Abstract
Background
Clinically node-negative breast cancer patients usually undergo sentinel lymph node (SLN) biopsy. When metastasis is identified, completion axillary lymph node dissection (CALND) is recommended. Newer data suggest that CALND may be omitted in some women as it does not improve local control or survival.
Methods
Women with a positive SLN diagnosed between 1999 and 2010 were included in this review and were stratified according to whether they did or did not undergo CALND. Primary endpoints included recurrence and breast cancer-specific mortality. Differences between the groups and in time to recurrence were compared and summarized.
Results
Overall, 276 women were included: 206 (79 %) women who underwent CALND (group 1) and 70 (21 %) women in whom CALND was omitted (group 2). Group 1 patients were younger, had more SLN disease, and received more chemotherapy (P <0.05 for each). The groups did not vary by tumor characteristics (P >0.05 for each). Median follow-up was 69 (range 6–147) and 73 (range 15–134) months for groups 1 and 2, respectively. Five (2 %) women in group 1 and three (4 %) women in group 2 died of breast cancer (P = 0.39). Local–regional or distant recurrence occurred in 20 (10 %) group 1 patients and in 10 (14 %) group 2 patients (P = 0.39). On multivariate analysis, only estrogen receptor negativity and lymphovascular invasion predicted for recurrence.
Conclusions
Omission of CALND in women with SLN disease does not significantly impact in-breast, nodal, or distant recurrence or mortality. Longer-term follow-up is needed to verify that this remains true with time.
Sentinel lymph node (SLN) biopsy is the standard of care for axillary staging in clinically node-negative breast cancer patients. Since the adoption of this technique, the consensus in the oncology community has been to proceed with completion axillary lymph node dissection (CALND) in the setting of a positive SLN, i.e., when tumor metastasis is found within the SLN.1 However, only about half of patients with macrometastasis in the SLN will have positive non-SLNs, and that likelihood lessens as the amount of SLN disease decreases.2–5 Recently, investigators have questioned whether patients with positive SLNs benefit from CALND, as axillary recurrence rates are low in these patients when CALND is omitted.6–10
To investigate the benefit of CALND following a positive SLN biopsy, the American College of Surgeons Oncology Group created a prospective trial, Z0011. This study randomized women with positive SLNs to CALND or to no further axillary surgery. The results were recently published, and no local control or survival advantage was seen with CALND.11,12 This paper has been practice-changing, but it has been criticized for its limited follow-up and for its failure to meet its accrual goal.
The purpose of our study is to review our SLN-positive patients, comparing local, regional, and distant recurrence rates in those who underwent CALND with those who did not, and to see whether our retrospective cohort produced results similar to those of Z0011.
METHODS
Institutional review board approval was obtained prior to the commencement of this retrospective study. Clinical, demographic, and pathologic data from all breast cancer patients treated at our institution are prospectively recorded in a database. We reviewed this database and identified 1,797 patients with breast cancer who underwent SLN biopsy at our institution between January 1, 1999 and August 31, 2010. Of this cohort, 356 women had positive SLNs. No follow-up information was available for 23 patients, and they were excluded. Fifty-seven patients had only isolated tumor cells (ITCs) in their sentinel nodes, and these patients were also excluded. Altogether, 276 women were included in the analysis. Data recorded included age at diagnosis, race, tumor size, histology, grade, estrogen receptor (ER) status, progesterone receptor (PR) status, Her-2neu status, type of surgical therapy [mastectomy versus breast-conservation therapy (BCT)], use of neoadjuvant or adjuvant systemic therapy, use of axillary-specific radiation therapy (radiation fields specifically designed to include axillary nodes), and the size of the SLN metastasis (micrometastatic versus macrometastatic). For patients who received neoadjuvant systemic therapy, pretreatment clinical tumor size was used.
