Abstract
Molecules that appear on the surface of tumor cells after their therapy treatment may have important roles either as damage-associated molecular patterns (DAMPs) or signals for phagocytes influencing the disposal of these cells. Treatment of SCCVII and CAL27 cells, models of mouse and human squamous cell carcinoma respectively, by photodynamic therapy (PDT) resulted in the presentation of ceramide and sphingosine-1-phposphate (S1P) on the cell surface. This was documented by anti-ceramide and anti-S1P antibody staining followed by flow cytometry. The exposure of these key sphingolipid molecules on PDT-treated tumor cells was PDT dose-dependent and it varied in intensity with different photosensitizers used for PDT. The above results, together with the finding that both ceramide and S1P can activate NFκB signaling in macrophages co-incubated with PDT-treated tumor cells, establish that these two sphingolipids can act as DAMPs stimulating inflammatory/immune reactions critical for tumor therapy response.
Keywords: Ceramide, Sphingosine-1-phosphate, Squamous cell carcinoma, Photodynamic therapy, Damage-associated molecular patterns
1. Introduction
The concept of damage-associated molecular patterns (DAMPs) was conceived by Polly Matzinger as a central element of danger model of the functioning of the immune system [1,2]. This model, which is based on the idea that the immune system recognizes altered self appearing as a consequence of injury, infection or oncogenic transformation, and the perception of DAMPs has brought vital insights into innate and adaptive immunity. According to the danger model, the immune response is mobilized by DAMPs alerting the host of impending danger of disturbed local homeostasis due to the appearance of distressed and dying/dead cells [3,4]. Normally, DAMPs are molecules performing non-immunological functions within cells/tissues that acquire immunomodulatory character once exposed on the cell surface, released from stressed cells, or dislocated in other way (e.g. extravasated). The sensors of exposed DAMPs are pattern-recognition receptors (PRRs). They are localized in the membrane or cytoplasm of immune cells, and include Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), and C-type lectin receptors (CLRs) [4]. Engagement of these receptors by DAMPs triggers the danger signaling processes leading to inflammatory and immune responses.
Tumor tissue injury induced by certain types of cancer therapy was found to result in the expression of DAMPs, which emerge as pivotal mediators of host response elicited by these treatments and subsequent therapy outcome [5,6]. Treatment of solid tumors by photodynamic therapy (PDT), a clinically established modality producing oxidative stress by localized photoactivation of administrated photosensitizing drug [7], is particularly effective in generating an abundance of various DAMPs. These include cell surface-expressed calreticulin and heat shock proteins, released high-mobility group box-1 (HMGB1) protein and ATP, extracellular matrix proteins, and extravasated fibrinogen [8–10]. In this report, it is shown that ceramide and sphingosine-1-phosphate (S1P), two key members of sphingolipid family, become engaged as DAMPs after PDT treatment. This is indicated by the detected appearance of these two sphingolipids on the surface of PDT-treated tumor cells, and the participation of ceramide and S1P from these cells in signaling leading to the activation of NFκB in neighboring tumor-associated macrophages (TAMs).
2. Materials and methods
2.1. Cell culture and chemicals
The used cells were derived from squamous cell carcinoma (SCC) tumors. Cultures of SCCVII cells, which originate from a cutaneous SCC that arose spontaneously in C3H mice [11], were maintained in alpha minimal essential medium (Sigma Chemical Co., St. Louis MO, USA) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan UT, USA). The same medium was used for culturing CAL-27 cells (human SCC of the tongue, ATCC CRL-2095) that were kindly provided by Dr. Rajan Saini. Both these cell lines are recognized as good models of human head and neck cancer [11,12]. Mitoxantrone (M6545, Sigma) was dissolved in ethanol (0.5 mg/ml) and this initial stock was then added to cell medium for the final concentration of 1 μg/ml. Neutral sphingomyelinase from B. cereus (S9396, Sigma) was added to serum-free cell medium for final concentration of 250 mU/ml in Petri dish containing 1×106 cells. The same conditions were applied for exposing cells to ceramidase, using the enzyme from Pseudomonas aeruginosa (a homolog of mammalian neutral ceramidases) that was cloned and expressed in Escherichia coli as described previously [13,14].
2.2. PDT treatment
Cells growing in 35-mm diameter Petri dishes were incubated with either Photofrin (20 μg/ml), Temoporfin (0.1 μg/ml, 0.15 μM), Pc4 (1 μg/ml, 1.4 μM) or ce6 (1.5 μg/ml, 2.5 μM). Cell exposure to Photofrin, Temoporfin and Pc4 was for 18 hours in complete growth medium, while ce6 exposure was in serum-free medium for 30 min. Photofrin was obtained from Axcan Pharma (Mont-Saint-Hilaire QC, Canada), Temoporfin (m-tetrahydrophenylchlorin, mTHPC, marketed as Foscan) was from Biolitec Research GmbH (Jena, Germany), ce6 (chlorin e6) was purchased from Frontier Scientific Inc. (Logan, UT, USA), while Pc4 was provided by Dr. Malcolm Kenney (Case Western Reserve University). After photosensitizer exposure, the medium was removed, the dishes (containing around 1 million cells) were washed, and cold PBS left during illumination. The light was produced by an integrated ellipsoidal reflector-equipped FB-QTH high throughput illuminator (Sciencetech, London ON, Canada) based on a 150 W QTH lamp and was delivered through an 8-mm core diameter liquid light guide (Oriel Instruments, Stratford CT, USA). Interference filters 630±10 and 650±10 nm were used for Photofrin and Foscan, respectively, and 665±10 nm for Pc4 and ce6. The fluence rate ranged from 30 mW/cm2 for ce6- and Pc4-PDT to 50 mW/cm2 for Photofrin-PDT.
