Summary
Endothelial progenitor cells (EPCs), which can be cultured in vitro from mononuclear cells in peripheral blood or bone marrow, express both hematopoietic stem cell and endothelial cell markers on their surface. They are believed to participate in endothelial repair and postnatal angiogenesis due to their abilities of differentiating into endothelial cells and secreting protective cytokines and growth factors. Mounting evidence suggests that circulating EPCs are reduced and dysfunctional in various diseases including hypertension, diabetes, coronary heart disease, and ischemic stroke. Therefore, EPCs have been documented to be a potential biomarker for vascular diseases and a hopeful candidate for regenerative medicine. Ischemic stroke, as the major cause of disability and death, still has limited therapeutics based on the approaches of vascular recanalization or neuronal protection. Emerging evidence indicates that transplantation of EPCs is beneficial for the recovery of ischemic cerebral injury. EPC‐based therapy could open a new avenue for ischemic cerebrovascular disease. Currently, clinical trials for evaluating EPC transfusion in treating ischemic stroke are underway. In this review, we summarize the general conceptions and the characteristics of EPCs, and highlight the recent research developments on EPCs. More importantly, the rationale, perspectives, and strategies for using them to treat ischemic stroke will be discussed.
Keywords: Angiogenesis, Endothelial progenitor cells, Ischemia stroke, Stem cell therapy, Transplantation
Introduction
Stroke is the fourth leading cause of death in the United States. According to the updated statistics reported by American Heart Association, there are about 795,000 new and recurrent stroke patients and 134,100 deaths each year in the United States 1. The burden of stroke is even higher in China, Africa, and South America 2. Ischemic stroke accounts for about 85% of all stroke events. Thrombogenesis and embolism in the intracranial artery are the two major causes of ischemic stroke. Earlier recanalization with following reperfusion constructs the foundation for conserving brain tissue under acute ischemia. Current recanalization therapies for acute ischemic stroke mainly include intravenous or intra‐arterial fibrinolysis 3, 4 and interventional treatments, such as percutaneous transluminal angioplasty and stenting (PTAS) and thrombectomy 5, 6. Although the fibrinolytics and interventional managements have achieved certain benefits, these therapies have several limitations. Intravenous thrombolysis with recombinant tissue‐type plasminogen activator (rt‐PA) or alteplase has a narrow therapeutic time window (3–4.5 h) 3, 7. The interventional PTAS has a high rate (20.0%) of re‐stroke within the first year 5. On the other hand, antiplatelets are also commonly used for treating ischemic stroke.
Although numerous animal studies on neuroprotective drugs have shown promising data in treating ischemic stroke, clinical trials testing these drugs revealed disappointing results 8. Current treatments for acute ischemic stroke mainly rely on vascular recanalization. However, approaches for promoting cerebral recovery following ischemic stroke are limited. Emerging studies document the beneficial role of different stem/progenitor cells in accelerating cerebral recovery after ischemic stroke, such as bone marrow (BM) stem cells 9, mesenchymal stem cells (MSCs) 10, neural stem cells 11, and endothelial progenitor cells (EPCs) 12. EPCs probably have a great potential in cerebrovascular disease because of their unique characteristics 13, 14, 15. This article reviews the general conceptions and recent research progress of EPCs. Furthermore, the rationale, perspectives, and strategies of EPC‐based therapy for ischemic stroke will be discussed.
Definition, Identification, and Characterization of EPCs
Endothelial progenitor cells were first isolated from human peripheral blood in 1997 and defined as BM‐derived immature cells with the ability to differentiate into mature endothelial cells (ECs) 16, 17. They are believed to originate from hematopoietic lineage, whereas their nonhematopoietic lineage origin is still in debate 18. EPCs have been identified through several methods, such as colony formation assay in combination with specific biomarkers, fluorescence detection of acetylated low‐density lipoprotein uptake and lectin binding, as well as flow cytometry technique based on their surface markers 16, 19. The biomarkers used for characterizing EPCs include both hematopoietic stem cell markers (CD34 and CD133) and EC markers, such as CD31, kinase insert domain receptor (KDR, VEGFR2), Von Willebrand factor (vWF), vascular endothelial cadherin (VE‐cadherin or CD 144), Tie2, c‐kit/CD117, and CD62E (E‐selectin) 19, 20, 21, 22. In addition, CD45, CXCR4, CXCR2, and CCR2 are also expressed on EPCs 23. The CD34+KDR+ antigenic combination appears to be of high sensitivity and specificity and has been often used for EPC identification 20. It was noticed that EPCs from different sources express different surface markers. For example, both bone‐marrow derived EPCs (BM‐EPCs) and cord‐blood derived EPCs (CB‐EPCs) have been shown to express the CD105, CD73, and CD34 markers 24. The markers CD31, CD144, CD146, and KDR are positive on CB‐EPCs, but are negative or weak on BM‐EPCs. Another study showed that peripheral blood derived EPCs (PB‐EPCs) expressed KDR, CD144, vWF, Tie‐2, CD31, CD11b, and CD14 13. Nevertheless, for therapeutic and diagnostic purposes, more exact identification of EPCs might be desired.
Based on the culture characters, EPCs are mainly divided into two types: early EPCs and late EPCs 20, 21. Early EPCs appear after short‐term (4–10 days) culture of mononuclear cells (MNCs) from peripheral blood. They are similar to colony‐forming unit ECs (CFU‐ECs). Early EPCs are spindle shape and display peak growth at 2–3 weeks and live up to 4 weeks. The late EPC or endothelial colony forming cells (ECFCs) can be found after long‐term culture (>14 days) of MNCs. Late EPCs exhibit cobblestone shape, rapid growth at 4–8 weeks, and survive until 12 weeks. Studies suggest that EPCs promote angiogenesis and neovascularization by producing diverse growth factors which may mainly be secreted by early EPCs 21, 25, 26, 27. The late EPCs have a higher expression level of VE‐cadherin and KDR and are able to physically contribute to vascular regeneration 21, 28. Genome‐wide transcriptional profiling and protein electrophoresis methods reveal that these two types of EPCs have different gene expression signatures 29. Early EPCs display a molecular phenotype linked to monocytes, whereas late EPCs highly express vascular development and angiogenesis‐related signaling genes (Tie2, eNOS, Ephrins).
