; IGF-1 and SP (see discussion above) proteins also were up-regulated. After infection, topical rHGF versus PBS increased mRNA levels of IL-12p40, IL-18, PI3KCα, and Akt; mTOR and IL-10 mRNA levels were down-regulated; furthermore, treatment of infected eyes with rIGF-1 increased HGF protein. In vitro, rHGF and lipopolysaccharide lowered RAW cell and macrophage mTOR levels; addition of a c-met inhibitor restored them. Thus, the data provided evidence of the growth factors and receptors tested; only HGF/c-met were similarly elevated. Moreover, rapamycin down-regulated mRNA for S6K1, a significant downstream signaling molecule of the mTOR pathway, and resulted in up-regulated IGF-1R and protein for both IGF-1 and SP; and rIGF-1 injection also was sufficient to up-regulate HGF protein. To test the importance of HGF protein in the overall pattern of disease, topical rHGF treatment was done and provided further confirmatory evidence that enhancement of HGF levels in infected cornea resulted in increased signaling through the c-met receptor. This, is turn, led to decreased mTOR levels, enhanced pro-inflammatory cytokines, decreased anti-inflammatory cytokines (namely IL-10), and provided novel evidence that HGF signaling is central to disease outcome. These data provide strong evidence that enhanced corneal HGF levels increases signaling through the c-met receptor, decreases mTOR levels, enhances pro-inflammatory cytokines while decreasing the anti-inflammatory cytokine IL-10, and that HGF signaling appears to be a central metabolic component which is critical to disease outcome (Fig. 1). It also suggests that control of this metabolic pathway, perhaps by manipulating levels of HGF and signaling through mTOR to favor IL-10 up-regulation during P. aeruginosa keratitis, would be a truly novel treatment for this and perhaps other diseases in which a similar pathway is involved in pathogenesis. It is also plausible that mTOR manipulation may require cell-specific targeting and kinetics during the disease response; that is, DC may be required to be targeted at a different time during disease than tissue macrophages.
FIG. 1.
Proposed signaling through mTOR by HGF/c-met, its downstream effects, and a putative feedback mechanism via S6K1. HGF, hepatocyte growth factor; mTOR, mechanistic target of rapamycin; SP, substance P. From Jiang et al.90
Acknowledgment
This study was supported by NEI R01 EY002986, R01 EY016058, and P30 EY004068.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Khatri S., Lass J.H., Heinzel F.P., et al. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and Toll-like Receptor 4. Invest. Ophthalmol. Vis. Sci. 43:2278–2284, 2002 [PubMed] [Google Scholar]
- 2.Pachigolla G., Blomquist P., and Cavanagh H.D.Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens. 33:45–49, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hazlett L.D.Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 23:1–30, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Zhou Z., Barrett R.P., McClellan S.A., et al. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci. 49:4458–4467, 2008 [DOI] [PubMed] [Google Scholar]
- 5.McClellan S.A., Huang X., Barrett R.P., van Rooijen N., and Hazlett L.D.Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 170:5219–5227, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Hazlett L.D., McClellan S.A., Barrett R.P., et al. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci. 48:797–807, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino D.F., Bond M.W., and Mosmann T.R.Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081–2095, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mege J.L., Meghari S., Honstettre A., Capo C., and Raoult D.The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 6:557–569, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Filippi C.M., and von Herrath M.G.IL-10 and the resolution of infections. J. Pathol. 214:224–230, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Moore K.W., Vieira P., Fiorentino D.F., Trounstine M.L., Khan T.A., and Mosmann T.R.Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 248:1230–1234, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Ejrnaes M., Filippi C.M., Martinic M.M., et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Kleij D., Latz E., Brouwers J.F.H.M., et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277:48122–48129, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Jang S., Uematsu S., Akira S., and Salgame P.IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J. Immunol. 173:3392–3397, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Netea M.G., Sutmuller R., Hermann C., et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172:3712–3718, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Brooks D.G., Trifilo M.J., Edelmann K.H., et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore K.W., de Waal Malefyt R., Coffman R.L., and O'Garra A.Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Gazzinelli R.T., Wysocka M., Hieny S., et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157:798–805, 1996 [PubMed] [Google Scholar]
