Abstract
Aims
The nature of the association of depression and anxiety with risk for acute myocardial infarction (AMI) remains unclear. We aimed to study the prospective association of single and recurrent self-reported symptoms of anxiety and depression with a risk of AMI in a large Norwegian population based cohort.
Methods and results
In the second wave of the Nord-Trøndelag Health Study (HUNT2, 1995–97) baseline data on anxiety and depression symptoms, sociodemographic variables, health status including cardiovascular risk factors and common chronic disorders were registered for 57 953 adult men and women free of cardiovascular disease. The cohort was followed up during a mean (SD) 11.4 (2.9) years for a first AMI from baseline through 2008. A total of 2111 incident AMIs occurred, either identified at hospitals or by the National Cause of Death Registry. The multi-adjusted hazard ratios were 1.31 (95% CI 1.03–1.66) for symptoms of depression and 1.25 (CI 0.99–1.57) for anxiety. Two episodes of mixed symptoms of anxiety and depression (MSAD), reported 10 years apart, increased the risk for AMI by 52% (11–108%). After exclusion of the first 5 years of follow-up, the association of depression symptoms with AMI risk was attenuated. Relative risk for AMI with anxiety symptoms and MSAD weakened when participants with chronic disorders were excluded.
Conclusion
Self-reported symptoms of depression and anxiety, especially if recurrent, were moderately associated with the risk of incident AMI. We had some indications that these associations might partly reflect reverse causation or confounding from common chronic diseases.
Keywords: Depression, Anxiety, Prospective, Risk, Acute myocardial infarction, Epidemiology
Introduction
Depression and anxiety, both highly prevalent conditions in the general population, are associated with elevated risk for acute myocardial infarction (AMI).1–7 However, the nature of this association remains controversial.
One of the greatest challenges in the research on the prospective association of anxiety and depression with AMI is that atherosclerosis, one of the main underlying pathophysiological mechanisms of AMI, is known to develop during decades before the first clinical symptoms.8 However, almost all studies of depression included middle aged or older adults, often with a short follow-up time.9 Thus, individuals free from clinical heart disease in the aforementioned prospective studies may not be free from atherosclerosis, which may facilitate depressive symptoms even before generating ischaemia.8,10
Furthermore, in most previous reports, the well-known cardiovascular risk factors were inadequately controlled for, and it is not clear to what extent these factors explain the observed association between depression and AMI.1,9,11 Among the most overlooked confounders are comorbid physical disorders.12 Depressive symptoms are highly correlated with the overall disease burden, and several common chronic disorders are also risk factors for AMI. Moreover, depression is known to have an episodic nature,13 yet in the great majority of previous studies, depression was assessed only once. Some studies, however, suggest that recurrent depression has considerably stronger association with AMI than single depressive episodes.14
Further, anxiety and depression are often considered separate psychopathological conditions, yet they share common symptoms and often overlap.15 Still, relatively little data are available on the prospective association between anxiety and risk for cardiovascular disorders (CAD), and similar methodological concerns as those presented for depression apply to these studies as well.16 Therefore, the aim of this study was to investigate the prospective association of single and recurrent self-reported symptoms of anxiety and depression with the risk of AMI controlled for potential confounders, including comorbidities in a large population-based cohort.
Methods
Study population and setting
All adult citizens in Nord-Trøndelag County, Norway, received a postal invitation to participate in the second wave of the Nord-Trøndelag Health Study (HUNT 2, 1995–97, http://www.ntnu.edu/hunt, last accessed 28 July 2013). In total, 94 187 individuals were invited and 65 215 (69%) participated in the HUNT 2 study. The participants filled in questionnaires (http://www.ntnu.edu/hunt/data/que, last accessed 28 July 2013) and attended a baseline clinical examination. Details about the study have been published elsewhere.17,18 Of the participants in HUNT 2, 47 316 also attended the first wave of the HUNT Study (HUNT 1, 1984–86).19
This study was approved by the regional ethics committee for research, by the National Directorate of Health, the Norwegian Data Inspectorate, and the HUNT data access committee. All participants gave written consent.
Exposure: depression and anxiety symptoms
The HUNT self-report questionnaire included a Norwegian version of the Hospital Anxiety and Depression Scale (HADS),20 which assesses core psychological symptoms of anxiety (HADS-A) and depression (HADS-D) during the last week. The seven anxiety questions mirror symptoms of worry and tension, while the seven depression items mirror mainly symptoms of anhedonia and loss of interest. All questions have a 4-point Likert scale response option from 0 (no symptom) to 3 points (highest symptom level). The HADS has shown good psychometric properties across various patient samples and settings.21
In total, 62 685 (96.1%) of the HUNT 2 participants had a valid HADS response, defined as response on 5 or more items on one subscale. Missing response among those who filled in five or six items were replaced based on the sum of completed items multiplied by 7/5 or 6/5, respectively. Both subscales range from 0 (no symptoms) to 21 points.
We categorized the participants according to their HADS-D and HADS-A scores, respectively, as having no symptoms of depression or anxiety (score < 8), having mild to moderate symptoms (score 8–10), and having severe symptoms (score ≥ 11).22 A combined total score (HADS-T) for assessing the impact of mixed symptoms of anxiety/depression (MSAD) was calculated by summing up valid HADS-A and HADS-D scores. We categorized the participants into three groups: no MSAD (score < 15), mild to moderate MSAD (score between 15 and 18), and severe MSAD (score ≥ 19).21
In HUNT 1 (1984–86), the Anxiety and Depression Index (ADI-4) was included. ADI-4 is an acceptable indicator for MSAD (sensitivity 0.51, specificity 0.93) and showed a high correlation (0.83) with the HADS-T score.23 ADI has been used as a crude baseline measure of MSAD in HUNT 1.23 ADI consists of four questions, two tapping into general anxiety and two into depression. Two of the questions have four response categories: nervousness ranging from never (1) to almost all the time (4) and calmness which range from almost all the time (1) to never (4). The two other questions had seven response categories; mood, ranging from very downhearted (7) to very happy (1) and vitality ranging from very strong and fit (1) to very tired and worn out (7).
