Abstract
Ovarian cancer is the deadliest of gynecologic cancers, largely due to the development of drug resistance in chemotherapy. Prostasin may have an essential role in the oncogenesis. In this study, we show that prostasin is decreased in an ovarian cancer drug-resistant cell line and in ovarian cancer patients with high levels of excision repair cross-complementing 1, a marker for chemoresistance. Our cell cultural model investigation demonstrates prostasin has important roles in the development of drug resistance and cancer cell survival. Forced overexpression of prostasin in ovarian cancer cells greatly induces cell death (resulting in 99% cell death in a drug-resistant cell line and 100% cell death in other tested cell lines). In addition, the surviving cells grow at a much lower rate compared with non-overexpressed cells. In vivo studies indicate that forced overexpression of prostasin in drug-resistant cells greatly inhibits the growth of tumors and may partially reverse drug resistance. Our investigation of the molecular mechanisms suggests that prostasin may repress cancer cells and/or contribute to chemoresistance by modulating the CASP/P21-activated protein kinase (PAK2)-p34 pathway, and thereafter PAK2-p34/JNK/c-jun and PAK2-p34/mlck/actin signaling pathways. Thus, we introduce prostain as a potential target for treating/repressing some ovarian tumors and have begun to identify their relevant molecular targets in specific signaling pathways.
Keywords: prostasin, chemoresistance, tumor repression, ovarian cancer
It is estimated that about 22 000 new cases of ovarian cancer and 15 000 deaths from this malignancy will occur in the United States in 2013.1 Chemotherapy after surging is the standard treatment for ovarian cancer, which may result in complete clinical remission in up to 75% of cases.2 However, a majority of responders will relapse within 18–28 months and only 20–40% of women will survive beyond 5 years.3, 4, 5 Ovarian cancer is the deadliest of gynecologic cancers, largely due to the development of drug resistance in chemotherapy and late-stage diagnosis.
Prostasin, also known as PRSS8 (protease serine 8), is a trypsin-like serine peptidase expressed in epithelial cells, with the highest expression in the normal prostate gland and seminal fluid and lesser amount in various tissues.6, 7 The expression of prostasin has been shown to malexpress in ovarian, prostate, breast, and gastric cancers8, 9, 10, 11 and to be a potential biomarker for ovarian cancer early detection alone or in combination with CA125.9, 12, 13 Prostasin has been suggested to inhibit cancer cell proliferation and invasion.14 Prostasin is also termed channel-activating protease 1, which is implicated in the regulation of sodium and fluid levels via proteolysis of the epithelial sodium channel, and thus has important functions in blood pressure being a target for regulating hypertension.15, 16, 17 In addition, prostasin has been found to have important roles in the epidermal barrier function, skin phenotypes, and embryonic viability.18, 19 Thus, prostasin is implicated in a wide spectrum of physiological and pathophysiological conditions.
P21-activated protein kinase (PAK) 2 is a serine/threoninekinase, which has critical roles in various cytoskeletal functions, such as cell motility and membrane blebbing during apoptosis.20, 21 Full-length PAK2 can be activated either by the small GTPases CDC42 and Rac or CASPs, which stimulates cell survival or induction of cell death in response to many apoptotic stimuli.21 PAK2-p34 is a constitutively active 34-kDa PAK2 C-terminal kinase fragment cleaved by several CASPs members.22 Recombinant expression of PAK-2p34 induced morphological changes characteristic of apoptotic cell death in a variety of cell lines and induced apoptotic cell death.23, 24 In addition, accumulation of PAK2-p34 by ubiquitin inhibits degradation results in a dramatic increase in cell death.25 Therefore, PAK2-p34 is involved in cellular death regulation and execution of programmed cell death, and caspase activation stimulated by apoptotic stimuli appears to turn the antiapoptotic activity of PAK2 into a proapoptotic activity of PAK2-p34. Aberrant activity/level of PAK2 or PAK2-p34 has been linked with human cancer, Alzheimer's, and Parkinson's disease.23
In this work, we focus on the functions of prostasin in chemoresistance and tumor repression. Our investigations revealed that prostasin has critical roles in chemoresistant development and cell survival. Forced overexpression of prostasin greatly reduces cancer cell survival, and may partially reverse chemoresistance. Therefore, prostasin may be a potential target for treating some ovarian tumors, including chemoresistant tumors. Investigations of the signaling pathways suggest that prostasin may repress cancer cells and/or contribute to chemoresistance by modulating the CASP/PAK2-p34 and thereafter PAK2-p34/JNK/c-jun and PAK2-p34/mlck/actin signaling pathways.
Results
Prostasin expression is decreased in ovarian cancer patients with high expression of excision repair cross-complementing 1 (ERCC1), a marker for chemoresistance in ovarian cancer
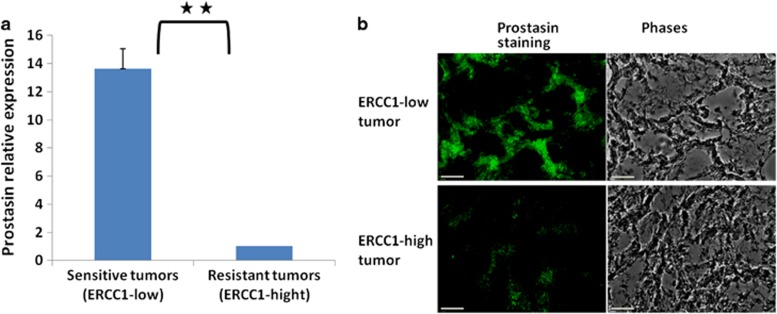
ERCC1, the essential nucleotide excision repair component, is a biomarker for chemoresistance and a target to overcome chemoresistance in cancer therapy.26, 27, 28, 29, 30 We proposed that prostasin may have role in chemoresistance because it is malexpressed in various types of cancers.8, 9, 10, 11 To investigate the possible association, we examined prostasin expression in ovarian tumor samples with known ERCC1 expression, representing chemoresistant phenotype.31 Real-time quantitative PCR data showed that prostasin expression was significantly lower in tumor samples from ERCC1-high group (n=18), compared with ERCC1-low group (n=31), and that prostasin mRNA is greatly reduced in potentially chemoresistant tumors compared with chemosensitive tumors (Figure 1a, P<0.01). We examined prostasin protein level in these tumor samples using immunohistochemistry. The data show that prostasin protein is decreased in chemoresistant/ERCC1-high tumors compared with chemosensitive/ERCC1-low tumors (Figure 1b), which is consistent with prostasin mRNA level. These results demonstrated that prostasin is decreased in ovarian tumors with high ERCC1 expression, the chemoresistant phenotype. Therefore, prostasin may have an important role in chemoresistance in ovarian cancer.