Patients were divided according to whether they underwent CALND (group 1) or had no further axillary surgery (group 2). Local, axillary, and distant recurrences were identified and recorded, as were deaths due to breast cancer and to non-breast cancer causes. Patients who underwent lumpectomy were followed by clinical examination and by mammography; routine axillary imaging surveillance was not performed. Computed tomography, positron emission tomography, and bone scans were performed at the discretion of the treating physicians. Differences between the two groups in demographic and clinical characteristics were compared using Fisher’s exact test, two-sample t test, or Mann–Whitney rank-sum test as appropriate. Differences between the groups in time to recurrence were summarized using Kaplan–Meier product limit method and compared by log-rank test. Univariate Cox proportional hazard models were used to assess the effects of patient demographic and clinical characteristics on time to recurrence. A multivariate Cox model was also developed via a backward selection procedure to identify factors independently predictive of recurrence. Due to relatively small number of recurrences, a preselection was performed first and only those factors showing P <0.30 on univariate analysis were included to undergo backward selection procedure. All statistical analyses were performed using a statistical package SAS (SAS Institutes, Cary, NC). p value under 0.05 was taken to indicate significance, and all statistical tests were two-sided.
RESULTS
Of 276 patients with SLN disease, 206 (79 %) underwent CALND (group 1) and 70 (21 %) had no further axillary surgery (group 2). The median age of group 1 patients was 51 (range 20–89) years. This was significantly younger than 57 (range 31–86) years, the median age of group 2 patients (P = 0.016). There was a trend toward larger tumor size in group 1, but this latter variable did not reach statistical significance. The groups did not differ significantly in terms of patient race, histology, receptor status, tumor grade, or presence of lymphovascular invasion. Patients in group 1 were more likely to have had a mastectomy compared with patients in group 2 (P <0.001), but there were no differences between the groups with respect to use of adjuvant endocrine therapy. Group 1 patients were significantly more likely to have received chemotherapy, including neoadjuvant chemotherapy (P = 0.002), and were more likely to have had macrometastatic SLN disease versus micrometastatic disease (P = 0.001). Patients in group 1 were less likely to have received axillary-specific radiation therapy, but this was not statistically significant. The characteristics of the two groups are summarized in Table 1.
TABLE 1.
Patient and tumor characteristics for group 1 (CALND after SLN biopsy) and group 2 (no CALND after SLN biopsy)
| Variable | Group 1 (n = 206), n (%) | Group 2 (n = 70), n (%) | P Value |
|---|---|---|---|
| Age | 0.016 | ||
| Range, years | 20–89 | 31–86 | |
| Median, years | 51 | 57 | |
| Mean, years | 53.2 | 57.7 | |
| Race | 0.569 | ||
| White | 158 (77) | 51 (73) | |
| Black | 43 (21) | 16 (23) | |
| Other | 5 (2) | 3 (4) | |
| Tumor size | 0.062 | ||
| Tis | 1 (0) | 1 (1) | |
| T1 | 122 (59) | 50 (71) | |
| T2 | 73 (35) | 19 (27) | |
| T3 | 9 (4) | 0 | |
| T4 | 1 (0) | 0 | |
| TX | 0 (0) | 0 | |
| Tumor histology | 0.483 | ||
| Ductal | 156 (76) | 58 (83) | |
| Lobular | 26 (13) | 5 (7) | |
| Other/mixed | 23 (11) | 7 (10) | |
| Unknown | 1 (0) | 0 | |
| ER status | 0.856 | ||
| Positive | 169 (82) | 57 (81) | |
| Negative | 35 (17) | 13 (19) | |
| Unknown | 2 (1) | 0 | |
| PR status | 0.656 | ||
| Positive | 139 (67) | 45 (64) | |