In total, 32 963 of the participants in the present study had valid MSAD scores both in HUNT 1 (ADI-4) and HUNT 2 (HADS-T). A variable to indicate the combined burden of MSAD in HUNT 1 and HUNT 2 was created in categories of never, one, and two episodes. MSAD in HUNT 2 was defined as cut off ≥19 on the HADS-T scale. We then defined previous episode of MSAD in HUNT 1 by scoring in the upper quartile on ADI, cut-off at ≥14 points. ‘Never MSAD’ was as defined as a scoring <14 on ADI in HUNT 1 and <19 on HADS in HUNT 2, ‘MSAD once’ was defined as scoring above cut-off in one of the studies, while ‘MSAD twice’ included those scoring above cut-off in both studies.
Follow-up and outcome ascertainment
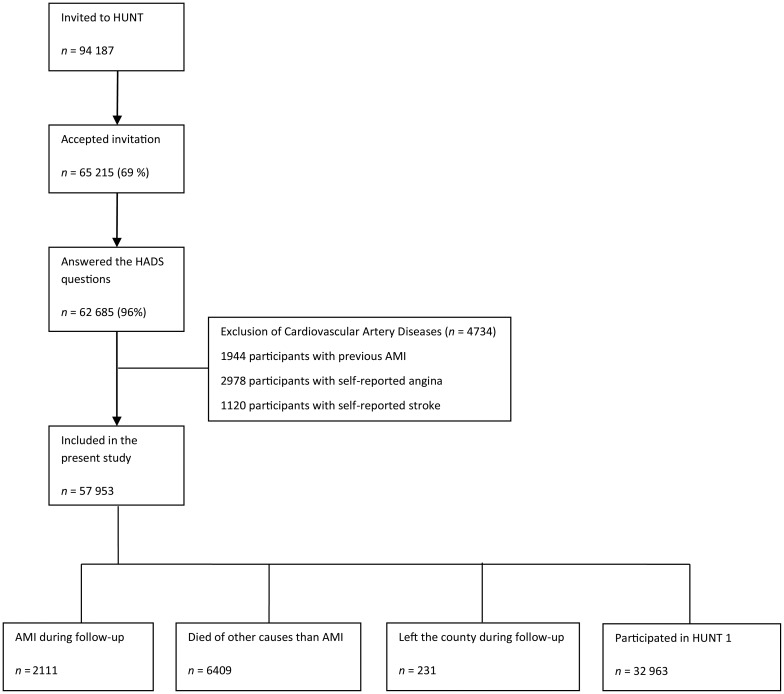
A total of 4734 participants were excluded at baseline because they reported a history of previous myocardial infarction (n = 1944), angina pectoris (n = 2978), or stroke (n = 1120). The remaining 57 953 participants were included in this study, and Figure 1 summarizes the recruitment of the participants.
Figure 1.
Flow chart for the participants.
Participants were followed-up for a first AMI from enrolment in HUNT 2 study until 31th of December 2008, either identified at the two hospitals in Nord-Trøndelag County or by the National Cause of Death Registry.24
Acute myocardial infarction was diagnosed according to the European Society of Cardiology/American College of Cardiology consensus guidelines.5 Criteria for AMI included: (i) specific clinical symptoms according to case history information, (ii) changes in blood levels of cardiac enzymes, and (iii) specified ECG changes. Fatal AMI incidents that never reached the emergency units at the hospitals were identified in the National Cause of Death Registry by the International Classification of Diseases codes (code no. 410 in the 9th revision and codes I21 and I22 in the 10th revision). During mean (SD) 11.4 (2.9) years of follow-up, 231 participants who left the county, and 6409 participants who died from other causes than AMI, were censored at the time of event (emigration or death) in the statistical analyses (see Figure 1 , flow chart).
Covariates
Demographic factors
Cohabitation status was dichotomized as living with a partner or alone. Education was categorized as low (≤ 9 years), medium (between 10 and 12 years), or high (over 12 years).
Life style factors
Smoking habits were self-reported and were categorized as current, previous, or never smoking. Physical activity was defined as light if it did not involve sweating or feeling of breathlessness, while hard physical activity was defined by sweating or breathlessness. The participants were categorized as inactive (less than 1 h of hard activity and less than 3 h of light physical activity per week), moderate active if they reported 1–3 h of hard activity or >3 h of light activity per week, and as physically active if they reported >3 h of hard physical activity per week.
Chronic somatic disorders
Participants self-reported if they had (or had ever had) cancer, asthma, diabetes mellitus, other endocrine disorders (hypothyreosis, hyperthyreosis, goitre, and thyroiditis), musculoskeletal disorders (osteoporosis, fibromyalgia, and arthrosis/arthritis), autoimmune disease (Bechterew disease, rheumatoid arthritis), epilepsy, or other chronic disorders (replying yes to the question ‘do you have or ever had any other chronic disease’ and no to the questions regarding previous specified diagnosis of chronic disease).
