Abstract
Objective
To pilot a protocol to evaluate acute cardiovascular effects in in-vehicle exposure to traffic air pollutants in people with diabetes.
Methods
Twenty-one volunteers with type 2 diabetes were passengers on 90- to 110-minute car rides on a busy highway. We measured in-vehicle particle number and mass (PM2.5) nitrogen dioxide, and carbon monoxide and heart rate, heart rate variability (HRV), and blood pressure.
Results
Compared with pre-ride measurements, we found a decrease in high frequency (HF) HRV from pre-ride to next day (ratio 0.66, 95% CI = 0.47 to 0.93) and an increase in low frequency to HF ratio at post-ride (ratio 1.92, 95% CI = 1.21 to 3.05) at post-ride. Interquartile range increases in measured pollutants were associated with next-day decreases in HR HRV.
Conclusions
This protocol appears useful for assessing acute adverse cardiovascular effects of in-vehicle exposures among people who have diabetes.
Numerous epidemiological studies have demonstrated consistent associations between cardiovascular (CV) health effects and ambient particulate matter (PM) air pollution, especially PM with aerodynamic diameter <2.5 μm (PM2.5).1 Two areas of emphasis in ongoing PM/CV research are the identification of susceptible subpopulations and the characterization of risks associated with specific sources of PM. Motor vehicles are a major source of PM that has been linked to CV effects,2–9 and studies suggest that people who have diabetes may be more susceptible to the health effects of PM.10–13
While commuting, drivers and passengers are exposed to a complex mixture of air pollutants, including emissions from other vehicles on the roadway, which consist of both particulate and gaseous emissions such as nitrogen oxides (NOx) and carbon monoxide (CO). Although they contribute little to the particle emissions on a mass basis, ultrafine particles (UFP, diameters <100 nm) account for >90% of the number of particles emitted from vehicles.14 A typical 1-hour commute on Los Angeles freeways contributes ~50% of daily UFP exposure to an individual whose residence is not near a major freeway.15 Compared with larger particles on a mass basis, UFP have greater lung deposition, higher surface area, and greater capacity to generate oxidative stress.15–17
Elevated levels of ambient PM may trigger acute myocardial infarction or atrial fibrillation or both within as little as 1 hour.18–20 Alterations in the activity of the autonomic nervous system may be one mechanism underlying these rapid-onset CV effects of ambient and traffic-related PM. Exposure to PM has been associated with dysrhythmia,3,20–23 exacerbation of congestive heart failure,24 and changes in blood pressure25–27 and heart rate.28,29 Changes in heart rate variability (HRV), an indicator of the relative balance of parasympathetic and sympathetic autonomic control of the heart rate, have been associated with ambient30,31,6 and traffic-related PM air pollution,2,7 most consistently with the high-frequency component of HRV. Decreased HRV has been shown to predict mortality in a number of patient groups.32,33 One observational study has suggested that polymorphisms in antioxidant genes and use of antioxidant statin medications may protect against PM-associated HRV changes, suggesting an important role for oxidative stress in these effects.8
People who have diabetes may be particularly susceptible to the CV effects of PM. Diabetes is associated with chronically elevated levels of oxidative stress, depleted antioxidant defenses, and already impaired autonomic function.34 Recent studies of people with diabetes have found associations among endothelial dysfunction, PM2.5, and black carbon, a marker of traffic-source air pollution.11,35 Previous studies of CV responses to on-road, traffic-source air pollution among people who have diabetes are not available.
We hypothesized that in-vehicle exposures cause rapid-onset adverse changes in autonomic activity in individuals who have type 2 diabetes. To test this hypothesis, we piloted a field-study protocol to evaluate acute changes in autonomic function (heart rate, HRV, and blood pressure) and endothelial function in a group of adults with diabetes before and after a 1.5- to 2-hour car ride on a major highway with heavy diesel truck traffic. Here, we report the effects on autonomic function, with vascular endpoints to be reported elsewhere. During this car ride, we measured in-vehicle concentrations of traffic-related air pollutants (UFP, PM2.5, NO2, and CO). We examined associations between these air pollutants and changes in the physiologic outcomes from before to immediately after the car ride, and from before to the next day following the car ride.
MATERIALS AND METHODS
Subjects
We recruited 21 nonsmoking adults with type 2 diabetes (median, age 61 years) from the endocrinology clinic at Robert Wood Johnson Medical School in New Brunswick, New Jersey. We excluded potential subjects with serious or unstable neurologic, cerebrovascular, CV, gastrointestinal, renal, or pulmonary disease including asthma. We did not include individuals with atrial flutter, atrial fibrillation, or pacemakers. All subjects lived within 20 km of the Clinical Center of the Environmental and Occupational Health Sciences Institute (EOHSI) in Piscataway, New Jersey. This study protocol was approved by the University of Medicine and Dentistry of New Jersey (UMDNJ) Institutional Review Board, and all subjects gave informed consent before participation in the study.