EPCs Generation, Mobilization, and Homing
Generally, EPCs are adult stem cells generated from BM 17. Most of EPCs quiescently lodge in a microenvironment within the BM, termed the stem cell niche 30. They can be mobilized into the circulation and are able to colonize in endothelium 31, 32. The mechanisms for this process have not been fully understood. The chemokine stromal‐derived factor 1 (SDF‐1)/CXCR4 axis has been well documented to play a key role in EPC mobilization in response to hypoxia or injury 33, 34. At basal conditions, the level of SDF‐1 is low in circulation, BM, and other tissues 31, 35. Upon tissue ischemia, hypoxia‐inducible factor‐1 (HIF‐1) is up‐regulated, which can activate its downstream factors, SDF‐1, and vascular endothelial growth factor (VEGF) 33, 36. Then, EPCs are mobilized from BM to circulation and migrate towards ischemic tissue following SDF‐1 gradients. VEGF also induces SDF‐1 expression which further promotes the process of EPC mobilization 37. On the other hand, matrix metalloproteinase‐9 (MMP‐9), which is up‐regulated by SDF‐1 and VEGF, partakes in the transformation of EPCs from quiescent to proliferative state in BM 38. MMP‐9 also promotes the mobilization of EPCs into the circulation by inducing the release of soluble Kit Ligand (sKitL), which can bind with the c‐Kit expressed on EPC for facilitating the mobilization 31. Granulocyte colony‐stimulating factor (G‐CSF) has been used to mobilize functional EPCs into the circulation of patients with coronary artery disease 39. G‐CSF induced EPC mobilization is associated with increased level of neutrophils in circulation, which could release VEGF 40. Another study showed that G‐CSF stimulates the mobilization of hematopoietic progenitor cells through BM‐neutrophils released elastase and cathepsin G, which trigger proteolytic cleavage of vascular cell adhesion molecule‐1 expressed by BM stromal cells 41. In addition, numerous physiopathological and pharmacological stimuli have been shown to mobilize EPCs (Table 1).
Table 1.
Factors affect the release, mobilization, and homing/recruitment of EPCs
| Mobilization and/or release | Homing/recruitment | |
|---|---|---|
| Chemokines/growth factors (GF) | Chemokines, GF and/or their receptor | |
| HIF‐1 33 SDF‐1 34 | ↑ | SDF‐1/CXCR4 34 |
| VEGF 51, IGF‐1 52 | ↑ | CCL5/CCR5 46 |
| G‐CSF 39 | ↑ | CXCL1 and CXCL7/CXCR2 45 |
| Angiopoietin‐2 53, PAR‐1 54 | ↑ | VEGF/VEGFR 81 |
| Drugs/protein/hormone | IL‐8/Gro CXCchemokines 82 | |
| Statin 55, ARB 56 | ↑ | IGF2/IGF2R 83 |
| ACEI 57 | ↑ | Other factors |
| Estrogen 58, EPO 59 | ↑ | Caspase‐8 84 |
| Phytoestrogen 60 | ↑ | Hyaluronic acid and thrombin 85 |
| Berberine 61 | ↑ | CD9 86 |
| Heme oxygenase‐1 62 | ↑ | Alpha6 integrin subunit 87 |
| NO and eNOS 63, 64 | ↑ | |
| Ang II 65 , Endostatin 66 | ↓ | |
| Morphine 67 | ↓ | |
| Aldosterone 68 | ↓ | |
| Physiologic/pathological factors | ||
| Physical training 69 | ↑ | |
| Wound 70 | ↑ | |
| Ischemic events 71, 72 | ↑ | |
| Aging 73, Obesity 74 | ↓ | |
| Smoking 75 | ↓ | |
| Hypertension 76 | ↓ | |
| Diabetes 77, 78 | ↓ | |
| Hypercholesterolemia 79 | ↓ | |
| Homocysteine 80 | ↓ | |
G‐CSF, granulocyte‐colony stimulating factor; IGF‐1, insulin‐like growth factor‐1; PAR‐1, protease‐activated receptor‐1; ARB, angiotensin II type 1 receptor blocker; ACEI, angiotensin‐converting enzyme inhibitor; EPO, Erythropoietin; eNOS, endothelial nitric oxide synthase; Ang II, Angiotensin II; IL‐8, Interleukin‐8; IGF2R, insulin‐like growth factor 2 receptor.
The homing or recruitment of circulating EPCs (cEPCs) into injury or ischemic sites is an important process for executing their angiogenic and repairing function 42. Both tissue factors and EPC surface receptors are involved in homing of EPCs (Table 1). For example, the SDF‐1/CXCR4 axis plays a significant role in mediating EPC homing in ischemic tissue 34, 43, 44. CXCR2 and its ligands, CXCL1 and CXCL7, have been shown to mediate EPC homing to injured arteries 23, 45. Recently, the interaction of chemokine ligand CCL5 and its receptor CCR5 is suggested as a signal for EPC recruitment into wounded tissue 46.
The mobilization and homing of EPCs to injured blood vessels and ischemic tissue are important for them to participate in endothelial repair and contribute to postnatal angiogenesis (see below). Although there is no evidence showing that EPCs directly induce malignant tumorigenesis, EPC migrating to tumor tissue may have a risk in supporting tumor vascularization 47, 48. The potential adverse effects of EPC‐based therapy are detailed in the section of “Safety Respects of EPC‐based Therapy”.
Several technologies have been developed for tracking EPCs in vivo. For example, EPCs stained with DiI‐Ac‐LDL or radiolabeled with 111In‐oxine have been used for tracking EPCs after injecting them into animals 13, 14, 49. A recent study has used Dex‐DOTA‐Gd3+ as a magnetic resonance imaging contrast agent for monitoring the anatomical migration and the survival period of transplanted EPCs in a rat model of hindlimb ischemia 50. Hence, these methods provide useful approaches for supporting preclinical and clinical research on EPC‐based therapy.