Clinical examination
Blood pressure, heart rate, weight, height, waist, and hip circumference were measured by specially trained nurses after a standardised protocol. Heart rate, and systolic and diastolic blood pressure were measured after the participant had been seated for at least 2 min with the cuff on, and the size of the cuff was adjusted for arm circumference.17 All blood pressure and heart rate recordings were performed using a Dinamap 845XT (Criticon) based on oscillometry and involved automatically three recordings at 1 min intervals. The first heart rate recording was in average 2 bpm lower than the second and third measurement and was thus used in order to mirror resting heart rate. For blood pressure, the value decreased for each recording, thus we used the average of the second and third measurements in our analysis. Height was measured without shoes to the nearest 1.0 cm and weight with light clothing to the nearest 0.5 kg.17 Body mass index (BMI) was computed as weight (in kg) divided by the squared value of height (in metres).
Blood sampling and laboratory measurements
A non-fasting whole blood sample was drawn from each participant, and the time between last meal and the venepuncture was recorded. Serum was separated by centrifugation at the screening site and immediately placed in a refrigerator (4°C). The samples were sent (same day or following Monday for samples drawn on Friday) for analyses at the central laboratory at Levanger Hospital, Norway, where they used a Hitachi 911 Autoanalyzer, applying reagents from Boehringer Mannheim. Serum total cholesterol was measured by an enzymatic colorimetric cholesterol esterase method. The day-to-day coefficient of variation for total cholesterol was 1.3–1.9%.17
Statistical analysis
Comparisons of continuous variables among those who developed AMI during follow-up and those who did not were made by two-sided t-test, and the χ2 test was used to compare categorical data. We used Cox proportional hazard models to examine the association of depression and anxiety symptoms and subsequent risk for AMI. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs). For test of trends, we assigned a numeric value of 0 to 2 to the HADS categories, with 0 having no, 1 having moderate, and 2 having severe anxiety or depression symptoms, treating the categories as a continuous variable. In a separate analysis, we calculated the risk for AMI associated with the presence of MSAD in HUNT 1 and HUNT 2 when those without symptoms of MSAD in both of the surveys constituted the reference group.
We adjusted all our models for sex and age as a continuous variable. In the second adjustment model, we added education and marital status as potentially confounding factors in our models. Established cardiovascular risk factors such as resting heart rate, high blood pressure, low physical activity, high BMI, smoking, dyslipidemia, and diabetes mellitus may act both as confounding and mediating factors for the association of depression and anxiety with the AMI risk. We therefore analysed the data both with and without the factors included in the analyses. We conducted several stratified analyses to assess whether the association of anxiety and depression and the risk for AMI could be modified by other factors.
We investigated the potential effect modification by sex, age (dichotomized at age 50 and age 65), BMI (dichotomized at ≥35 kg/m2), total cholesterol (dichotomized at 6.5 mmol/L), education (dichotomized at 12 years), blood pressure (systolic blood pressure dichotomized at >140 mm Hg, diastolic at >90 mm Hg), and smoking status (current vs. no current smoking). We also formally tested the homogeneity of stratum-specific relative risks. For these tests of interaction, we used the trend variable as defined above.
We present event-free survival curves using the Kaplan–Meier method according to symptom categories.
We performed several sensitivity analyses to assess the robustness of our findings. First, we excluded participants with cancer, asthma, diabetes mellitus, other endocrine disorders, musculoskeletal disorders, and autoimmune diseases or other reported chronic disorders. We also restricted the analysis to AMI cases that were confirmed at the hospital, thus cases whose AMI diagnosis were based on death certificates alone were excluded from the analyses. Finally, in order to address the possibility of reverse causation as an explanation for possible associations, we excluded the first 5 years of follow-up and repeated the analyses. All sensitivity analyses were run with adjustments for socioeconomic status and traditional cardiovascular risk factors (Model 3).
We tested the proportionality of hazard using log–log curves and formal tests of interaction with time or log-time. There was no evidence against the proportionality assumption (all P > 0.10).
Statistical analyses were performed in Stata IC/12.1 for windows (© Stata Corp LP).
Results
