Abstract
The amount of postnatal experience for perinatal rats was manipulated by delivering pups one day early (postconception day 21; PC21) by cesarean delivery and comparing their motor behavior to age-matched controls on PC22 (the typical day of birth). On PC22, pups were tested on multiple measures of motor coordination: leg extension response (LER), facial wiping, contact righting, and fore- and hindlimb stepping. The LER and facial wiping provided measures of synchronous hind- and forelimb coordination, respectively, and were sensory-evoked. Contact righting also was sensory-evoked and provided a measure of axial coordination. Stepping provided a measure of alternated forelimb and hindlimb coordination and was induced with the serotonin receptor agonist quipazine. Pups that were delivered prematurely and spent an additional day in the postnatal environment showed more bilateral limb coordination during expression of the LER and facial wiping, as well as a more mature righting strategy, compared to controls. These findings suggest that experience around the time of birth shapes motor coordination and the expression of species-typical behavior in the developing rat.
Keywords: Neonatal, motor development, maternal care, postnatal experience, premature birth
1. Introduction
1.1 Experiential Effects in Sensory Systems
Developmental and psychobiological research during the perinatal period has shown that manipulating sensory experiences, either by deprivation or enhancement, can profoundly affect developmental outcomes [1]. Classic studies on vision deprivation in kittens demonstrated cortical reorganization after sewing one or both eyes shut [2, 3]. Congenitally deaf kittens fitted with cochlear implants showed increased auditory cortex activity compared with naïve deaf and hearing controls [4]. Deprivation studies of the whisker barrel cortex in developing rats also have shown cortical plasticity in the somatosensory system due to experience [5].
Other research has shown that premature enhancement of sensory input in a later developing modality can inhibit the development of other modalities [6, 7, 8]. For example, premature visual stimulation in young rats interferes with the normal development of olfaction [9] and facilitates exploratory and hindlimb rearing behaviors in young rats whose eyes were surgically opened one week early [10]. This shift in the timing of visual input had an effect on the development of other sensory and motor systems. A great deal of research is available on activity-dependent plasticity in sensory systems. However, less is known about the effects of experience on motor systems, particularly during early development.
1.2 Experiential Effects in Motor Systems
Some evidence for the plasticity of motor systems during early development has been demonstrated in animal and human research. For example, changes in interlimb motor coordination in fetal rats has been shown to occur following interlimb yoke training [11]. Training consisted of yoking (physically linking) the fetus’s hindlimbs together so that movement was constrained to in-phase, conjugate trajectories for a 30-minute training session. After training, the interlimb yoke was cut so that the hindlimbs could move independently. Overall hindlimb activity increased, as well as conjugate movements, both during yoking and afterwards (as if the yoke were still constraining movement). Additional research with the yoke training paradigm has demonstrated modification of limb patterns between forelimbs, ipsilateral hind and forelimbs, as well as hindlimbs [12]. Yoke training increased conjugate limb movement for trained limbs during the yoked and unyoked post-training periods. These results suggest that fetal rats can adapt their motor behavior in response to proprioceptive feedback to learn new motor patterns.
Viala, Viala, and Fayein [13] determined that locomotor behavior in young spinalized rabbits is dependent on hindlimb experience. Infant rabbits were suspended in a sling with their hind paws secured to motor-driven pedals that moved in a synchronous pattern, an alternating pattern, or both. Animals trained on the synchronous or alternating pattern showed respective hopping or walking locomotor gaits exclusively, reflecting their training regimen. Animals trained on both patterns displayed both hopping and walking gaits. In addition, recent evidence from studies of human infants suggests that babies within the first year of life respond to sensory information, such as trip-inducing stimuli [14] and load [15], during brief stepping trials when tested on a treadmill.
Muir and Chu [16] showed that, even in precocial animals such as chicks, experience is necessary for the development of adult patterns of upright walking. Chicks trained on a treadmill showed more mature locomotor development than either the experience-restricted or the normal experience groups. Experience-restricted chicks showed shortened stride lengths and decreased horizontal head movements (characteristic of chick locomotion) compared to age-matched controls. Sindhurakar and Bradley [17] demonstrated that chick embryos exposed to increased amounts of light showed accelerated locomotor development due to early hatching, without impacting motor performance. However, extreme amounts of light exposure may have accelerated the development of neural circuitry used in locomotion. This suggests that motor systems utilize species-typical experiences in order to develop properly, just as sensory systems.
1.3 Purpose of Current Study
The studies described above provide examples of changes in motor behavior that result from providing immature animals with atypical experiences (e.g., yoke motor training, stepping on a treadmill, etc.). In contrast, the current study addresses the effects of typical kinds of experiences (e.g., labor, delivery, exposure to gravity, maternal-infant interaction, etc.) on the development of species-typical action patterns. The current study examined the effect of an additional 24 hours of postnatal experience on motor behavior in the in vivo perinatal rat, thus exposing rats prematurely to gravitation, variations in maternal care, skin-to-skin contact with siblings, and so on. Our hypothesis was that pups that had an extra day of postnatal experience would show more coordinated behavior during expression of species-typical action patterns, compared to age-matched pups with less postnatal experience. Motor coordination was examined in four different motor tasks/action patterns: the LER (a synchronous hindlimb task), facial wiping (a synchronous forelimb task), contact righting (axial coordination) and quipazine-induced stepping (an alternated forelimb and hindlimb task). Each of these action patterns has been shown to occur in the perinatal rat using the appropriate evoking stimulus (see section 1.5 Action Patterns).
1.4 Perinatal Development in the Rat
Although they are born furless, blind, and deaf, neonatal rats have some sensory capacities and behavioral abilities that are adaptive to and functional in the terrestrial environment. For instance, they have olfaction and are able to root for, attach to, and suckle at the dam’s nipple. They also can emit ultrasonic vocalizations to solicit care from the dam. Rooting and suckling are good examples of coordinated behavior that involve coordinated movement of the head, mouth, and forelimbs. Such nascent behaviors start developing before birth and can be studied directly in the rat fetus. Over the past few decades, such research has demonstrated that the expression of coordinated behavior in the newborn emerges from initial spontaneous movements in the fetus [18].
1.5 Action Patterns
In addition to expressing spontaneous motor activity, perinatal rats exhibit several action patterns, such as facial wiping, suckling, rooting, and forelimb treadling. Evidence for the development of these action patterns shows that the neural circuitry for these behaviors begins developing before birth. The action patterns of concern for this study are the leg extension response (LER), facial wiping, contact righting, and alternated fore- and hindlimb stepping.
1.5.1 The Leg Extension Response
First described in newborn rats, the LER is expressed when the hind legs move from a resting posture to an immobile and stiffly extended posture with the hindquarters lifted [19]. Moore and Chadwick-Dias [19] demonstrated the LER occurs in rat pups in response to stimulation of the anogenital region and not in response to stimulation of other areas of the body, and continues into about the third postnatal week. This action pattern occurs in the neonate when the dam licks the pup’s anogenital region to necessitate urination and defecation. In addition, the dam benefits by reclaiming fluids and nutrients lost through nursing [20, 21]. The proximal function of the LER seems to be to provide better access to the pup’s anogenital region in order to promote micturition and defecation. Rat fetuses tested two days before birth reliably show the LER when stimulated by a soft camelhair brush (mimicking stimulation by the dam), thus providing prenatal evidence of neural control of the response [22].
1.5.2 Facial Wiping
Facial wiping is another action pattern expressed by the perinatal rat; it is the act of drawing one or both paws along the side of the face from the ears downward toward the nose. In adults, the action pattern is part of the stereotypical grooming repertoire and provides a good measure of interlimb coordination expressed by the forelimbs. Rat fetuses typically do not express the facial wiping response during spontaneous activity, although the response can be evoked in E20 (embryonic day 20; 2 days before birth) rats by infusing an aversive stimulus, such as lemon extract, into the mouth of the fetus through an intraoral cannula [23]. This same method has been used to evoke facial wiping in newborn rats [24].
1.5.3 Contact Righting
Contact righting involves full body (axial) coordination and is used to test vestibular and tactile function [25]. Animals are typically tested by placing them onto a surface in a supine position, and observing the latency and motor strategy used to return to a prone posture. Pellis et al. [25] have shown that newborn rats are capable of righting, and that the righting strategy typically used changes during the early postnatal period. From the day of birth (postnatal day 0; P0) to P2, pups typically use the u-shaped posture to right themselves from supine to prone. When pups are held supine in contact with the ground, they raise the head and hindlimbs skyward, thus forming a “U” posture. This posture allows the pup to fall to one side and then rotate to a fully prone position. The corkscrew pattern develops subsequently and is used by rotating the head, neck, and shoulders in one direction while the pelvis rotates in the opposite direction. The percentage of pups using the corkscrew pattern increases from P0–P2, as the u-shaped strategy decreases during the same period.