| Negative | 64 (31) | 24 (34) | |
| Unknown | 3 (1) | 1 (1) | |
| Her-2neu status | 0.497 | ||
| Amplified | 40 (19) | 17 (24) | |
| Nonamplified | 160 (78) | 53 (76) | |
| Unknown | 6 (3) | 0 | |
| Tumor grade | 0.937 | ||
| I | 55 (27) | 21 (30) | |
| II | 89 (43) | 30 (43) | |
| III | 57 (28) | 19 (27) | |
| Unknown | 5 (2) | 0 | |
| Lymphovascular invasion | 0.195 | ||
| Present | 45 (22) | 21 (30) | |
| Absent/unknown | 161 (78) | 49 (70) | |
| Micrometastatic SLN | 0.001 | ||
| Yes | 47 (23) | 31 (44) | |
| No | 159 (77) | 39 (56) | |
| Surgical therapy | <0.001 | ||
| Breast conservation | 98 (48) | 52 (74) | |
| Mastectomy | 108 (52) | 18 (26) | |
| Chemotherapy | 0.002 | ||
| Yes, adjuvant | 134 (65) | 47 (67) | |
| Yes, neoadjuvant | 35 (17) | 4 (6) | |
| No | 22 (11) | 18 (26) | |
| Unknown | 15 (7) | 1 (1) | |
| Endocrine therapy | 0.207 | ||
| Yes, adjuvant | 150 (73) | 50 (71) | |
| Yes, neoadjuvant | 2 (1) | 2 (3) | |
| No | 7 (3) | 0 | |
| Unknown | 18 (9) | 5 (7) | |
| Not indicated (ER negative)a | 29 (14) | 13 (19) | |
| Axillary radiation therapy | 0.151 | ||
| Yes | 50 (24) | 27 (39) | |
| No | 56 (27) | 17 (24) | |
| Unknown | 100 (49) | 26 (37) |
CALND completion axillary lymph node dissection, SLN sentinel lymph node, ER estrogen receptor, PR progesterone receptor
Some women with tumors categorized as ER− were sometimes considered weakly ER+ by the oncologists and were given endocrine therapy
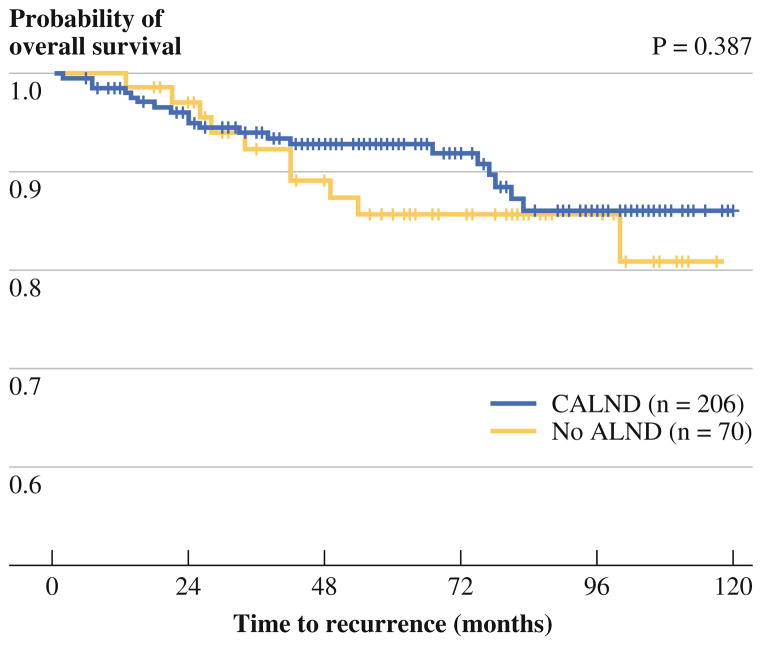
The median follow-up for group 1 was 69 (range 6–147) months, and the median follow-up for group 2 was 73 (range 15–134) months. During the follow-up period for group 1, 20 women (10 %) experienced recurrence: there were 7 in-breast recurrences, 4 regional nodal recurrences, and 14 distant recurrences. Five women in group 1 (2 %) died of breast cancer and six women (3 %) died of non-breast cancer causes. The median time to a breast cancer-related event (recurrence or death) was 24 (range 2–83) months. During the follow-up period for group 2, 10 women (14 %) experienced recurrence: there were 3 in-breast recurrences, 0 regional nodal recurrences, and 9 distant recurrences. Three women (4 %) in group 1 died of their disease and three (4 %) died of other causes. The median time to a breast cancer-related event in group 2 was 38 (range 13–100) months. The number of breast cancer recurrences and deaths did not differ significantly between the two groups (P >0.05 for each), and these follow-up data are summarized in Table 2. Figure 1 illustrates the disease-free survival curves of the two groups (P = 0.387).
TABLE 2.
Subsequent events and follow-up of group 1 (CALND after SLN biopsy) and group 2 (no CALND after SLN biopsy)
| Group 1 (n = 206) n (%) |
Group 2 (n = 70) n (%) |
P Value | |
|---|---|---|---|
| Follow-up period | 0.349 | ||
| Range in months | 6–147 | 15–123 | |
| Median in months | 69 | 73 | |
| Mean in months | 69.9 | 73.5 | |
| Breast cancer recurrence | |||
| In-breast recurrence, number of events (events/n, %) | 7 (4) | 3 (4) | 0.718 |
| Regional nodal recurrence, number of events (events/n, %) | 4 (2) | 0 | 0.575 |
| Distant recurrence, number of events (events/n, %) | 14 (7) | 9 (13) | 0.133 |
| All cancer recurrences together | 20 (10) | 10 (14) | 0.276 |
| Deaths | |||
| Breast cancer-related deaths (deaths/n, %) | 5 (2) | 3 (4) | 0.422 |
| Breast cancer-related hospice or unknown living status (events/n, %) | 0 | 2(3) | 0.064 |
| Non-breast cancer deaths (deaths/n, %) | 6 (3) | 3 (4) | 0.697 |
| Total deaths, all causes | 10 (5) | 6 (9) | 0.248 |
Some patients had more than one subsequent event (breast/chest wall, nodal, or distant recurrence or death) and therefore count multiple times in this table
FIG. 1.