Study Procedure
Each subject participated in one exposure session during the study, consisting of a car ride in the rear seat of a sport utility vehicle for 1.5 to 2 hours during morning rush-hour traffic. Physiologic outcomes were measured at sessions immediately before (pre-exposure), immediately after (post-exposure), and 24 hours after (next day) the start of the car ride. Subjects were asked to fast after midnight and withhold diabetes medications on the evening before the morning car ride, so as to avoid potential effects of food ingestion, consistent with previous studies of endothelial function.36 Each subject reported to the EOHSI Clinical Center at 7:00 AM for the pre-exposure session and was given a finger-stick blood-glucose test to rule out hypoglycemia. All subjects’ blood glucose levels were ≥90 mg/dL, and thus none were excluded. Each subject then completed a general health history form including current medications and a stress symptom rating (SSR).37 Subjects rated how they were currently feeling on a five-point modified Likert scale connecting two pairs of antonyms for “stress” (tense to relaxed, stressed to at ease) and “anxiety” (nervous to calm, jittery to tranquil). The subject then sat quietly for HRV measurement (method described below). After HRV measurement, the subject underwent a peripheral venous blood draw. The subject then rested quietly for at least 5 minutes before a trained technician measured systolic and diastolic blood pressure (SBP and DBP) with a mercury sphygmomanometer (CE0050; Hokanson, Indianapolis, IN), with the mean of three successive blood pressure measurements used in all analyses. After blood pressure measurements, each subject underwent noninvasive studies of endothelial function. The results of the endothelial function tests and venous blood markers are reported elsewhere.
Following the pre-exposure session, the subject was escorted to a waiting UMDNJ vehicle (either a 2005 Ford Explorer or a 2004 Dodge Durango) with a professional UMDNJ driver and a study technician. The subject was then driven from the EOHSI Clinical Center to and from the northernmost point on the New Jersey Turnpike (NJTPK; 79 miles roundtrip of which 57 miles were on the NJTPK). Car trips lasted 90 to 110 minutes, depending on traffic conditions. The NJTPK is a divided highway with six traffic lanes in each direction. Along the study route, truck traffic is required to use the right-most two traffic lanes. The study vehicle remained in the right-most lane of traffic averaging 65 mph. The vehicle ventilation settings were maintained with the fan on and the vent in the open position during the car ride. Air conditioning or heater temperature settings were adjusted for subject comfort. About midway through the car ride, the subject completed another SSR questionnaire.
For the post-exposure session, the subject returned to the EOHSI clinic immediately after the car ride, where the same physiologic measurements were made using the same protocol as the pre-exposure session. After the study session was completed, the subject was permitted to eat a meal and resume a normal diabetes medication schedule. For the next day session, subjects again reported to the EOHSI clinic at 7:00 AM and followed the same clinical protocol with the same physiologic measurements as the pre-exposure and post-exposure sessions.
HRV Analysis
By using a two-channel, five-lead, Holter monitor (Trillium 3000; Forest Medical, East Syracuse, NY), we recorded ECG during the pre-exposure, post-exposure, and next day sessions. The subject sat comfortably in a chair for 12 minutes during ECG recording. For HRV analysis, the best quality (minimum artifact) continuous 5-minute period was selected from the last 7 minutes of the 12-minute period. We processed the digital ECG signal, sampled at 256 Hz, and calculated the HRV parameters using PC-based software (Trillium Gold for MS Windows; Forest Medical). The software automatically detected heart beats and labeled ectopic beats and artifacts. An experienced technician then visually reviewed the ECG tracing to correct mislabeled beats or artifacts. We included all normal to normal beat intervals in the 5-minute recording in computations of the standard deviation of normal to normal intervals (SDNN), square root of the mean of the squared differences between adjacent NN intervals (r-MSSD), high-frequency power (HF; 0.15 to 0.4 Hz), low-frequency power (LF; 0.04 to 0.15 Hz), and LF:HF ratio.
Exposure Measurements
During the car ride, we measured pollutant concentrations, temperature, and humidity. The instrument sampling inlets were positioned in the back passenger seat area, near the subject’s breathing zone. Mass concentrations of PM with median cut point of 2.5 μ m (PM2.5) were measured continuously at 1-minute intervals using a TSI SidePak model AM510 aerosol monitor (TSI, Inc, Shoreview, MN) with the calibration factor set at 0.32 (based on collocated gravimetric analysis of ambient PM in Piscataway). As a proxy for UFP, number concentrations of particles with aerodynamic diameter ≥0.01 to 1.0 μm were measured at 1-minute intervals using a condensation particle counter TSI model 3007 (TSI, Inc). (Strictly speaking, we measured PM1.0 that includes UFP and particles with sizes between 0.1 and 1 μm. Nevertheless, the contribution of particles in the size range of 0.1 to 1 μm is negligible.) Integrated air samples for nitrogen dioxide were collected on triethanolamine-coated Sep-Pak cartridges (Waters, Corp, Millford, MA) with an SKC model 224-XR air pump (Eighty Four, PA) calibrated daily. The cartridges were extracted with a ratio of 6:1 water:ethanol and analyzed by high pressure liquid chromatography with an ultraviolet detector.38 CO concentration and air temperature were measured continuously with a Langan T15v monitor (Langan, Inc, San Francisco, CA). Relative humidity was monitored continuously using a HOBO 8 Pro Series monitor (Onset, Bourne, MA).
Statistical Analysis
After log transforming each HRV measure (SDNN, r-MSSD, HF, and LF), heart rate, SBP, and DBP, we calculated the geometric mean and 95% CI for each metric at each visit (pre-exposure, post-exposure, and next day). For each subject, we calculated the change in each metric from pre-exposure to post-exposure and again from pre-exposure to next day. Next, we calculated means and 95% CI for these differences and then exponentiated each to estimate the relative change in geometric means. We then used a signed rank test to test whether each change (pre-exposure to post-exposure for all subjects or pre-exposure to next day for all subjects) was statistically different from zero.