Characteristics of the study population, by AMI status, are shown in Table 1. Among the 59 953 participants, 2111 (3.5%) had a first AMI during follow-up. Of these, 1632 cases were diagnosed at a hospital and 479 cases were registered by the National Cause of Death Registry alone.
Table 1.
Baseline characteristics of participants in the total population and according to AMI vs. no AMI during follow-up
| Variable | No. of subjects | Total population | AMI during follow-up | No AMI during follow-up | P-value |
|---|---|---|---|---|---|
| Total % (n) | 57 953 | % | 3.6 (2111) | 96.7 (55 854) | |
| Variables, % (n) | |||||
| Male sex | 26 551 | 45.8 | 62.5 (1312) | 45.2 (25 239) | <0.0001 |
| Diabetes mellitus | 1292 | 2.2 | 8.3 (176) | 2.0 (1 116) | <0.0001 |
| Smoking | <0.0001 | ||||
| Never | 26 669 | 46.2 | 34.0 (714) | 46.6 (25 955) | |
| Former | 13 748 | 23.8 | 28.0 (608) | 23.6 (13 140) | |
| Current | 17 334 | 30.0 | 36.0 (775) | 29.8 (16 569) | |
| Physical activity | <0.0001 | ||||
| Inactive | 20 333 | 38.1 | 46.9 (796) | 37.8 (19 537) | |
| Moderately active | 27 574 | 51.7 | 46.5 (788) | 51.9 (26 786) | |
| Physically active | 5434 | 10.2 | 6.6 (112) | 10.3 (5322) | |
| Living alone | 23 009 | 39.8 | 35.9 (757) | 39.9 (22 252) | <0.0001 |
| Education | <0.0001 | ||||
| ≤9 years | 18 930 | 33.7 | 63.2 (1468) | 32.8 (17 763) | |
| 10–12 years | 25 311 | 45.1 | 27.3 (632) | 45.7 (24 765) | |
| >12 years | 11 857 | 21.1 | 9.0 (133) | 21.5 (11 661) | |
| Mean (SD) | |||||
| Age, years | 57 953 | 47.7 (16.3) | 64.9 (12.7) | 47.0 (16.0) | <0.0001 |
| Body mass index, kg/m2 | 57 498 | 26.2 (4.1) | 27.4 (4.0) | 26.2 (4.1) | <0.0001 |
| Systolic blood pressure, mmHg | 57 586 | 123.5 (21.1) | 153.9 (23.5) | 135.8 (20.7) | <0.0001 |
| Diastolic blood pressure, mmHg | 57 586 | 79.9 (12.0) | 87.1 (12.7) | 79.7 (11.9) | <0.0001 |
| Resting heart rate, bpm | 57 673 | 71.6 (13.0) | 73.4 (13.5) | 71.6 (13.0) | <0.0001 |
| Total cholesterol, mmol/L | 57 644 | 5.8 (1.2) | 6.7 (1.2) | 5.8 (1.2) | <0.0001 |
| HADS—depression score | 57 819 | 3.4 (3.0) | 4.1 (3.0) | 3.4 (3.0) | <0.0001 |
| HADS—anxiety score | 57 023 | 4.2 (3.3) | 3.9 (3.4) | 4.3 (3.3) | <0.0001 |
| HADS—total score | 56 889 | 7.6 (5.6) | 8.7 (5.8) | 8.6 (5.4) | 0.3555 |
| ADI score (HUNT 1) | 32 963 | 13.5 (1.2) | 13.6 (1.3) | 13.5 (1.2) | 0.0036 |
Using the highest cut off (≥11), the prevalence rates of HADS-defined anxiety and depression were 5.2 and 3.0, respectively. These estimates were comparable with the corresponding values (6.4 and 4.2%) found in the general European population.25
In our study, women reported higher symptom levels of anxiety and MSAD, while depression symptom levels were the same in both genders.
A total of 2111 participants had a first AMI during a mean (SD) follow-up of 11.4 (2.9) years. The prevalence of current smoking, diabetes, physical inactivity, and low education was higher among those who developed AMI during follow-up.
Table 2 presents the HRs for AMI in relation to symptoms of anxiety, depression, and MSAD. Risk for AMI was moderately elevated for participants reporting symptoms of depression, anxiety, and MSAD. The point estimates were fairly robust in multivariable models for socioeconomic and cardiovascular risk factors. Additional adjustment for resting heart rate in model 3 did not alter these results considerably (data not shown). Symptoms of anxiety were associated with higher risks for non-fatal AMI (HR 1.33, 95% CI 1.03–1.71) than for fatal AMI (HR 0.93, 95% CI 0.70–1.24), and this pattern was similar regarding symptoms of depression and MSAD (data not shown).
Table 2.
Hazard ratios (95% confidence intervals) for AMI during follow up according to symptoms of depression (HADS-D) and anxiety (HADS-A) and mixed anxiety/depression symptoms (MSAD measured by HADS-T) in HUNT 2 (1995–97)
| Variable | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| HADS-D | Events/person-time | 2096/656 582 | 1896/636 445 | 1561/590 365 |
| 0–7 | Reference | Reference | Reference | |
| 8–10 | 1.08 (0.94–1.24) | 1.03 (0.88–1.19) | 0.91 (0.77–1.09) | |
| ≥11 | 1.32 (1.07–1.61) | 1.25 (1.01–1.56) | 1.31 (1.03–1.66) | |
| P for trend | 0.008 | 0.074 | 0.253 | |
| HADS-A | Events/person-time | 2012/648 777 | 1847/630 642 | 1533/586 848 |
| 0–7 | Reference | Reference | Reference | |
| 8–10 | 1.02 (0.87–1.20) | 1.00 (0.84–1.18) | 0.91 (0.76–1.00) | |
| ≥11 | 1.30 (1.06–1.60) | 1.30 (1.06–1.61) | 1.25 (0.99–1.57) | |
| P for trend | 0.027 | 0.049 | 0.309 | |
| HADS-T | Events/person-time | 1997/647 414 | 1836/629 490 | 1528/586 132 |
| 0–12 | Reference | Reference | Reference | |
| 15–19 | 1.02 (0.85–1.21) | 1.00 (0.83–1.20) | 0.87 (0.70–1.07) | |
| ≥19 | 1.26 (1.04–1.51) | 1.24 (1.02–1.51) | 1.22 (0.98–1.51) | |
| P for trend | 0.032 | 0.066 | 0.345 |
HR indicates hazard ratio; CI, confidence interval; AMI, acute myocardial infarction.
Model 1: adjusted for calendar age and sex. Model 2: Model 1 + marital status, education.
Model 3: Model 2 + smoking, physical activity, body mass index, total cholesterol, diabetes mellitus, systolic blood pressure.