Figure 1.
Reduced prostasin expression in patients with high expression of ERCC1, a marker for chemoresistance in ovarian cancer. (a) Prostasin mRNA levels were compared in tumors with high ERCC1 levels (ERCC1 average=1.54, n=18) representing the chemoresistant phenotype, and in tumors with low ERCC1 levels (ERCC1 average=0.44, n=31) representing the chemosensitive phenotype26, 27 by real-time qPCR analysis. Relative levels of prostasin in chemoresistant versus chemosensitive tumors are shown, respectively. **P<0.01. Mean±s.d. are given, and P values were calculated using the two-sided Student's t-test. (b) Expression of prostasin by fluorescent immunohistochemistry in (patient-derived) ovarian tumor samples. Prostasin is decreased in ovarian ERCC1-high tumors compared with the ERCC1-low tumors
Prostasin is decreased, has key roles in chemoresistance and cell survivals in ovarian cancer cells
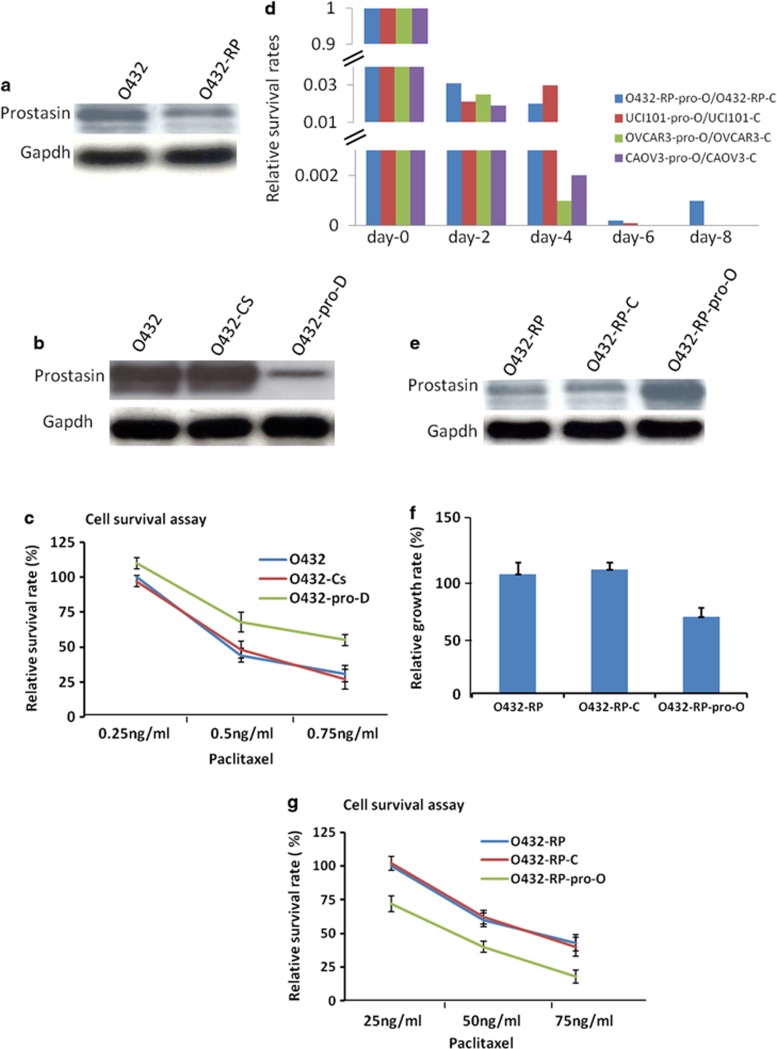
To study the function of prostasin in chemoresistance, we examined prostasin expression in ovarian cancer paclitaxel-resistant cell line O432-RP (it also cross-resistant to cisplatin), a cell line we generated previously from ovarian cancer cell Ovca432 (O432),32 and performed the functional investigations. Western blot analysis showed that prostasin is deceased in drug-resistant O432-RP cell line, compared with the parental-sensitive O432 cell line (Figure 2a), suggesting prostasin may be involved in acquired chemoresistance in ovarian cancer cells. We then examined chemoresistant phenotype after knockdown prostasin in sensitive O432 cell and overexpression of prostasin in resistant cell O432-RP. siRNA knockdown prostasin in O432 cells obtained greatly reduced prostasin cells (O432-pro-D: Figure 2b) compared with the control cells. These cells were treated with paclitaxel at several doses and drug sensitivity was assessed by cell survival assays. We observed that O432-pro-D cells have a higher survival rate compared with O432 cells, which transfected with no-targeting siRNA or reagent (Figure 2c). The data demonstrated that O432-pro-D cells are more resistant to paclitaxel treatment compared with the control cells, suggesting that downregulation of prostasin alone may result in increasing chemoresistance. In contrast, we overexpressed prostasin by transfecting prostasin cDNA containing vector into O432-RP cells. To our surprise, we observed that forced overexpression of prostasin resulted in inducing cell death dramatically, with more than 99.99% cells dead after successful transfection (Figure 2d). Less than 0.01% of the surviving cells (named O432-RP-pro-O), which expressed higher prostasin (Figure 2e), were found to have a very low growth rate compared with the control cells O432-RP and O432-RP-C (transfected with empty vector; Figure 2f). These three cell sub-lines were then treated with paclitaxel at several doses and were assessed for the drug sensitivity by cell survival assays. The survival rate of O432-RP-pro-O was dramatically reduced compared with the control cells (Figure 2g), suggesting that chemoresistance may be reversed. We also tried to overexpress prostasin in other ovarian cancer cell lines, such as Uci101, OVCAR-3, and Caov-3. As seen in the O432-RP cell line, cell death was greatly induced upon prostasin overexpression. However, we did not detect any surviving cells in these cell lines after prostasin cDNA was successfully transfected, which was assessed by geneticin selection and control transfections (Figure 2e). These results indicate that forced overexpression of prostasin in ovarian cancer cells dramatically induces cell death – 100% in some cell lines and more than 99.99% in the other cell line – and represses the cell growth of surviving cells, and may partially reverse chemoresistance. These functional studies indicate prostasin may have critical roles in cell survival and chemoresistance in the studied cell models.