To determine if any change in each metric was associated with in-vehicle air pollution concentrations, we regressed the UFP number concentration against the change in each metric (eg, pre-exposure to next day) using robust regression and Huber weights39 to mitigate the influence of outliers for this small sample. In this method, residuals greater than a certain size are assigned weights proportional to the inverse of the size of the residual. We then scaled this percent change in each metric (eg, HF) to the interquartile range increase in UFP number concentration observed during the study and divided this by the mean pre-exposure level of the corresponding metric across all subjects. We then repeated this for each pollutant (PM2.5, NO2, and CO). Next, to control for potential confounding by temperature, we included a linear term for in-vehicle mean temperature and ambient mean temperature, as measured at the nearby Liberty Airport, Newark, NJ, monitoring station from 8:00 AM through 11:59 AM, during the car ride and reran our robust regression analysis.
Finally, we examined sensitivity of these analyses to confounding by perceived stress by excluding those subjects who scored a 4 or 5 (highest two levels of “stress”) on either of the antonym pairs for “stress” at any of the four stress questionnaire time points (pre-exposure, in-vehicle, post-exposure, and next day) and then repeating our analyses. Similarly, we excluded subjects who reported an “anxiety level” value of one or two (highest two levels of “anxiety”) to control for confounding by perceived anxiety and repeated our analyses. In stratified analyses, we also evaluated effect modification of these associations by statins, β-blockers, and angiotensin converting enzyme (ACE) inhibitors. All analyses were performed using SAS v.9.1® (SAS, Inc, Cary, NC).
RESULTS
Twenty of the 21 subjects completed the pre-exposure, post-exposure, and next day sessions and had analyzable Holter monitor data. The median age of these 20 subjects was 61 years (range, 46 to 70 years), and 60% were men. Two subjects (10%) had a history of myocardial infarction, and 12 (60%) were hypertensive. All subjects were overweight (body mass index ≥25), and 15 (75%) were obese (body mass index ≥30). The participants reported taking a variety of medications including insulin, oral hypoglycemic agents, and a number of antihypertensive medications including ACE inhibitors, β-blockers, calcium channel blockers, and diuretics (Table 1). Thirteen (65%) of the subjects were taking HMG-CoA reductase inhibitors (statins).
TABLE 1.
Characteristics of Study Panel (n = 20 Subjects With HRV Measurements)
| Characteristic | N | % |
|---|---|---|
| Gender | ||
| Male | 12 | 60 |
| Female | 8 | 40 |
| Age (yr) | ||
| <50 | 3 | 15 |
| 50–59 | 6 | 30 |
| 60–69 | 9 | 45 |
| ≥70 | 2 | 10 |
| Race/ethnicity | ||
| White non-Hispanic | 13 | 65 |
| Black non-Hispanic | 6 | 30 |
| Hispanic | 1 | 5 |
| Education | ||
| <12 (<HS education) | 0 | 0 |
| 12 (HS education) | 5 | 25 |
| ≥12 (some college or more) | 15 | 75 |
| Smoking status | ||
| Current | 0 | 0 |
| Past | 10 | 50 |
| Never | 10 | 50 |
| Comorbidity | ||
| Past myocardial infarction | 2 | 10 |
| Hypertension | 12 | 60 |
| Body mass index (kg/m2) | ||
| 25–30 | 5 | 25 |
| >30 | 15 | 75 |
| Duration of diabetes (yrs since diagnosis) | ||
| 0–9 | 11 | 55 |
| 10–19 | 5 | 25 |
| >20 | 4 | 20 |
| Employment status | ||
| Employed | 9 | 45 |
| Unemployed | 2 | 10 |
| Retired | 9 | 45 |
| Prescribed diabetic medications (self-reported) | ||
| Exenatide (Byetta®) | 2 | 10 |
| Glyburide/metformin | 4 | 20 |
| Insulin | 9 | 45 |
| Metformin | 10 | 50 |
| Pioglitazone (Actos®) | 1 | 5 |
| Replaglinide (Prandin®) | 1 | 5 |
| Rosiglitazone (Avandia®) | 1 | 5 |
| Sitagliptin metformin (Janumet®) | 2 | 10 |
| Sitagliptin phosphate (Januvia®) | 2 | 10 |
| Other prescribed medications/drug classes (self-reported) | ||
| ACE inhibitors | 9 | 45 |
| Alpha-adrenergic blocker | 2 | 10 |
| Angiotensin II receptor antagonist | 6 | 30 |
| Aspirin | 8 | 40 |
| β-blockers | 5 | 25 |
| Calcium channel blockers | 5 | 25 |
| Cholesterol lowering agent | 3 | 15 |
| Digoxin | 2 | 10 |
| Diuretic | 5 | 25 |
| Nitrates | 1 | 5 |
| Statin | 13 | 65 |
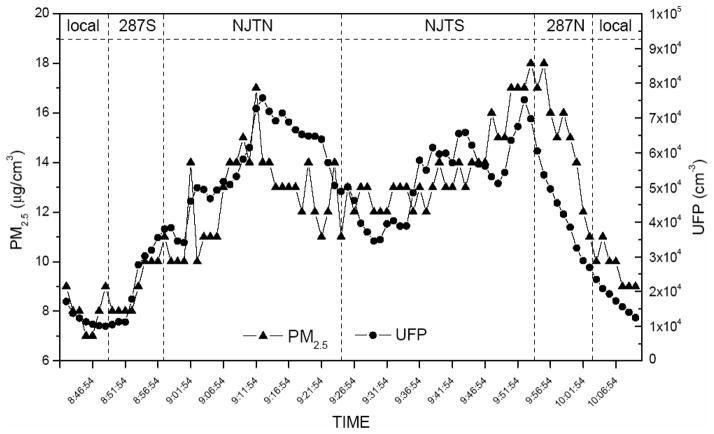
The UFP number and PM2.5 mass concentrations during an example car ride are shown in Fig. 1. By comparing the NJTPK segments with local road segments, the mean UFP number concentration for all rides increased fivefold, whereas PM2.5 mass concentration and CO increased by ~30% (data not shown). The distribution of in-vehicle mean pollutant concentrations and physical measurements (temperature and humidity levels) averaged over the car rides are shown in Table 2. UFP number concentration was not highly correlated with any of the other measured pollutants, PM2.5, NO2, and CO (Table 3).
