Abstract
Clinical researchers have tracked patients with early life trauma and noted generalized anxiety disorder, unipolar depression, and risk-taking behaviors developing in late adolescence and into early adulthood. Animal models provide an opportunity to investigate the neural and developmental processes that underlie the relationship between early stress and later abnormal behavior. The present model used repeated exposure to 2,3,5-trimethyl-3-thiazoline (TMT), a component of fox feces, as an unconditioned fear-eliciting stimulus in order to induce stress in juvenile rats aged postnatal day (PND) 23 through 27. After further physical maturation characteristic of the adolescent stage (PND 42), animals were tested using an elevated plus maze (EPM) for anxiety and plantar test (Hargreaves method) for pain to assess any lingering effects of the juvenile stress. To assess how an additional stress later in life affects anxiety and pain nociception, PND 43 rats were exposed to inescapable shock (0.8 mA) and again tested on EPM and plantar test. A final testing period was conducted in the adult (PND 63) rats to assess resulting changes in adult behaviors. TMT-exposed rats were significantly more anxious in adolescence than controls, but this difference disappeared after exposure to the secondary stressor. In adulthood, but not in adolescence, TMT-exposed rats demonstrated lower pain sensitivity than controls. These results suggest that early life stress can play a significant role in later anxiety and pain nociception, and offer insight into the development and manifestation of anxiety- and trauma-related disorders.
Keywords: trimethylthiazoline, predator odor, juvenile stress, analgesia, anxiety, animal models
1. Introduction
It is widely accepted that stress and trauma in early life can predispose an individual to stress and anxiety disorders later in life (McCormick & Green, 2012). In their meta-analysis Kessler et al. (2005) reported lifetime prevalence of anxiety and mood disorders to be 28.8% and 20.8%, respectively and found that these disorders typically emerge in adolescence. Among these disorders are post-traumatic stress disorder (PTSD) and non-suicidal self-injury (NSSI), both of which are linked to stress (McCormick & Green, 2012; Bloom, Holly, & Miller, 2012).
A large body of work has been published regarding the importance of developmental time periods when considering the anxiogenic and cognitive effects of stress exposure. In their study of the effect of a time lapse between two stressors and the consequent behavioral outcomes, Avital and Ricther-Levin (2005) concluded that the developmental period at which stressors occur is more predictive of later emotional and cognitive dysfunction than the length of time between two stressors. When exposed to a stressor first in juvenility and then again in adulthood, rats had lower anxiety-like behaviors and poorer spatial learning than rats exposed to both stressors in adulthood but with the same intervening amount of time between stressors. These results suggest a dynamic effect of early stress insofar as it correlates with lower anxiety-like behaviors but at the cost of poorer cognitive learning. Although it is well agreed-upon that stress affects anxiety-like behaviors, the direction of that effect is a matter of some controversy. Tsoory and Richter-Levin (2006) reported that stress-coping behaviors exhibited in adulthood are significantly influenced by the period of early development at which stress was experienced. Rats stressed in juvenility (post-natal day 28; PND) and rats stressed in adolescence (PND 34) showed equal deficits in avoidance learning compared to controls, but only juvenile-stressed rats also demonstrated significant learned helplessness-like behavior in adulthood. These findings suggest that juvenility is a more impactful stress-sensitive period than adolescence and that stress during juvenility has severe anxiogenic, rather than anxiolytic, effects in adulthood (Tsoory & Richter-Levin, 2006). Regardless of the direction of effect, the most prevalent hypothesis is that stress during development significantly impacts the immature neuroanatomy.
Neuroanatomical research has found that the hypothalamic-pituitary-adrenal (HPA) axis is an essential structure in mediating stress responses. McCormick, Matthews, Thomas, and Waters (2010) have shown that stress during adolescence has longer lasting behavioral effects than similar stress experienced in adulthood, perhaps because of the HPA’s sensitivity to glucocorticoids and the general abundance of hormones present during adolescence. The medial prefrontal cortex (mPFC), which undergoes significant development from juvenility to adolescence, may also be important as it is suggested that it could be responsible for the relationship between the developmental period at which stress is endured and the subsequent anxiogenic effect (Chan et al., 2011).
In an effort to further elucidate which developmental time periods are more susceptible than others to stressors and how they relate to later anxiety, the current experiment further investigates the role of early life stress on later emotional functioning but includes aspects of pain nociception to study whether stress-induced analgesia has similar developmental contingencies. Animals in the experimental group were exposed to 2,3,5-trimethyl-3-thiazoline (TMT) odor, a derivative of fox-feces, repeatedly during juvenility. During adolescence, anxiety and pain sensitivity levels were measured before and after exposure to an inescapable shock (IS). Animals were tested again in adulthood to assess any long-term changes as a result of stressors experienced earlier in their development.