Figure 2.
Prostasin has important roles in chemoresistance and cell death in cell culture model. (a) Prostasin decreased in paclitaxel-resistance cancer cell line. O432: ovarian cancer cell line Ovca432 (sensitive to paclitaxel); O432-RP: paclitaxel resistance cell line generated from Ovca432. The prostasin protein levels in O432 and O432-RP cells are shown in immunoblots with specific antibodies. (b) Prostasin siRNAs transfection reduced prostasin. O432-pro-D cells (transfected with prostasin siRNA) express lower prostasin, compared with control cells of O432 (transfected with reagent only) and O432-Cs (transfected with no-targeting siRNA). The prostasin protein levels are shown in immunoblots with specific antibodies. (c) Downregulation of prostasin in O432 cells resulted in increase of chemoresistant activity. Cells were treated with paclitaxel at different concentrations for 24 h (starting 48 h after siRNAs transfection) and cultured with normal medium for an additional 7 to 10 days before cell survival was assayed. Relative cell survival rates of each cell line are shown. (d) Overexpression of prostasin greatly induces cell death in ovarian cancer cells. The cell survival rates are shown after forced overexpression of prostasin in several cell lines from day-0 to day-8, respectively. (e) Prostasin cDNA transfection resulted in overexpression of prostasin in chemoresistant O432-RP cells. O432-RP-pro-O cells (transfected with prostasin cDNA) express higher prostasin compared with control cells O432-RP and O432-RP-C (transfected with control vector). The prostasin protein levels are shown in immunoblots with specific antibodies. (f) Forced overexpression of prostasin represses growth of chemoresistant cells. Relative cell growth rates are shown for O432-RP-pro-O and control cells O432-RP and O432-RP-C. (g) Forced overexpression of prostasin in O432-RP cells re-sensitizes chemoresistant cells. Cells were plated at about 10–20% confluence and treated with paclitaxel at different concentrations for 24 h, cultured with normal medium for additional 7 to 10 days, then assayed for cell survival. Relative survival rates of cell lines are shown
Overexpression of prostasin represses chemoresistant tumors
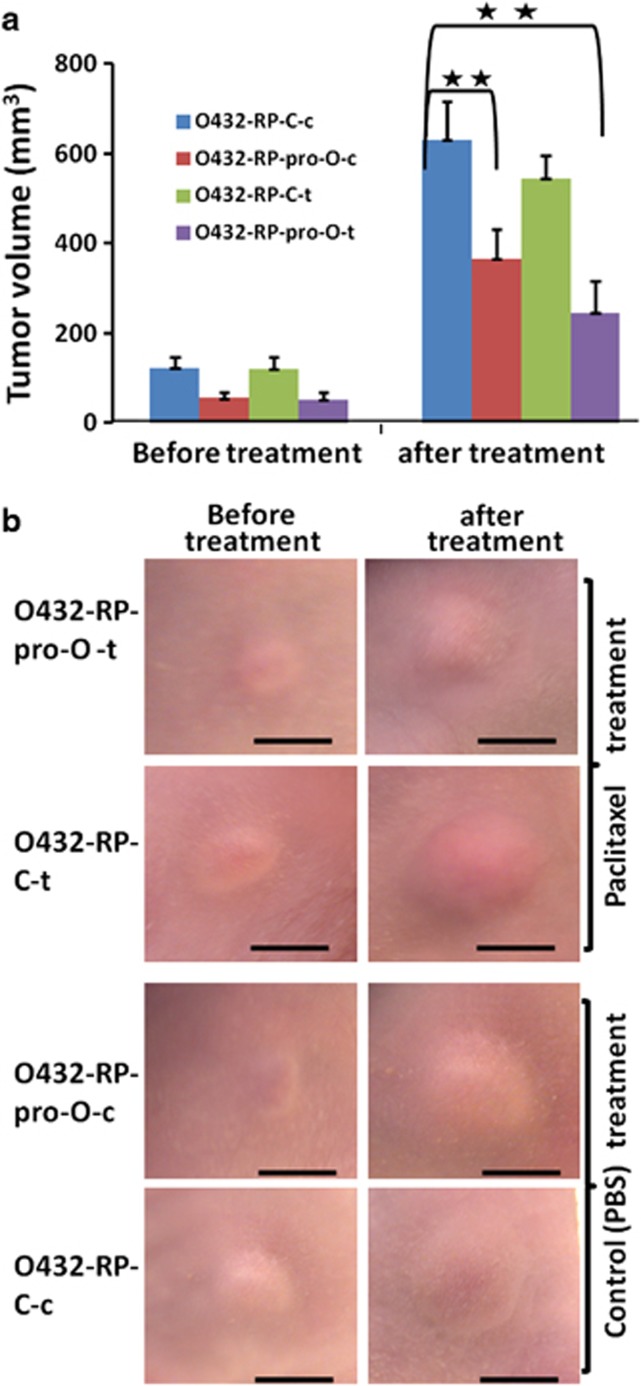
We extended our in vitro finding to a mouse tumor model to explore the potential of prostasin as a therapeutic target for treating/repressing some ovarian tumors. The tumor cells of O432-RP-pro-O and O432-RP-C were implanted into each flank of mice. These tumor-bearing animals were treated with paclitaxel or vehicle PBS when the tumor volume reaches about 100 mm3 (the tumor volume of O432-RP-C is about 100 mm3at the time of treatment; however, the volume of O432-RP-pro-O tumor is only about 50 mm3 because of low growth rate of cells). At the end of treatment, we compared the tumor volumes of O432-RP-C and O432-RP-pro-O in the presence and absence of paclitaxel, respectively. In the absence of paclitaxel treatment (e.g., just vehicle PBS), we observed that O432-RP-pro-O tumors were significantly smaller compared with that of O432-RP-C control tumors (P<0.01, Figures 3a and b). We believe this is probably due to the low growth rate of this cell line, which overexpressed prostasin. In addition, we observed that the O432-RP-pro-O tumors became further smaller when treated with paclitaxel (P<0.001, Figures 3a and b), and paclitaxel-treated O432-RP-pro-O tumors were slightly smaller than PBS-treated O432-RP-pro-O tumors (P<0.1, Figures 3a and b). The data suggest that restoration of prostasin in chemoresistant cells inhibits growth of these tumors and may partially reverse chemoresistance, which is consistent with our in vitro findings.