FIGURE 1.
PM2.5 mass and UFP number concentrations during a single car ride session.
TABLE 2.
Number and Distribution of In-Vehicle Mean Pollutant Concentrations and Temperature and Relative Humidity Levels
| Pollutant/Weather | N | Minimum | 25th Percentile | 50th Percentile | 75th Percentile | Maximum | Interquartile Range |
|---|---|---|---|---|---|---|---|
| PM2.5 (μ g/m3) | 20 | 7.7 | 10.7 | 12.3 | 20.2 | 40.6 | 9.5 |
| UFP (particles/cm3) | 19 | 18,929 | 25,925 | 43,099 | 67,976 | 140,429 | 42,051 |
| NO2* (ppb) | 20 | 3.0 | 17.7 | 25.9 | 32.8 | 61.1 | 15.1 |
| CO (ppm) | 20 | 0.90 | 1.40 | 1.85 | 2.35 | 4.30 | 0.95 |
| In-vehicle temperature (°C) | 20 | 14.5 | 20.8 | 22.2 | 24.9 | 29.2 | 4.1 |
| RH (%) | 19 | 12.2 | 27.8 | 37.4 | 40.7 | 52.8 | 12.9 |
| Ambient temperature (°C) | 18 | 2.2 | 13.3 | 20.8 | 25.6 | 29.8 | 12.2 |
All pollutant and weather characteristics (except NO2) were measured continuously and then averaged over the entire car ride.
NO2 concentration was measured for the whole car ride only.
RH, relative humidity.
TABLE 3.
Pearson Correlation Coefficients for In-Vehicle Mean Pollutant Concentrations, Temperature and Relative Humidity, and Ambient Temperature
| PM2.5 | UFP | CO | NO2 | In-Vehicle Temperature | RH | Ambient Temperature | |
|---|---|---|---|---|---|---|---|
| PM2.5 | — | 0.07 | −0.09 | 0.66 | 0.04 | 0.01 | 0.22 |
| UFP | 0.07 | — | 0.15 | 0.20 | −0.53 | 0.06 | −0.77 |
| CO | −0.09 | 0.15 | — | 0.04 | −0.21 | 0.32 | 0.09 |
| NO2* | 0.66 | 0.20 | 0.04 | — | −0.09 | 0.02 | −0.01 |
| In-vehicle temperature | 0.04 | −0.53 | −0.21 | −0.09 | — | −0.44 | 0.14 |
| RH (%) | 0.01 | 0.06 | 0.32 | 0.02 | −0.44 | — | 0.27 |
| Ambient temperature | 0.22 | −0.77 | 0.09 | −0.01 | 0.14 | 0.27 | — |
All pollutant and weather characteristics (except NO2) were measured continuously and then averaged over the entire car ride.
NO2 concentration was measured for the whole car ride only.
RH, relative humidity.
We observed large decreases in r-MSSD and HF from pre-exposure to the next day (pre-:post-exposure ratios 0.81, 95% CI = 0.68 to 0.96 and 0.66, 95% CI = 0.47 to 0.93), and smaller decreases from pre- to post-exposure (Table 4). We did not observe changes in SDNN, heart rate, SBP, DBP, or blood glucose at either the post-exposure or the next day time point (Table 4). The LF/HF ratio increased from pre- to post-exposure (ratio 1.92, 95% CI = 01.21 to 3.05), likely reflecting the observed increase in LF (1.64, 95% CI = 1.05 to 2.56). Because we found the largest changes in pre-exposure to next day HF and r-MSSD, and pre- to post-exposure LF:HF ratio only, further analyses were restricted to these endpoints.
TABLE 4.
HRV, Heart Rate, Blood Pressure, and Glucose Levels at Each Session, With Ratios of Pre-Exposure, Post-Exposure, and Next Day GM’S and P-Values From Signed Rank Tests of Changes From Pre-Exposure
| Endpoint | Pre-Exposure GM (95% CI) | Post-Exposure GM (95% CI) | Next Day GM (95% CI) | Ratioa of Post-Exposure GM to Pre-Exposure GM (95% CI) P-Value* | Ratiob of Next Day GM to Pre-Exposure GM (95% CI) P-Value** |
|---|---|---|---|---|---|
| SDNN (ms) | 37 (29–46) | 39 (32–47) | 33 (27–40) | 1.05 (0.85–1.31) 0.74 | 0.90 (0.73–1.11) 0.26 |
| r-MSSD (ms) | 30 (21–42) | 28 (21–36) | 24 (18–33) | 0.93 (0.78–1.11) 0.83 | 0.81 (0.68–0.96) 0.05 |
| Low frequency (ms2) | 79 (51–121) | 129 (92–180) | 86 (54–136) | 1.64 (1.05–2.56) 0.06 | 1.09 (0.68–1.76) 0.90 |
| High frequency (ms2) | 138 (73–261) | 118 (70–198) | 91 (50–166) | 0.85 (0.59–1.23) 0.45 | 0.66 (0.47–0.93) 0.02 |
| Ratio of low frequency to high frequency | 0.57 (0.35–0.92) | 1.10 (0.77–1.56) | 0.95 (0.61–1.48) | 1.92 (1.21–3.05) 0.02 | 0.86 (0.60–1.24) 0.62 |
| Heart rate (beats/min) | 68 (63–73) | 67 (62–73) | 70 (66–75) | 0.99 (0.96–1.02) 0.73 | 1.03 (1.00–1.07) 0.11 |
| SBP (mm Hg) | 132 (125–138) | 133 (126–142) | 133 (125–142) | 1.01 (0.99–1.04) 0.57 | 1.01 (0.98–1.04) 0.24 |
| DBP (mm Hg) | 78 (74–82) | 79 (75–83) | 77 (73–81) | 1.02 (0.99–1.04) 0.23 | 0.99 (0.96–1.02) 0.56 |
| Blood glucose (mg/dL) | 142 (123–163) | 137 (97–195) | 147 (95–230) | 0.97 (0.90–1.04) 0.43 | 1.04 (0.91–1.19) 0.89 |
P-value for signed rank test comparing pre-exposure with post-exposure.