2.3. Survival Assay
Survival of PDT-treated SCCVII cells was determined by the conventional survival assay [15]. After photosensitizer exposure, cells were washed, trypsinized, and exposed to light while suspended in PBS. Immediately after illumination, the cells were counted and plated for colony growth. The colonies were stained with malachite green six days later and counted. The surviving fraction was calculated as a fraction of plating efficiency of PDT-treated cells.
2.4. Flow cytometry analysis
After PDT treatment, the Petri dishes with cells were returned to the incubator and kept in culture conditions with complete growth medium until they were collected for antibody staining. The exception was the exposure to sphingomyelinase or ceramidase, which was done for 15 minutes in serum-free medium. Intracellular staining for ceramide and S1P was performed as described previously [16]. Briefly, fixed and permeabilized cells were first incubated with anti-ceramide monoclonal antibody 15B4 (Enzo Life Sciences, Plymouth Meeting, PA, USA), anti-S1P monoclonal antibody NHS1P (Cosmo Bio USA, Carlsbad, CA, USA) or with normal mouse IgM (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) as their isotype control. This was followed by exposure to phycoerythrin (PE)-conjugated goat anti-mouse IgM antibody conjugated with (Santa Cruz Biotechnology). The same antibodies were used for detecting cell surface exposed ceramide and S1P, but the procedure was with non-fixed cells. Staining of calreticulin on cell surface was done with PE-conjugated polyclonal antibody to calreticulin (US Biological, Swampscott, MA, USA) with PE-conjugated normal rabbit IgG (Santa Cruz Biotechnology) used as the isotype control. To identify viable and nonviable cells, they were also stained with either 7-aminoactinomycin D (7-AAD, BD Biosciences, San Jose CA, USA) or SYTOX AADvanced (Molecular probes, Eugene, OR, USA). Flow cytometry was performed using a Coulter Epics Elite ESP (Coulter Electronics, Hialeah FL, USA). Ceramide, S1P or calreticulin levels were identified in viable cells based on antibody-associated fluorescence (mean fluorescence intensity in arbitrary units per cell), which was corrected by values obtained with the isotype controls. It should be emphasized that the fluorescence level measured by flow cytometry is in arbitrary units (entirely dependent on the instrument voltage settings used in taking the measurement) that should not be considered comparable between different experiments.
2.5. NFκB ELISA
For preparing primary cultures of TAMs, cells obtained by enzymatic disaggregation of SCCVII tumors and suspended in cell growth medium containing 20% FBS and 1% dispase II (Roche Diagnostics GmbH, Mannheim, Germany) were placed in 35-mm Petri dishes and left in a 37°C incubator. After 30 minutes, non-attached cells were washed away leaving highly enriched populations of attached TAMs [17] as confirmed by flow cytometry documenting their positive staining with anti-mouse FITC-conjugated GR1 and PE-conjugated F480 antibodies (both from eBioscience Inc., San Diego CA, USA). Culture 30-mm inserts with growing SCCVII cells that were treated by Photofrin-PDT (Photofrin 20 μg/ml 18 hours followed by 1 J/cm2) were immediately transferred to Petri dishes containing freshly-selected TAMs for a 3-hour co-incubation at 37°C in complete growth medium. In control samples, TAMs were co-incubated with untreated SCCVII cells. In some samples, anti-ceramide antibody (15B4) or anti-S1P antibody (NHS1P) were present at 20 μg/ml (azide-free) in the co-incubation medium. After the co-incubation, the inserts and culture medium were removed, ice-cold lysis buffer (Cell Signaling Technology, Danvers MA, USA) containing 1 mM phenylmethylsulfonyl fluoride (PMSF, obtained from Sigma) was added to the dishes, and TAMs collected by cell scraper to be stored at −80°C until further use. The activity of NFκB in the TAMs was determined using ELISA kit® PathScan Phospho-NF-κB p65 (Ser536) manufactured by Cell Signaling. Briefly, TAM lysate samples were added to the wells coated with Phospho-NF-κB p65 (Ser536) mouse monoclonal antibody. The Phospho-NF-κB p65 protein captured by the antibody was detected by a rabbit NF-κB p65 detection antibody and recognized by peroxide-linked anti-rabbit IgG antibody through 3, 3′, 5, 5′-tetramethylbenzydine-based colorimetric reaction at 450 nm.
2.6. Statistical analysis
Mann-Whitney test was used for data evaluation and the significance level threshold of 5% (two-tailed test) was set for determining whether the groups were statistically different.