Kaplan–Meier curves of recurrence-free survival for women undergoing completion axillary lymph node dissection (CALND) compared with women in whom CALND was omitted. Vertical bars in each curve represent distribution of censored cases in the corresponding group. Curves are based on univariate analysis
Of the 20 women in group 1 who experienced any recurrence, 5 had axillary radiation, 5 did not, and data were unknown for 10. Of the four women in group 1 who had an axillary recurrence, two had axillary-specific radiation, one did not, and data were unknown for one. Ten (50 %) of the women who recurred were ER negative. Five of the women in group 1 who experienced a recurrence were Her-2neu-amplified (25 %). Receptor status was unknown for one of the women who recurred. Of the 10 women in group 2 who experienced any recurrence, 4 had axillary-specific radiation, 2 did not, and data were unknown for 4. None of these recurrences were in axillary nodes. Five of these 10 women were ER negative (50 %). Two of the 10 were Her-2neu-amplified (20 %).
The effects of demographic and clinical factors on recurrence-free survival were also evaluated in the whole sample (Table 3). On univariate analysis, ER negativity, PR negativity, higher tumor grade, and lymphovascular invasion were associated with recurrence (P <0.05 for each). Patient age, race, tumor size, histology, Her-2neu status, and type of surgical therapy (mastectomy versus BCT) were not associated with recurrence (P >0.05 for each). Use of axillary-specific radiation therapy was included in this final analysis, but data were unknown for a significant number of patients in both groups and therefore no significant conclusion can be drawn regarding its effect on recurrence. After backward selection, ER negativity and lymphovascular invasion were included in multivariate analysis. Both remained predictors of recurrence; the effect of ER negativity was significant (P <0.0001), and the presence of lymphovascular invasion approached significance (P = 0.051). The between-group differences remained insignificant (P = 0.643) after adjusting for ER status and lymphovascular invasion; women in whom CALND was omitted were not likelier to experience recurrence.
TABLE 3.
Hazard ratios (HR) and 95 % confidence intervals (CI) for the effects of listed variables on recurrence-free survival
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | |
| Group | ||||
| No CALND vs. CALND | 1.40 (0.65–2.99) | 0.387 | 1.20 (0.55–2.62) | 0.643 |
| Age | ||||
| Per 1-year increase | 0.97 (0.94–1.00) | 0.089 | ||
| Race | ||||
| Black vs. White | 1.23 (0.52–2.89) | 0.639 | ||
| Other vs. White | 2.47 (0.58–10.6) | 0.221 | ||
| Tumor size | ||||
| T2–4 vs. T1 | 1.83 (0.89–3.74) | 0.098 | ||
| Tumor histology | ||||
| Lobular vs. ductal | 0.25 (0.03–1.86) | 0.177 | ||
| Others vs. ductal | 0.22 (0.03–1.63) | 0.139 | ||
| ER status | ||||
| Positive vs. negative | 0.19 (0.09–0.40) | <0.0001 | 0.20 (0.10–0.41) | <0.0001 |
| PR status | ||||
| Positive vs. negative | 0.26 (0.12–0.56) | 0.0005 | ||
| Her-2neu status | ||||
| Amplified vs. not | 1.34 (0.60–3.04) | 0.478 | ||
| Tumor grade | ||||
| Grade II vs. grade I | 7.85 (1.02–60.30) | 0.048 | ||
| Grade III vs. grade I | 19.70 (2.62–147.94) | 0.004 | ||
| Lymphovascular invasion | ||||
| Present vs. absent | 2.13 (1.02–4.42) | 0.043 | 2.11 (1.00–4.47) | 0.051 |
| Micrometastatic SLN | ||||
| Micro- vs. macrometastatic | 0.66 (0.27–1.60) | 0.353 | ||
| Surgical therapy | ||||
| Mastectomy vs. BCT | 1.49 (0.73–3.06) | 0.276 | ||
| Axillary radiation therapy | ||||
| Yes vs. no | 1.63 (0.59–4.47) | 0.347 | ||
CALND completion axillary lymph node dissection, ER estrogen receptor, PR progesterone receptor, SLN sentinel lymph node, BCT breast-conservation therapy
DISCUSSION
SLN biopsy has supplanted CALND for axillary staging in clinically node-negative breast cancer patients. Current American Society of Clinical Oncology guidelines recommend proceeding with CALND when tumor deposits greater than 0.2 mm are identified within the SLN.1 It has been assumed that CALND provides better disease control, and better local control has been shown to improve survival.13–20 More recent studies, however, have questioned the disease control advantage of CALND, especially in the setting of effective systemic therapy.6–10,21