P-value for signed rank test comparing pre-exposure with next day exposure.
A ratio >1 indicates that the post-exposure measure was higher than the pre-exposure measure, and a ratio <1 indicates that the post-exposure measure was lower than the pre-exposure measure.
A ratio >1 indicates that the next day measure was higher than the pre-exposure measure, and a ratio <1 indicates that the next day measure was lower than the pre-exposure measure.
GM, geometric mean.
Elimination of stressed and anxious subjects (n = 3 and n = 2, respectively) had little effect on either the magnitude of next day decrease in r-MSSD and HF or the increase in LF/HF ratio (Table 5). There was no clear effect modification of these associations by statin, β-blocker, or ACE inhibitor use (Table 5).
TABLE 5.
Sensitivity Analyses of Heart Rate Variability at Each Session With Ratios of Pre-Exposure, Post-Exposure and Next Day GM’S, and P-Values for Changes From Pre-Exposure to Post-Exposure or Pre-Exposure to Next Day
| Endpoint | Pre-Exposure GM (95% CI) | Post-Exposure GM (95% CI) | Next Day GM (95% CI) | Ratioa of Post-Exposure GM to Pre-Exposure GM (95% CI) P* | Ratiob of Next Day GM to Pre-Exposure GM (95% CI) P** |
|---|---|---|---|---|---|
| Removing three subjects with stress (n = 17) | |||||
| r-MSSD (ms) | 31 (22–45) | 28 (22–36) | 24 (17–34) | 0.90 (0.74–1.10) 0.67 | 0.76 (0.64–0.91) 0.03 |
| High frequency (ms2) | 150 (79–285) | 125 (81–195) | 87 (44–172) | 0.83 (0.55–1.26) 0.46 | 0.58 (0.42–0.81) 0.01 |
| Ratio of low frequency to high frequency | 0.60 (0.38–0.95) | 1.09 (0.76–1.57) | 0.93 (0.61–1.40) | 1.82 (1.09–3.06) 0.06 | 0.85 (0.57–1.26) 0.64 |
| Removing two subjects with anxiety (n = 18) | |||||
| r-MSSD (ms) | 30 (20–43) | 28 (21–37) | 25 (18–35) | 0.93 (0.76–1.13) 0.97 | 0.84 (0.69–1.01) 0.15 |
| High frequency (ms2) | 135 (66–274) | 114 (64–204) | 95 (49–186) | 0.85 (0.57–1.26) 0.50 | 0.71 (0.49–1.02) 0.07 |
| Ratio of low frequency to high frequency | 0.58 (0.35–0.97) | 1.16 (0.79–1.69) | 0.95 (0.58–1.56) | 1.98 (1.24–3.16) 0.02 | 0.82 (0.56–1.21) 0.52 |
| Statin users only (n = 13) | |||||
| r-MSSD (ms) | 32 (22–46) | 30 (22–39) | 25 (17–37) | 0.93 (0.73–1.17) 0.91 | 0.77 (0.64–0.93) 0.06 |
| High frequency (ms2) | 153 (75–315) | 135 (83–218) | 89 (40–202) | 0.88 (0.55–1.40) 0.74 | 0.58 (0.41–0.83) 0.02 |
| Ratio of low frequency to high frequency | 0.55 (0.32–096) | 0.97 (0.66–1.43) | 0.70 (0.42–1.16) | 1.76 (0.97–3.18) 0.13 | 0.72 (0.44–1.18) 0.34 |
| Nonstatin users only (n = 7) | |||||
| r-MSSD (ms) | 26 (13–53) | 24 (14–42) | 23 (14–37) | 0.93 (0.70–1.23) 0.81 | 0.89 (0.61–1.29) 0.47 |
| High frequency (ms2) | 113 (30–418) | 91 (26–316) | 94 (37–236) | 0.81 (0.44–1.50) 0.69 | 0.83 (0.39–1.75) 0.47 |
| Ratio of low frequency to high frequency | 0.61 (0.23–1.57) | 1.37 (0.67–2.81) | 1.66 (0.80–3.43) | 2.27 (1.06–4.87) 0.16 | 1.21 (0.83–1.78) 0.47 |
| Excluding five subjects prescribed β-blockers | |||||
| r-MSSD (ms) | 28 (20–40) | 26 (19–35) | 24 (17–34) | 0.93 (0.75–1.14) 0.71 | 0.86 (0.71–1.04) 0.19 |
| High frequency (ms2) | 122 (59–252) | 102 (56–187) | 94 (48–187) | 0.84 (0.53–1.32) 0.49 | 0.77 (0.52–1.14) 0.14 |
| Ratio of low frequency to high frequency | 0.60 (0.34–1.08) | 1.21 (0.85–1.72) | 1.04 (0.60–1.81) | 2.01 (1.21–3.33) 0.04 | 0.86 (0.58–1.28) 0.60 |
| ACE inhibitor users (n = 9) | |||||
| r-MSSD (ms) | 32 (20–50) | 28 (20–39) | 27 (16–45) | 0.87 (0.64–1.19) 0.84 | 0.84 (0.67–1.04) 0.25 |
| High frequency (ms2) | 169 (75–384) | 116 (62–216) | 109 (41–289) | 0.68 (0.40–1.16) 0.36 | 0.64 (0.42–0.99) 0.16 |
| Ratio of low frequency to high frequency | 0.46 (0.25–0.86) | 1.15 (0.64–2.07) | 0.76 (0.41–1.42) | 2.49 (1.07–5.79) 0.10 | 0.66 (0.35–1.27) 0.30 |
| Non-ACE users (n = 11) | |||||
| r-MSSD (ms) | 28 (17–46) | 27 (18–41) | 22 (16–32) | 0.97 (0.80–1.19) 0.97 | 0.79 (0.60–1.03) 0.15 |
| High frequency (ms2) | 116 (44–308) | 119 (52–272) | 78 (64–96) | 1.02 (0.63–1.67) 0.90 | 0.67 (0.39–1.15) 0.15 |
| Ratio of low frequency to high frequency | 0.68 (0.33–1.38) | 1.05 (0.67–1.65) | 1.13 (0.60–2.13) | 1.55 (0.96–2.51) 0.24 | 1.07 (0.75–1.53) 0.41 |
P-value for Signed Rank test comparing pre-exposure to post-exposure.
P-value for Signed Rank test comparing pre-exposure to next day exposure.