3. Results
3.1. Mitoxantrone and PDT induce cell surface exposure of calreticulin, ceramide and S1P
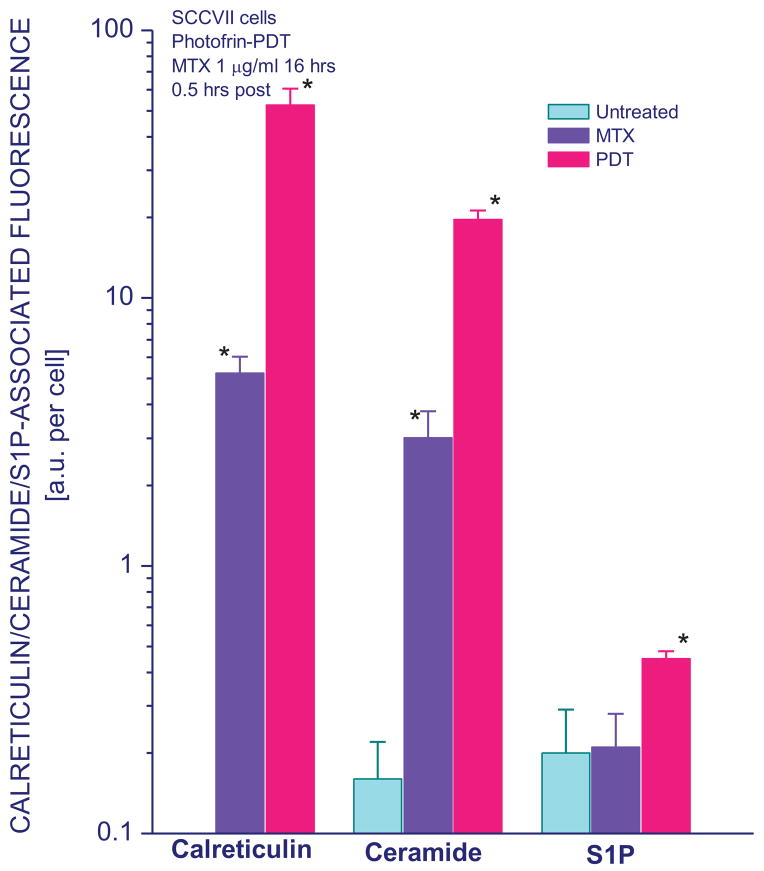
Calreticulin is a protein residing primarily in endoplasmic reticulum but it can be found exposed on the cell surface in response to specific stress stimuli, which include the exposure to anthracyclines and similar drugs such as mitoxantrone or PDT treatment [9,18]. Hence, surface staining with calreticulin-specific antibody of mouse tumor SCCVII cells 30 minutes after their treatment by either Photofrin-PDT or 16-hour exposure to 1 μg/ml mitoxantrone confirmed the appearance of calreticulin exposed on the exterior of these cells that was not detectable on untreated cells (Fig. 1). Counterstaining for dead cells with 7-AAD or SYTOX AADvanced dyes allowed determining calreticulin levels for viable cells. Comparative intracellular staining using the same antibody with permeabilized cells gave around 60% higher values than surface staining (not shown). The results also reveal that cell surface expression of two important sphingolipid molecules, ceramide and S1P, which were barely detectable on untreated cells, changed markedly after PDT treatment. This change was more pronounced with ceramide. The levels of ceramide exposed on cell surface dramatically increased after mitoxantrone treatment as well while no significant change was found with S1P (Fig. 1).
Figure 1. Cell surface expression of calreticulin, ceramide, and S1P on SCCVII cells treated by mitoxantrone or Photofrin-PDT.
After mitoxantrone treatment (1 μg/ml for 16 hours) or PDT treatment (Photofrin 20 μg/ml for 18 hours followed by 1 J/cm2 of 630±10 nm light), cells were left in culture for 30 minutes before they were collected for 7-AAD and surface antibody staining. For the latter, cells were either directly stained with PE-conjugated anti-calreticulin or isotype control rabbit polyclonal antibody, while in other cases anti-ceramide, anti-S1P and or isotype control IgM exposure was followed by PE-conjugated secondary antibody. The results of flow cytometry analysis are presented for 7-AAD negative cells and shown as fluorescence (in arbitrary units per cell) directly corresponding to the levels of calreticulin, ceramide or S1P on the surface of cells. The bars denote SD, n = 4; * = statistically significant difference from untreated group levels.
3.2. Cell surface and integral levels of ceramide and S1P after Photofrin-PDT
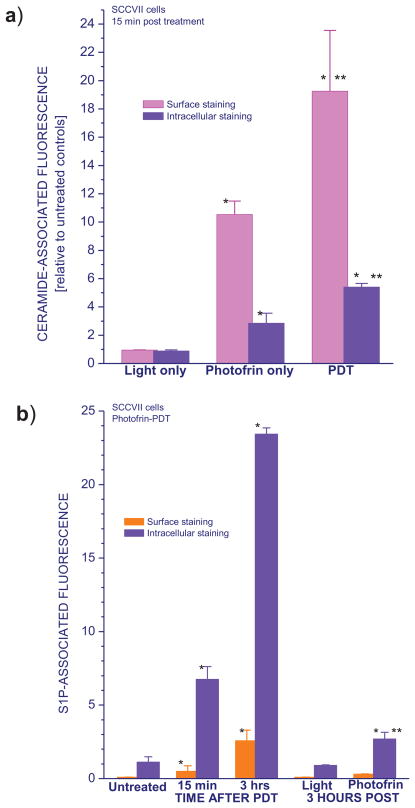
Comparative intracellular and surface staining with the same anti-ceramide antibody was performed to assess integral cellular ceramide levels as well as on the surface of same SCCVII cells. These integral cellular levels of ceramide also increased following PDT treatment as reported earlier [16] but the rise was not as striking as with the surface-exposed ceramide (as shown by the relative values depicted in Fig. 2a). Cell surface-exposed ceramide was detectable already at 15 minutes after Photofrin-PDT, while light alone treatment had no significant impact on either surface or total ceramide levels. Interestingly, the exposure to Photofrin resulted in a rise of surface levels and to a lesser extent integral levels of ceramide even without illumination. Compared to PDT, however, the effect of Photofrin alone was considerably less pronounced indicating that the induction of ceramide surface exposure is largely PDT-specific.
Figure 2. Ceramide and S1P levels in SCCVII cells treated by Photofrin-PDT determined by surface and intracellular antibody staining.