Functional Characteristics of EPCs
EPCs Participate in Endothelial Homeostasis and Repair
The abilities of EPCs to differentiate into mature ECs and secrete different protective cellular factors indicate that they play a significant role in endothelial homeostasis and repair. This notion is supported by solid evidence. For one thing, EPCs presenting in both vascular intima and circulation have been shown to participate in endothelialization and replacement of dysfunctional ECs 32, 65, 88, 89. Secondly, reduction of cEPCs can independently predict the progress of atherosclerotic disease 20, 88. More directly, transfusion of EPCs has been reported to reduce neointima formation in a vascular injury model 90, and to inhibit platelet activation and thrombogenesis in an arterial thrombosis model 91.
EPCs Contribute to Angiogenesis
Angiogenesis is necessary for blood vessel reconstruction and collateral circulation establishment, which are important to deliver nutrients and protectants to the jeopardized tissue for repair. The first finding of EPCs by Asahara et al. has initiated a new era in angiogenesis research 16, 17. Thereafter, mounting evidence confirms the role of EPCs in angiogenesis. Both early and late EPCs have been suggested to participate in the process of angiogenesis. Early EPCs are involved in angiogenesis by secreting an array of growth factors and cytokines, such as VEGF, SDF‐1, IGF‐1, and G‐CSF, which can enhance EC proliferation, reduce cell apoptosis, and recruit endogenous progenitor cells 13, 21, 26. Later evidence suggests that late EPCs may also have the ability to secrete soluble factors to contribute to these processes 14. These findings help to explain why EPC‐conditioned medium promotes neovascularization 25. Moreover, late EPCs contribute to neovasculogenesis by differentiating into ECs 20, 21. EPCs have been shown to account for up to 26% of all ECs in neovascularization 92. On the other hand, the contribution of EPCs in angiogenesis has also been documented in the recovery processes of various diseases, such as myocardial ischemia 93, 94, limb ischemia 16, 34, ischemic stroke 12, 13, and wounds 95. All these researches in animal models prelude the physiological function of EPCs and highlight the potential of EPCs as a cell candidate for regenerative therapy.
EPCs for Treating Ischemic Stroke
Pathophysiology of Ischemic Stroke
The pathophysiology of ischemic stroke involves complex processes such as energy failure, loss of cellular ion homeostasis, free radical‐mediated and cytokine‐mediated toxicities, inflammation, disruption of the blood‐brain barrier (BBB), and infiltration of leukocytes. These events are interrelated and coordinated 96. Upon ischemic stroke, cerebral damage occurs early and in a progressive fashion. Based on the time course, ischemic stroke can be roughly derived into acute (hours), subacute (hours to days), and chronic (days to months) phases 97. The acute phase is manifested with BBB disruption and vascular tonus. Neutrophils adhere to the endothelium and produce superoxide anions by reacting with NO and can further trigger tissue damage and inflammation. Within the subacute phase, frank edema and injury appear. Multiple genes such as MMP‐9, IL‐1, VEGF, angiopoietin‐2 are activated. In the chronic phase, limited endogenous angiogenesis and neurogenesis attempting for recovery are proceeding. Pathologically, ischemic areas include an infarct core and penumbra (peri‐infarct area). Dead cells constitute the infarct core, which represents irreversible damage, whereas the penumbra is the rescuable area where the angiogenesis can develop 98, 99. Thus, the penumbra is the target for reducing acute damage.
Rationale for Using EPCs to Treat Ischemic Stroke
Level of cEPCs Correlates with Ischemic Stroke
Mounting evidence advocates that the level of cEPCs is reduced in various stroke risk factors such as hypertension 76, hypercholesterolemia 79, diabetes 77, 78, and atherosclerosis 88. The level of cEPCs has been manifested as an important biological marker to predict endothelial dysfunction, cardiovascular risk 88, 89, 100, and cerebrovascular events 101, 102. Clinical studies show that acute stroke induces a transient increase of cEPCs 103, and the level of cEPCs negatively correlates with severity of ischemic damage 104, 105. A higher level of CFU‐ECs during the first week of stroke is shown to independently associate with a better outcome 106. Current evidence supports that EPCs not only serve as biomarker but also might offer a new therapeutic strategy for ischemic stroke 19, 42.
EPCs Contribute to Neurovascular Protection, Angiogenesis and Neurogenesis
As stated above, EPCs have been suggested to maintain endothelial protection/repair and angiogenesis. Further studies provide evidence that angiogenesis is coupled with neuroprotection and neurogenesis following ischemic injury 14, 107. The underlying mechanisms include that the regenerated blood vessels provide nutritive blood flow and that EPCs, by secreting factors such as SDF‐1 and VEGF, create a microenvironment for neural regeneration and survival 108, 109. Furthermore, neuroblasts migrate along these regenerated vessels to achieve neurogenesis in peri‐infarct area 107, 110, 111. Therefore, suppression of angiogenesis substantially reduces migration of neuroblasts from the subventricular zone to the ischemic region 111.
Transplantation of EPCs Accelerates Cerebral Repair Following Ischemic Stroke
The involvement of endogenous EPCs in cerebral neovascularization after ischemic stroke was first reported by Zhang et al. in 2002 12. However, EPCs are usually reduced in number and dysfunctional in disease conditions. Therefore, transfusion of exogenous EPCs could accelerate the repairing processes. Several transplantation studies on CD34+ cells (EPC‐rich fraction) have shown their therapeutic effect in promoting new vessel formation and neurogenesis after ischemic stroke 112, 113. Lately, injection of human ECFCs was shown to decrease cell apoptosis, promote angiogenesis and neurogenesis, and improve functional recovery 14. It is also suggested that administration of EPCs can increase regional cortical blood flow, reduce infarct volume, and neurological deficits in 2 days after stroke 114. Our study demonstrates that EPCs are reduced in quantity and dysfunctional in db/db type‐2 diabetic mice, which might account for decreased cerebral microvascular density and enlarged ischemic damage 15. Infusion of functional EPCs reduces ischemic cerebral damage in db/db diabetic mice, which is associated with improvement in angiogenesis. A recent study demonstrates that labeled EPCs were found around microvessels in the cerebral ischemic boundary 24 h after EPC transplantation, and improved long‐term neurobehavioral outcomes of ischemic stroke 13. Several studies demonstrated that EPCs could replace dysfunctional endothelium at the site of denuding injury 115, 116, 117. All these studies indicate that EPCs could serve as a cellular reservoir for the replacement/repair of dysfunctional ECs in stroke and are promising stem cells for the treatment of ischemic stroke.