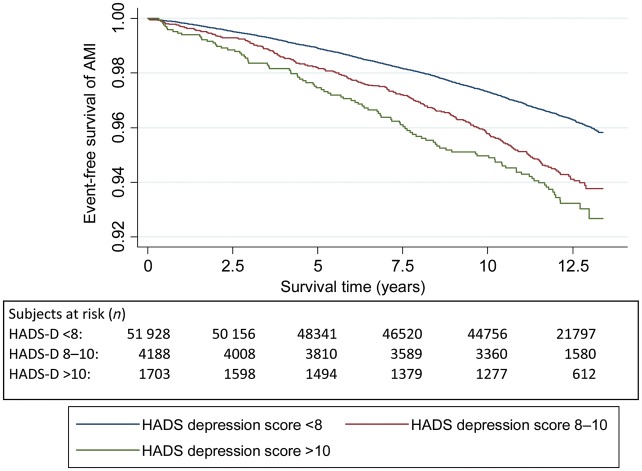
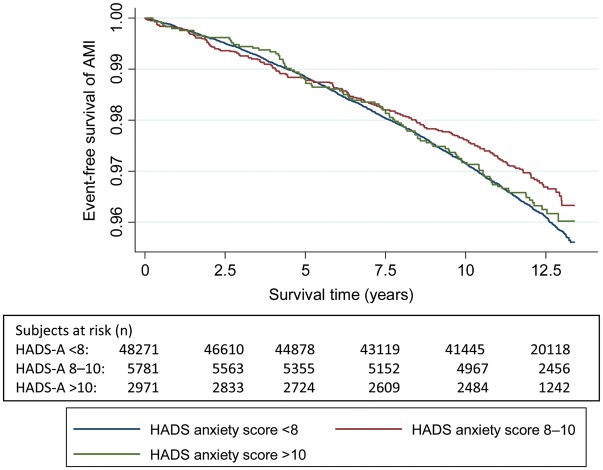
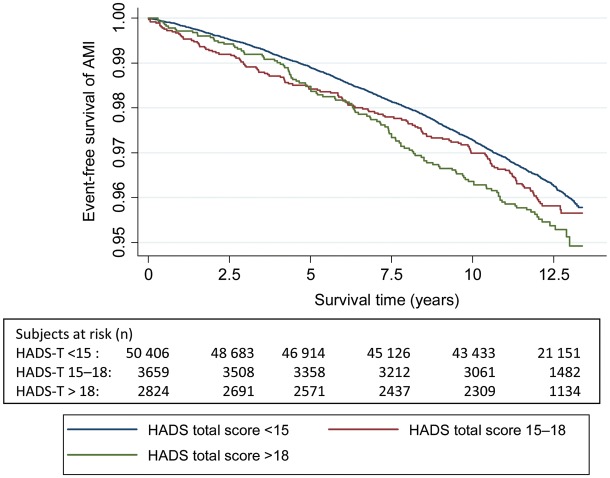
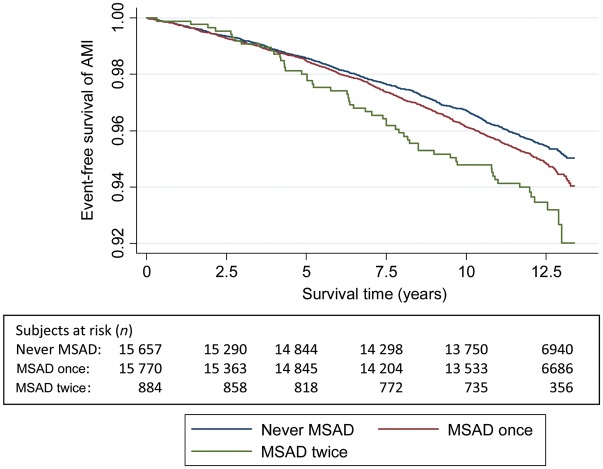
Figures 2–5 show Kaplan–Meier curves for event-free survival according to symptom categories and number of subjects at risk for each 2.5 years of follow-up.
Figure 2.
The Kaplan–Meier curve of incident acute myocardial infarction and subjects at risk during follow-up according to HADS-Depression symptom categories.
Figure 3.
The Kaplan–Meier curve of incident acute myocardial infarction and number of persons at risk during follow-up according to HADS-Anxiety symptom categories.
Figure 4.
The Kaplan–Meier curve of incident acute myocardial infarction and number of persons at risk during follow-up according to HADS-Total symptom categories.
Figure 5.
The Kaplan–Meier curve of incident acute myocardial infarction and number of persons at risk during follow-up according to mixed anxiety and depression symptom categories in HUNT 1 and 2.
Table 3 displays the HRs for AMI according to the presence of MSAD combining data from HUNT 1 and 2. In the study population, 48.4% (n = 15 657) reported no episodes of MSAD, 48.9% (n = 15 770) reported one MSAD episode, and 2.7% (n = 884) experienced two MSAD episodes. There was a trend towards higher HRs in those with two episodes of MSAD compared with those who did not report MSAD at HUNT 1 or HUNT 2 and HRs were 30% higher than for those reporting MSAD only in HUNT 2.
Table 3.
The association between episodes of mixed symptoms of anxiety and depression (MSAD) in HUNT 1 and 2, and risk of subsequent acute myocardial infarction
| Number of episodes MSAD | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Events/person years | 1491/372 505 | 1368/361 051 | 1145/333 405 |
| Never MSAD (n = 15 657) | Reference | Reference | Reference |
| MSAD once (n = 15 770) | 1.07 (0.97–1.19) | 1.06 (0.96–1.19) | 1.02 (0.99–1.14) |
| MSAD twice (n = 884) | 1.63 (1.24–2.19) | 1.65 (1.24–2.20) | 1.52 (1.11–2.08) |
| P for trend | 0.009 | 0.016 | 0.184 |
Results presented as HR's (95% CIs).
Model 1: adjusted for calendar age and sex.
Model 2: Model 1 + marital status, education.
Model 3: Model 2 + smoking, physical activity, body mass index, total cholesterol, diabetes mellitus+ systolic blood pressure.
Sensitivity analyses
Table 4 presents the multivariable adjusted HRs for AMI in relation to depression, anxiety, and MSAD in different sensitivity analyses.
Table 4.