1.5.4 Alternated Fore- and Hindlimb Stepping
Locomotor-like stepping is an action pattern that involves antiphase coordination between the limbs. Newborns rats typically do not show walking locomotion until approximately two weeks after birth, mainly due to muscle weakness and poor postural control [26]. Thus locomotor behavior is often studied in these young animals by using a paradigm called air-stepping. In air-stepping, the pup is suspended in a sling so that the limbs hang freely. The animal is then injected with a drug (e.g., L-DOPA [27]; or a 5-HT agonist [28]) or exposed to strong sensory stimulation (e.g., [29], [30]) that induces stepping.
Recently we showed that quipazine-induced stepping in P1 rats is modulated by sensory feedback [28], suggesting that developing locomotor circuits are influenced by peripheral events. Alternated limb movement also has been observed in naturalistic environments as well as in the air-stepping paradigm [31]. As with the LER and facial wiping, evidence suggests that the neural mechanisms supporting locomotor behavior begin developing before birth [32, 33]. Thus, in this study, we examined how stepping behavior, in addition to the LER, facial wiping, and contact righting, in prematurely delivered rats compared to that of age-matched controls. These action patterns were examined because they provided measures of different patterns of motor coordination, they can be evoked in perinatal rats, and they are species-typical patterns of motor coordination. All animals were tested at the same post-conceptional age, but differed in the amount of time that they had in the prenatal vs. postnatal environment.
2. Methods
2.1 Subjects
Subjects were 96 post-conception day 22 (PC22) male Sprague-Dawley rats bred from 24 litters in the vivarium at Idaho State University (ISU). Adult animals were obtained from Simonsen Laboratories. The day of conception was determined by the presence of sperm from vaginal smears during a 4-day breeding period. The day of conception was designated embryonic day 0 (E0), the day before birth was E21, and the day of birth was E22 (PC22). Pregnant females were pair housed until E19, when they were housed individually. Animals were kept on a 12:12-hour light:dark cycle (lights on at 0700) with food and water ad libitum. Adult and newborn rats were maintained in accordance with guidelines for animal care established by the National Institutes of Health and by the Institutional Animal Care and Use Committee at ISU.
2.2 Delivery of Pups
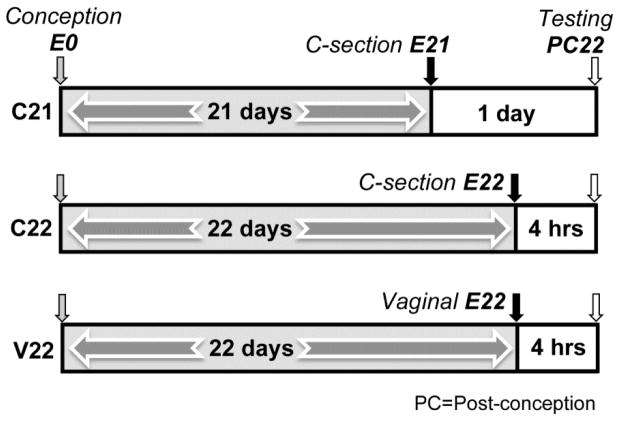
Subjects (males only) were delivered in one of three birth conditions (Figure 1). Subjects in the experimental group, C21, were delivered by cesarean section on post-conception day 21 (one day before birth: E21). Subjects in the other two groups were delivered on the timed day of birth (PC22). One group, the C22 group, was delivered by c-section on PC22, before any evidence that the pregnant female was in labor (e.g., contractions or the presence of blood). This condition was the control group for the effects of c-section that was necessary to deliver the C21 group one day early. The other control group, V22, delivered vaginally. This unmanipulated control group experienced the normal birth process and stayed in the home cage until testing. Both control groups (C22 and V22) were exposed to 4–7 hours (n = 16 litters; M = 4.86, SD = 0.71) of postnatal experience before behavioral testing compared to 25–27 hours (n = 8 litters; M = 26.23, SD = 1.51) for the experimental (C21) group. Statistical analysis showed no difference in amount of postnatal experience (4–7 hrs) between the two control groups [M = 4.8, F(1, 14) = .10, p = n.s.]. All subjects were delivered by 1300 hrs.
Figure 1.
C21, C22, and V22 groups are shown in individual timelines. Gray shaded arrows indicate number of days in a prenatal environment. Small box areas in timeline show hours spent in a postnatal environment. Arrows indicate day of conception (E0), day of delivery (on E21 or E22) and corresponding method of delivery (Cesarean delivery or Vaginal), and day testing occurs, Post-conception Day 22 (PC22).
On E21 or E22, the pregnant females in the cesarean delivery groups (C21 and C22) were euthanized by cervical dislocation [34] following a light anesthesia with isoflurane [35]. Isoflurane was administered through a calibrated rodent inhalation anesthesia delivery system (Matrx) using a concentration of 5.0%/L/min in 100% oxygen. C-section dams were placed in the induction chamber (23.5 cm × 12 cm × 12 cm) until behavioral measures of Stage 3, Level 2, anesthesia were observed.
Immediately after cervical dislocation, an incision was made in the dam’s abdominal wall and the uterus was externalized [34]. The individual fetuses were removed from the uterus and placed on a saline soaked paper towel. Researchers gently used cotton swabs to remove the amniotic membranes, and then tied and cut the umbilical cord near the abdomen. These procedures took place at room temperature, thus allowing the pups to experience ambient cooling following delivery. Each pup was stimulated with a small, soft paintbrush to facilitate respiration [36] and then placed inside a warm (35°C) incubator to recover for approximately 30 minutes until its color was pink (indicating good oxygenation), and it showed regular respiration and typical newborn behavior. All c-sections were performed between 0800 and 1200.
Pups in the cesarean birth conditions were culled to 8–10 pups and fostered to surrogate dams that had recently given birth. The foster dam’s entire litter was removed from the nest. First, two biological pups were reintroduced to the cage to see how readily the dam retrieved her own pups. After the dam showed consistent retrieval behavior, the two biological pups were removed and two foster pups were introduced to the cage. After the dam gathered the two foster pups and deposited them into the nest, two additional foster pups were placed in the cage. Fostering has been shown to have no effect on pup-directed licking and arched-backed nursing [37].
Vaginal birth litters (V22) were culled to 8–10 pups but were not fostered. V22 subjects were born between 0800 and 1200 and were not manipulated before testing (4–7 hours after birth). These pups were not manipulated or fostered so as to provide a non-manipulated control group that would presumably show evidence of “typical” newborn behavior. Figure 1 shows a schematic of the three birth conditions.
2.3 Design
There were 32 subjects per birth condition group. Subjects in each birth condition were randomly assigned to and tested on one of four behavioral measures of motor coordination (n = 8 subjects per test): the LER (leg extension response), facial wiping, contact righting, and stepping. Each subject within a group was selected from a different dam to avoid litter effects, and males were used to avoid confounding group effects with sex effects. That is, no subject was tested on more than one behavioral test.
2.4 Behavioral Testing
The LER, facial wiping, and stepping tests occurred inside a testing incubator (35° C). All subjects had fed recently as indicated by a milk band on the abdomen. Subjects were voided by stimulating the anogenital area with a soft paintbrush until urination and/or defecation occurred. This was done to equate bladder fullness across subjects. Then subjects were placed in the incubator inside a small plastic container with three littermates, to permit 30 minutes of acclimation to testing conditions. Test sessions were recorded with a microcamera located inside the incubator that was connected to an outside DVD recording unit. Due to the brief nature of the contact righting test, righting subjects were tested outside of the incubator. Contact righting was recorded using a video camera (Sanyo VP-FH1) suspended above the subjects. Time codes were generated at 30 frames per second for all behavioral tests.