The beneficial effects of EPC‐based therapy might come from several aspects (as shown in Figure 1). At the early stage of ischemic stroke, both injected and endogenous EPCs could protect cells (ECs and neurons) from ischemia‐induced death/damage because EPCs secrete various growth factors such as VEGF, SDF‐1, IGF‐1. These factors also assist to recruit more EPCs and support their survival, while alleviating acute injury via protecting the function of neurovascular units and/or existing collateral blood vessels. In the later stage, EPCs working together with their secreted factors promote neovascularization and neurogenesis, which functionally and structurally rebuild the BBB, blood vessels, and neuron networks; in turn, contributing to the recovery.
Figure 1.
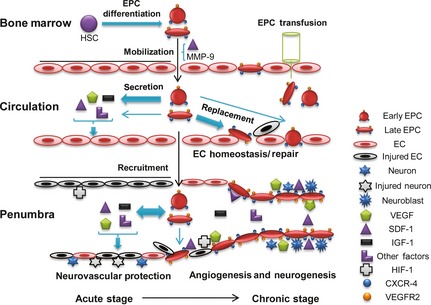
EPC function and therapeutic mechanism of EPCs for ischemic stroke.
Strategies of EPC‐based Therapy for Ischemic Stroke
Administration of EPCs
The optimal starting time point for administration of EPCs following ischemic stroke may be important for the therapeutic efficacy. However, there is limited research on this aspect. Based on the ability of EPCs to secrete various growth factors which have protective effects on ECs and neurons, their application at the earlier stage of stroke may have better efficacy. However, it should be pointed out that inflammation, free radical‐mediated, and cytokine‐mediated toxicities occurring in the acute phase of stroke may limit the function and survival of transplanted EPCs 96, 97, 118. EPCs obtained from patients in the subacute phase of ischemic stroke have showed greater vasculogenic capacity than those from patients in the acute phrase 119. It remains to be determined whether administration of autologous EPCs in the subacute period is more effective. In regards to EPC administration in clinical settings, intravenous infusion should be the optimal route because intra‐arterial infusion is inconvenient and could cause embolism, and direct injection of stem cells into the brain is complex and might cause local hemorrhaging 120. As for the dosing, administration of EPCs with the range of 0.2–3.0 × 104 per gram body weight has shown satisfactory efficacies in various animal models 13, 14, 18, 112. The first on‐going clinical trial on EPC‐based therapy for ischemic stroke (Identifier: NCT01468064) is designed to intravenously apply 2.5 × 106 EPCs per kilogram body weight. It is also unclear regarding the ideal frequencies of EPC administration. The current clinical trial adopts two EPC transplantations 1 week after initial dosing.
A recent study on late EPCs raises perspective for the use of late EPCs as an optimal EPC‐based therapy 14. However, in this study, transplantation of early EPCs also led to similar improvement in modified neurological severity score and somatosensory scores up to 14 days after stroke. Another study showed infusion of early EPCs significantly reduced ischemic infarct volume at 3 days following stroke and enhanced the long‐term outcome 13. Which type of EPCs is more effective should be further investigated as the data from comparison of early or late EPCs are still elusive. Currently, coadministration of different types of progenitor/stem cells may constitute a novel therapeutic strategy for ischemic diseases 121.
Ex Vivo Modification of EPCs before Administration
In order to enhance the therapeutic effect, EPC modifications such as gene transfection, ischemia preconditioning and pre‐treatment have been investigated. EPCs transduced with vectors over‐expressing diverse genes such as CXCR4 122, VEGF 123, IGF‐1 52, HIF‐1 124, and eNOS 125 have shown positive results. In a carotid artery injury model, transplantation of EPCs over‐expressing CXCR4 was able to further enhance the reendothelialization capacity of EPCs 122. In a hind limb ischemic model, combination of intravenous infusion of EPCs over‐expressing VEGF with local SDF‐1 application showed to be more efficient in improving local blood supply than either of them used alone 123. Interestingly, VEGF over‐expression on EPCs could increase the expression of CXCR4 which could further enhance EPC homing. Transplantation of EPCs over‐expressing IGF‐1 has led to inhibition of cardiac apoptosis, enhancement of cardiomyocyte proliferation, and increment of capillary numbers in the peri‐infarct area 52. On the other hand, hypoxia preconditioning enhances VEGFR2 expression on EPCs, and accordingly, augments the neovascularization efficacy of EPCs after administration 126. In addition, preincubating EPCs with SDF‐1 enhances their pro‐angiogenic potential in treating hind‐limb ischemia 127. The mechanism is mainly due to the up‐regulation of α4 and αM integrin subunits, which are involved in the homing of EPCs, and secretion of FGF‐2 and MMP‐2 which are involved in enhancing cell invasion. All these studies indicating the advantages of modified EPCs advocate the new directions of EPC‐based therapy for ischemic stroke.
Modulation of Endogenous EPC Mobilization and Function by Drugs
Drugs that can affect endogenous EPC behavior are summarized in Table 1. G‐CSF is one of the early drugs discovered to be able to enhance EPC mobilization into the circulation and augments EPC colony‐forming capacity after venous administration 39. Afterwards, Ang II was shown to induce pro‐apoptotic signaling pathways through Ang II type 1 receptor (AT1‐R) expressed on EPCs, and impairs colony‐forming and migratory capacities of EPCs 65. By decreasing Ang II production or blockade of AT1‐R, the drugs targeting the renin‐angiotensin system such as ACEI and ARB are shown to increase the number and functional activity of EPCs in vitro or in vivo 56, 57. Furthermore, statins have also been shown to promote the mobilization, clonal growth ability of cEPCs, and may consequently increase myocardial capillary density in the chronically ischemic heart 55, 128. The underlying molecular mechanisms may relate to the activation of AKT signaling and inhibition of TNF‐alpha‐induced apoptosis pathway. As these drugs are commonly used in clinical treatment of cardiovascular diseases, all these data may help to interpret the beneficial effects of these drugs on top of their known pharmacological actions. Further studies in this area could facilitate the discovery of new drugs targeting EPCs.