Risk for acute myocardial infarction associated with symptoms of anxiety and depression in sensitivity analyses
| Exclusion of all chronic diseasesa | Restriction to hospital verified AMI | Exclusion of first 5 years of follow-up | |
|---|---|---|---|
| HADS—depression | |||
| Events/ person years | 768/384 975 | 1228/51 442 | 1082/341 485 |
| Cut off 8–10 | 0.95 (0.74–1.23) | 0.94 (0.44–1.15) | 0.96 (0.78–1.18) |
| Cut off ≥11 | 1.25 (0.81–1.91) | 1.33 (1.02–1.75) | 1.14 (0.83–1.57) |
| P for trend | 0.632 | 0.203 | 0.673 |
| HADS—anxiety | |||
| Events/person years | 762/384 068 | 1209/586 848 | 1060/345 716 |
| Cut off 8–10 | 1.08 (0.82–1.43) | 0.97 (0.79–1.19) | 0.87 (0.69–1.10) |
| Cut off ≥11 | 1.14 (0.74–1.75) | 1.33 (1.03–1.71) | 1.32 (1.00–1.73) |
| P for trend | 0.439 | 0.099 | 0.321 |
| HADS—total | |||
| Events/person years | 761/383 732 | 1205/586 132 | 1057/339 216 |
| Cut off 15–19 | 0.75 (0.53–1.05) | 0.89 (0.70–1.31) | 0.80 (0.61–1.05) |
| Cut off ≥19 | 1.07 (0.72–1.58) | 1.27 (1.00–1.61) | 1.23 (0.95–1.59) |
| P for trend | 0.559 | 0.202 | 0.563 |
| MSAD (HUNT 1 and 2) | |||
| Events/person years | 587/204 678 | 908/333 405 | 787/27 215 |
| One episode MSAD | 0.93 (0.79–1.09) | 1.07 (0.93–1.22) | 1.06 (0.92–1.23) |
| Two episode MSAD | 1.38 (0.79–2.40) | 1.81 (1.29–2.52) | 1.75 (1.21–2.53) |
| P for trend | 0.688 | 0.022 | 0.053 |
MSAD, mixed symptoms of anxiety and depression.
aJoint effect of persons that reported asthma, diabetes, other endocrine disorders, musculoskeletal disorders, autoimmune diseases, epilepsy, cancer, and other chronic diseases.
There were 1582 AMI cases among 49 330 participants that completed the questionnaire on common chronic somatic disorders. Of these, 13 515 persons (27.4%) reported to have at least one chronic disease. The frequency of chronic disorders was: diabetes mellitus n = 902, other endocrine disorders n = 1998, autoimmune diseases n = 1330, muscle/skeletal disorders n = 4184, asthma n = 3898, cancer n = 1323, epilepsy n = 652, and ‘other chronic disorders’ n = 1793. After exclusion of common chronic disorders, the effect sizes were generally attenuated (Table 4). No single group of chronic disorders appeared to be responsible for this effect.
When we restricted follow-up to AMI cases that were confirmed in hospital (1632) events, we obtained similar or even higher hazard ratios for anxiety, depression, and MSAD than in the main analyses.
After the fifth year of follow-up, we observed 1443 AMI events. Exclusion of the first 5 years substantially weakened the observed association between depression and AMI risk. Risk for AMI associated with anxiety symptoms and MSAD remained fairly stable (Table 4).
Discussion
In this large population-based cohort study, participants with symptoms of anxiety, depression and mixed symptoms (MSAD) had a 20–30% increased risk for first AMI. Participants reporting two episodes of MSAD, 10 years apart, had >50% increased risk for AMI. All effects were robust to extensive adjustments for socioeconomic and traditional cardiovascular risk factors. For depression symptoms, the effect was only evident the first 5 years of follow-up, which raises a possibility for a reverse causation. For anxiety symptoms and MSAD, AMI risk attenuated with exclusion of participants with co-existent chronic physical illnesses at baseline, which probably reflects confounding.
Comparisons with previous studies
Although anxiety and depression symptoms tend to overlap, these conditions have to date mostly been investigated separately in terms of risk for AMI.10 A recent meta-analysis of 23 prospective studies including a wide range of anxiety measures, found a pooled effect-size associated with incident cardiac heart disease of 1.26 (95% 1.15–1.38).26 Only 22% of the anxiety studies included in the meta-analysis consisted of both men and women.26 In our study, the relative risks for AMI in relation to anxiety and depression symptoms were similar in the two sexes and the overall effect was comparable to previous studies.26
For depression, another meta-analysis including 21 prospective studies reported a pooled effect of 1.95 (95% CI 1.51–2.51) on CAD death and AMI,9 considerably higher than the point estimates found in our study. We can think of several explanations for this discrepancy; first, studies using self-report instruments have generally found smaller effects than studies relying on clinical diagnosis or psychiatric interviews.9 Second, adjustment for cardiovascular risk factors and comorbid physical illness(es) was limited in most previous studies. Third, large population-based studies tended to report lower point estimates than smaller studies probably due to publication bias.9 Finally, we shall emphasize that previous studies used diagnostic interviews or self report instruments that included a wide depressive symptom spectrum, such as sleep problems, lack of energy and disturbed appetite. These symptoms greatly overlap with symptoms of common physical illnesses, including subclinical coronary artery disease.13 As a result, the genuine contribution of core psychological and cognitive symptoms of anxiety and depression to cardiovascular morbidity has been uncertain. Importantly, the tool used in the current study, i.e. HADS, was originally designed for detection of anxiety and depression in patients with physical illness, and systematically excludes somatic symptoms which can mimic heart disease.22 Hence, our study is one of the first to link core psychological and cognitive symptoms of anxiety and depression to a moderately increased AMI risk. Nevertheless, by using this approach, some participants with predominantly somatic symptoms of depression might go undetected. As a consequence, we are likely to have underestimated, rather than overestimated, the association between depression and AMI in our study.
Most studies confirm that comorbid anxiety and depression are associated with more severe illness, worse outcome, and increased mortality compared with single conditions.27 This was not confirmed in our study, as we found no excess risk for AMI associated with MSAD in HUNT 2.