2.4.1 Leg Extension Response
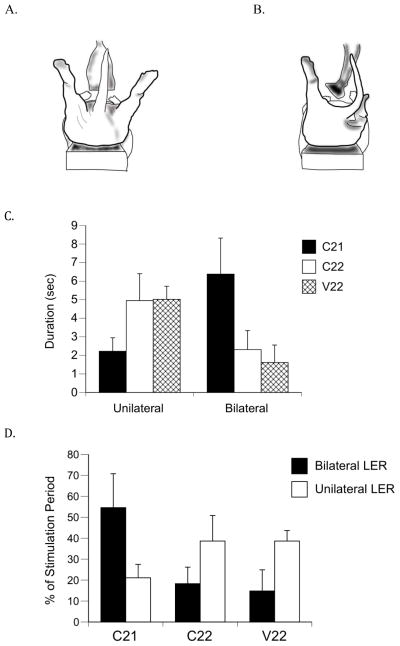
The LER was defined as the motion of one or both of the pup’s hind legs from a resting position to hyperextension and immobile position at a right angle to the body. The LER was evoked by vibrotactile stimulation, using an electronic vibration device (Penthouse Cyber Flicker; 120 Hz) that had a strip of latex (8 mm long; 1.5 mm wide) attached to the tip. Subjects were secured in the supine posture on a soft vinyl-covered bar using a jacket with an adjustable elastic strap across the abdomen and were able to move their limbs freely. A 1-min baseline (pre-stimulation) period, stimulation period (mean time: 15.19 sec, p = n.s.), and a 1-min post-stimulation period were recorded. Subjects were stimulated in the anogenital region with the vibrotactile device until 5 sec after they first showed the LER, or a maximum of ~25 sec if they did not show the LER. The LER is typically seen as a response to stimulation in the anogenital area and was used here as a test of synchronous hindlimb coordination. This study examined expression of both the unilateral and bilateral LER. Figures 2A and 2B illustrate pups expressing bilateral and unilateral LERs, respectively, during anogenital stimulation.
Figure 2.
Unilateral and bilateral expressions of the LER. (A) Illustration of the bilateral and (B) unilateral LERs expressed during anogenital stimulation. (C) Absolute duration in seconds of unilateral and bilateral LERs and (D) mean percentages of unilateral and bilateral LER durations during the 15-sec stimulation period. C21 subjects showed a significantly longer absolute bilateral LER duration and significantly longer bilateral LERs as a percentage of stimulation time compared to subjects in the control groups (C22 and V22). Bars show means; vertical lines depict SEM. There were 8 subjects each in the C21 and V22 groups, and 6 subjects in the C22 group that showed the LER.
For subjects that expressed the LER, the latency to express the response (time from stimulation to expression of the response), and duration of the LER expression (as a function of time stimulated) were scored. Both the unilateral and bilateral response latencies and durations were examined. Maximum LER responses (defined as the maximal hyperextension of one or both hindlimbs as measured against a lined reference card positioned behind the pup) also were scored and compared among groups. These dependent measures were analyzed by one-way ANOVAs with birth condition group as the independent variable.
2.4.2 Facial Wiping Test
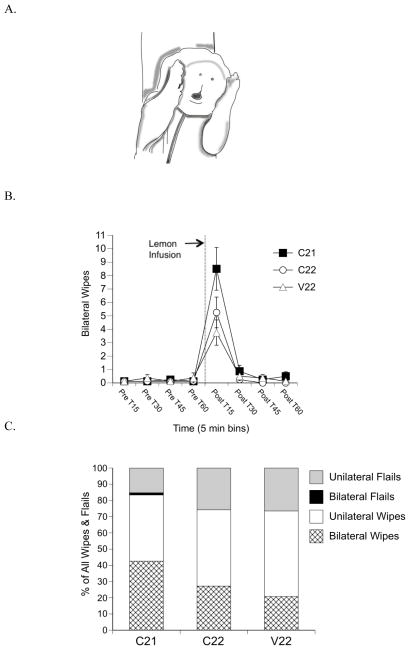
Facial wiping was defined as one or both paws in contact with the side of the face near the ears that are then drawn down toward the nose (Figure 3A). Facial wiping was induced with a lemon infusion into the subject’s mouth through a cannula inserted through the tongue and lower jaw. Before subjects were put in the incubator to begin the acclimation period, the cannula was placed by inserting a wire under the jaw and through the tongue, placing the cannula over the wire, and pulling both down through the tongue and jaw until the flange of the cannula rested on the midline of the tongue [38]. After the acclimation period, the subject was secured supine on a vinyl-covered bar and held in place by soft tape across the abdomen under the forelimbs that allowed the forelimbs to be free to express the facial wiping response [24].
Figure 3.
Facial wiping behavior. (A) Illustration of bilateral facial wiping following intraoral lemon infusion. (B) Mean frequencies of bilateral wipes in 15-sec time bins during the pre- and post-infusion periods. C21 subjects showed significantly more bilateral wipes 15-sec post-infusion compared to control subjects. Points show means; vertical lines depict SEM. Dotted line and arrow indicate lemon infusion. (C) Mean percentage of the total unilateral and bilateral wipes and unilateral and bilateral flails as a function of total wipes and flails during the 15-sec post-infusion period.
Undiluted lemon extract (1:0) was infused into the subject’s mouth at a volume of 10 μl at a 2-sec infusion pulse rate. This concentration, volume, and pulse time have been shown to reliably elicit facial wiping in E20 fetal rats [23]. After a 1-min baseline, the test session began with an infusion of lemon extract into the mouth of the pup. Behavior was recorded for an additional 60 seconds.
The facial wiping response was categorized as unilateral (single paw) or bilateral (both paws) and was used here as a measure of synchronous forelimb coordination. Unilateral wipes were counted when one limb was moving in the wiping pattern with the other limb not touching the face. A bilateral wipe occurred when both paws were simultaneously in contact with the face during the wiping cycle and nearly synchronous [39]. Figure 3A illustrates a pup showing a bilateral facial wipe. Other limb movements that resembled wiping but without contact on the side of the face were termed “flails.” Flails generally occurred when the animal’s head moved away from the wiping motion of the paws, thus preventing contact with the side of the face.
Frequencies of unilateral and bilateral wipes and flails and the percentage of bilateral wipes were summarized across the entire test session and analyzed using a repeated measures ANOVA, with time in 15 sec time bins as the repeated measure and birth condition group as the independent variable.
2.4.3 Contact Righting
Contact righting is a motor response by which the animal moves from a supine to prone position, and was used here as a measure of axial coordination. Subjects were placed supine using the method described by Moore and Chadwick-Dias [19]. The experimenter steadied the pup by holding it between the thumb and forefinger on either side of the pup’s shoulders. The tip of a cotton swab was gently placed against the pup’s chest. As soon as the pup stopped moving, the hand was removed, followed shortly thereafter by the swab, thus allowing the pup the exhibit the righting response. The testing platform was flanked by two mirrors (~55° angle) that provided side views of the behavior. Subjects showed the righting response on at least one of three total righting attempts or were replaced by a littermate.
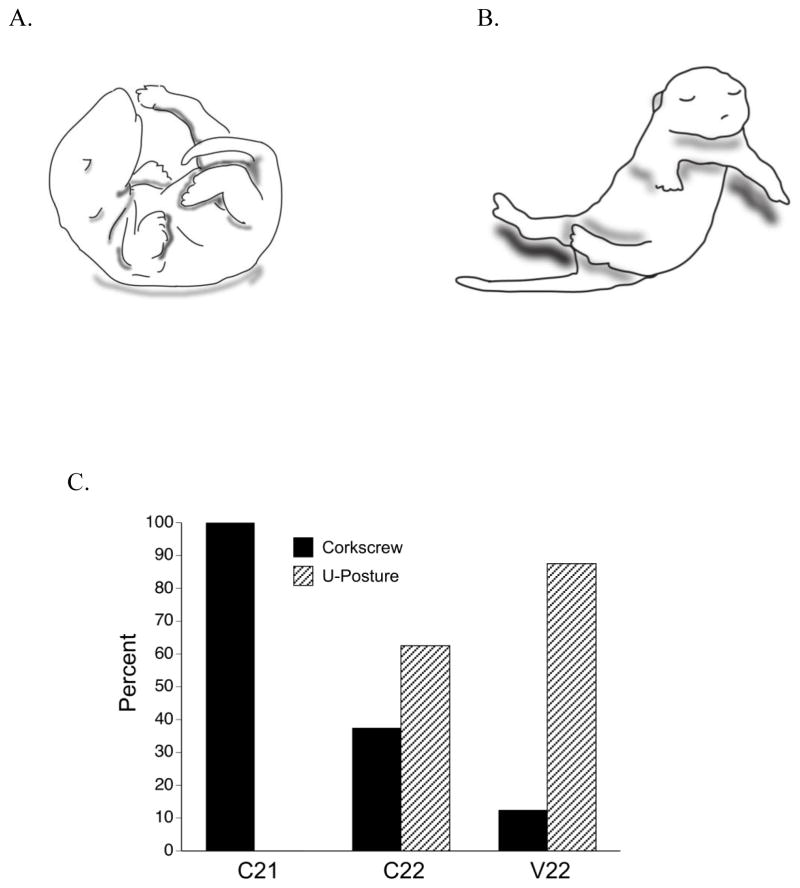
Latencies and strategy used to successfully right were scored from video records, using frame-by-frame analysis. Latencies were scored for touching the horizontal surface with each fore paw and hind paw. Strategies were distinguished between the u-shape or corkscrew postures [25] (see Figures 4A and 4B). The pup used the u-shape posture by ventroflexion of the body into a “u” shape until it fell to one side. The pup then tucked its limbs and rolled to a prone position. The corkscrew posture was seen when the pup twisted its upper body in one direction as the lower body twisted laterally in the opposite direction until it rolled prone. Figures 4A and 4B illustrate pups showing the u-shaped and corkscrew strategies, respectively.