Risk Factor Management
The risk factors for stroke such as hypertension, diabetes, or hypercholesterolemia could reduce the number and biological activity of EPCs (Table 1). It can be logically speculated that environment of circulation is essential for the living of EPC, which would raise the perspective on the demand in managing the risk factors of stroke.
Promising Strategies Relate to EPCs
A recent study showed that a collagen patch seeded with EPCs promotes angiogenesis and arteriogenesis when placed on cryo‐injured rat heart 129. This may offer a new strategy to increase the local number of EPCs in ischemic area through interventional therapy for stroke. In addition, application of a bio‐engineered EPC‐capture stent, which accelerates re‐endothelialization and reduces thrombogenicity, may reduce the rate of restenosis after PTAS in the future 130.
Safety Respects of EPC‐based Therapy
Translational research (from laboratory to clinic) on stem cell‐based therapeutics for stroke has been explored in recent years. The studies have been guided by the research recommendations from Stem Cell Therapeutics as an Emerging Paradigm for Stroke (STEPS) in order to enhance therapeutic safety and efficacy 131. The pioneering pilot studies have been conducted in stroke patients to explore the feasibility and safety of autologous BM stem cell and MSC transplantations 9, 10, 120, 132, 133. Intravenous infusion of autologous human MSCs has not shown any treatment‐related abnormal cell growth or tumorigenesis, neurological deterioration, and venous thromboembolism during 1–5 years of follow‐up 10, 120. Intra‐arterial transplantation of BM stem cells at 5–9 days after stroke onset has also been demonstrated to be safe and has a trend to improve the Barthel Index, positively correlating with the number of CD34+ cells 133. However, these pilot studies had a relatively small size of samples. Larger clinical trials are in need to further warrant the results of those studies.
The safe aspects of EPC transfusion have been explored in recent years. The level of cEPCs has been found higher in patients with lung, hepatocellular, breast, and colorectal cancers 47. BM‐EPCs have been shown to present in the early phase of tumor angiogenesis, and ablation of EPCs results in delay of tumor growth which is associated with decreased vessel density 48. This evidence indicates that EPCs participate in the neovascularization of tumors and that EPC transfusion to patients with tumors should be avoided. In addition, EPCs might aggravate ischemia by increasing the ischemic inflammation because they could produce inflammatory factors such as interleukin‐8, monocyte chemotactic protein‐1, and recruit monocytes and macrophages 14, 21, 134. By contrast, transplantation of EPCs was shown to decrease inflammation and enhance neovascularization in a rat model of myocardial infarction 135. A study of EPC transfusion in patients with acute myocardial infarction showed that EPC therapy did not affect the serum levels of C‐reactive protein and leukocytes 136 and did not cause any tumorigenesis during the 5‐year follow‐up 137. Currently, two clinical trials (clinicaltrials.gov identifier: NCT01468064; NCT00535197) are undergoing to evaluate the safety and efficacy of autologous EPC and CD34+ stem cell transplantation for treating ischemic stroke.
Conclusion
To sum up, there is no doubt of the angiogenic ability of EPCs, which is probably the most distinguishable characteristic over other stem cells. Accumulating evidence suggests the great therapeutic potential of EPCs for ischemic stroke. It remains to clarify if EPC‐based therapy is the safest and has the greatest efficacy over other types of stem/progenitor cells. How to improve the strategies in order to maximize the therapeutic application of EPCs deserves further investigation. Besides the hope of therapy, the potential of EPC‐based prevention for ischemic stroke may also present a future direction.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Michelle Durrant for proofreading the manuscript. This work was supported by the National Institutes of Health (NIH, HL098637), the National Natural Science Foundation of China (NSFC, 81271214, 81270195). The authors apologize to all whose original work could not be cited due to the limitation of the space.
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, et al. Executive summary: Heart disease and stroke statistics–2012 update: A report from the American Heart Association. Circulation 2012;125:188–197. [DOI] [PubMed] [Google Scholar]
- 2. Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 2011;124:314–323. [DOI] [PubMed] [Google Scholar]
- 3. Brott T, Broderick J, Kothari R, et al. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 4. Lee M, Hong KS, Saver JL. Efficacy of intra‐arterial fibrinolysis for acute ischemic stroke: Meta‐analysis of randomized controlled trials. Stroke 2010;41:932–937. [DOI] [PubMed] [Google Scholar]
- 5. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castano C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the solitaire AB device in large artery occlusions of the anterior circulation: A pilot study. Stroke 2010;41:1836–1840. [DOI] [PubMed] [Google Scholar]
- 7. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 8. Adams HP Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;115:e478–e534. [DOI] [PubMed] [Google Scholar]
- 9. Suarez‐Monteagudo C, Hernandez‐Ramirez P, Alvarez‐Gonzalez L, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci 2009;27:151–161. [DOI] [PubMed] [Google Scholar]
- 10. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long‐term follow‐up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–1106. [DOI] [PubMed] [Google Scholar]
- 11. Andres RH, Horie N, Slikker W, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011;134:1777–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow‐derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res 2002;90:284–288. [DOI] [PubMed] [Google Scholar]
- 13. Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long‐term stroke outcome in mice. Ann Neurol 2010;67:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moubarik C, Guillet B, Youssef B, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev 2011;7:208–220. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Chen S, Chen Y, et al. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: Possible implications in cerebral ischemic damage. Am J Physiol Endocrinol Metab 2011;301:E62–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 17. Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–228. [DOI] [PubMed] [Google Scholar]