Limited previous evidence suggested that information on anxiety or depressive symptoms provided by repeated measures enable a considerably better risk prediction when compared with the information derived from a single measurement.1,3,28,29 Our study supports these earlier findings and suggests that persistent or recurrent symptoms might be associated with increased cardiovascular risk. One can hypothesize that a single measurement could reflect a normal reaction to stressful events, while multiple episodes may better capture anxiety and depressive disorders,30 such as bipolar disorders, known for poorer adherence with lifestyle and medical advice.31
Our sensitivity analysis regarding depressive symptoms, but not anxiety or mixed symptoms, indicate a higher risk for AMI in the first 5 years of follow-up. This might reflect a reverse causation. The possibility that both depressive symptoms and subsequent AMI are caused by subclinical manifestations of atherosclerosis is among the greatest challenges for research on the prospective association between depression and AMI.12,13 Individuals free from clinical heart disease included in prospective studies may not be free from atherosclerosis, which is known to develop during decades before first symptoms.8,10 Thus, individuals free from clinical heart disease included in prospective studies may not be free from atherosclerosis. Atherosclerosis may facilitate depressive symptoms even before generating cardiac ischaemia creating a spurious association between depression and atherosclerosis. One possible pathway is the depressogenic actions of the increased inflammatory activity.32,33 Moreover, neuroimaging and neuropathology studies suggest that late onset depression often has a cerebrovascular origin.34 Cerebral atherosclerosis may thus facilitate depressive symptoms even before coronary atherosclerosis generates cardiac ischaemia.
Several epidemiological findings supported the reverse causality hypothesis. Studies with a shorter follow-up generally show stronger association with AMI.9 In the PRIME study, depressive symptoms were associated with coronary heart disease only in the first 5 years of follow-up,35 a finding which is confirmed in our study. There were some studies with null findings which were conducted in young populations, where vascular depression and subclinical CAD are less likely. In a study of healthy US army personnel 39–45 years of age,36 no correlation was found between depression and subclinical coronary artery disease identified by electron-beam computed tomography. Further, in a large prospective cohort study of men who were 19–21 years old at baseline, only anxiety but not depression was associated with future CAD risk.37
While adjustment for established cardiovascular risk factors was extensive in several studies regarding anxiety,26 many prospective studies of depression and AMI risk failed to adjust even for well-known cardiovascular risk factors such as smoking and physical activity.9 Furthermore, comorbid physical disorders are among the most overlooked confounders in previous prospective studies of anxiety or depression and subsequent heart disease.12 Depressive symptoms are highly correlated with the overall disease burden, and several common chronic disorders are risk factors for AMI.13 In the present study, AMI risk in participants with symptoms of anxiety and MSAD at baseline was attenuated considerably after excluding all participants with comorbid chronic diseases from the analysis. In contrast, the excess AMI risk in those with depression symptoms and recurrent MSAD was more robust throughout these analyses.
Strengths and limitations
Compared with prior studies, we had an ample statistical power to address the prospective association between symptoms of depression and anxiety, and the risk for AMI. The population-based nature of the study, the high stability (less than 0.3% net migration/year), and relatively high genetic and socioeconomic homogeneity of the population17 along with reliable hospital and register information covering the entire county, ensured close to complete follow-up and minimized the possibility for the misclassification of endpoints or for a selection bias.24 Moreover, in contrast to many previous studies, we could extensively control for potentially confounding factors.
Yet, the present work has some important limitations. Attendance in HUNT 2 was generally high, but considerably lower in the age group of 70 years and older.19 Further, we had no information on treatment for depression. SSRIs, the most commonly used medication for anxiety and depression, are known to reduce platelet adhesion38 and increase BMI39 and therefore are potential mediators for the observed associations. However, in the present study, it was not possible to separate the effect of depression and its eventual treatment.
Conclusion
Our findings indicate that self-reported core psychological symptoms of depression and anxiety, especially if recurrent, are moderately associated with the AMI risk. We had some indication that these associations might partly reflect reverse causation (depression) or confounding from common chronic diseases (anxiety and mixed anxiety and depression).
Funding
The study is funded by the Liason Committee between the Central Norway Regional Health Authority and The Norwegian University of Science and Technology. The funding providers played no role in study design; in the collection, analysis or interpretation of data; in writing of the report; or in decision to submit this article.
Acknowledgements
The Nord-Trøndelag Health Study (The HUNT Study) is collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health. I.J. and L.T.G. are supported by the Liaison Committee between the Central Norway Regional Health Authority. I.J. is also supported by the Norwegian University of Science and Technology and by the Swedish Council of Working Life and Social Research. We also thank the Department for Research and Development and the Department of Internal Medicine, at Nord Trøndelag Hospital Trust, for extracting the data from hospital records and allocating time to develop this manuscript.
Conflict of interest: The authors have no financial, professional, or personal conflict of interest relevant to the study and herby certifies compliance with ethical principles of publishing.