Shown are changes in ceramide (a) and S1P levels (b) after light alone, photosensitizer alone, or PDT treatment followed by a post-incubation of 15 min (ceramide) or either 15 min or 3 hours (S1P). Cells were treated with Photofrin-PDT as described for Fig. 1, with the 1 J/cm2 and 20 μg/ml doses used also for the light alone and Photofrin alone treatments. Ceramide and S1P levels determination was either as described for Fig. 1 (surface staining) or with fixed and permeabilized cells using the same antibody (intracellular staining). The results are presented either as relative values compared to those obtained with corresponding untreated control samples (a) or the extent of S1P-associated fluorescence per cell (b). The bars denote SD, n = 4; * = statistically significant difference from corresponding untreated group levels, ** = statistically significant difference from values obtained with corresponding light only and Photofrin only groups.
The exposure of S1P on the surface of SCCVII was also detectable at 15 minutes after Photofrin-PDT (Fig. 2b). Its levels, as well as integral S1P, increased even more at 3 hours post treatment. No significant changes in S1P were induced by the light treatment alone, whereas a small rise in integral S1P levels was registered after exposure to Photofrin alone.
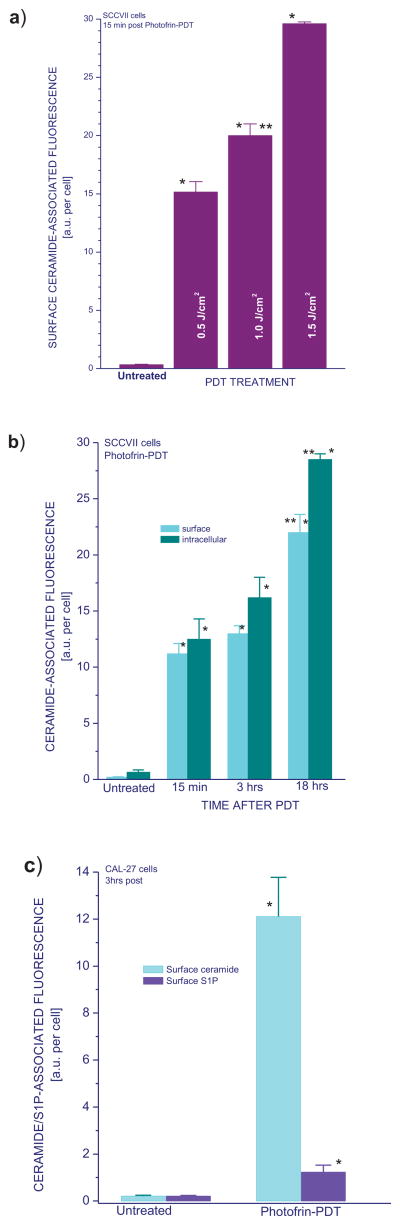
Further experiments revealed that the extent of increase in surface ceramide levels depend on PDT dose (Fig. 3a), but the expression of ceramide on the cell surface was highly pronounced even with the lowest tested PDT dose.
Figure 3. Ceramide levels in SCCVII and CAL-27 cells treated by Photofrin-PDT with dependence on PDT dose or time interval after treatment.
To determine the changes in ceramide levels dependent on PDT-dose (a), cells were treated with Photofrin-PDT as described for Fig. 1, except for additional light doses (0.5 and 1.5 J/cm2). For post-treatment time-dependence analysis (b), cells were after PDT light treatment left in the 37°C incubator for either 15 min, 3 hours, or 18 hours before collected for antibody staining and flow cytometry. Using CAL-27 instead of SCCVII cells (c) served to demonstrate the PDT-induced surface ceramide and S1P induction in a different cell line. The post-incubation time interval used with these cells was 3 hours. Ceramide and S1P levels determination was as described for Figs. 1 and 2. The bars denote SD, n = 4; * = statistically significant difference from corresponding untreated group levels, ** = statistically significant difference from 15-min group.
Examined next was the time-dependence of the appearance of ceramide on the SCCVII cell surface after PDT treatment. While detectable already at 15 minutes after Photofrin-PDT, the surface exposure of ceramide persisted at similar levels at 3 hours post treatment and became further elevated at 18 hours after PDT (Fig. 3b). A similar kinetics was observed for the integral ceramide levels.
3.3. Cell surface ceramide and S1P exposure on PDT-treated CAL27 cells
To confirm with different cultures the above findings on mouse tumor SCCVII cells, human squamous cell carcinoma cells, CAL-27, were treated by Photofrin-PDT. Cell surface levels of ceramide and S1P were assessed at 3 hours post treatment. The appearance of these sphingolipids was clearly evident following Photofrin-PDT, although the effect was much more pronounced for ceramide (Fig. 3c). No evidence was detected of difference between SCCVII and CAL-27 cells.