Figure 4.
Contact righting behavior. (A) U-shaped righting and (B) Corkscrew righting strategies. (C) The percentage of subjects in each group showing the u-shaped and corkscrew righting strategy. The occurrence of corkscrew righting was significantly higher in the C21 group compared to the control groups.
2.4.4 Stepping Test
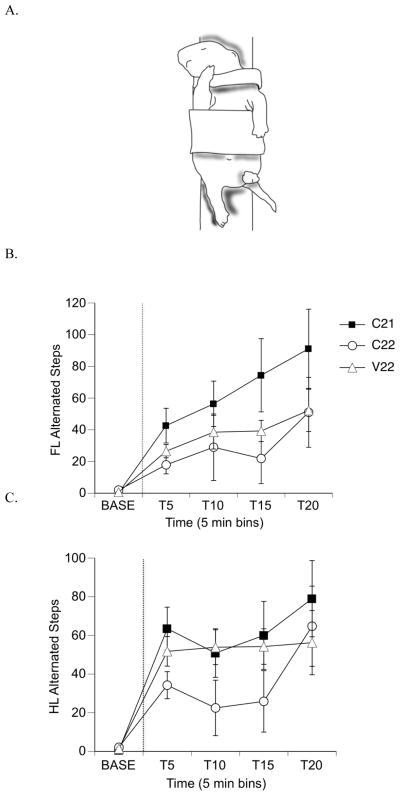
Stepping was used here as a measure of alternated forelimb and hindlimb coordination. The pup was held securely, with a soft jacket that did not impede limb movement, to a vinyl-covered bar in the supine position. Subjects were recorded for a 5 min baseline, followed by a 50 μl intraperitoneal injection of quipazine (3.0 mg/kg) that started a 20-min test session. Quipazine is a serotonin receptor agonist that has been shown to reliably induce locomotor-like stepping in perinatal rats [27], at the dose used here [28][32]. Figure 5A illustrates a pup in the testing apparatus and exhibiting stepping behavior.
Figure 5.
Alternated fore- and hindlimb stepping following injection of 3.0 mg/kg quipazine. (A) Illustration of alternated stepping. Notice that one forelimb/hindlimb is flexed while the other is extended. (B) Mean frequencies of forelimb (FL) alternated steps and (C) mean frequencies of hindlimb (HL) steps. Note that in both graphs, C22 frequencies are lower at most time points during the test session compared to the C22 and V22 groups. Points show means; vertical lines depict SEM. Dotted line separates the baseline and test periods.
All alternated stepping and non-stepping forelimb and hindlimb movements were scored during the 25-min test session (5-min baseline plus 20-min post-injection of quipazine). One alternated step-cycle was defined as two consecutive and alternating extensions and flexions of homologous limb pairs; thus the term “step” is used here to indicate a bilaterally alternating pattern of coordination [32]. We did not score synchronous steps separately (but rather included them in non-stepping behavior), as we have previously shown that quipazine does not evoke many synchronous steps in newborn rats [28]. Frequencies of steps and non-stepping limb movements and the percentage of steps were summarized in 5-min bins and analyzed using repeated measures ANOVA, with time as the repeated factor and birth condition group as the independent variable. Following a significant time x group interaction effect, one-way ANOVAs were performed to determine main effects.
2.5 Data Analysis
Scoring of different behavioral criteria was done during normal or reduced speed playback of the DVD recordings of the test sessions. Data were scored with JWatcher™, an event recorder program that records the category of behavior and time of entry (± 0.01 s). The scorer was blind to subjects’ birth conditions, and intrarater scoring reliability of the same video clip was ≥ 90% for each behavior. StatPlus® (AnalystSoft) was used for statistical analyses of the data. A 5% significance level was adopted for all tests, and Tukey’s HSD was employed in post hoc comparisons.
3. Results
All subjects were tested on PC22. Thus, in this study we controlled for age, and only varied the amount of time that subjects spent in the prenatal vs. postnatal environment. Subjects in the C21 group had one full day of exposure to the postnatal environment, whereas subjects in the C22 and V22 groups had only 4 hours of exposure to the postnatal environment. There was no difference in body weight among pups from the different groups. The mean body weight for pups in this study was 6.04 ± 0.7 grams.
3.1 Leg Extension Response
Subjects in the C21 group displayed either bilateral only (n = 2) or unilateral and bilateral LERs (n = 6) during the stimulation period. In contrast, no subjects from the C22 or V22 groups showed the bilateral response only. Two subjects in the V22 group displayed the unilateral LER only. Five of the six C22 responders showed the unilateral response prior to responding with the bilateral LER; two subjects in the C22 group did not respond with either the unilateral or bilateral LER.
3.1.1 Latency and Duration of the LER
Latency to respond to anogenital stimulation with an LER and the duration of the response (either unilaterally or bilaterally) were examined. Latency to express the LER was examined from the beginning of anogenital stimulation to the moment the subject expressed the first LER (unilateral or bilateral) and maximum expression of the LER. The ANOVAs yielded no significant differences in the latency to the first LER [F(2, 19)] = 1.85, p = .18], the latency to the maximum expression of the LER [F(2, 19) = .66, p = .53], or the difference between the two latencies [F(2, 19)] = 1.31, p = .29].
To normalize the stimulation period (M = 15.2 s; range: 6.04 – 23.54 s), subjects’ behaviors were scored for a maximum of 15.2 seconds (the mean stimulation period for all subjects) during AG stimulation. Mean durations for unilateral and bilateral LERs were analyzed as absolute times and percentages of the normalized stimulation periods, for all subjects.
A one-way ANOVA for mean absolute duration of bilateral LERs revealed significant differences among groups [F(2, 21) = 3.48, p = .049; Figure 2C]. As can be seen in Figure 2C, the mean bilateral duration for group C21 was significantly longer than the mean for C22 and V22 groups. There were no group differences in absolute duration of unilateral LERs.
Additionally, LER durations were examined as a percentage of time from the first LER expression to the end of the stimulation period. Figure 2D shows the unilateral duration and bilateral duration percentages of the stimulation period. A one-way ANOVA for the percentage of bilateral duration revealed statistically significant differences among groups [F(2, 21) = 4.45, p = .024, Figure 2D]. The percentage of bilateral LER duration was significantly longer for the C21 group than the C22 and V22 groups. However, the percentage of unilateral LER duration was not statistically significantly different among the groups.
3.2 Facial Wiping
Facial wiping responses typically occurred within the first few seconds following intraoral lemon infusion. Thus, frequencies of unilateral and bilateral wipes and the percentage of bilateral wipes were summarized across the entire test session and analyzed using a repeated measures ANOVA, with time in 15 sec time bins as the repeated measure (to capture the quick response) and birth condition group as the independent variable. Analyses were conducted on unilateral and bilateral wipes (in contact with the face) and unilateral and bilateral flails (wiping motions not in contact with face). To normalize the data, the mean percentage of bilateral wipes as a function of total wipes and flails was calculated.
The repeated measures ANOVA for frequency of bilateral wipes revealed a time x group interaction [F(14, 147) = 6.17, p = .0001, Figure 3B]. A one-way ANOVA at Post T15 (15 seconds immediately following lemon infusion) revealed a main effect of group [F(2, 21) = 3.90, p = .036]. Post hoc comparisons indicated that C21 pups showed significantly more bilateral wipes than V22 subjects. Additionally, a priori contrasts comparing the C21 group with the control groups (C22 and V22) revealed significant differences for the frequency of bilateral wipes [t(21) = 2.64, p = .015].