- 18.Li C., Corraliza I., and Langhorne J.A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect. Immun. 67:4435–4442, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Garra A., and Vieira P.TH1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 7:425–428, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G.Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 204:239–243, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kühn R., Löhler J., Rennick D., Rajewsky K., and Müller W.Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 75:263–274, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Leon L.R., Kozak W., and Kluger M.J.Role of IL-10 in inflammation. Studies using cytokine knockout mice. Ann. N. Y. Acad. Sci. 856:69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Sellon R.K., Tonkonogy S., Schultz M., et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224–5231, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajeer A.H., Lazarus M., Turner D., et al. IL-10 gene promoter polymorphisms in rheumatoid arthritis. Scand. J. Rheumatol. 27:142–145, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Lim S., Crawley E., Woo P., and Barnes P.J.Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 352:113, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Crawley E., Kay R., Sillibourne J., et al. Polymorphic haplotypes of the interleukin-105’ flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum. 42:1101–1108, 1999 [DOI] [PubMed] [Google Scholar]
- 27.VanBoxel-Dezaire A.H.H., Hoff S.C.J., VanOosten B.W., et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann. Neurol. 45:695–703, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Gibson A.W., Edberg J.C., Wu J., et al. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J. Immunol. 166:3915–3922, 2001 [DOI] [PubMed] [Google Scholar]
- 29.O'Garra A., and Vieira P.Regulatory T cells and mechanisms of immune system control. Nat. Med. 10:801–805, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Roncarolo M.G., Gregori S., Battaglia M., et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212:28–50, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Maynard C.L., and Weaver C.T.Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol. Rev. 226:219–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloy K.J., and Powrie F.Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Hawrylowicz C.M., and O'Garra A.Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat.e Rev. Immunol. 5:271–283, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R.Recognition of microorganisms and activation of the immune response. Nature 449:819–826, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., and O'Garra A.IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815–3822, 1991 [PubMed] [Google Scholar]
- 36.de Waal Malefyt R., Abrams J., Bennett B., Figdor C.G., and de Vries J.E.Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209–1220, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber J.S., and Mosser D.M.Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J. Immunol. 166:6861–6868, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Boonstra A., Rajsbaum R., Holman M., et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF dependent TLR signals, and TLR-independent signals. J. Immunol. 177:7551–7558, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Chang E.Y., Guo B., Doyle S.E., and Cheng G.Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J. Immunol. 178:6705–6709, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Edwards A.D., Manickasingham S.P., Spörri R., et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652–3660, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Agrawal S., Agrawal A., Doughty B., et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen activated protein kinase and c-Fos. J. Immunol. 171:4984–4989, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Dillon S., Agrawal A., Van Dyke T., et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172:4733–4743, 2004 [DOI] [PubMed] [Google Scholar]
- 43.McGuirk P., McCann C., and Mills K.H.Pathogen specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221–231, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers N.C., Slack E.C., Edwards A.D., et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 22:507–517, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Geijtenbeek T.B., Van Vliet S.J., Koppel E.A., et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7–17, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akbari O., DeKruyff R.H., and Umetsu D.T.Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725–731, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Siewe L., Bollati-Fogolin M., Wickenhauser C., et al. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur. J. Immunol. 36:3248–3255, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Zhang X., Majlessi L., Deriaud E., Leclerc C., and Lo-Man R.Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 31:761–771, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Sica A., and Mantovani A.Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122:787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon S., and Martinez F.O.Alternative activation of macrophages: mechanism and functions. Immunity. 32:593–604, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Chawla A.Control of macrophage activation and function by PPARs. Circ. Res. 106:1559–1569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 447:1116–1120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang K., Reilly S.M., Karabacak V., et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7:485–495, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van den Bossche J., Lamers W.H., Koehler E.S., et al. Pivotal Advance: Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J. Leukoc. Biol. 91:685–699, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Murray P.J., and Wynn T.A.Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11:723–737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cramer T., Yamanishi Y., Clausen B.E., et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 112:645–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vats D., Mukundan L., Odegaard J.I., et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4:13–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benoit M., Desnues B., and Mege J.L.Macrophage polarization in bacterial infections. J. Immunol. 181:3733–3739, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Mills C.D., Kincaid K., Alt J., Heilman M., and Hill A.M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166–6173, 2000. 10843666 [Google Scholar]