3.4. Use of sphingomyelinase and ceramidase for validation of ceramide detection
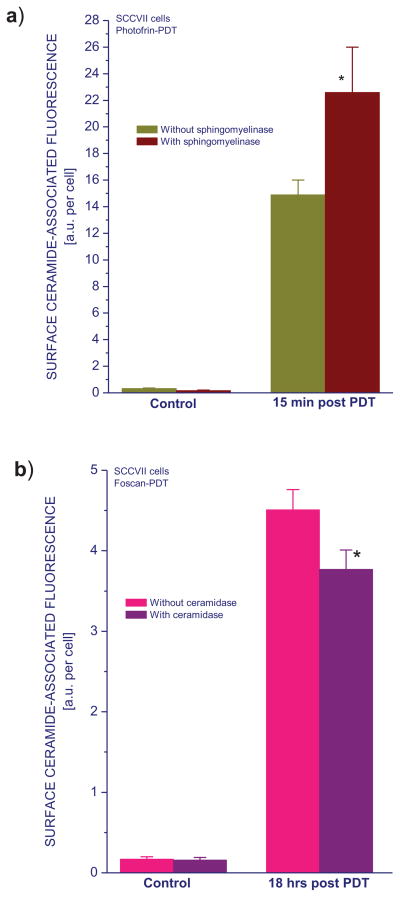
Since it would be of benefit if the 15B4 antibody used cellular ceramide detection could be more fully validated, this was addressed using sphingomyelinase and ceramidase, the enzymes involved in ceramide metabolism. Sphingomyelinase (250 mU/ml in serum-free medium) was added to Photofrin-PDT treated SCCVII cells immediately after the termination of light treatment and left in contact with cells until they were collected 15 minutes later. This neutral enzyme cleaves membrane-bound sphingomyelin to yield ceramide and phosphocholine [19]. As expected, ceramide levels detected on the surface of PDT-treated cells were significantly higher in the presence of sphingomyelinase (Fig. 4a). For testing the effect of ceramidase, cells were after PDT left in culture for 18 hours. During the last 15 minutes of this period, cell medium was replaced with a fresh serum-free medium containing ceramidase (250 mU/ml). The presence of this enzyme, that cleaves the N-acyl linkage in ceramide to form sphingosine and free fatty acid [20], caused a decrease in the levels of detected surface ceramide levels (Fig. 4b).
Figure 4. The effect of sphingomyelinase and ceramidase treatment on cell surface ceramide expression following PDT.
SCCVII cells were treated by Photofrin-PDT as described for Fig. 1. For Temoporfin-PDT, photosensitizer exposure (0.2 μg/ml for 18 hours) was followed by 1 J/cm2 light dose. After PDT, cells were then kept in culture for 15 minutes (Photofrin-PDT) or 18 hours (Temoporfin-PDT). During the last 15 minutes of this post-incubation, the cells were exposed to either sphingomyelinase (a) or ceramidase (b), both at 250 mU/ml, before their collection. Subsequent cell surface ceramide staining and flow cytometry analysis were as described for Fig. 1. The results are presented as surface ceramide-associated fluorescence intensity per cell. The bars denote SD, n = 4; * = statistically significant difference from untreated group levels, ** = statistically significant difference from corresponding sphingomyelinase/ceramidase untreated group levels.
3.5. Cell surface ceramide exposure after PDT with various photosensitizers
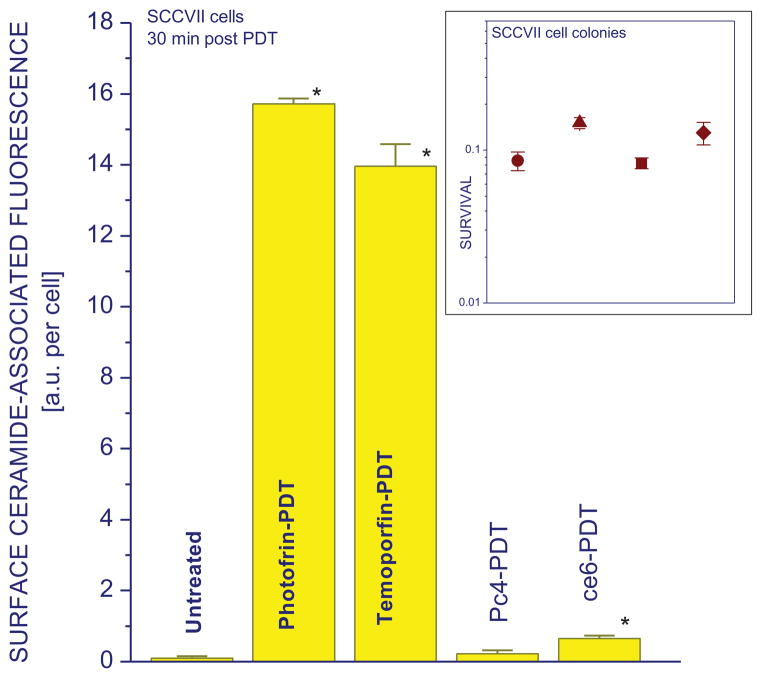
Further investigation revealed that the induction of surface ceramide expression can differ considerably with different photosensitizers used for PDT. The doses of Photofrin-PDT, Temoporfin-PDT, ce6-PDT, and Pc4-PDT were chosen that attain a similar degree of cell killing with around 10% surviving fraction based on colony-forming assay. The results for the chosen time interval (30 min post treatment) show that similar levels of surface-exposed ceramide can be detected on cells appearing viable (7-AAD unstained) after Photofrin- and Temoporfin-PDT. A considerably less surface ceramide was found after ce6-PDT, while the surface ceramide induction appeared not significant after Pc4-PDT treatment (Fig. 5).
Figure 5. Cell surface ceramide levels induced by PDT mediated by different photosensitizers.
SCCVII cells were treated either Photofrin-PDT as described for Fig. 1 or by PDT mediated by Temoporfin (0.2 μg/ml – 24 hrs), chlorin e6 (1.5 μg/ml – 30 min), or Pc4 (1 μg/ml – 18 hrs), with light dose 1 J/cm2 in all cases. These PDT treatments produced a similar level of cell killing, as determined by the colony formation assay (insert; ● = Photofrin-PDT, ▲ = Temoporfin-PDT, ■ = Pc4-PDT, ◆ = ce6-PDT). For surface ceramide analysis, cells were in all cases collected after 30 min post-PDT incubation for antibody staining as described for Fig. 1. The results are presented as means of surface ceramide-associated fluorescence intensity per cell. The bars denote SD, n = 4; * = statistically significant difference from untreated group levels.