The mean percentage of bilateral wipes as a function of total movements (all wipes and flails) revealed differences that approached significance [F(2, 21) = 3.16, p = .06, Figure 3C]. At Post T15, a priori contrasts comparing the C21 group with the control groups (C22 and V22) indicated significant differences for the percentage of bilateral wipes [t(21) = 2.41, p = .025].
Figure 3C shows the percentages of bilateral wipes, unilateral wipes, unilateral flails, and bilateral flails as a function of total movements at Post T15, the 15-sec time period immediately following lemon infusion. A priori contrasts revealed differences between the C21 group and the control groups for the percentage of bilateral flails [t(21) = 2.14, p = .044] and differences that approached significance for the percentage of unilateral flails [t(21) = −2.02, p = .058]. There were no significant interaction effects of group x time or simple main effects of groups for total movements.
3.3 Righting Response
Latencies to touch the floor for each paw were measured, as was the total time for the pups to move from a supine to prone posture. One-way ANOVA tests indicated no significant differences among groups for any of these measures (all ps < 0.05). The average time for pups to achieve the prone posture was 9.92 sec (± 8.8 SD).
However, an overall chi square test revealed a significant difference among the groups in the strategy used to right [X2 (2, N=24) = 13, p = 0.002]. As Figure 4C shows, all C21 pups achieved the prone posture using the corkscrew righting strategy, whereas only 3 out of 8 and 1 out 8 pups in the C22 and V22 groups, respectively, used that strategy. Instead of the corkscrew strategy, the majority of pups in the control groups (C22 and V22) used the more immature u-shape posture to right themselves. The occurrence of corkscrew righting was significantly higher when comparing the C21 group to each control group (both ps < 0.05), but not different between the control groups.
3.4 Stepping Behavior
Analyses were conducted on forelimb and hindlimb activity to determine group differences in the frequencies of alternated steps and non-stepping movements during the 5-min baseline and 20-min test session (following treatment with quipazine). Overall frequencies were compared in two factor ANOVAs (birth condition x time), with time in 5-min bins as a repeated measure. To account for possible overall activity differences among groups, we analyzed alternated steps and non-stepping movements as a percentage of total behavior.
3.4.1 Forelimbs
Total forelimb movement (alternated steps and non-stepping movements) was analyzed among the groups and there was no interaction of group x time [F(8, 84) = 1.1, p = .36] or a main effect of group [F(2, 21) = 1.5, p = .25]. At all time points during the test session, the frequency of alternated forelimb steps was higher for the C21 group than the control groups; however, the results were not significant [F(2, 21) = 1.85, p = .18, Figure 5B], and the interaction of group and time was not significant [F(8, 84) = 1.15, p = .34]. The percentage of alternated steps as a function of total movements was higher in all time bins for the C21 group as well, but also not significantly different from the control groups [F(2, 21) = 1.58, p = .14]. Both the frequency and the percentage of alternated steps for the C22 group were lower than the C21 and V22 groups in all but the last 5-min time bin.
For the frequency of non-stepping forelimb movements, there was an interaction of group x time [F(8, 84) = 4.08, p = .0004], and a main effect of group [F(2, 21) = 4.35, p = .026]. Examination at each time period using one way ANOVAs revealed differences among groups at T10 that approached significance [F(2, 21) = 3.07, p = .06] and significant differences at T15 [F(2, 21) = 4.39, p = .02], with the V22 group showing the highest frequency of non-stepping forelimb movements (C21: 74.4 ± 23.1; C22: 21.88 ± 15.8; V22: 39.25 ± 6.7). The interaction of group x time for percentage of non-stepping movements as a function of total movements approached significance [F(8, 84) = 2.03, p = .052], but there was no main effect of group [F(2, 21) = 2.8, p = .127].
3.4.2 Hindlimbs
Total hindlimb movement was analyzed among the groups and there was no interaction of group x time [F(8, 84) = 1.2, p = .36] or a main effect of group [F(2, 21) = 1.5, p = .24]. As can be seen in Figure 5C, there was no main effect of group [F(2, 21) = 1.36, p = .28] or a group x time interaction [F(8, 84) = 1.07, p = .39] on alternated hindlimb step frequency. For the percentage of alternated steps as a function of total movement, there was no interaction of group x time [F(8, 84) = .97, p = .45] or main effect of group [F(2, 21) = 1.07, p = .38].
For the frequency of non-stepping hindlimb movements, there was no interaction of group x time [F(8, 84) = 1.0, p = .43] and no main effect of group [F(2, 21) = .66, p = .5], or a group x time interaction [F(8, 84) = 1.00, p = .44]. There was an interaction of group x time for percentage of non-stepping movements as a function of total movements [F(8, 84) = 2.15, p = .039] but no main effect of group [F(2, 21) = 2.13, p = .14]. Post hoc tests showed that at T10 indicated that subjects in the C22 group expressed fewer non-steps than subjects in the V22 group (C21: 49.38 ± 5.7; C22: 33.5 ± 12.2; V22: 49.0 ± 12.5). However, the group mean for the C21 subjects was not significantly different from the control groups.
4. Discussion
We examined the effect of early delivery on the development of motor coordination in perinatal rats. Pups were prematurely exposed to the typical postnatal environment (C21 group) and compared to aged-matched controls (C22 and V22 groups) on four behavioral measures of coordination—the LER, facial wiping, contact righting, and quipazine-induced stepping. Specific environmental contributions to motor coordination were not manipulated in this study, but rather, exposure to the entire complement of the postnatal environment was increased by one day. Three sensory-evoked measures of motor coordination—the LER, facial wiping, and righting—were shown to be sensitive to the birth manipulation in this study.
4.1 Experience Affects the LER, Facial Wiping, and Contact Righting
Pups with more postnatal experience (C21 group) showed more bilateral coordination during expression of the LER and longer durations of the LER during anogenital stimulation than subjects with less postnatal experience (C22 and V22 groups). This effect may be due to an increased amount of maternal licking and subsequent licking-induced LER expression that C21 pups experienced, as maternal licking has been shown to be a significant source of sensory stimulation [40]. Pup-directed licking and grooming is cyclic and occurs most frequently during the dark phase [37], which the C22 and V22 groups did not experience before testing. C21 pups had one extra day of maternal-infant interactions, which prominently includes pup-directed anogenital licking (AGL) by the dam [41]. Therefore, C21 pups also should have expressed the LER more frequently than pups in the control groups, by mere exposure to anogenital stimulation. Thus, C21 subjects may be showing a “practice effect,” or a type of experience-dependent learning, from typical AGL received in the nest environment. A recent study in our lab [42] showed that the likelihood to express the LER increases significantly from P0 to P1. Thus, it is suggested that the first 24 hours of postnatal experience facilitates acquisition of interlimb coordination for the LER.
Postnatal experience also may contribute to interlimb coordination expressed during facial wiping. Results from the current study showed that C21 pups showed more bilateral facial wiping as measured in absolute amounts and as a percentage of all wipes and flails, compared to pups in the other groups. As fetal rats rarely show spontaneous facial wiping [43], C21 pups may have had more opportunities to encounter stimuli in the postnatal environment that could trigger a wiping response. In fact, mere exposure to the dam’s ventrum is sufficient to elicit facial wiping in P0–P2 pups [31]. Being part of the huddle in the postnatal nest could have facilitated facial wiping practice; C21 pups may have had opportunities to use littermates as a biomechanical support for one limb as it expressed facial wiping behavior with the other limb, as has been shown in neonatal mice [44]. Another possible source of experience could be generalization resulting from other coordinated forelimb behaviors that newborn rats show, such as treadling during suckling episodes [31].
In addition to possible effects of maternal care, sibling stimulation, and exposure to behavior-evoking stimuli, C21 pups were prematurely exposed to a ubiquitous environmental stimulus—gravity. Premature exposure to the direct effects of gravity may have contributed to the expression of a more mature righting strategy for the C21 pups. The C21 pups in this study showed a righting strategy typically seen by older (P1–P2) pups [25]. Research on rats gestated in micro- and hypergravity has shown altered righting responses in these pups [45, 46, 47, 48]. For example, young rats gestated in microgravity display diminished responses to gravity when tested in a water immersion tank [49]. Additionally, 24 hours in a postnatal environment changes the way newborn rat pups respond to a unilateral limb weight, suggesting that experience in a gravitational and limb-loading environment has effects on the motor system [50].