This past year, the American College of Surgeons Oncology Group published results of the Z0011 trial, and recommendations regarding use of CALND in this setting are changing as a result of this study.22 The Z0011 trial was a prospective, randomized noninferiority trial that compared CALND with no CALND in women with positive SLNs. Eligible patients were women with early-stage disease (tumor size <5 cm, clinically node negative) who were found to have SLN disease. All patients were treated with lumpectomy, and all were expected to receive whole-breast radiation therapy. Women with three or more positive SLNs, with extranodal extension, with matted nodes, or who underwent neoadjuvant systemic therapy were excluded. Women were randomized to CALND or to no CALND and were stratified by age, tumor size, and ER status. Systemic therapy was determined by the treating physicians. The study was expected to accrue 1,900 patients but closed early to due poor enrollment and due to a very low number of deaths. Altogether, 891 patients were included in the analysis. Median follow-up was 6.3 years. Almost 90 % of the women in both cohorts did receive standard whole-breast radiation therapy. Of note, over 95 % of the women in Z0011 received adjuvant systemic therapy. Overall survival, disease-free survival, and local–regional recurrence rates did not differ significantly between the two groups.11,12
The authors did comment that their data apply specifically to women meeting the Z0011 inclusion criteria, and therefore should not be extrapolated to women treated with mastectomy or neoadjuvant systemic therapy, women in whom adjuvant radiation is omitted, and women undergoing partial-breast radiation.12 National Comprehensive Cancer Network guidelines reflect these exclusions.22 Our study included a significant number of women who would have been excluded from Z0011; for instance, a significant proportion of our patients underwent mastectomy (52 % of group 1 patients and 26 % of group 2 patients). As discussed above, the type of breast surgery (mastectomy versus BCT) did not significantly affect disease recurrence. Our study also included a significant minority of patients treated with neoadjuvant chemotherapy or endocrine therapy (18 % of group 1 patients and 9 % of group 2 patients). Women in both groups who recurred were more likely to have had adjuvant or neoadjuvant chemotherapy when compared with women who did not recur, likely reflecting more aggressive or advanced disease. Overall, however, only five patients in group 1 and two patients in group 2 treated with neoadjuvant chemotherapy recurred, and given these small numbers, no meaningful statistical analysis could be performed to evaluate the effect of neoadjuvant chemotherapy on recurrence. No recurrences were seen with neoadjuvant endocrine therapy in either group. Women in Z0011 were expected to receive whole-breast radiation, which typically includes the low axilla and which may therefore add local control benefit. In our study, women in group 2 were more likely to have received axillary-specific radiation, but this was not statistically significant, and no differences in axillary radiation rates were seen within the groups between women who did and did not recur. As discussed above, the large proportion of women with unknown axillary radiation status prohibited a strong conclusion regarding the effect of radiation on recurrence. In addition, because there were no axillary recurrences in group 2, it is impossible to comment on whether axillary radiation can substitute for CALND for regional disease control.
In our study, only ER negativity predicted for axillary recurrence on univariate and multivariate analysis. The proportion of our cohort with ER-negative disease was similar to that seen in Z0011, but ER negativity was only a predictor of recurrence in Z0011 on univariate, and not multivariate, analysis.11 In other series reporting on outcomes for SLN-positive patients in whom CALND was omitted, recurrence events were infrequent and little comment could be made on the impact of ER status. Pepels et al. reported a significantly higher risk of regional recurrence with hormone receptor negativity, but the authors commented that the majority of their patients did not receive systemic therapy, and a significant proportion underwent mastectomy (and therefore did not have whole-breast radiation therapy).23 When we controlled for ER status, no significant difference in recurrence was seen between our two groups; recurrence in ER-negative women seems more attributable to disease biology than to omission of surgical therapy.