A ratio >1 indicates that the post-exposure measure was higher than the pre-exposure measure, and a ratio <1 indicates that the post-exposure measure was lower than the pre-exposure measure.
A ratio >1 indicates that the next day measure was higher than the pre-exposure measure, and a ratio <1 indicates that the next day measure was lower than the pre-exposure measure.
GM, geometric mean.
Although none of the associations between individual pollutants and HRV changes were statistically significant, inter-quartile range increases in measured pollutants were associated with decreases in HF at next day and LF:HF ratio at post-exposure (Table 6).
TABLE 6.
Changes in HF From Pre-Exposure to Next Day, and Changes in LF/H Ratio From Pre-Exposure to Post-Exposure Associated With Interquartile Range Increases in In-Vehicle Air Pollutants
| Pollutant | Interquartile Range | % Change in HF From Pre-Exposure to Next Day (95% CI) | % Change in LFHR Ratio From Pre-Exposure to Post-Exposure (95% CI) |
|---|---|---|---|
| UFP (particle/cc) | 42,051 | −25 (−147 to 96) | −26 (−275 to 222) |
| PM2.5 (μ g/m3) | 9.5 | −20 (−87 to 47) | −97 (−225 to 31) |
| NO2 (ppb) | 15.1 | −9 (−79 to 61) | −81 (−225 to 63) |
| CO (ppm) | 0.95 | −1 (−44 to 42) | −5 (−94 to 84) |
DISCUSSION
In this study, we successfully piloted a novel approach to studying the effects of exposure to traffic in potentially susceptible individuals. In a group of volunteers with type 2 diabetes, we examined acute autonomic responses to traffic exposure that may underlie previously reported associations between traffic pollution and adverse CV outcomes. Immediately following the car ride, we observed an increase in LF:HF ratio, whereas 22 hours after the car ride we observed a decrease in HF HRV (HF, r-MSSD). These HRV changes were independent of our perceived stress and anxiety measures, and there was little difference in responses among those subjects taking and not taking statins, β-blockers, and ACE inhibitors. Although this pilot study was underpowered to evaluate associations between individual pollutants and the outcomes, the decrease in HF HRV at Next-Day was associated with increasing in-vehicle UFP number concentration, with similar, but smaller effects for PM2.5, NO2, and CO.
Our finding of next day decreases in HF HRV is consistent with PM-associated decreases in HF HRV observed in a number of other studies. Gold et al30 found inverse associations between ambient PM2.5 levels and SDNN and rMSSD (a metric highly correlated with HF) in a panel of elderly subjects. Park et al6 found that decreases in HF and increases in LF/HF were associated with increasing 48 hours average ambient PM2.5 in an elderly cohort. In contrast, another study of HRV responses to in-vehicle pollution exposure found increases in HF associated with increased PM2.5 exposure among state troopers.7 In addition to differences in PM characteristics between the studies, these disparate results may be because of different responses among young physically fit subjects compared with elderly subjects, with most having several comorbidities.
Our findings are somewhat consistent with results of a previous study among elderly adults during 24-hour periods that included two trips aboard a diesel-powered bus (and control days without the bus trip).2 In this study, fine PM and black carbon, an indicator of traffic particles, were negatively associated with several HRV parameters including HF and positively associated with LF/HF. Statistically significant decreases in HF were associated with 5-minute, 30-minute, 1-hour, 4-hour, and 24-hour averages of PM2.5. Our observed decrease in mean HF at the next day session is consistent with a similar effect from air pollution, although the associations with in-vehicle air pollutant measurements were not statistically significant in our small pilot study. In contrast to Adar et al,2 we found large negative associations between LF/HF and increases in several measured traffic-related air pollutants measured in the vehicle (UFP number concentration, PM2.5 mass concentration, and NO2). In addition to the difference in proportion of people with diabetes in our study compared with Adar et al2 (100% vs 18%), there were other differences in age (median age 61 vs 80 years), obesity (75% vs 34%), and other comorbidities such as hypertension (60% vs 82%) that may account for some of the differences in observed responses.