Several studies have demonstrated the effects of micro- and macro-gravity on motor development in perinatal rats, so it seems self-evident that gravity would play a part in typical motor development. In this study, gravity is part of the postnatal environment that may have contributed to the differences in behavior demonstrated. Brocard et al. [30] showed that significant development occurs in the major hindlimb extensor muscles during the first postnatal week in the rat, with the most rapid changes taking place within the first two postnatal days. How development of these anti-gravity muscles are related to experience moving in a gravitational environment independent of age remains to be determined. Findings from the current study suggest that experience or time spent in the postnatal environment may be a contributing factor to early developmental changes in the motor system and behavior.
4.2 No Effect on Quipazine-Induced Stepping
The serotonin receptor agonist quipazine effectively elicits alternated stepping in both fetal and newborn rats [27, 28, 43]. However, in the current study there were no meaningful statistical differences between the experimental group and the control groups on alternated stepping behavior in the hind- or forelimbs. However, results were in the predicted direction for alternated stepping of the forelimbs, with C21 pups showing more forelimb stepping than pups in the control groups. Perhaps at this age, drug-induced alternated limb movement is more variable than sensory-evoked behavior or less sensitive to the birth manipulation than synchronous limb movements (i.e. facial wiping, LER). Greater variability results in greater difficulty in detecting differences among groups. Future studies could include the use of kinematic analyses of limb trajectories to examine more subtle differences in limb coordination.
4.3 Pups’ Differential Abilities to Solicit Care
This study makes an assumption that the physical postnatal environment for a gestational day 21 (E21) rat is not markedly different from the environment of an E22 rat. However, a pup born on E21 may be endowed with an altered behavioral repertoire, that is, different abilities, to solicit care from the dam compared to a pup born on the typical day of birth (E22). In general, neonates have the ability to make ultrasonic vocalizations, orient to the dam, root and suckle, and produce chemical attractants in their urine and feces. These types of behaviors result in modulating maternal behavior and depend on experience with the dam [51]. For example, arched-back nursing (a measure of maternal care) results from effective suckling behavior, which in turn is dependent on pups’ abilities to orient and attach to the nipple [52]. It is the quality of the pups’ suckling abilities that stimulate the dam’s quiescence, nursing posture, and milk production.
Pups produce mother-directed behavior, such as ultrasonic vocalizations, that result in more AGL [53] and pup retrieval [54]. However, vocalizations alone are not sufficient to induce maternal care. Research has shown that additional olfactory cues are necessary for pup retrieval [55] and for AGL [53]. Pup urine and feces contain the chemical, dodecyl propionate (DP), an important olfactory cue known to promote maternal pup-directed AGL and maternal responsiveness [56, 57]. Thus, it may be possible that C21 pups produced higher or lower concentrations of DP than control pups. If pups delivered on E21 produced more DP, AGL would have increased, resulting in more opportunities for pups to express the LER. If the pups (due to birth condition) solicit differing amounts of maternal licking or nursing from the dam, they have also contributed to the variability in the postnatal environment. Alternatively, if pups’ ability to solicit maternal care is assumed to be no different on E21 than E22, subjects born one day early still had more opportunities (one full postnatal day) to emit ultrasonic calls, or otherwise solicit maternal care, than pups born on the typical day of birth and tested only after 4 hours of postnatal experience.
4.4 Effects of Cesarean Section
Cesarean section may have affected motor behavior for pups in the C21 and C22 groups and may present a limitation for this study. The standard practice of stroking cesarean born pups to facilitate breathing is sufficient for survival, but may not be optimal stimulation to overcome the immediate effects of cesarean section [58]. Likewise, any residual effects of general anesthesia may have affected pup behavior. However, isoflurane anesthesia has been shown to have no significant effects on breathing frequency or blood pH within 2 hr of cesarean delivery of fetal rats [59], and there is no significant difference between cesarean-delivered and vaginal-delivered pups on measures of pO2, brain lactate, blood pH, pCO2, and brain ATP levels within 4 hr after delivery [60]. Further, the E21 rat has been shown to be able to induce protective lung antioxidant enzymes and lung surfactant responses that are comparable to the full-term rat [61]. Thus, there appears to be little concern for respiratory compromise in the current experiment.
Cesarean-delivered pups in this study also were fostered to another dam. Although recently parturient rat dams readily provide maternal care for foster pups, differences in the natural time course of maternal-infant interactions (e.g., time to first suckling episode, time to first AGL episode) may have occurred, that subsequently influenced motor coordination.
It was found that the C22 group (control group born at term) showed suppressed overall motor activity in the stepping test compared to the C21 and V22 groups. It is possible that the cesarean delivery procedure produced effects in the C22 group that were not apparent in the C21 group, as the C21 group had an extra 24 hours to recover from the c-section procedure. Birth by cesarean delivery has been shown to alter later dopamine levels [62, 63]. The typical vaginal birth process provides a surge of protective cholinergic hormones that does not occur during cesarean delivery and may affect motor behavior. Research suggests that developing spinal circuits are affected by cholinergic activity [64, 65], which may have been reduced or absent in both the C21 and C22 groups. Any of the above factors could contribute to suppressed motor activity for the C22 subjects.
To ameliorate any potential immediate effects of birth by cesarean delivery, future research could manipulate the groups in the same manner as the current study but conduct behavioral testing on developmental day P1 (PC23). On P1, the C22 group should have sufficient time to recover, but the difference in overall postnatal experience would be 24 hours. Another possible solution for mitigating any deleterious effects cesarean delivery could include the administration of a single injection of epinephrine at birth [63], or providing stimulation to pups that more closely mimics peristaltic contractions that is experienced as the pup moves down the birth canal [66]. However, research has primarily examined the long term, rather than acute, effects of protective effects of drugs on brain function and behavioral outcomes.
4.5 Implications for Motor Development
The possibility that experience or practice effects may contribute to the early expression and development of action patterns remains to be fully explored. However, the findings from this study show that the immature motor system can be influenced by perinatal experience and that motor coordination is sensitive to the environment. Such findings imply that experience during early motor development may normally influence the development of motor behavior and their mechanisms, even for species-typical action patterns. Hence, some developmental patterns of behavior may actually be due to species-typical experiential effects that masquerade as the results of neural maturational processes.
For example, this was shown to be the case with the disappearance of the neonatal stepping response in humans. Originally thought to disappear soon after birth because of the development of brain mechanisms inhibiting primitive spinal circuits, Thelen and colleagues [67] elegantly demonstrated that stepping behavior disappears during development because increases in leg muscle strength lag behind increases in leg muscle mass. Thus, the stepping response went away because the babies did not have enough muscle strength to lift their legs. However, when newborns were given daily stepping practice [68], their stepping reflex did not disappear.
In addition to motor practice, other kinds of environmental stimulation (including non-obvious forms of experience) also may contribute to motor development, as the current study suggests. For instance, close body contact between the neonate and its mother, regulates the infant’s physiology and behavior in diverse and important ways [69], which may result in long-term benefits for motor development. Gottlieb suggested that experience may play maintenance, facilitative, or inductive roles in the development of behavior [70, 71]. We may conceptualize the findings of the current study as early postnatal experience providing a facilitation of newborn motor coordination, in that motor coordination appeared to increase with more postnatal experience. However, a more carefully documented case has to be made to identify the changes that are indicative of advanced stages of development. Additionally, changes in motor behavior during ontogeny can lead to different opportunities for social interactions and cognitive experiences, thus contributing to the development of social, emotional, and cognitive development as well [72].
5. Conclusion
The effect of experience on sensory systems is well documented; however, less is known about experiential effects on motor systems. Here we examined four motor patterns—the leg extension response, facial wiping, contact righting, and alternated stepping—in neonatal rats. Results demonstrated that early exposure to the postnatal environment resulted in more coordinated behavior as measured by facial wiping, contact righting, and the leg extension response. Pups were prematurely exposed to the postnatal environment, which included variations in maternal care, sibling interactions, and natural variations in the physical environment. Any of these naturally occurring sources of stimuli may have contributed partly or collectively to the developmental changes in motor coordination.
If offspring benefit from a shorter gestation period, what prohibits this? Studies have shown that premature exposure in one sensory system can disrupt the development of an earlier developing sensory system (e.g., [73]). Therefore, early birth may prematurely expose the animal to postnatal stimuli in ways that are detrimental to its overall survival, even while enhancing motor coordination. There is abundant evidence that preterm birth in humans can lead to atypical developmental trajectories, including functional differences (often deficits) in fine motor skills [74], visual and attentional processing, memory, and IQ scores, among other things [for review see 75]. In the present case, care should be taken in drawing parallels to the effects of early birth on the development of motor coordination in human infants. The preterm rats in this study were delivered 4.5% earlier than a normative birth, whereas mildly preterm human infants experience a 20% earlier delivery. [Note that we could not deliver our rats any earlier than E21, otherwise they would not have survived.] This study merely illuminates the role of the perinatal environment in shaping the development of motor coordination.