- 18. Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011;29:1650–1655. [DOI] [PubMed] [Google Scholar]
- 19. Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, Tervaert JW, Lodder J. Endothelial progenitor cell research in stroke: A potential shift in pathophysiological and therapeutical concepts. Stroke 2008;39:2158–2165. [DOI] [PubMed] [Google Scholar]
- 20. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 2012;110:624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004;24:288–293. [DOI] [PubMed] [Google Scholar]
- 22. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 2003;23:1185–1189. [DOI] [PubMed] [Google Scholar]
- 23. Walenta KL, Bettink S, Bohm M, Friedrich EB. Differential chemokine receptor expression regulates functional specialization of endothelial progenitor cell subpopulations. Basic Res Cardiol 2011;106:299–305. [DOI] [PubMed] [Google Scholar]
- 24. Liu JW, Dunoyer‐Geindre S, Serre‐Beinier V, et al. Characterization of endothelial‐like cells derived from human mesenchymal stem cells. J Thromb Haemost 2007;5:826–834. [DOI] [PubMed] [Google Scholar]
- 25. Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell‐free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE 2009;4:e5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 2005;39:733–742. [DOI] [PubMed] [Google Scholar]
- 27. Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003;107:1164–1169. [DOI] [PubMed] [Google Scholar]
- 28. Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medina RJ, O'Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 2010;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lapidot T, Petit I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 2002;30:973–981. [DOI] [PubMed] [Google Scholar]
- 31. Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: Mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol 2009;68:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall‐derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005;105:2783–2786. [DOI] [PubMed] [Google Scholar]
- 33. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med 2004;10:858–864. [DOI] [PubMed] [Google Scholar]
- 34. Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell‐derived factor‐1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003;107:1322–1328. [DOI] [PubMed] [Google Scholar]
- 35. Lapidot T, Dar A, Kollet O. How do stem cells find their way home. Blood 2005;106:1901–1910. [DOI] [PubMed] [Google Scholar]
- 36. Kelly BD, Hackett SF, Hirota K, et al. Cell type‐specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia‐inducible factor 1. Circ Res 2003;93:1074–1081. [DOI] [PubMed] [Google Scholar]
- 37. Grunewald M, Avraham I, Dor Y, et al. VEGF‐induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 2006;124:175–189. [DOI] [PubMed] [Google Scholar]
- 38. Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP‐9 mediated release of kit‐ligand. Cell 2002;109:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Powell TM, Paul JD, Hill JM, et al. Granulocyte colony‐stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2005;25:296–301. [DOI] [PubMed] [Google Scholar]
- 40. Ohki Y, Heissig B, Sato Y, et al. Granulocyte colony‐stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J 2005;19:2005–2007. [DOI] [PubMed] [Google Scholar]
- 41. Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule‐1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony‐stimulating factor. Blood 2001;98:1289–1297. [DOI] [PubMed] [Google Scholar]
- 42. Lapergue B, Mohammad A, Shuaib A. Endothelial progenitor cells and cerebrovascular diseases. Prog Neurobiol 2007;83:349–362. [DOI] [PubMed] [Google Scholar]
- 43. Wu Q, Shao H, Darwin ED, et al. Extracellular calcium increases CXCR4 expression on bone marrow‐derived cells and enhances pro‐angiogenesis therapy. J Cell Mol Med 2009;13:3764–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hill WD, Hess DC, Martin‐Studdard A, et al. SDF‐1 (CXCL12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 2004;63:84–96. [DOI] [PubMed] [Google Scholar]
- 45. Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res 2007;100:590–597. [DOI] [PubMed] [Google Scholar]
- 46. Ishida Y, Kimura A, Kuninaka Y, et al. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest 2012;122:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dome B, Timar J, Ladanyi A, et al. Circulating endothelial cells, bone marrow‐derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: From biology to therapy. Crit Rev Oncol Hematol 2009;69:108–124. [DOI] [PubMed] [Google Scholar]
- 48. Nolan DJ, Ciarrocchi A, Mellick AS, et al. Bone marrow‐derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 2007;21:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 2003;107:2134–2139. [DOI] [PubMed] [Google Scholar]
- 50. Agudelo CA, Tachibana Y, Hurtado AF, Ose T, Iida H, Yamaoka T. The use of magnetic resonance cell tracking to monitor endothelial progenitor cells in a rat hindlimb ischemic model. Biomaterials 2012;33:2439–2448. [DOI] [PubMed] [Google Scholar]
- 51. Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow‐derived endothelial progenitor cells. EMBO J 1999;18:3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sen S, Merchan J, Dean J, et al. Autologous transplantation of endothelial progenitor cells genetically modified by adeno‐associated viral vector delivering insulin‐like growth factor‐1 gene after myocardial infarction. Hum Gene Ther 2010;21:1327–1334. [DOI] [PubMed] [Google Scholar]
- 53. Gill KA, Brindle NP. Angiopoietin‐2 stimulates migration of endothelial progenitors and their interaction with endothelium. Biochem Biophys Res Commun 2005;336:392–396. [DOI] [PubMed] [Google Scholar]
- 54. Smadja DM, Laurendeau I, Avignon C, Vidaud M, Aiach M, Gaussem P. The angiopoietin pathway is modulated by PAR‐1 activation on human endothelial progenitor cells. J Thromb Haemost 2006;4:2051–2058. [DOI] [PubMed] [Google Scholar]
- 55. Wang W, Lang JK, Suzuki G, Canty JM Jr., Cimato T. Statins enhance clonal growth of late outgrowth endothelial progenitors and increase myocardial capillary density in the chronically ischemic heart. PLoS ONE 2011;6:e24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Honda A, Matsuura K, Fukushima N, Tsurumi Y, Kasanuki H, Hagiwara N. Telmisartan induces proliferation of human endothelial progenitor cells via PPARgamma‐dependent PI3K/Akt pathway. Atherosclerosis 2009;205:376–384. [DOI] [PubMed] [Google Scholar]
- 57. Muller P, Kazakov A, Jagoda P, Semenov A, Bohm M, Laufs U. ACE inhibition promotes upregulation of endothelial progenitor cells and neoangiogenesis in cardiac pressure overload. Cardiovasc Res 2009;83:106–114. [DOI] [PubMed] [Google Scholar]