Compared with NO2 and CO, UFP was associated with a larger decrease in HF at next day (Table 6), but none of the associations were statistically significant. This is somewhat consistent with suggestions that UFP may be the best proxy of traffic-related air pollution mixture in predicting cardiopulmonary health effects.38 The lack of statistically significant associations between the HRV changes and measured concentrations of traffic-related air pollutants during the car rides was not unexpected because of the small size of this pilot study (n = 20). Our finding of an increase in the LF:HF ratio from pre-exposure to post-exposure is inconsistent with our finding of a decrease in the LF:HF ratio associated with interquartile range increases in in-vehicle pollutant concentrations (Table 6). It is not clear why these two analyses are inconsistent, but may be partly because of residual confounding. The pre-exposure to post-exposure LF:HF ratio results may be confounded by acute stress and circadian rhythm, whereas the pre-exposure to next day analyses may not be (discussed below).
The low correlation between UFP number concentration and other measured pollutants makes confounding by the other pollutants less likely. The low correlation between UFP number concentration and PM2.5 is not surprising, because PM2.5 in New Jersey is strongly influenced by long-range transport. In addition, we expect a large difference in (outdoor to in-vehicle) penetration fractions between UFP and PM2.5. The low correlation between UFP number concentration and CO might be explained by different ride-to-ride contributions to in-vehicle pollutant levels from diesel engine and gasoline engine vehicles, from which relative emissions of UFP and CO differ substantially. Also, the relative emissions of UFP and CO from the same vehicle vary with speed and other conditions.40 The low correlation between UFP and NO2 may be explained by the fact that the majority of NOx emitted near the tailpipe is nitric oxide, whereas NO2 is influenced by more distant sources.
A limitation of the quasi-experimental study design used in this pilot study was that we did not have a control exposure. Thus, we were unable to control for several factors that might have confounded associations between the car ride and/or PM exposure and changes in HRV, especially changes in HRV across the pre-exposure to post-exposure time period. These factors included circadian rhythm, psychological or physical stress not captured by our SSR questionnaire, and the experimental procedures’ effects on the subjects, including fasting and temporary withholding of diabetes medications. HRV has a well-established circadian rhythm that includes a decreasing HF component from waking to the late morning hours,41,42 which may have contributed to the decrease in HF and significant increase in LF:HF ratio from pre-exposure to post-exposure (about 8:00 AM to 11:00 AM). Nevertheless, circadian rhythm does not explain the next day decrease in HF HRV, since we measured HRV at the same time of day at the pre-exposure and next day sessions.
Psychological or physical stress during the car ride may have contributed to the increase in the LF factor in the LF:HF ratio at the post-exposure session. In contrast to HF HRV, LF is affected by sympathetic and parasympathetic tone and may be increased by stress-related increases in sympathetic tone.43 However, stress seems an unlikely explanation for the changes observed for two reasons. First, the participants were seated as passengers in the back seat of the vehicle and, thus, were not subjected to the stress of driving. Second, we obtained similar results when we excluded subjects that reported higher stress or anxiety levels on the stress questionnaire at any of the four time points (pre-exposure, in-vehicle, post-exposure, and or next day). However, we cannot rule out some confounding by more subtle stress that was not measured by the questionnaire. The increase in LF:HF ratio at the post-exposure session did not appear to be because of exposure to the measured air pollutants, because regression analysis showed large, but not statistically significant, inverse associations between pollutant levels and the LF:HF ratio.
Hyperglycemia has been associated with decreased HRV,44 but there were no substantial differences in mean blood glucose concentration across the three data collection sessions (pre-exposure, post-exposure, and next day). Changes in diabetes medications and other experimental procedures are unlikely to explain the pre-exposure to next day decrease in HF HRV, because subjects underwent the same protocol for the pre-exposure and next day sessions. Although unlikely, it remains possible that some additive effect of the second day of repeated fasting or diabetes medication withholding or both may have contributed to the next day decrease in HF HRV.
The subjects in this pilot study were a small group of people with diabetes who were largely overweight, hypertensive, older, white, and male, which may limit the generalizability of study findings. However, people with diabetes, who represent a growing segment of the US general population, often have multiple CV risk factors. Almost two thirds of our subjects were taking statins. Although a previous observational study reported larger reductions in HF HRV among nonstatin users than among statin users,8 we found larger reductions among those taking statins. Nevertheless, our study was not sufficiently powered to address effect modification by and medication use, and thus a larger confirmatory study is needed to properly examine this.
CONCLUSIONS
We successfully piloted a novel protocol for examining CV effects of traffic pollutant exposure during automobile commuting in susceptible individuals. Consistent with our hypothesis, adult volunteers with type 2 diabetes demonstrated changes in HRV immediately after and 24 hours after a passenger car ride on a major highway with heavy truck traffic. Although not statistically significant, associations between measured air pollutants and decreased HF HRV were in the hypothesized direction, suggesting that the change in HRV may be partly because of in-vehicle pollutant exposure. Changes in HRV while commuting may have public health importance, considering the number of people who commute to and from work. This pilot study provides a framework for future studies to determine which commuting-related factors are responsible for these associations.
Acknowledgments
The authors thank the contribution of Eric Lauer, MPH, for his contributions to the statistical analysis of the results.