Highlights.
Manipulating the amount of postnatal experience in perinatal rats resulted in increased motor coordination. Premature exposure to the postnatal environment contributed to increased motor coordination during expression of the leg extension response and facial wiping, as well as a more mature strategy for contact righting, compared to age-matched controls. Data suggest that environmental factors are involved in the development of motor coordination.
Acknowledgments
This research was supported by NIH grant R15HD062980-01 to MRB and grant S09-2 from the WeLEAD Project which is funded by NSF grant SBE0620073. The authors wish to thank Brian Livesay, Misty Strain, Valerie Mendez-Gallardo, and Mary Ann Vineyard for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lickliter RJ. Atypical perinatal sensory stimulation and early perceptual development: Insights from developmental psychobiology. Perinatol. 2000;20:S45–S54. doi: 10.1038/sj.jp.7200450. [DOI] [PubMed] [Google Scholar]
- 2.Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28(6):1029–40. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 3.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206(2):419–36. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: Central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12(8):797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- 5.Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404(6780):876–81. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 6.Turkewitz G, Kenny PA. The role of developmental limitations of sensory input on sensory/perceptual organization [Abstract] J Dev Behav Pediatr. 1985;6(5):302–6. [PubMed] [Google Scholar]
- 7.Lickliter R, Lewkowicz DJ. Intersensory experience and early perceptual development: Attenuated prenatal sensory stimulation affects postnatal auditory and visual responsiveness in bobwhite quail chicks (Colinus virginianus) Dev Psychol. 1995;31(4):609–18. [Google Scholar]
- 8.Casey MB, Lickliter R. Prenatal visual experience facilitates the development of spatial turning bias in bobwhite quail chicks. Dev Psychobiol. 1998;32:327–38. [PubMed] [Google Scholar]
- 9.Kenny PA, Turkewitz G. Effects of unusually early visual stimulation on the development of homing behavior in the rat pup. Dev Psychobiol. 1986;19(1):57–66. doi: 10.1002/dev.420190107. [DOI] [PubMed] [Google Scholar]
- 10.Foreman N, Altaha M. Exploration and spontaneous alternation in hooded rat pups: Effects of unusually early eyelid opening. Dev Psychobiol. 1991;24(7):521–37. doi: 10.1002/dev.420240706. [DOI] [PubMed] [Google Scholar]
- 11.Robinson SR, Kleven GA, Brumley MR. Prenatal development of interlimb motor learning in the rat fetus. Infancy. 2008;13(3):204–28. doi: 10.1080/15250000802004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson SR. Conjugate limb coordination after experience with an interlimb yoke: Evidence for motor learning in the rat fetus. Dev Psychobiol. 2005;47(4):328–44. doi: 10.1002/dev.20103. [DOI] [PubMed] [Google Scholar]
- 13.Viala D, Viala G, Fayein N. Plasticity of locomotor organization in infant rabbits spinalized shortly after birth. In: Goldberger ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Liviana Press; Padova: 1986. pp. 301–10. [Google Scholar]
- 14.Pang MYC, Yang JF. Interlimb co-ordination in human infant stepping. J Physiol. 2003;533(2):617–25. doi: 10.1111/j.1469-7793.2001.0617a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musselman KE, Yang JF. Loading the limb during rhythmic leg movements lengthens the duration of both flexion and extension in human infants. J Neurophysiol. 2007;97(2):1247–57. doi: 10.1152/jn.00891.2006. [DOI] [PubMed] [Google Scholar]
- 16.Muir GD, Chu TK. Posthatching locomotor experience alters locomotor development in chicks. J Neurophysiol. 2002;88(1):117–23. doi: 10.1152/jn.2002.88.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Sindhurakar A, Bradley NS. Kinematic analysis of overground locomotion in chicks incubated under different light conditions. Dev Psychobiol. 2010;52(8):802–12. doi: 10.1002/dev.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smotherman WP, Robinson SR, Robertson SS. Cyclic motor activity in the fetal rat (Rattus norvegicus) J Comp Psych. 1988;102(1):78–82. doi: 10.1037/0735-7036.102.1.78. [DOI] [PubMed] [Google Scholar]
- 19.Moore CL, Chadwick-Dias A-M. Behavioral responses of infant rats to maternal licking: Variations with age and sex. Dev Psychobiol. 1986;19(5):427–38. doi: 10.1002/dev.420190504. [DOI] [PubMed] [Google Scholar]
- 20.Friedman MJ, Bruno JP, Alberts JR. Physiological and behavioral consequences in rats of water recycling during lactation. J Comp Physio Psych. 1981;95:26–35. doi: 10.1037/h0077753. [DOI] [PubMed] [Google Scholar]
- 21.Gubernick DJ, Alberts JR. Maternal licking by virgin and lactating rats: Water transfer from pups. Physiol Behav. 1985;34:501–6. doi: 10.1016/0031-9384(85)90040-x. [DOI] [PubMed] [Google Scholar]
- 22.Smotherman WP, Robinson SR. Fetal expression of the leg extension response to anogenital stimulation. Physiol Behav. 1988;43(2):243–4. doi: 10.1016/0031-9384(88)90246-6. [DOI] [PubMed] [Google Scholar]
- 23.Brumley MR, Robinson SR. Facial wiping in the rat fetus: Variation of chemosensory stimulus parameters. Dev Psychobiol. 2004;44(4):219–29. doi: 10.1002/dev.20005. [DOI] [PubMed] [Google Scholar]
- 24.Méndez-Gallardo V, Robinson SR. Opioid mediation of amniotic fluid effects on chemosensory responsiveness in the neonatal rat. Dev Psychobiol. 2010;52(8):740–54. doi: 10.1002/dev.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellis VC, Pellis SM, Teitelbaum P. A descriptive analysis of the postnatal development of contact-righting in rats (Rattus norvegicus) Dev Psychobiol. 1991;24(4):237–63. [Google Scholar]
- 26.Geisler HC, Westerga J, Gramsbergen A. Development of posture in the rat. Acta Neurobiol Exp. 1993;53:517–23. [PubMed] [Google Scholar]
- 27.McEwen ML, Van Hartesveldt C, Stehouwer DJ. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behav Neurosci. 1997;111(4):825–33. doi: 10.1037//0735-7044.111.4.825. [DOI] [PubMed] [Google Scholar]
- 28.Brumley MR, Roberto ME, Strain MM. Sensory feedback modulates quipazine-induced stepping behavior in the newborn rat. Behav Brain Res. 2012;229(1):257–64. doi: 10.1016/j.bbr.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamon M, Maloum I, Riviere G, Bruguerolle B. Air-stepping in neonatal rats: A comparison of L-dopa injection and olfactory stimulation. Behav Neurosci. 2002;116(6):1014–21. doi: 10.1037//0735-7044.116.6.1014. [DOI] [PubMed] [Google Scholar]
- 30.Brocard F, Vinay L, Clarac F. Development of hindlimb postural control during the first postnatal week in the rat. Brain Res Dev Brain Res. 1999;117(1):81–9. doi: 10.1016/s0165-3806(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 31.Polan HJ, Milano D, Eljuga L, Hofer MA. Development of rats’ maternally directed orienting behaviors from birth to day 2. Dev Psychobiol. 2001;40(2):81–103. [PubMed] [Google Scholar]
- 32.Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A, and alpha-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behav Neurosci. 2005;119(3):821–33. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- 33.Bekoff A, Lau BJ. Interlimb coordination in 20-day old rat fetuses. Exp Zool. 1980;214(2):173–5. doi: 10.1002/jez.1402140207. [DOI] [PubMed] [Google Scholar]
- 34.Smotherman WP, Robinson SR, La Vallee PA, Hennessy MB. Influences of the early olfactory environment on the survival, behavior and pituitary-adrenal activity of caesarean delivered preterm rat pups. Dev Psychobiol. 1987;20(4):415–23. doi: 10.1002/dev.420200406. [DOI] [PubMed] [Google Scholar]
- 35.Vaillancourt C, Berger N, Boksa P. Effects of vaginal birth versus caesarean section birth with general anesthesia on blood gases and brain energy metabolism in neonatal rats. Exp Neurol. 1999;160(1):142–50. doi: 10.1006/exnr.1999.7201. [DOI] [PubMed] [Google Scholar]