Our study has several limitations. First, it is a retrospective review. We do not know why the 70 women in group 2 did not undergo CALND, but they were older, had less SLN disease, were more likely to have had BCT, and were less likely to have received chemotherapy. Although not statistically significant, they had smaller tumors. This suggests selection bias and that group 2 patients had more favorable prognosis compared with group 1. Second, we were unable to find documentation regarding the use of axillary-specific radiation for a significant number of patients. Therefore, we cannot comment on whether nodal radiation had a significant effect on recurrence, specifically for women in group 2; axillary radiation is often used as a replacement therapy when CALND is omitted.24–26 Third, our follow-up period is similar to Z0011. For the majority of women who are ER positive and who would have been prescribed antiestrogen therapy, many would have only recently completed that therapy; it is possible that longer-term follow-up may reveal higher recurrence rates.
Despite these limitations, our study demonstrates no axillary recurrences at a median follow-up of 73 months in women with a positive SLN who did not undergo CALND. Our findings are similar to those of other studies reporting low axillary recurrence rates when CALND is omitted despite the presence of SLN disease, as summarized in Table 4. These studies, and ours, support the idea that CALND is of limited value for local control. In fact, women in whom CALND was omitted were not significantly more likely to experience recurrence at any site and did not have higher disease-specific mortality. Increased recurrence risk is instead seen with higher grade, ER and PR negativity, and lymphovascular invasion, suggesting that tumor biology more than surgical therapy is a predictor of recurrence. Future studies should include women not meeting the inclusion criteria for Z0011; if biology and adequate systemic therapy are more important than surgical therapy for decreasing disease-related mortality, then CALND would likely provide little advantage to women undergoing mastectomy, partial-breast radiation, and neo-adjuvant therapy. Longer-term data are also needed to prove that these results are consistent with time, especially for women with ER-positive disease who are maintained on antiestrogen therapy for 5 years posttreatment.
TABLE 4.
Summary of studies reporting axillary recurrence rates when axillary lymph node dissection is omitted despite the presence of sentinel lymph node metastasis6,8–11,21,23,27–30
| Author | Years | N | Micro- or macrometastatic sentinel node disease | Axillary recurrence rate (%) | Median follow-up (months) |
|---|---|---|---|---|---|
| Fant | 2003 | 31 | Botha | 0 | 30 |
| Hwang | 2007 | 196 | Botha | 0 | 29.5 |
| Jeruss | 2005 | 73 | Both | 0 | 27.6 |
| Naik | 2004 | 210 | Botha | 1.4 | 31 |
| Giuliano | 2011 | 891 | Both | 0.9 | 75 |
| Bilimoria | 2009 | 20,217 | Both | 1 | 63 |
| Yi | 2010 | 2,185 | Both | 0.2b | 50 |
| Milgrom | 2012 | 234 | Both | 1.5–2.5c | 57.8 |
| Pepels | 2012 | 141 | Micrometastatic | 5.6 | 61 |
| Rayhanabad | 2010 | 33 | Micrometastatic | 1.6 | 70 |
| Yegiyants | 2010 | 47 | Both | 4.3 | 82 |
Studies included sentinel node metastases identified with immunohistochemistry
Axillary recurrence was inferred from “ipsilateral regional events” as described in the Surveillance, Epidemiology, and End Results (SEER) database
Breast conservation was associated with a regional recurrence rate of 1.5 %; mastectomy was associated with a regional recurrence rate of 2.5 %
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Biostatistics Core. The Core is supported in part by the National Cancer Institute Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center. We also thank Marsha Woodall for her maintenance of the surgical breast cancer database.
Footnotes
FINANCIAL DISCLOSURES This project was unfunded, and the authors have no financial disclosures regarding this topic.