- 58. Iwakura A, Luedemann C, Shastry S, et al. Estrogen‐mediated, endothelial nitric oxide synthase‐dependent mobilization of bone marrow‐derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 2003;108:3115–3121. [DOI] [PubMed] [Google Scholar]
- 59. Yip HK, Tsai TH, Lin HS, et al. Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit Care 2011;15:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chan YH, Lam TH, Lau KK, et al. Dietary intake of phytoestrogen is associated with increased circulating endothelial progenitor cells in patients with cardiovascular disease. Eur J Cardiovasc Prev Rehabil 2011;18:360–368. [DOI] [PubMed] [Google Scholar]
- 61. Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine‐induced mobilization of circulating endothelial progenitor cells improves human small artery elasticity. J Hum Hypertens 2008;22:389–393. [DOI] [PubMed] [Google Scholar]
- 62. Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase‐1/carbon monoxide enhances re‐endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost 2009;7:1401–1408. [DOI] [PubMed] [Google Scholar]
- 63. Aicher A, Heeschen C, Mildner‐Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 2003;9:1370–1376. [DOI] [PubMed] [Google Scholar]
- 64. Ozuyaman B, Ebner P, Niesler U, et al. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost 2005;94:770–772. [DOI] [PubMed] [Google Scholar]
- 65. Endtmann C, Ebrahimian T, Czech T, et al. Angiotensin II impairs endothelial progenitor cell number and function in vitro and in vivo: Implications for vascular regeneration. Hypertension 2011;58:394–403. [DOI] [PubMed] [Google Scholar]
- 66. Schuch G, Heymach JV, Nomi M, et al. Endostatin inhibits the vascular endothelial growth factor‐induced mobilization of endothelial progenitor cells. Cancer Res 2003;63:8345–8350. [PubMed] [Google Scholar]
- 67. Lam CF, Chang PJ, Huang YS, et al. Prolonged use of high‐dose morphine impairs angiogenesis and mobilization of endothelial progenitor cells in mice. Anesth Analg 2008;107:686–692. [DOI] [PubMed] [Google Scholar]
- 68. Thum T, Schmitter K, Fleissner F, et al. Impairment of endothelial progenitor cell function and vascularization capacity by aldosterone in mice and humans. Eur Heart J 2011;32:1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004;109:220–226. [DOI] [PubMed] [Google Scholar]
- 70. Fox A, Smythe J, Fisher N, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg 2008;95:244–251. [DOI] [PubMed] [Google Scholar]
- 71. Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001;103:2776–2779. [DOI] [PubMed] [Google Scholar]
- 72. Takahashi T, Kalka C, Masuda H, et al. Ischemia‐ and cytokine‐induced mobilization of bone marrow‐derived endothelial progenitor cells for neovascularization. Nat Med 1999;5:434–438. [DOI] [PubMed] [Google Scholar]
- 73. Rauscher FM, Goldschmidt‐Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003;108:457–463. [DOI] [PubMed] [Google Scholar]
- 74. Muller‐Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction. Eur Heart J 2008;29:1560–1568. [DOI] [PubMed] [Google Scholar]
- 75. Kondo T, Hayashi M, Takeshita K, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004;24:1442–1447. [DOI] [PubMed] [Google Scholar]
- 76. Umemura T, Soga J, Hidaka T, et al. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am J Hypertens 2008;21:1203–1209. [DOI] [PubMed] [Google Scholar]
- 77. Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199. [DOI] [PubMed] [Google Scholar]
- 78. Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 2005;45:1449–1457. [DOI] [PubMed] [Google Scholar]
- 79. Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. [DOI] [PubMed] [Google Scholar]
- 80. Alam MM, Mohammad AA, Shuaib U, et al. Homocysteine reduces endothelial progenitor cells in stroke patients through apoptosis. J Cereb Blood Flow Metab 2009;29:157–165. [DOI] [PubMed] [Google Scholar]
- 81. Rabbany SY, Heissig B, Hattori K, Rafii S. Molecular pathways regulating mobilization of marrow‐derived stem cells for tissue revascularization. Trends Mol Med 2003;9:109–117. [DOI] [PubMed] [Google Scholar]
- 82. Kocher AA, Schuster MD, Bonaros N, et al. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL‐8/Gro CXC chemokines. J Mol Cell Cardiol 2006;40:455–464. [DOI] [PubMed] [Google Scholar]
- 83. Maeng YS, Choi HJ, Kwon JY, et al. Endothelial progenitor cell homing: Prominent role of the IGF2‐IGF2R‐PLCbeta2 axis. Blood 2009;113:233–243. [DOI] [PubMed] [Google Scholar]
- 84. Scharner D, Rossig L, Carmona G, et al. Caspase‐8 is involved in neovascularization‐promoting progenitor cell functions. Arterioscler Thromb Vasc Biol 2009;29:571–578. [DOI] [PubMed] [Google Scholar]
- 85. Shirvaikar N, Marquez‐Curtis LA, Ratajczak MZ, Janowska‐Wieczorek A. Hyaluronic acid and thrombin upregulate MT1‐MMP through PI3K and Rac‐1 signaling and prime the homing‐related responses of cord blood hematopoietic stem/progenitor cells. Stem Cells Dev 2011;20:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86. Leung KT, Chan KY, Ng PC, et al. The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood 2011;117:1840–1850. [DOI] [PubMed] [Google Scholar]
- 87. Bouvard C, Gafsou B, Dizier B, et al. alpha6‐integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2010;30:1569–1575. [DOI] [PubMed] [Google Scholar]
- 88. Schmidt‐Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005;111:2981–2987. [DOI] [PubMed] [Google Scholar]
- 89. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 90. Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 2003;93:e17–e24. [DOI] [PubMed] [Google Scholar]
- 91. Abou‐Saleh H, Yacoub D, Theoret JF, et al. Endothelial progenitor cells bind and inhibit platelet function and thrombus formation. Circulation 2009;120:2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Murayama T, Tepper OM, Silver M, et al. Determination of bone marrow‐derived endothelial progenitor cell significance in angiogenic growth factor‐induced neovascularization in vivo. Exp Hematol 2002;30:967–972. [DOI] [PubMed] [Google Scholar]
- 93. Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103:634–637. [DOI] [PubMed] [Google Scholar]
- 94. Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107:461–468. [DOI] [PubMed] [Google Scholar]
- 95. Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 2008;10:1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener 2011;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke 2004;35:2220–2225. [DOI] [PubMed] [Google Scholar]
- 98. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994;25:1794–1798. [DOI] [PubMed] [Google Scholar]
- 99. Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood‐brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab 2002;22:379–392. [DOI] [PubMed] [Google Scholar]