Figure 3.
Forced restoration of prostasin represses and re-sensitizes chemoresistant tumors. (a) Overexpression of prostasin represses and re-sensitizes chemoresistant tumors. O432-RP-pro-O cells (stably transfected with prostasin cDNA) or control cells O432-RP-C (stably transfected with control pCI-neo vector) were injected into the left and right flanks of mice, respectively. The animals were treated with paclitaxel (15 mg/kg/week) or control vehicle PBS for 2 weeks after the tumors reached about 100 or 50 mm3. Tumor volumes before and after treatment are shown. O432-RP-psp-O-c and O432-RP-psp-O-t: PBS- and paclitaxel-treated O432-RP-psp-O tumors; O432-RP-C-c and O432-RP-C-t: PBS- and paclitaxel-treated O432-RP-C tumors. n=6 per group, **P<0.01. Mean±s.d. are given, and the P-values were calculated using the two-sided Student's t-test. (b) Tumors in mice before and after treatments. Scale bar=5 mm
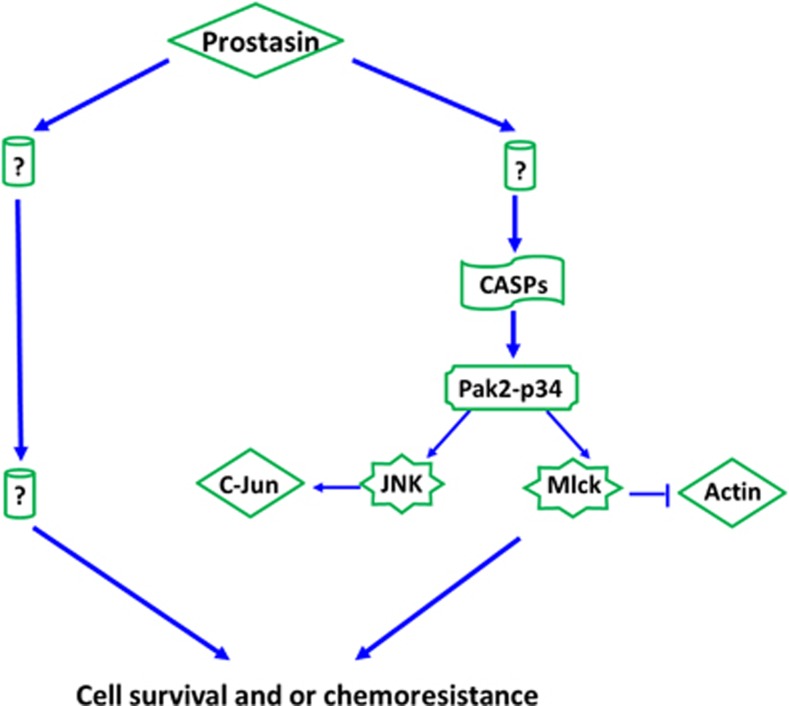
Prostasin regulates a network involving CASP/PAK2-p34 and thereafter mlck/actin, and JNK/c-jun pathways in ovarian cancer cells
To uncover the signaling pathways for prostasin in cell survival and chemoresistance, we compared the expression profile of O432-RP-pro-O and O432-RP-C cells using PCR arrays analysis.
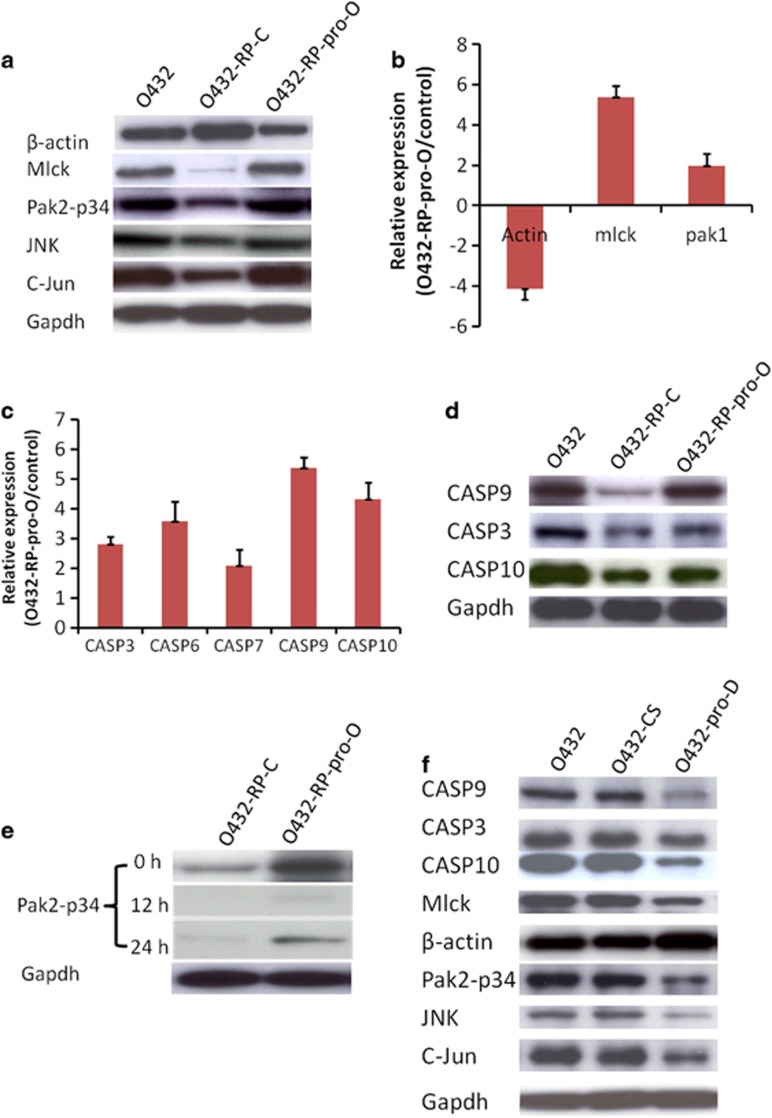
β-Actin was found to be decreased in O432-RP cells compared with O432 cells in our study (we initially used β-actin as a loading control for western blot analysis; however, we found β-actin is not consistant when we used GAPDH as loading control for equal loading of samples (Figure 4a)). With the previous finding that β-actin expression changed in breast cancer drug resistance cells, this prompted us to hypothesize that β-actin and cytoskeletal genes may be involved in the prostasin-directed chemoresistance development as actin gene is believed to be a central player of cell shape and movement and a key component of cytoskeleton.33 We examined gene expression of cytoskeleton pathway using PCR array in these cells. The PCR array data showed that PAK and mlck increased, and β-actin decreased in O432-RP-pro-O cells, compared with control O432-RP-C cells (Figure 4b). Western blot analysis further demonstrated that protein levels of mlck and β-actin changed, which were consistent with mRNA levels (Figure 4a). However, we did not see significant difference for PAK proteins. Instead, we observed that PAK2-p34, a 34KD C-terminal fragment of PAK2, which is cleaved by CASPs,34, 35 is increased in O432-RP-pro-O cells. PAK2-p34 has been shown to regulate JNK expression during apoptosis,20 so we examined JNK and thereafter target c-jun expression. The western blot analysis showed that JNK and c-Jun both increased in O432-RP-pro-O cells. The data suggested that prostasin regulates PAK2-p34 and thereafter JNK and c-jun signaling in these cells. In addition, mlck has been shown to be a downstream target of PAK2/PAK2-p34, and upstream target of actin. Thus, PAK2-p34 seems to be an important mediator of prostasin in these cells and appears to regulate JNK/c-jun and mlck/act sub-pathways.