3.7. Effect on NFκB activity
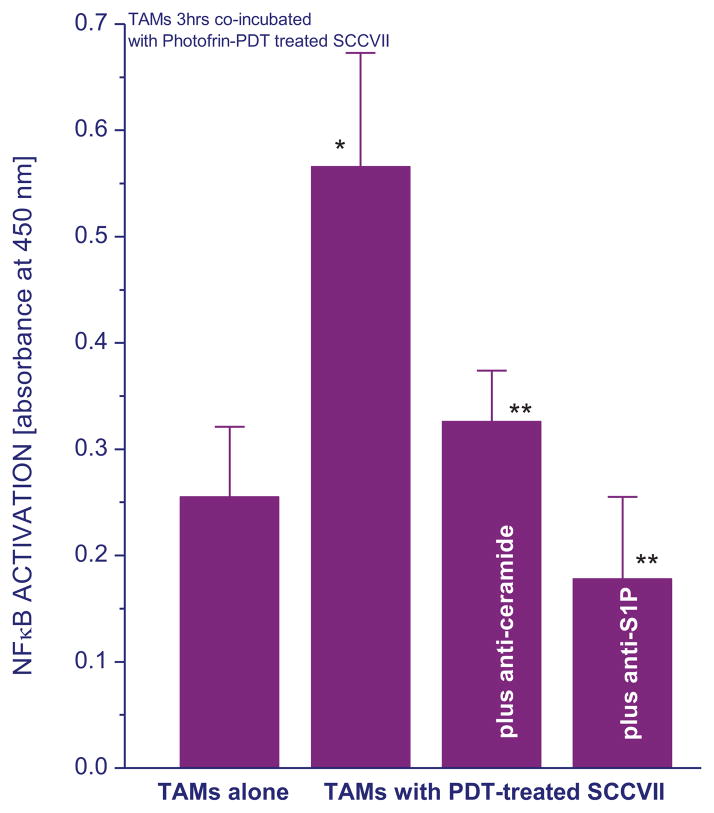
Since NFκB is a major regulator of transcriptional activity of genes involved in inflammatory/immune responses as well as cell proliferation and death [21], its status in TAMs was examined upon their co-incubation for 3 hours with PDT-treated SCCVII cells. The TAM populations were selected from cell suspensions obtained from untreated SCCVII tumors using a standard selective attachment protocol (see Materials and Methods). The results confirm that NFκB activity in these TAMs increased considerably compared to that in TAMs incubated alone (Fig. 6) or co-incubated with untreated SCCVII cells (not shown). We have shown that the rise in NFκB activity is followed by the production of cytokines such as TNFα by these TAMs [22]. Evidence of NFκB activation in TAMs induced in the presence of PDT-treated tumor cells was already document in our previous work [22,23]. To test whether the presence of SLs in the co-incubation medium may be involved in the observed activation of NFκB, additional samples were prepared with antibodies neutralizing either ceramide or S1P in the co-incubation medium. The results reveal that the NFκB activation in TAMs was completely prevented by neutralizing S1P and strongly diminished by blocking ceramide (Fig. 6).
Figure 6. Activation of NFκB in TAMs induced by co-incubation with PDT-treated SCCVII cells is blocked by ceramide- or S1P-neutralizing antibodies.
Cultures of TAMs obtained from SCCVII tumors were incubated alone or co-incubated with cultured SCCVII cells treated by PDT as described for Fig. 1. Some samples contained anti-ceramide or anti-S1P antibody (20 μg/ml) in the co-incubation medium. After 3 hours, TAMs were collected and their NFκB activity measured using PathScan® Phospho-NF-κB p65 (Ser536) ELISA. The results are shown as colorimetric readouts (absorbance at 450 nm) reflecting levels of phospho-NFκB p56 protein at Ser536. The bars denote SD; * = statistically significant difference from the value in TAMs incubated alone, ** = statistically significant difference from the value in TAMs co-incubated with PDT-treated SCCVII cells in the absence of corresponding antibody.
4. Discussion
The results of the present study demonstrate that ceramide and S1P become exposed on the surface of cells treated by PDT. This was found with cultures of mouse and human SCC cells, SCCVII and CAL27 respectively, both serving as well established models for human head and neck tumors [11,12]. Such surface expression post PDT is known to occur with calreticulin, a well known DAMP [9,10], as confirmed in Fig. 1. Moreover, as shown in the same Figure, mitoxantrone treatment also known to induce calreticulin surface expression [18] proved effective in prompting surface ceramide exposure as well.
The antibodies used for detecting ceramide and S1P both inside cells and on their surface were described as highly specific under physiological conditions and have proven valuable in our earlier work [16]. The anti-ceramide antibody was reported not react with sphingomyelin, cholesterol or other phospholipids [24], while anti-S1P antibody showed no cross-reactivity with ceramide, sphingosine, sphingomyelin and other phospholipids [25]. Additional testing of anti-ceramide antibody reliability was done by incubating cells with either sphingomyelinase or ceramidase, the enzymes that release ceramide or break it down, respectively. The detected changes with PDT-treated cells had the expected trend, i.e. an increase in ceramide levels found with sphingomyelinase treatment and an opposite effect with ceramidase treatment (Fig. 2). However, it is not quite clear why there was no increase in ceramide signal on untreated cells exposed to sphingomyelinase because this should have also resulted in the production of sphingomyelin-derived ceramide. A possible explanation is that the generated ceramide was removed after washing cells repeatedly during the procedure for antibody staining. Ceramide exposed on the surface of PDT-treated cells appears not to be attached that loosely. After PDT treatment there are changes in the cell membrane including possible inner membrane sections flopping outward to the outer surface (that could not be washed out)[22], which could be responsible for ceramide exposure. In ongoing studies, we continue investigating the characteristics of ceramide and S1P exposure on cell surface and reliability of their detection by available antibodies.