- 60.Joshi A.D., Raymond T., Coelho A.L., Kunkel S.L., and Hogaboam C.M.A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to Toll-like receptor agonists. J. Leuk. Biol. 83:314–324, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Mantovani A., Sozzani S., Massimo L., Allavena P., and Sica A.Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23:549–555, 2002 [DOI] [PubMed] [Google Scholar]
- 62.O'Neill L.A.SIGIRR puts the brakes on Toll-like receptors. Nat. Immunol. 4:823–824, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Liew F.Y., Xu D., Brint E.K., and O'Neill L.A.Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5:446–458, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Brint E.K., Xu D., Liu H., et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 5:373–379, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Wilhelmus K.R.Bacterial keratitis. In: Pepose J.S., Holland G.N., and Wilhelmus K.R., eds. Ocular Infection and Immunity. St. Louis: Mosby; 1995; p. 970–1031 [Google Scholar]
- 66.Rattanatam T., Heng W.J., Rapuano C.J., Laibson P.R., and Cohen E.J.Trends in contact lens-related corneal ulcers. Cornea. 20:290–294, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Hazlett L.D., McClellan S., Kwon B., and Barrett R.Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest. Ophthalmol. Vis. Sci. 41:805–810, 2000 [PubMed] [Google Scholar]
- 68.Lighvani S., Huang X., Trivedi P.P., Swanborg R.H., and Hazlett L.D.Substance P regulates NK cell IFN-γ production and resistance to Pseudomonas aeruginosa infection. Eur. J. Immunol. 35:1567–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Marriott I., and Bost K.L.Expression of authentic substance P receptors in murine and human dendritic cells. J. Neuroimmunol. 114:131–141, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Marriott I., and Bost K.L.IL-4 and IFN-gamma up-regulate substance P receptor expression in murine peritoneal macrophages. J. Immunol. 165:182–191, 2000 [DOI] [PubMed] [Google Scholar]
- 71.Muller L.J., Marfurt C.F., Kruse F., and Tervo T.M.Corneal nerves: structure, contents and function. Exp. Eye Res. 76:521–542, 2003 [DOI] [PubMed] [Google Scholar]
- 72.McClellan S.A., Zhang Y., Barrett R.P., and Hazlett L.D.Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators down-regulated. Invest. Ophthalmol. Vis. Sci. 49:1502–1511, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Foldenauer M.E., McClellan S.A., Barrett R.P., Zhang Y., and Hazlett L.D.Substance P affects growth factors in Pseudomonas aeruginosa-infected mouse cornea. Cornea. 31:1176–1188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong H.S., Lee J., Lee E.A., et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat. Med. 15:425–435, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Delgado A.V., McManus A.T., and Chambers J.P.Exogenous administration of substance P enhances wound healing in a novel skin-injury model. Exp. Biol. Med. (Maywood). 230:271–280, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Howell J.J., and Manning B.D.mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 22:94–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duvel K., Yecies J.L., Menon S., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex. Mol. Cell. 39:171–183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tee A.R., Fingar D.C., Manning B.D., et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. U. S. A. 99:13571–13576, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang J., and Manning B.D.A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 37:217–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chi H.Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12:325–338, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weichhart T., Costantino G., Poglitsch M., et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 29:565–577, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Baker A.K., Wang R., Mackman N., and Luyendyk J.P.Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-α expression in macrophages by reducing IL-10 expression. Mol. Immunol. 46:2249–2255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheekatla S.S., Aggarwal A., and Naik S.mTOR signaling pathway regulates the IL-12/IL-10 axis in Leishmania donovani infection. Med. Microbiol. Immunol. (Berl). 201:37–46, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Thomson A.W., Turnquist H.R., and Raimondi G.Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 9:324–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Q., and Guan K.L.Expanding mTOR signaling. Cell Res. 17:666–681, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Webster A.C., Lee V.W., Chapman J.R., and Craig J.C.Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 81:1234–1248, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Saemann M.D., Haidinger M., Hecking M., Horl W.H., and Weichhart T.The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am. J. Transplant. 9:2655–2661, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Schmitz F., Heit A., Dreher S., et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur. J. Immunol. 38:2981–2992, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Foldenauer M.E.B., McClellan S.A., Berger E.A., and Hazlett L.D.Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J. Immunol. 190:5649–5658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang X., McClellan S.A., Barrett R., Foldenauer M., and Hazlett L.D.HGF signaling impacts severity of P. aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. pii: , 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]