- 36.Smotherman WP, Robinson SR. Cryptopsychobiology: The appearance, disappearance, and reappearance of a species-typical action pattern during early development. Behav Neurosci. 1989;103(2):246–53. doi: 10.1037//0735-7044.103.2.246. [DOI] [PubMed] [Google Scholar]
- 37.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79(3):359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 38.Kehoe P, Blass EM. Gustatory determinants of suckling in albino rats 5–20 days of age. Devel Psychobiol. 1985;18(1):67–82. doi: 10.1002/dev.420180106. [DOI] [PubMed] [Google Scholar]
- 39.Korthank AJ, Robinson SR. Effect of amniotic fluid on opioid activity and fetal responses to chemosensory stimuli. Devel Psychobiol. 1998;33(3):235–48. doi: 10.1002/(sici)1098-2302(199811)33:3<235::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 41.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psych. 1979;93(4):677–84. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 42.Vineyard MA, Strain MM, Roberto ME, Brumley MR. Developmental changes in hindlimb coordination during the leg extension response evoked by anogenital stimulation [Abstract] Dev Psychobiol. 2010;52(7):721. [Google Scholar]
- 43.Robinson SR, Brumley MR. Chapter 24: Prenatal Behavior. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat: A Handbook with Tests. Oxford Press; New York, NY: 2005. pp. 257–265. [Google Scholar]
- 44.Golani I, Fentress JC. Early ontogeny of face grooming in mice. Dev Psychobiol. 1985;18(6):529–44. doi: 10.1002/dev.420180609. [DOI] [PubMed] [Google Scholar]
- 45.Ronca AE, Fritzsch B, Bruce LL, Alberts JR. Orbital spaceflight during pregnancy shapes function of mammalian vestibular system. Behav Neurosci. 2008;122(1):224–32. doi: 10.1037/0735-7044.122.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouët V, Wubbels RJ, de Jong HA, Gramsbergen A. Behavioural consequences of hypergravity in developing rats. Brain Res Dev Brain Res. 2004;153(1):69–78. doi: 10.1016/j.devbrainres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Ronca AE, Alberts JR. Effects of prenatal spaceflight on vestibular responses in neonatal rats. J Appl Physiol. 2000;89:2318–24. doi: 10.1152/jappl.2000.89.6.2318. [DOI] [PubMed] [Google Scholar]
- 48.Wubbels RJ, de Jong HAA. Vestibular-induced behaviour of rats born and raised in hypergravity. Brain Res Bull. 2000;52(5):349–56. doi: 10.1016/s0361-9230(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 49.Alberts JR, Ronca AE. Development as adaptation: A paradigm for gravitational and space biology. Adv Space Biol Med. 2005;10:175–207. doi: 10.1016/s1569-2574(05)10007-0. [DOI] [PubMed] [Google Scholar]
- 50.Brumley MR, Robinson SR. Sensory feedback alters spontaneous limb movements in newborn rats: Effects of unilateral forelimb weighting. Dev Psychobiol. 2013;55:323–333. doi: 10.1002/dev.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eljuga L, Mathew E, Hofer MA, Polan HJ. Evidence that newborns’ differential responding to maternal cues depends on the first day’s experience [Abstract] Devel Psychobiol. 2002;41(1):76. [Google Scholar]
- 52.Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47:993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 53.Brouette-Lahlou I, Vernet-Maury E, Vigouroux M. Role of pups’ ultrasonic calls in a particular maternal behavior in Wistar rat: pups’ anogenital licking. Behav Brain Res. 1992;50(1–2):147–54. doi: 10.1016/s0166-4328(05)80296-7. [DOI] [PubMed] [Google Scholar]
- 54.White NR, Adox R, Reddy A, Barfield RJ. Regulation of rat maternal behavior by broadband pup vocalization. Behav Neural Biol. 1992;58:131–7. doi: 10.1016/0163-1047(92)90363-9. [DOI] [PubMed] [Google Scholar]
- 55.Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- 56.Moore CL. An olfactory basis for maternal discrimination of sex of offspring in rats (rattus norvegicus) Anim Behav. 1981;29(2):383–6. [Google Scholar]
- 57.Brouette-Lahlou I, Amouroux R, Chastrette F, Cosnier J, Stoffelsma J, Vernet-Maury EJ. Dodecyl Propionate, attractant from rat preputial gland: Characterization and identification. Chem Ecol. 1991;17(7):1343–54. doi: 10.1007/BF00983767. [DOI] [PubMed] [Google Scholar]
- 58.Ronca AE, Abel RA, Ronan PJ, Renner KJ, Alberts JR. Effects of labor contractions on catecholamine release and breathing frequency in newborn rats. Behav Neurosci. 2006;120(6):1308–14. doi: 10.1037/0735-7044.120.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronca AE, Abel RA, Alberts JR. Maternal anesthesia via isoflurane or ether differentially affects pre- and postnatal behavior in rat offspring. Dev Psychobiol. 2007;49:467–84. doi: 10.1002/dev.20242. [DOI] [PubMed] [Google Scholar]
- 60.Vaillancourt C, Berger N, Boksa P. Effects of vaginal birth versus caesarean section birth with general anesthesia on blood gases and brain energy metabolism in neonatal rats. Exp Neurol. 1999;160:142–50. doi: 10.1006/exnr.1999.7201. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Whitney PL, Frank L. Comparative responses of premature versus full-term newborn rats to prolonged hyperoxia. Ped Res. 1994;35:233–37. doi: 10.1203/00006450-199402000-00023. [DOI] [PubMed] [Google Scholar]
- 62.Boksa P, Zhang Y, Bestawros A. Dopamine D1 receptor changes due to caesarean section birth: Effects of anesthesia, developmental time course, and functional consequences. Exp Neurol. 2002;175(2):388–97. doi: 10.1006/exnr.2002.7896. [DOI] [PubMed] [Google Scholar]
- 63.Boksa P, Zhang Y. Epinephrine administration at birth prevents long-term changes in dopaminergic parameters caused by Cesarean section birth in the rat. Psychopharmacology. 2008;200(3):381–91. doi: 10.1007/s00213-008-1213-9. [DOI] [PubMed] [Google Scholar]
- 64.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11(1):18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19(8):3007–22. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ronca AE, Lamkin CA, Alberts JR. Maternal contributions to sensory experience in the fetal and newborn rat (Rattus norvegicus) J Comp Psychol. 1993;107(1):61–74. doi: 10.1037/0735-7036.107.1.61. [DOI] [PubMed] [Google Scholar]
- 67.Thelen E, Fisher DM, Ridley-Johnson R. The relationship between physical growth and a newborn reflex. Infant Behav Dev. 1984;7:479–93. [Google Scholar]
- 68.Zelazo NA, Zelazo PR, Cohen KM, Zelazo PD. Specificity of practice effects on elementary neuromotor patterns. Dev Psychobiol. 1993;29(4):686–91. [Google Scholar]
- 69.Winberg J. Mother and newborn baby: Mutual regulation of physiology and behavior— A selective review. Dev Psychobiol. 2005;47(3):217–29. doi: 10.1002/dev.20094. [DOI] [PubMed] [Google Scholar]
- 70.Gottlieb G. Conceptions of prenatal development: behavioral embryology. Psychol Rev. 1976;83:215–34. [PubMed] [Google Scholar]
- 71.Gottlieb G. The roles of experience in the development of behavior and the nervous system. In: Gottlieb G, editor. Development of neural and behavioral specificity. NY: Academic Press; 1976. [Google Scholar]
- 72.Ulrich DA, Lloyd MC, Tiernan CW, Looper JE, Angulo-Barroso RM. Effects of intensity of treadmill training on developmental outcomes and stepping in infants with Down syndrome: A randomized trial. Phys Ther. 2008;88(1):114–22. doi: 10.2522/ptj.20070139. [DOI] [PubMed] [Google Scholar]
- 73.Radell PL, Gottlieb G. Developmental intersensory interference: Augmented prenatal sensory experience interferes with auditory learning in duck embryos. Dev Psychol. 1992;28(5):795–803. [Google Scholar]
- 74.Bos AF, Van Braekel KNJA, Hitzert MM, Tanis JC, Roze E. Development of fine motor skills in preterm infants. Dev Med & Child Neurol. 2013;55:1–4. doi: 10.1111/dmcn.12297. [DOI] [PubMed] [Google Scholar]
- 75.Sansavini A, Guarini A, Caselli MC. Preterm birth: neuropsychological profiles and atypical developmental pathways. Dev Disabil Res Rev. 2011;17:102–13. doi: 10.1002/ddrr.1105. [DOI] [PubMed] [Google Scholar]