- 100. Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005;353:999–1007. [DOI] [PubMed] [Google Scholar]
- 101. Chu K, Jung KH, Lee ST, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 2008;39:1441–1447. [DOI] [PubMed] [Google Scholar]
- 102. Ghani U, Shuaib A, Salam A, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke 2005;36:151–153. [DOI] [PubMed] [Google Scholar]
- 103. Zhou WJ, Zhu DL, Yang GY, et al. Circulating endothelial progenitor cells in Chinese patients with acute stroke. Hypertens Res 2009;32:306–310. [DOI] [PubMed] [Google Scholar]
- 104. Bogoslovsky T, Chaudhry A, Latour L, et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology 2010;75:2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bogoslovsky T, Spatz M, Chaudhry A, et al. Stromal‐derived factor‐1[alpha] correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 2011;42:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 2007;38:2759–2764. [DOI] [PubMed] [Google Scholar]
- 107. Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long‐term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007;38:3032–3039. [DOI] [PubMed] [Google Scholar]
- 108. Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell‐derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 2004;101:18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schanzer A, Wachs FP, Wilhelm D, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 2004;14:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol 2009;8:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006;26:13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004;114:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shyu WC, Lin SZ, Chiang MF, Su CY, Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin‐mediated angiogenesis in chronic stroke rats. J Neurosci 2006;26:3444–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ohta T, Kikuta K, Imamura H, et al. Administration of ex vivo‐expanded bone marrow‐derived endothelial progenitor cells attenuates focal cerebral ischemia‐reperfusion injury in rats. Neurosurgery 2006;59:679–686; discussion 679‐686. [DOI] [PubMed] [Google Scholar]
- 115. Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res 2004;95:343–353. [DOI] [PubMed] [Google Scholar]
- 116. Croce G, Passacquale G, Necozione S, Ferri C, Desideri G. Nonpharmacological treatment of hypercholesterolemia increases circulating endothelial progenitor cell population in adults. Arterioscler Thromb Vasc Biol 2006;26:e38–e39. [DOI] [PubMed] [Google Scholar]
- 117. Valgimigli M, Rigolin GM, Fucili A, et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation 2004;110:1209–1212. [DOI] [PubMed] [Google Scholar]
- 118. Locatelli F, Bersano A, Ballabio E, et al. Stem cell therapy in stroke. Cell Mol Life Sci 2009;66:757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Navarro‐Sobrino M, Rosell A, Hernandez‐Guillamon M, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res 2010;80:317–323. [DOI] [PubMed] [Google Scholar]
- 120. Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum‐expanded autologous mesenchymal stem cells in stroke. Brain 2011;134:1790–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Foubert P, Matrone G, Souttou B, et al. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell‐based therapy. Circ Res 2008;103:751–760. [DOI] [PubMed] [Google Scholar]
- 122. Chen L, Wu F, Xia WH, et al. CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc Res 2010;88:462–470. [DOI] [PubMed] [Google Scholar]
- 123. Yu JX, Huang XF, Lv WM, et al. Combination of stromal‐derived factor‐1alpha and vascular endothelial growth factor gene‐modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg 2009;50:608–616. [DOI] [PubMed] [Google Scholar]
- 124. Jiang M, Wang B, Wang C, et al. In vivo enhancement of angiogenesis by adenoviral transfer of HIF‐1alpha‐modified endothelial progenitor cells (Ad‐HIF‐1alpha‐modified EPC for angiogenesis). Int J Biochem Cell Biol 2008;40:2284–2295. [DOI] [PubMed] [Google Scholar]
- 125. Kaur S, Kumar TR, Uruno A, Sugawara A, Jayakumar K, Kartha CC. Genetic engineering with endothelial nitric oxide synthase improves functional properties of endothelial progenitor cells from patients with coronary artery disease: An in vitro study. Basic Res Cardiol 2009;104:739–749. [DOI] [PubMed] [Google Scholar]
- 126. Akita T, Murohara T, Ikeda H, et al. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest 2003;83:65–73. [DOI] [PubMed] [Google Scholar]
- 127. Zemani F, Silvestre JS, Fauvel‐Lafeve F, et al. Ex vivo priming of endothelial progenitor cells with SDF‐1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol 2008;28:644–650. [DOI] [PubMed] [Google Scholar]
- 128. Henrich D, Seebach C, Wilhelm K, Marzi I. High dosage of simvastatin reduces TNF‐alpha‐induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL‐1beta in vitro. J Surg Res 2007;142:13–19. [DOI] [PubMed] [Google Scholar]
- 129. Pozzobon M, Bollini S, Iop L, et al. Human bone marrow‐derived CD133(+) cells delivered to a collagen patch on cryoinjured rat heart promote angiogenesis and arteriogenesis. Cell Transplant 2010;19:1247–1260. [DOI] [PubMed] [Google Scholar]
- 130. Larsen K, Cheng C, Tempel D, et al. Capture of circulatory endothelial progenitor cells and accelerated re‐endothelialization of a bio‐engineered stent in human ex vivo shunt and rabbit denudation model. Eur Heart J 2012;33:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke 2011;42:825–829. [DOI] [PubMed] [Google Scholar]
- 132. Friedrich MA, Martins MP, Araujo MD, et al. Intra‐arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant 2012;21(Suppl 1):S13–S21. [DOI] [PubMed] [Google Scholar]
- 133. Moniche F, Gonzalez A, Gonzalez‐Marcos JR, et al. Intra‐arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke 2012;43:2242–2244. [DOI] [PubMed] [Google Scholar]
- 134. van der Strate BW, Popa ER, Schipper M, et al. Circulating human CD34+ progenitor cells modulate neovascularization and inflammation in a nude mouse model. J Mol Cell Cardiol 2007;42:1086–1097. [DOI] [PubMed] [Google Scholar]
- 135. Schuh A, Liehn EA, Sasse A, et al. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol 2008;103:69–77. [DOI] [PubMed] [Google Scholar]
- 136. Assmus B, Schachinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI). Circulation 2002;106:3009–3017. [DOI] [PubMed] [Google Scholar]
- 137. Leistner DM, Fischer‐Rasokat U, Honold J, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI): Final 5‐year results suggest long‐term safety and efficacy. Clin Res Cardiol 2011;100:925–934. [DOI] [PubMed] [Google Scholar]