The presented results also reveal that ceramide and S1P exposure on cell surface is not only PDT dose-dependent, but also varies in its intensity depending of the photosensitizer. Comparing the results with four photosensitizers at the doses producing a similar rate of cell kill reveals that the strong effect seen with Photofrin and Temoporfin is less pronounced but still evident with ce6, whereas it was not detectable after Pc4-PDT (Figs.5 and additional unpublished data). This pertains to cells remaining viable at 30 min after PDT. In contrast, nonviable cells from the same samples had greatly reduced ceramide staining after Photofrin- and Temoporfin-PDT but substantially higher than with viable cells after Ce6- and Pc4-PDT (not shown). However, surface staining of damaged cells is not reliable because they can become permeabilized for antibodies or normally internal ceramide-containing membrane sections can become exposed.
The above differences with photosensitizes could be related to the sites of their intracellular distribution because PDT-induced oxidative stress is restricted to these sites [7]. One of critical locations is the endoplasmic reticulum (ER), which is the site of calreticulin accumulation as well as ceramide and S1P biosynthesis. Among the tested four photosensitizers, Temoporfin tends to localize mostly in the ER and Golgi apparatus, Photofrin is found in various cellular membrane structures including ER, while mitochondrial membranes and ER are the predominant sites for Pc4, and lysosomes for ce6 [7,31,32]. Despite this diversity, the presence of at least minor fractions of all these photosensitizers in the ER could be sufficient to trigger ER stress pathways, which were suggested to activate a specific danger signaling module required to emit immune response-defining DAMPs [29,30]. Hence, it seems unlikely that, the intracellular localization profile of photosensitizers is a predominant determinant of PDT-induced ceramide/S1P externalization.
One of the factors likely contributing to a pronounced surface exposure of ceramide after Photofrin-PDT is the relatively high photosensitizer concentration needed to be used because of lower potency of Photofrin. As shown in Fig. 3, at the used concentration (20 μg/ml) Photofrin induces the expression of both ceramide and S1P on cell surface even without light treatment. It is known from the literature that Photofrin treatment can have significant effects in the dark because of its porphyrin nature it can significantly affect cellular metabolism [26,27]. Such drug alone effect was not observed with Temoporfin, ce6 and Pc4 at the concentrations used for PDT. The above differences between Photofrin- and Pc4-PDT in vitro may have relevance for the therapy outcome in vivo, as evidenced by superior control of SCCVII tumors by Photofrin-PDT compared to Pc4-PDT when combined with sphingolipid modulating agents [28].
Comparison of ceramide and S1P levels detected by surface and intracellular antibody staining suggests that the size of surface-exposed fraction is much greater in case of ceramide than with S1P (Fig. 2). Several explanations are possible for this difference. It is possible that the rate of the induced translocation from ER to the cell surface is more efficient for ceramide than S1P. Even more likely possibility is that the surface-exposed S1P is easier to liberate from cells than ceramide, because S1P is known to be efficiently secreted from cells in order to act in both paracrine and autocrine manner [33].
After PDT, both ceramide and S1P exposed on the surface of treated tumor cells can get in contact with neighboring immune effector cells like macrophages and dendritic cells that are equipped with surface receptors capable of binding these sphingolipids. It was reported that ceramide can serve as an agonist of Toll-like receptor-4 (TLR4) and activate signaling associated with this receptor [34]. On the other hand, S1P receptors are ubiquitously expressed on macrophages and other cells. Our data indicate that both ceramide and S1P could be involved in triggering NFκB signaling in macrophages co-incubated with PDT-treated SCCVII cells (Fig. 6). Interestingly, genes involved in de novo ceramide biosynthesis are also activated through TLR4-NFκB signaling [35].
In conclusion, this report establishes that both ceramide and S1P can act as DAMPs. Their engagement is elicited by insults such as oxidative stress, particularly when involving the ER as is the case with PDT mediated by various photosensitizers. This type of ER perturbation prompts the activation of unfolded protein response [30]. This response developed for reestablishing ER homeostasis was found to also serve as a specific danger signaling module required to emit DAMPs [4]. We suggest that, similarly to the well established ER-originating DAMP calreticulin, ceramide and S1P could be launched by the ER stress pathway to act as DAMPs activating inflammatory/immune processes that can culminate in immune rejection of DAMP-emitting tumor cells.
Highlights.
Ceramide and sphingosine-1-phosohate (S1P) cell levels change after photodynamic therapy (PDT)
Both ceramide and S1P appear on the surface of tumor cells treated by PDT
Cell surface-exposed ceramide and S1P on PDT-treated tumor cells allows them to act as DAMPs
Acknowledgments
This work was made possible by support from the Canadian Cancer Society (grant # 2012-701132) and from the US Public Health Service Grant R01 CA77475 from the National Cancer Institute (NCI), National Institutes of Health (NIH) and NCI Grant IPO1CA097132.