This study was funded by NIEHS Center ES005022, K08 ES135202, and USEPA STAR R832144.
Footnotes
The authors declare that they have no competing financial or nonfinancial interests.
References
- 1.Brook RD, Franklin B, Cascio W, et al. Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 2.Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18:95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- 3.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Particulate air pollution and nonfatal cardiac events. Part II. Association of air pollution with confirmed arrhythmias recorded by implanted defibrillators. Res Rep Health Eff Inst. 2005:83–126. discussion 127–148. [PubMed] [Google Scholar]
- 4.Gold DR, Litonjua AA, Zanobetti A, et al. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect. 2005;113:883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen KL, Larson TV, Koenig JQ, et al. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environ Health Perspect. 2005;113:1741–1746. doi: 10.1289/ehp.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riediker M, Cascio WE, Griggs TR, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169:934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz J, Park SK, O’Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roselund M, Bellander T, Nordquist T, Alfredsson L. Traffic-generated air pollution and myocardial infarction. Epidemiology. 2009;20:265–271. doi: 10.1097/EDE.0b013e318190ea68. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg MS, Burnett RT, Bailar JC, III, et al. The association between daily mortality and ambient air particle pollution in Montreal, Quebec 2 Cause-specific mortality. Environ Res. 2001;86:26–36. doi: 10.1006/enrs.2001.4243. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Ruddy TD, Dalipaj M, et al. Influence of personal exposure to particulate air pollution on cardiovascular physiology and biomarkers of inflammation and oxidative stress in subjects with diabetes. J Occup Environ Med. 2007;49:258–265. doi: 10.1097/JOM.0b013e31803220ef. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 13.Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13:588–592. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Kuhn T, Mayo P, Hinds WC. Comparison of daytime and nighttime concentration profiles and size distributions of ultrafine particles near a major highway. Environ Sci Technol. 2006;40:2531–2536. doi: 10.1021/es0516514. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Eiguren-Fernandez A, Hinds WC, Miguel AH. In-cabin commuter exposure to ultrafine particles on Los Angeles freeways. Environ Sci Technol. 2007;41:2138–2145. doi: 10.1021/es0618797. [DOI] [PubMed] [Google Scholar]
- 16.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 17.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 19.Peters A, von Klot S, Heier M, et al. Particulate air pollution and nonfatal cardiac events. Part I. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study. Res Rep Health Eff Inst. 2005:1–66. discussion 67–82, 141–148. [PubMed] [Google Scholar]
- 20.Rich DQ, Mittleman MA, Link MS, et al. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114:120–123. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 22.Rich DQ, Schwartz J, Mittleman MA, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- 23.Rich DQ, Kim MH, Turner JR, et al. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occup Environ Med. 2006;63:591–596. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am J Cardiol. 2006;97:404–408. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 25.Urch B, Silverman F, Corey P, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 27.Auchinclos AH, Diez Roux AV, Dvonch JT, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Perz S, Doring A, Stieber J, Koenig W, Wichmann HE. Increases in heart rate during an air pollution episode. Am J Epidemiol. 1999;150:1094–1098. doi: 10.1093/oxfordjournals.aje.a009934. [DOI] [PubMed] [Google Scholar]
- 29.Pope CA, III, Dockery DW, Kanner RE, Villegas GM, Schwartz J. Oxygen saturation, pulse rate, and particulate air pollution: a daily time-series panel study. Am J Respir Crit Care Med. 1999;159:365–372. doi: 10.1164/ajrccm.159.2.9702103. [DOI] [PubMed] [Google Scholar]
- 30.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 31.Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation. 2001;104:986–991. doi: 10.1161/hc3401.095038. [DOI] [PubMed] [Google Scholar]
- 32.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 33.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 34.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Schneider A, Neas L, Herbst MC, et al. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. 2008;116:1666–1674. doi: 10.1289/ehp.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peretz A, Sullivan JH, Leotta DF, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–942. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naliboff BD, Benton D, Solomon GF, et al. Immunological changes in young and old adults during brief laboratory stress. Psychosom Med. 1991;53:121–132. doi: 10.1097/00006842-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 38.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 39.Huber PJ. Robust regression: asymptotics, conjectures and Monte Carlo. Ann Stat. 1973;1:799–821. [Google Scholar]
- 40.Pekkanen J, Kulmala M. Exposure assessment of ultrafine particles in epidemiologic time-series studies. Scand J Work Environ Health. 2004;30(suppl 2):9–18. [PubMed] [Google Scholar]
- 41.Aronson D, Weinrauch LA, D’Elia JA, Tofler GH, Burger AJ. Circadian patterns of heart rate variability, fibrinolytic activity, and hemostatic factors in type I diabetes mellitus with cardiac autonomic neuropathy. Am J Cardiol. 1999;84:449–453. doi: 10.1016/s0002-9149(99)00331-8. [DOI] [PubMed] [Google Scholar]
- 42.Burger AJ, Charlamb M, Sherman HB. Circadian patterns of heart rate variability in normals, chronic stable angina and diabetes mellitus. Int J Cardiol. 1999;71:41–48. doi: 10.1016/s0167-5273(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 43.Cohen H, Benjamin J. Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Auton Neurosci. 2006;128:1–8. doi: 10.1016/j.autneu.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Burger AJ, Weinrauch LA, D’Elia JA, Aronson D. Effect of glycemic control on heart rate variability in type I diabetic patients with cardiac autonomic neuropathy. Am J Cardiol. 1999;84:687–691. doi: 10.1016/s0002-9149(99)00417-8. [DOI] [PubMed] [Google Scholar]