References
- 1.Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–1779. doi: 10.1161/01.CIR.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 2.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28:1339–1345. doi: 10.2337/diacare.28.6.1339. [DOI] [PubMed] [Google Scholar]
- 3.Janszky I, Ahlbom A, Hallqvist J, Ahnve S. Hospitalization for depression is associated with an increased risk for myocardial infarction not explained by lifestyle, lipids, coagulation, and inflammation: the SHEEP Study. Biol Psychiatry. 2007;62:25–32. doi: 10.1016/j.biopsych.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Gardner CO, Fiske A, Gatz M. Major depression and coronary artery disease in the Swedish twin registry: phenotypic, genetic, and environmental sources of comorbidity. Arch Gen Psychiatry. 2009;66:857–863. doi: 10.1001/archgenpsychiatry.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Shitti-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 8.Elovainio M, Keltikangas-Jarvinen L, Kivimaki M, Pulkki L, Puttonen S, Heponiemi T, Juonala H, Viikari JS, Raitakari OT. Depressive symptoms and carotid artery intima-media thickness in young adults: the Cardiovascular Risk in Young Finns Study. Psychosom Med. 2005;67:561–567. doi: 10.1097/01.psy.0000170340.74035.23. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 10.Seldenrijk A, van Hout HP, van Marwijk HW, de Groot E, Gort J, Rustemeijer C, Diamant M, Penninx BW. Carotid atherosclerosis in depression and anxiety: associations for age of depression onset. World J Biol Psychiatry. 2011;12:549–558. doi: 10.3109/15622975.2011.583942. [DOI] [PubMed] [Google Scholar]
- 11.Kubzansky LD, Kawachi I, Spiro A, III, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation. 1997;95:818–824. doi: 10.1161/01.CIR.95.4.818. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M, Peterson JC. Medical comorbidity and late life depression: what is known and what are the unmet needs? Biol Psychiatry. 2002;52:226–235. doi: 10.1016/S0006-3223(02)01422-1. [DOI] [PubMed] [Google Scholar]
- 13.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/S0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 14.Lesperance F, Frasure-Smith N, Talajic M. Major depression before and after myocardial infarction: its nature and consequences. Psychosom Med. 1996;58:99–110. doi: 10.1097/00006842-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Smith PJ. Risk factors: anxiety and risk of cardiac events. Nat Rev Cardiol. 2010;7:606–608. doi: 10.1038/nrcardio.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White KS. Cardiovascular disease and anxiety. In: Zvolensky MJ, Smits JA, editors. Anxiety in Health Behaviors and Physical Illness. New York: Springer; 2008. pp. 279–315. [Google Scholar]
- 17.Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, Vatten L, Lund-Larsen PG. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiol. 2003;13:19–32. [Google Scholar]
- 18.Nauman J, Janszky I, Vatten LJ, Wisloff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579–2587. doi: 10.1001/jama.2011.1826. [DOI] [PubMed] [Google Scholar]
- 19.Krokstad S, Langhammer A, Hveem K, Holmen T, Midthjell K, Stene T, Bratberg G, Heggland J, Holmen J. Cohort profile: The HUNT Study, Norway. Int J Epidemiol. 2012;42:1–10. doi: 10.1093/ije/dys095. [DOI] [PubMed] [Google Scholar]
- 20.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Bjelland I, Krokstad S, Mykletun A, Dahl AA, Tell GS, Tambs K. Does a higher educational level protect against anxiety and depression? The HUNT study. Soc Sci Med. 2008;66:1334–1345. doi: 10.1016/j.socscimed.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–2081. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 25.Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, de Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lépine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, Arbabzadeh-Bouchez S, Autonell J, Bernal M, Buist-Bouwman MA, Codony M, Domingo-Salvany A, Ferrer M, Joo SS, Martínez-Alonso M, Matschinger H, Mazzi F, Morgan Z, Morosini P, Palacín C, Romera B, Taub N, Vollebergh WA. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;0:21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 26.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JM, Monson RR, Olivier DC, Sobol AM, Leighton AH. Affective disorders and mortality. A general population study. Arch Gen Psychiatry. 1987;44:473–480. doi: 10.1001/archpsyc.1987.01800170095012. [DOI] [PubMed] [Google Scholar]
- 28.Penninx BW, Guralnik JM, Mendes de Leon CF, Pahor M, Visser M, Corti MC, Wallace RB. Cardiovascular events and mortality in newly and chronically depressed persons > 70 years of age. Am J Cardiol. 1998;81:988–994. doi: 10.1016/S0002-9149(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 29.Wassertheil-Smoller S, Applegate WB, Berge K, Chang CJ, Davis BR, Grimm R, Jr, Kostis J, Pressel S. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systoloc Hypertension in the elderly) Arch Intern Med. 1996;156:553–561. doi: 10.1001/archinte.1996.00440050111012. [DOI] [PubMed] [Google Scholar]
- 30.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 31.Cerimele JM, Katon WJ. Associations between health risk behaviors and symptoms of schizophrenia and bipolar disorder: a systematic review. Gen Hosp Psychiatry. 2013;35:16–22. doi: 10.1016/j.genhosppsych.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjerkeset O, Romild U, Smith GD, Hveem K. The associations of high levels of C-reactive protein with depression and myocardial infarction in 9258 women and men from the HUNT population study. Psychol Med. 2011;41:345–352. doi: 10.1017/S0033291710000887. [DOI] [PubMed] [Google Scholar]
- 33.Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behav Immun. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- 35.Majed B, Arveiler D, Bingham A, Ferrieres J, Ruidavets JB, Montaye M, Appleton K, Haas B, Kee F, Amouyel P, Ducimetiere P, Empana JP. Depressive symptoms, a time-dependent risk factor for coronary heart disease and stroke in middle-aged men: the PRIME Study. Stroke. 2012;43:1761–1767. doi: 10.1161/STROKEAHA.111.645366. [DOI] [PubMed] [Google Scholar]
- 36.O'Malley PG, Jones DL, Feuerstein IM, Taylor AJ. Lack of correlation between psychological factors and subclinical coronary artery disease. N Engl J Med. 2000;343:1298–1304. doi: 10.1056/NEJM200011023431803. [DOI] [PubMed] [Google Scholar]
- 37.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol. 2010;56:31–37. doi: 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, van Zyl LT, Finkel MS, Krishnana KR, Gaffney M, Harrison W, Califf RM, O'Connor CM. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003;108:939–944. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 39.Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahra S, Rush AJ. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40:41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]