2. Methods
All procedures remained in compliance with the animal safety regulations and guidelines set forth by Providence College IACUC.
2.1 Animals
Twenty male Sprague-Dawley rats were delivered from Charles River Laboratories (Wilmington, MA) at age 22 days and individually housed in 25×25×35 cm Plexiglas cages maintained in temperature controlled (21.6 °C) quarters with a 12-h light-dark schedule (lights on 8:00—20:00 h). Ten rats were randomly assigned to each of the control and experimental conditions. Animals were allowed 24-h acclimation before the start of the experimental procedure, and had ad libitum access to standard solid-pellet rat food (LabDiet ProLab® RMH 3000, Wausau, WI) and water throughout the experiment.
2.2 Behavioral Procedures
2.2.1 Scent exposure in metabolic cage
On PND 23 through 26, rats were individually placed in small (48×28×36 cm) metabolic cages (Harvard Apparatus, Holliston, MA) and exposed to 0.5 mL of either 10% TMT (Contech Enterprises, Delta, B.C., Canada) or water vapor, which was pipetted into a cotton ball and placed below the metal grid floor of the cage. The control group (n = 10) was exposed first on all four days to ensure that their testing environment was not affected by any TMT odor that may have lingered in the room. Rats remained in the exposure chamber for 30 min, after which boli were counted as a measure of fear and anxiety. The cages were then thoroughly cleaned and the experimental group (n = 10) was exposed to TMT in the same manner.
2.2.2 Scent exposure in open field
On PND 27, rats were exposed in an open field apparatus in order to analyze motion as a measure of fear and anxiety. Contained in a sound-attenuating chamber, the open field consisted of an open-top Plexiglas box (43.2×43.2×30.5 cm) with infrared motion sensors lining the walls (Med Associates Inc., St. Albans, VT). Activity Monitor MDB software recorded the amount of time the rat spent in the center of the field (13.2×13.2 cm; Med Associates Inc., St. Albans, VT). Animals were individually exposed and the control rats were run first, again to prevent any lingering scent of TMT. A cotton ball with 0.5 mL of either 10% TMT or water vapor was placed on a small glass directly outside of the open field, but within the chamber in which the field was contained. Rats were exposed for 30 min and motion was detected with the Activity Monitor software within the open field hardware (Med Associates Inc., St. Albans, VT).
2.2.3 Plantar test and EPM
At age PND 42, pain sensitivity and anxiety-like behavior were measured with the plantar test (Hargreaves Method; IITC Life Science, Inc., Woodland Hills, CA) and an elevated plus maze (EPM; custom-built, Providence, RI), respectively.
In a protocol adapted from Hargreaves et al. (1988), the glass surface of the plantar test apparatus was allowed to reach a uniform 29 °C, at which point rats were placed five at a time into plastic cubicles (9×22×25 cm) atop the surface. After a 10 min habituation period, a focused, high-intensity projector lamp beam was shone below onto the mid-plantar surface of the left hind paw of the first rat. Initially at an idle intensity of 10%, the lamp was then activated, at which point it gradually increased to a maximum of 50% active intensity. When the paw was withdrawn from the surface, or after a maximum of 25 s, the lamp was switched off and latency from the start point was recorded. In identical fashion, latency for withdrawal of the left hind paw was recorded for the remaining rats, followed by a 2 min inter-trial interval, and then latency for each rat’s right hind paw was recorded. For every rat, three trials of each hind foot were recorded, the median latency for each paw was kept, and the two median values were averaged.
The rats were then transferred back to home cages for a period ranging from 10–30 minutes acclimation period before undergoing the EPM test, during which time the next five rats began the plantar test. Following the acclimation period, subjects were tested on EPM. The custom-built maze consisted of four 50 cm arms made of polished particle board mounted on a medium-density fiberboard frame. Two of the arms were surrounded by 30 cm-high walls, and the entire maze sat 50 cm above the floor. Rats were placed in the center of the maze and allowed to freely explore the enclosed and open arms. Suspended from the ceiling was an EthoVision camera which recorded the amount of time spent on open and closed arms (Noldus Information Technology, Wageningen, Netherlands). After five min, the rat was removed from the maze, returned to its home cage, and the process was then repeated for the next rat.
2.2.4 Inescapable shock
On PND 43, 24 h after the first set of plantar and EPM testing, all rats were subjected to IS to examine the effect of a stressor in adolescence—both alone and in conjunction with juvenile stress—on pain tolerance and anxiety-like behavior. Rats were placed five at a time in individual operant chambers (30.5×24.1×29.2 cm) with metal grid flooring connected to an electric shocker controlled by MED-PC IV (Med Associates Inc., St. Albans, VT) software. Subjects were shocked at 0.8 mA intensity for one second every minute for 30 min. After the 30 min exposure period, the five rats were moved to the plantar test and EPM where they were tested for pain sensitivity and anxiety-like behavior under the same protocols as described in 2.2.3.