Figure 4.
Prostasin regulates CASPs-PAK2-p34 axis and thereafter downstream signaling. (a) Immunoblot of mlck, β-actin, PAK2-p34, JNK, C-Jun in O432-RP-pro-O (O432-RP cells transfected with prostasin cDNA which express higher levels of prostasin) control O432-RP-C cells (O432-RP cells transfected with pCI-neo vector). Mlck, PAK2-p34, JNK, C-Jun expressions increase and β-actin decreases in O432-RP-pro-O cells when compared with control O432-RP-C. (b) Comparison of actin, mlck, and pak1 expression between O432-RP-pro-O and control O432-RP-C cells by real-time qPCR. mRNA levels of actin decreases and mlck and pak1 increase in O432-RP-pro-O cells compared with control O432-RP-C cells. (c) mRNA levels of CASPs detection by real-time qPCR in O432-RP-pro-O cells and control O432-RP-C cells. Several CASP expression increase in O432-RP-pro-O cells compared with the controls. (d) Immunoblot of CASP3, 9, and 10 in O432-RP-pro-O and O432-RP-C cells. CASP3, 9, and 10 protein levels increase in O432-RP-pro-O cells compared with control O432-RP-C cells. (e) CASP inhibitors block CASP activity and thus PAK2 cleavage. PAK2-p34 protein was examined by immunoblot after CASP inhibitors were added to the medium. PAK2-p34 was seen decreased or lost when CASPs were inhibited at several time points. (f) Immunoblot of mlck, β-actin, PAK2-p34, JNK, C-Jun, CASP3, 9, and 10 in prostasin knockdown O432-pro-D cells and controls O432 (mock transfected with reagent only) and O432-Cs (transfected with no-targeting siRNA). Reverse expression patterns for these genes are revealed in O432-pro-D cells and controls compared with O432-RP-pro-O cells and controls
To explore the upstream targets of PAK2-p34, we examined expression of several CASP genes, as PAK2-p34 is specifically cleaved by CASPs.34, 35 Interestingly, several CASPs were found increased in O432-RP-pro-O cells compared with O432-RP-C cells (Figures 4c and d). To confirm that PAK2-p34 is cleaved by CASPs, we blocked CASPs activity using CASPs inhibitors. We observed that the cleavage was blocked (PAK2-p34 decrease or disappear) as early as 12 h after incubating the cell with CASPs inhibitor (Figure 4e). Thus, CASPs are upstream targets of PAK2-p34 and downstream target of prostasin in our experimental system. We tried to identify the upstream signaling of CASPs in these cells, but were unsuccessful. We also checked the expression level of these prostasin downstream targets in prostasin knockdown cells O432-pro-D compared with the control, and a reverse pattern was revealed (Figure 4f). The data suggest that prostasin may affect chemoresistance and/or cell survival through regulating the CASPs/PAK2-p34 axis and thereafter JNK/c-jun and mlck/actin signaling pathways (Figure 5).
Figure 5.
Proposed model of prostasin-regulated signaling network affecting cell survival and/or chemoresistance. Prostasin appears to regulate cell survival and/or chemoresistance may be through CASPs/Pak2-p34 axis and thereafter PAK2-p34/JNK/c-jun and PAK2-p34/mlck/actin signaling pathways
Discussion
Dramatic advances have been made in understanding cancer's basic mechanisms during recent decades. Despite these advances, ovarian cancer is still a deadly disease with a less than 50% 5-year survival rate.1 Antitumor drug resistance or non-response to chemotherapy is one of the major challenges to successful treatment of ovarian cancer. In this study, we show that prostasin expression is significantly decreased in both ovarian cancer chemoresistant cells and in tumor tissues of high ERCC1-expressing ovarian cancer patients. ERCC1 has been well-described as a potential biomarker for drug resistance.26, 27, 28, 29 Prostasin appears to have an important role in chemoresistance. The functional investigations demonstrate that forced overexpression of prostasin in ovarian cancer cells induces more than 99.99% cell death and represses the cell growth of surviving cells, and may partially reverse chemoresistance. This indicates that prostasin may have very strong power in repressing cancer cells and have roles in chemoresistance. Thus, may be a target for treating/repressing some ovarian tumors in gene therapy.