Footnotes
Competing interests: The authors declare no existence of competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 2.Seong S, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates immune responses. Nature Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 3.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Cecic I, Stott B, Sun J, Korbelik M. Relevance of innate immunity recognition of altered self in the induction of host response associated with photodynamic therapy. Recent Res Devel Cancer. 2004;6:153–161. [Google Scholar]
- 6.Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:e631. doi: 10.1038/cddis.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn S, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA: A Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38:500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- 9.Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cell death: origins, plasticity and regulation. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.48. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korbelik M, Zhang W, Merchant S. Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol Immunother. 2011;60:1431–1437. doi: 10.1007/s00262-011-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana D, Martin EA, Kasperbauer JL, O’Malley BW, Jr, Salomao DR, Chen L, Strome SE. Characterization of a spontaneously arising murine squamous cell carcinoma (SCC VII) as a prerequisite for head and neck cancer immunotherapy. Head Neck. 2001;23:899–906. doi: 10.1002/hed.1130. [DOI] [PubMed] [Google Scholar]
- 12.Gioanni J, Fischel J-L, Lambert J-C, Demard F, Mazeau C, Zanghellini E, Ettore F, Formento P, Chauvel P, Lalanne C-M, Courdi A. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol. 1988;24:1445–1455. doi: 10.1016/0277-5379(88)90335-5. [DOI] [PubMed] [Google Scholar]
- 13.Wu BX, Snook CF, Tani M, Büllesbach EE, Hannun YA. Large-scale purification and characterization of recombinant Pesudomonas ceramidase: regulation by calcium. J Lipid Res. 2007;48:600–608. doi: 10.1194/jlr.M600423-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Canals D, Jenkins RW, Roddy P, Henandez-Corbacho MJ, Obeid LM, Hannun YA. Differential effects of ceramide and sphingosine-1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane. J Biol Chem. 2010;285:32476–32485. doi: 10.1074/jbc.M110.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:636–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korbelik M, Zhang W, Separovic D. Monitoring ceramide and sphingosine-1-phosphate levels in cancer cells and macrophages from tumours treated by photodynamic therapy. Photochem Photobiol Sci. 2012;11:779–784. doi: 10.1039/c2pp05384e. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty DJ, Allen CA, Hogg NM. Applications of immunological techniques to the study of tumor-host relationship. In: Weir DM, Herzenberg LA, Blackwell C, Herzenberg LA, editors. Handbook of Experimental Immunology. Vol. 4. Oxford: Blackwell; 1986. pp. 125.1–125.12. [Google Scholar]
- 18.Obeid M, Tesniere S, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S. Neutral sphingomyelinase: past, present and future. Chem Phys Lipids. 1999;102:79–96. doi: 10.1016/s0009-3084(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 20.Merrill AH, Jr, Wang E. Enzymes of ceramide biosynthesis. Methods Enzymol. 1992;209:427–437. doi: 10.1016/0076-6879(92)09053-6. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar FH, Li Y, Wang Z, Kong D. NF-κB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- 22.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 23.Korbelik M. Complement upregulation in photodynamic therapy-treated tumors: Role of Toll-like receptor pathway and NFκB. Cancer Lett. 2009;281:232–238. doi: 10.1016/j.canlet.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 24.Cowart LA, Szulc Z, Bielawska A, Hannun YA. Structural determinants of sphingolipid recognition by commercially available anti-ceramide antibodies. J Lipid Res. 2002;43:2042–2048. doi: 10.1194/jlr.m200241-jlr200. [DOI] [PubMed] [Google Scholar]
- 25.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phorphate receptor-2 enhances migration of neural progenitor cells toward an area of brain infarction. Stroke. 2008;39:3411–3417. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 26.Canti G, Franco P, Marelli O, Ricci L, Nicolin A. Hematoporphyrin derivative rescue from toxicity caused by chemotherapy or radiation in a murine leukemia model (L1210) Cancer Res. 1984;44:1551–1556. [PubMed] [Google Scholar]
- 27.Bressoud D, Jomini V, Tyrell RM. Dark induction of haem oxygenase messanger RNA by haematoporphyrin derivative and zinc phthalocyanine; agents for photodynamic therapy. J Photochem Photobiol B: Biol. 1992;14:311–318. doi: 10.1016/1011-1344(92)85110-g. [DOI] [PubMed] [Google Scholar]
- 28.Korbelik M, Zhang W, Saw KM, Szulc ZM, Bielawska A, Separovic D. Cationic ceramides and analogues, LCL30 and LCL85, as adjuvants to photodynamic therapy of tumors. J Photochem Photobiol B: Biol. 2013;126:72–77. doi: 10.1016/j.jphotobiol.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. The emergence of phox—ER stress induced immunogenic apoptosis. Oncoimmunology. 2012;1:786–788. doi: 10.4161/onci.19750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Letters. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Marchal S, Bezdatnaya L, Guillemin F. Modality of cell death induced by Foscan-based photodynamic treatment in human colon adenocarcinoma cell line HT29. Biochemistry (Mosc) 2004;69:45–49. doi: 10.1023/b:biry.0000016350.61894.be. [DOI] [PubMed] [Google Scholar]
- 32.Calzavara-Pinton PG, Venturini M, Sala R. Photodynamic therapy: update 2006. Part 1: Photochemistry and photobiology. J Eur Acad Dermatol Venereol. 2007;21:293–302. doi: 10.1111/j.1468-3083.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- 33.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer H, Ellström P, Ekström K, Gustafsson L, Gustafsson M, Svanborg C. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell Microbiol. 2007;9:1239–1251. doi: 10.1111/j.1462-5822.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 35.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]