2.2.5 Adulthood
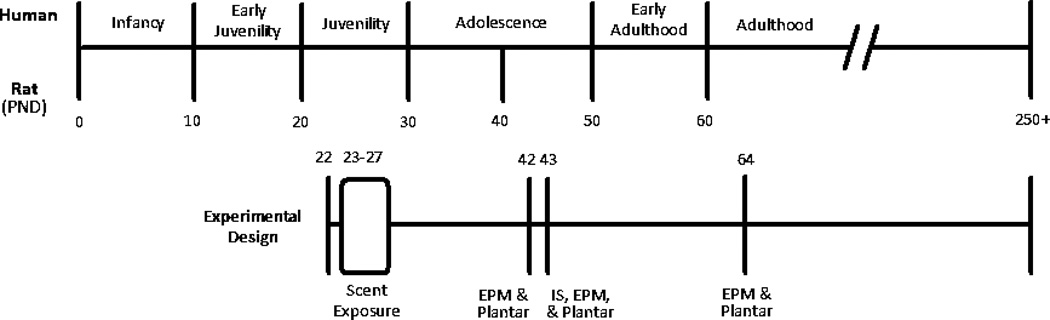
On PND 64, all rats were tested again on plantar and EPM under the same protocols as previously described in 2.2.3 to examine long-term effects of early life stressor on pain tolerance and anxiety-like behavior. A summary timeline of rat development and experimental manipulations is represented in Fig. 1.
Figure 1.
A representation of rat and human development parallels and their relation to the experimental design. Adapted from Andersen (2003).
3. Results
3.1 Juvenile Exposure
3.1.1 Day 1–4: Bolus count
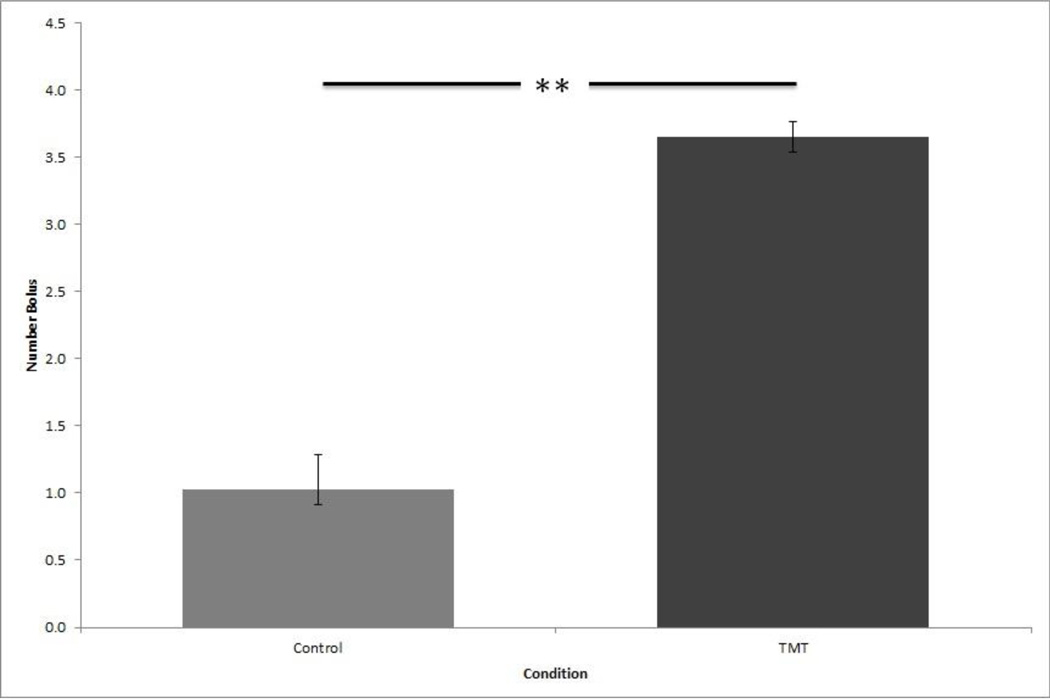
As shown in Fig. 2, the mean number of boli per day excreted during scent exposure differed by condition across the four days of exposure in the metabolic cages. An independent samples t-test was used to compare by condition. The boli count over four days of exposure was significantly different, with exposed rats excreting more boli than control rats, t(18) = −5.010, p < 0.001. Control rats exposed to distilled water had a total M = 1.03, S = .515 boli per day whereas experimental rats exposed to TMT had a total M = 3.65, S = .232 boli per day.