Prostasin may have an important role in the development of various types of cancers, including ovarian, prostate, breast, and gastric cancers, and expression of prostasin has been shown to be changed in these cancer patients.8, 9, 10, 11 In addition, restoration of prostasin in prostate cancer cells results in the inhibition of cell proliferation and invasion.14 Therefore, prostasin is believed to be a tumor suppressor in prostate and breast cancers. For ovarian cancer, the role of prostasin is complex. It is overexpressed in ovarian cancer patients suggesting it is an oncogene.9 Our findings show that prostasin is decreased in potentially chemoresistant ovarian cancer patients and chemoresistant cell line, and forced overexpression of prostasin in ovarian cancer cells greatly induces cells death, which behaves like a tumor suppressor. Prostasin is overexpressed in ovarian cancer patients with all stages. However, the levels change significantly from early to late stages. Prostasin increases and reaches a peak level at stage II and III and then decreases sharply at stage IV (the level is still slightly higher than in normal tissue).9 Therefore, decreased prostasin (advanced stage compared with early stage patients) may be required for advanced ovarian cancer cells, or prostasin has different roles in different stage cancer cells. Prostasin is a glycosylphosphatidylinositol-anchored membrane protein as well as a secreted protein from epithelial cells, where it is bound to the cell surface, secreted, or both.10 Studies showed that the membrane-anchored form of prostasin has important roles in tumor or invasion suppression of prostate or breast cancers, but not the secreted or recombinant prostasin.10 Our finding is consistent with these observations, which demonstrate that forced overexpression of prostasin inhibits cancer cells growth, but the recombinant prostasin has no effect on cell growth or death in ovarian cancer cells (data not shown). Therefore, membrane-anchored prostasin has critical roles in cell survival and/or chemoresistance, and overexpression of prostasin triggers cell death in some ovarian cancer cells. We propose that prostasin is a potential target for treating some ovarian tumors in gene therapy, which overexpress prostasin in cells, but not by infusion of secreted/recombinant prostasin. Overexpression of prostasin can be done either by traditional transgene methods or newly identified gene-regulation mechanisms to enhance gene expression through transfection of promoter-associated siRNAs.36
The signaling pathways that we investigated show that prostasin controls the downstream axis of CASPs/PAK2-p34, and thereafter mlck/actin and JNK/c-jun sub-signalings, in ovarian cancer cells. The finding suggests a new mechanism by which prostasin induces cell death and/or contribute to chemoresistance through regulating these pathways (Figure 5). The CASPs are believed to be the core effectors of apoptosis.37 PAK2-p34 has been shown to induce cell death may be by increasing signal of JNK and regulating cytoskeletal dynamics of mlck pathways.20 All these genes and signaling pathways33, 38, 39 have essential roles in apoptosis and transcription. Therefore, prostasin may contribute to chemoresistance and repress cancer cells through these mechanisms. Study showed that overexpression of PAK2-p34 in cancer cells induced apoptosis and cell death.20 We also observed that forced overexpression of PAK2-p34 in chemoresistant cell O432-RP greatly inhibited cell survival (Supplementary Figure 1). We propose that PAK2-p34 is a key executer of prostasin, and prostasin induces cancer cell death and/or contributes to chemoresistance by regulating these signaling pathways in our experimental system. Further functional studies should build the direct link between prostasin and these pathways. Our pathway analysis is focused on the apoptosis. Surely, the functions of prostasin in cell death and chemoresistance may involve in other pathways, such as cell cycle or stem cell development, and future study may focus on these topics.
PAK2 has been shown to contribute to chemoresistance in breast cancer cells by decreasing levels of active caspase-3 and thereafter PAK2-p34 (PAK2 and PAK2-p34 have opposing role in cell apoptosis). β-Actin was also found to be altered in these cells.36 We proposed that alteration of β-actin should result from CASP/PAK2 (PAK2-p34)/mlck regulation, as mlck is one main target of PAK2-p34.15 Together with our findings, the data suggest CASP/PAK2-p34 and sub-pathways thereafter may have an important role in chemoresistance in different cancer types (Supplementary Figure 2). In addition, changes in β-actin have been shown both in breast cancer drug-resistant (cisplatin and paclitaxel) cells36 and our ovarian cancer drug-resistant cells. Involvement of β-actin in drug resistance in different types of cancer precludes it as an internal control in the future study.
In summary, our study points to prostasin as a potential target for treatment/repress of some ovarian cancers, including chemoresistant tumors. The pathway and mechanism findings suggest prostasin may regulate cancer cell survival and/or chemoresistance by controlling CASPs/PAK2-p34 and thereafter signaling through apoptosis mechanisms.
Meterials and Methods
Human samples and ethics statement
Frozen GOG (Gynecologic Oncology Group) samples from ovarian cancer patients were obtained from the Cooperative Human Tissue Network, Pediatric Division (Children's Hospital, Columbus, Ohio). All patient identifiers of these samples had been stripped of before shipping to ensure that the presented molecular laboratory data cannot be linked with any subject in this study. The tumor specimens were collected at primary surgery, fresh frozen in liquid nitrogen, and stored at −80 °C until RNA/DNA extraction. All samples were evaluated by pathologists.
Cell lines, cell culture and generation of drug-resistance sub-line
Cell lines, cell culture and generation of drug-resistance sub-line are described as previously.32 All cell lines were propagated as an adherent monolayer in MEM (Invitrogen, Life Technologies, Inc., Carlsbad, CA, USA) at 37 °C in a humidified atmosphere of 5% CO2 supplemented with 10% heat inactivated FBS, 100 μg/ml penicillin, and 100 μg/ml streptomycin. Human ovarian cancer cell lines of Ovca432 and Uci101were provided by Dr. Liu T from the Chinese Academy of Sciences. OVCAR-3 and Caov-3 were purchased from ATCC (Manassas, VA, USA). The Ovca432-RP drug-resistance sub-cell line was generated from Ovca432 cells with paclitaxel (Sigma-Aldrich, Inc., St. Louis, MO, USA) treatment at increasing concentrations for several cycles (each cycle including 24-h exposure to the drug, and subsequent recovery culture with normal medium for about 10 days) until cells were found resistant to treatment.
RNA extraction and cDNA generation
Total RNA from tumors of ovarian cancer patients was partially purified by hot phenol/chloroform extraction as previously reported.40 Through reverse transcription, using the Super Script Preamplification System (Life Technologies, Inc.), cDNA was generated with oligo-dT primers from 5 μg of total RNA per sample (Life Technologies, Inc.). Total RNA was extracted from cells using Trizol. The cDNA was synthesized using Taqman Reverse Transcription (PE Applied Biosystems, Foster City, CA, USA) from 1 μg of total RNA. cDNAs were used for the gene expression profiling.
Real-time reverse transcription quantitative-PCR
Real-time quantitative PCR was performed with an ABI PRISM 7900 or 7500 instrument (PE Applied Biosystems) according to the manufacturer's instructions as previously described.32 PCR cycling conditions were set as follows: 50 °C, 2 min; 95 °C, 10 min; and 40 cycles for the melting (95 °C, 15 s) and annealing/extension (60 °C for 1 min) steps. qPCR reactions for each template were carried out in duplicate or triplicate in 96-well plates. Comparative Ct (PE Applied Biosystems) determined relative expression in each sample using 18S or GAPDH as endogenous controls.