References
- 1.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Cserni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91:1245–52. doi: 10.1002/bjs.4725. [DOI] [PubMed] [Google Scholar]
- 3.Van Zee KJ, Manasseh DME, Bevilacqua JLB, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Reed J, Rosman M, Verbanac KM, Mannie A, Cheng Z, Tafra L. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center sentinel node multicenter study. J Am Coll Surg. 2009;208:333–40. doi: 10.1016/j.jamcollsurg.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Chu KU, Turner RR, Hansen NM, Brennan MB, Bilchik A, Giuliano AE. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229:536–41. doi: 10.1097/00000658-199904000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fant JS, Grant MD, Knox SM, Livingston SA, Ridl K, Jones RC, Kuhn JA. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–30. doi: 10.1245/aso.2003.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Grube BJ, Giuliano AE. Observation of the breast cancer patient with a tumor-positive sentinel node: Implications of the ACO-SOG Z0011 trial. Semin Surg Oncol. 2001;20:230–7. doi: 10.1002/ssu.1038. [DOI] [PubMed] [Google Scholar]
- 8.Hwang RF, Gonzalez-Angulo AN, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–30. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 9.Jeruss JS, Sener SF, Brinkmann EM, et al. Axillary recurrence after sentinel node biopsy. Ann Surg Oncol. 2005;12:34–40. doi: 10.1007/s10434-004-1164-2. [DOI] [PubMed] [Google Scholar]
- 10.Naik AM, Fey JV, Gemignani M, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection. Ann Surg. 2004;240:462–71. doi: 10.1097/01.sla.0000137130.23530.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–32. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher B, Wolmark N, Bauer M, Redmond C, Gebhardt M. The accuracy of clinical nodal staging and of limited axillary dissection as a determinant of histologic nodal status in carcinoma of the breast. Surg Gynecol Obstet. 1981;152:765–72. [PubMed] [Google Scholar]
- 14.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–62. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard M, Hansen PS, Ovegaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 16.Quan ML, McCready D. The evolution of lymph node assessment in breast cancer. J Surg Oncol. 2009;99:194–8. doi: 10.1002/jso.21201. [DOI] [PubMed] [Google Scholar]
- 17.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:1087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 18.Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 19.Sosa JA, Diener-West M, Gusez Y, et al. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann Surg Oncol. 1998;5:140–9. doi: 10.1007/BF02303847. [DOI] [PubMed] [Google Scholar]
- 20.Hayward J, Caleffi M. The significance of local control in the primary treatment of breast cancer. Arch Surg. 1987;122:1244–7. doi: 10.1001/archsurg.1987.01400230030004. [DOI] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–53. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Breast Cancer. Version 1.2012. Available: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 23.Pepels MJ, de Boer M, Bult P, et al. Regional recurrence in breast cancer patients with sentinel node micrometastases and isolated tumor cells. Ann Surg. 2012;255:116–21. doi: 10.1097/SLA.0b013e31823dc616. [DOI] [PubMed] [Google Scholar]
- 24.Chua B, Ung O, Boyages J. Treatment of the axilla in early breast cancer: past, present, and future. ANZ J Surg. 2001;71:729–36. doi: 10.1046/j.1445-1433.2001.02271.x. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht MR, Zink K, Busch W, Ruhl U. Dissection or irradiation of the axilla in postmenopausal patients with breast cancer? Long-term results and long-term effects in 655 patients. Strahlenther Onkol. 2002;178:510–6. doi: 10.1007/s00066-002-1035-3. [DOI] [PubMed] [Google Scholar]
- 26.Bansal M, Mohanti BK. Sentinel lymph node biopsy, axillary dissection and breast cancer: a radiation oncologist’s viewpoint. Natl Med J India. 2002;15:154–7. [PubMed] [Google Scholar]
- 27.Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17:343–51. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milgrom S, Cody H, Tan L, et al. Characteristics and Outcomes of Sentinel Node-Positive Breast Cancer Patients after Total Mastectomy without Axillary-Specific Treatment. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2386-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Rayhanabad J, Yegiyants S, Putchakayala K, Haig P, Romero L, Difronzo LA. Axillary recurrence is low in patients with breast cancer who do not undergo completion axillary lymph node dissection for micrometastases in sentinel lymph nodes. Am Surg. 2010;76:1088–91. [PubMed] [Google Scholar]
- 30.Yegiyants S, Romero LA, Haigh PI, DiFronzo LA. Completion axillary lymph node dissection not required for regional control in patients with breast cancer who have micrometastases in a sentinel node. Arch Surg. 2010;145:564–9. doi: 10.1001/archsurg.2010.84. [DOI] [PubMed] [Google Scholar]