Western blot
Western blot analysis was performed as previously described.32 Briefly, cells were rinsed twice with PBS and total proteins were solubilized in lysis buffer (150 mM sodium chloride; 50 mM Tris hydrochloride, pH 7.5;1% glycerol; 1% Non-idetp-40 substitute solution). Equal amounts of proteins were loaded and separated by SDS-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene difluoride membrane, blocked by 5% skim milk, and probed with the specific (primary) antibodies, followed by treatment with secondary antibody conjugated to horseradish peroxidase (1 : 5000). Proteins were visualized by (enhanced) chemiluminescence detection (Pierce Biotechnology, Rockford, IL, USA) and exposure to X-ray film. β-Actin or Gapdh proteins were detected by normal chemiluminescence detection (Pierce Biotechnology) and exposed to less-sensitive X-ray film, which may result in a relatively weaker signal. All antibodies used in this study were obtained from Abnova (Taipei, Taiwan) or Abcam (Cambridge, MA, USA).
siRNA and cDNA transfection
O432 cells were transfected with prostasin siRNA duplexes (Ambion, Austin, TX, USA) and O432-RP cells with prostasin or PAK2-p34 cDNA-containing vectors (cDNAs were amplified by reverse transcription PCR and then cloned into a pCI-neo vector) using Lipofectamine 2000 (Invitrogen) according the manufacturer's instructions. For siRNA experiments, mock transfections and no-targeting siRNA were used as negative controls. Cells were treated for 72 h to allow for maximum knockdown, after which they were lysed for western blot analysis, RNA preparation or other assays. For cDNA transfection, a pCI-neo vector was used as a negative control. Geniticin was used to select stably expressing cells.
Cell survival assay
Cell survival assay was performed as previously described.32 Cells were counted and plated in culture dishes at about 10–20% confluence on the day before treatment. Paclitaxel or control PBS was added for about 20 h and removed, then the cells were cultured with normal medium for recovery and continuous propagation for 10–14 days. To quantify final cell numbers, cells were stained with 0.25% crystal violet/20% ethanol and counted, or the proliferation rate was measured using a Cell Proliferation Assay kit (Promega, Madison, WI, USA) according to manufacturer's instructions. Briefly, MTS/PMS solution (at final concentrations of 333 μg/ml MTS and 25 μM PMS) was added to each well and cells were incubated for 2–3 h at 37 °C. The absorbance was determined at 490 nm using a 96-well plate ELISA reader. Culture medium was used as background control. The experiments were repeated at least three times.
Immunofluorescence
The tumor sections were deparaffinized in Histo Clear II (Electron Microscopy Sciences, Hatfield, PA, USA), hydrated in gradient alcohol (passed through gradient alcohol washes), and antigen retrieval treated in a microwave oven for 10 min at ‘Power 20%' in citrate buffer (0.01 M, pH=6.0,0.05% Tween-20), then cooled for 20 min. The sections were incubated with blocking buffer (2% goat serum, 5% BSA, 0.1% cold fish skin gelatin, 0.1% Triton X-100, 0.05% Tween-20, 0.05% sodium azide, pH 7.2) for 2 h. Thereafter, the slides were incubated with mouse monoclonal anti-prostasin antibody (1 : 20 dilution) overnight at 4 °C. The primary antibody was detected using anti-mouse IgG Alexa Fluor 488 (Life Technologies, Inc.). Slides were mounted with the SlowFade Gold AntiFade reagent with DAPI (Life Technologies, Inc.). In the negative control tissue sections, the primary antibody was replaced by isotype-specific non-immune mouse IgG. Immunoreactivity was visualized and photographed using a Nikon confocal microscope (Nikon Elipse90i, Deutschland, Germany) at the appropriate wavelength.
Tumor mouse model study
We performed mouse experiments as previously described32 and in compliance with the published guidelines of Laboratory Animal Care (NIH Publication No. 85-23, revised 1985; http://grants1.nih.gov/grants/olaw/references/phspol.htm) and the Care and Use of Laboratory Animals (National Research Council, 1996). Four- to six-week-old female BALB/cnu mice (Charles Rivers Laboratories, Wilmington, MA, USA and Laboratory Animal Research Center, Shanghai, China) were used to generate xenograft tumors. 1.0 × 107 cells were suspended in 200 μl saline solution and injected into both flanks of mice. The mice were treated with paclitaxel (15 mg/kg) or vehicle PBS by intratumoral injection for 2 weeks when tumor volume reached about 100 or 50 mm3. Tumor volume and mouse weight were monitored accordingly.
CASPs inhibition experiment
CASP family inhibitor (MBL International, Woburn, MA, USA) was added to the culture medium at 1000-fold dilution to inhibit caspase activity. Cells were lysed after 12 and 24 h post inhibitor treatment and subjected to western blot analysis.
Statistical analysis
Statistical analysis was conducted using Student's t-test. Statistical significance was defined as P<0.05. All statistical tests and corresponding P-values were two-sided.
Acknowledgments
We acknowledge the Molecular Medicine Core Facility, Mary Babb Randolph Cancer Center, West Virginia University, and Icesnow Yanyan Bioscience Association, Beijing, for supporting this study.
Glossary
- O432 (Ovca432)
human ovarian cancer parental cell line
- O432-RP (Ovca432-RP)
drug-resistant sub-line generated by repeatedly treated Ovca432 cells with paclitaxel
- ERCC1
excision repair cross-complementing 1
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Thigpen T. First-line therapy for ovarian carcinoma: what's next. Cancer Invest. 2004;22 (Suppl 2:21–28. doi: 10.1081/cnv-200030115. [DOI] [PubMed] [Google Scholar]
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, et al. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst Rev. 2010;9:CD007414. doi: 10.1002/14651858.CD007414.pub2. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Kita T, Takano M, Kudoh K, Yamamoto K. Treatment options in the management of ovarian cancer. Expert Opin Pharmacother. 2005;6:743–754. doi: 10.1517/14656566.6.5.743. [DOI] [PubMed] [Google Scholar]
- Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem. 1994;269:18843–18848. [PubMed] [Google Scholar]
- Yu JX, Chao L, Chao J. Molecular cloning, tissue-specific expression, and cellular localization of human prostasin mRNA. J Biol Chem. 1995;270:13483–13489. doi: 10.1074/jbc.270.22.13483. [DOI] [PubMed] [Google Scholar]
- Chen LM, Hodge GB, Guarda LA, Welch JL, Greenberg NM, Chai KX. Down-regulation of prostasin serine protease: a potential invasion suppressor in prostate cancer. Prostate. 2001;48:93–103. doi: 10.1002/pros.1085. [DOI] [PubMed] [Google Scholar]
- Mok SC, Chao J, Skates S, Wong K, Yiu GK, Muto MG, et al. Prostasin, a potential serum marker for ovarian cancer: identification through microarray technology. J Natl Cancer Inst. 2001;93:1458–1464. doi: 10.1093/jnci/93.19.1458. [DOI] [PubMed] [Google Scholar]
- Chen LM, Chai KX. Prostasin serine protease inhibits breast cancer invasiveness and is transcriptionally regulated by promoter DNA methylation. Int J Cancer. 2002;97:323–329. doi: 10.1002/ijc.1601. [DOI] [PubMed] [Google Scholar]
- Sakashita K, Mimori K, Tanaka F, Tahara K, Inoue H, Sawada T, et al. Clinical significance of low expression of Prostasin mRNA in human gastric cancer. J Surg Oncol. 2008;98:559–564. doi: 10.1002/jso.21158. [DOI] [PubMed] [Google Scholar]
- Lu KH, Mimori K, Tanaka F, Tahara K, Inoue H, Sawada T, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res. 2004;10:3291–3300. doi: 10.1158/1078-0432.CCR-03-0409. [DOI] [PubMed] [Google Scholar]
- Costa FP, Batista EL, Jr, Zelmanowicz A, Svedman C, Devenz G, Alves S, et al. Prostasin, a potential tumor marker in ovarian cancer-a pilot study. Clinics (Sao Paulo) 2009;64:641–644. doi: 10.1590/S1807-59322009000700006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- Rickert KW, Kelley P, Byrne NJ, Diehl RE, Hall DL, Montalvo AM, et al. Structure of human prostasin, a target for the regulation of hypertension. J Biol Chem. 2008;283:34864–34872. doi: 10.1074/jbc.M805262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, et al. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med. 2010;2:26–37. doi: 10.1002/emmm.200900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NF, Zhang JH, Chang JH, Yang J, Wang HM, Zhou L, et al. Association of genetic variations of the prostasin gene with essential hypertension in the Xinjiang Kazakh population. Chin Med J (Engl) 2011;124:2107–2112. [PubMed] [Google Scholar]
- Frateschi S, Keppner A, Malsure S, Iwaszkiewicz J, Sergi C, Merillat AM, et al. Mutations of the Serine Protease CAP1/Prss8 Lead to Reduced Embryonic Viability, Skin Defects, and Decreased ENaC Activity. Am J Pathol. 2012;181:605–615. doi: 10.1016/j.ajpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–496. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas GL, Corvi MM, Plummer GJ, Seime AM, Lambkin GR, Berthiaume LG, et al. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc Natl Acad Sci USA. 2006;103:6542–6547. doi: 10.1073/pnas.0600824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Koeppel MA, McCarthy CC, Moertl E, Jakobi R. Identification and characterization of PS-GAP as a novel regulator of caspase-activated PAK-2. J Biol Chem. 2004;279:53653–53664. doi: 10.1074/jbc.M410530200. [DOI] [PubMed] [Google Scholar]
- Marlin JW, Chang YW, Ober M, Handy A, Xu W, Jakobi R. Functional PAK-2 knockout and replacement with a caspase cleavage-deficient mutant in mice reveals differential requirements of full-length PAK-2 and caspase-activated PAK-2p34. Mamm Genome. 2011;22:306–317. doi: 10.1007/s00335-011-9326-6. [DOI] [PubMed] [Google Scholar]
- Lee N, MacDonald H, Reinhard C, Halenbeck R, Roulston A, Shi T, et al. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc Natl Acad Sci USA. 1997;94:13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobi R, McCarthy CC, Koeppel MA, Stringer DK. Caspase-activated PAK-2 is regulated by subcellular targeting and proteasomal degradation. J Biol Chem. 2003;278:38675–38685. doi: 10.1074/jbc.M306494200. [DOI] [PubMed] [Google Scholar]
- Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994;94:703–708. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yu JJ, Mu C, Yunmbam MK, Slavsky D, Cross CL, et al. Association between the level of ERCC-1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res. 2012;2000:645–652. [PubMed] [Google Scholar]
- Olaussen KA, Mountzios G, Soria JC. ERCC1 as a risk stratifier in platinum-based chemotherapy for nonsmall-cell lung cancer. Curr Opin Pulm Med. 2007;13:284–289. doi: 10.1097/MCP.0b013e32816b5c63. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil EM, Melton DW. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012;40:9990–10004. doi: 10.1093/nar/gks818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E, Yu JJ, Davies A, Gannon J, Armentrout SL. Clear cell tumors have higher mRNA levels of ERCC1 and XPB than other histological types of epithelial ovarian cancer. Clin Cancer Res. 2003;9:5299–5305. [PubMed] [Google Scholar]
- Yan BX, Ma JX, Zhang J, Guo Y, Riedel H, Mueller MD, et al. PSP94 contributes to chemoresistance and its peptide derivative PCK3145 represses tumor growth in ovarian cancer Oncogene 2013. e-pub ahead of print 4 November 2013; doi: 10.1038/onc.2013.466 [DOI] [PubMed]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Stroh C, Schulze-Osthoff K. Unique and overlapping substrate specificities of caspase-8 and caspase-10. Oncogene. 2006;25:152–159. doi: 10.1038/sj.onc.1209015. [DOI] [PubMed] [Google Scholar]
- Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Yan BX, Ma JX. Promoter-associated RNAs and promoter-targeted RNAs. Cell Mol Life Sci. 2012;69:2833–2842. doi: 10.1007/s00018-012-0953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326 (Pt 1:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, et al. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci USA. 2009;106:8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Dabholkar M, Bennett W, Welsh J, Mu C, Bostickbruton F, et al. Platinum-sensitive and platinum-resistant ovarian cancer tissues show differences in the relationships between mRNA levels of p53, ERCC1 and XPA. Int J Oncol. 1996;8:313–317. doi: 10.3892/ijo.8.2.313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.