Figure 2.
Mean number of daily boli per day. Error bar denotes standard deviation. ** p < 0.001.
3.1.2 Day 5: Open field
Although rats were exposed to control or TMT scent in the open field for 30 min, they appeared to have habituated to the chamber over time resulting in no significant correlation of time spent in the center zone between the first and second fifteen-min periods, r(18) = 0.176, p > .05. Thus, only the first 15 min were used for analysis. Data were analyzed by a one-way between groups ANOVA. Rats did not differ by condition in the amount of time spent in the center zone of the open field during the first 15 min of exposure, F(1, 18) = 0.899, p > .05. Rats exposed to TMT spent M = 34.49, S = 22.82 s in the center and rats exposed to distilled water spent M = 19.85, S = 14.92 s in the center.
3.2 Adolescence and Adulthood Testing
3.2.1 Elevated plus maze
The anxiety-like behavior level of adolescent rats was evaluated by time spent in the open arms of an EPM before (PND 42) and after (PND 43) exposure to IS (phases 1 and 2) and again in adulthood (PND 64; phase 3). The data were analyzed by a 2×3 mixed-model ANOVA and the results of the EPM test are reported in Fig. 3 for a measure of anxiety-like behavior.
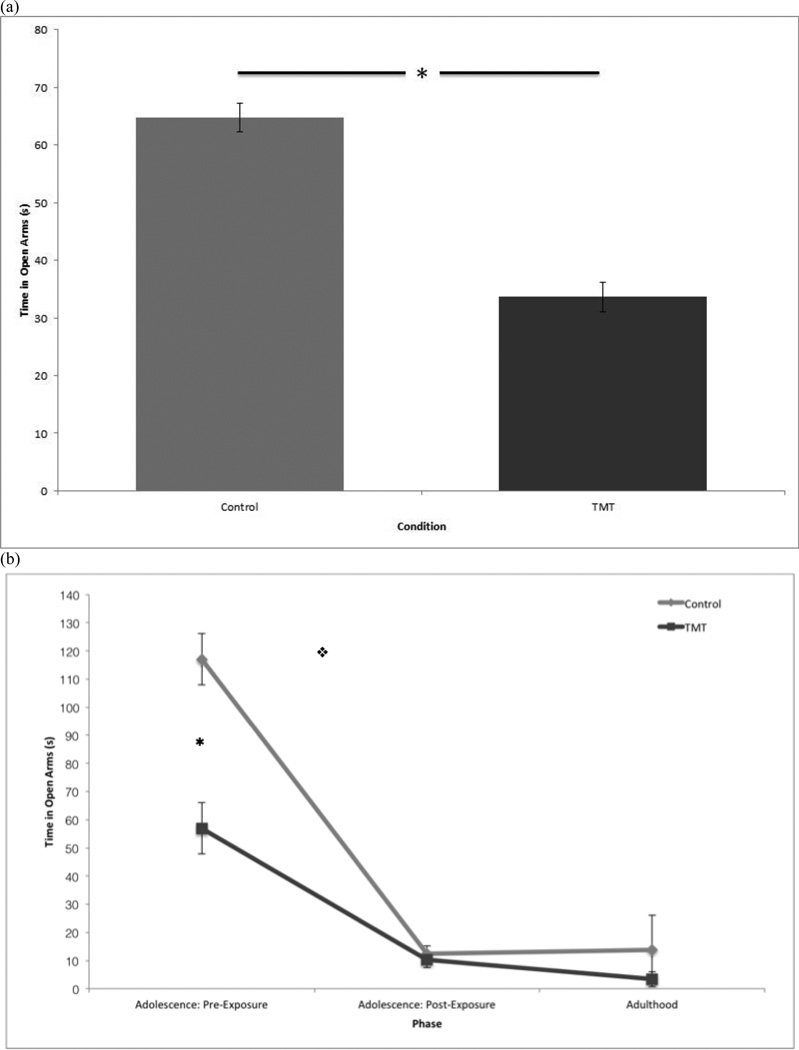
Figure 3.
(a) Main effect of condition on mean time spent in open arm of EPM, regardless of phase. (b) Time spent on open arms of EPM by condition across phases. Error bars denote standard deviation. *p < 0.05, ❖p < 0.05 * Denotes significant difference between conditions pre-IS ❖Denotes significant interaction between condition and phase for pre- and post-IS
As displayed in Fig. 3a, control rats spent significantly more time in open arms compared to treated rats, F(1, 18) = 4.691, p = 0.044. Control rats spent M = 64.745, S = 5.077 s in open arms while treated rats were in open arms for M = 33.644, S = 5.077 s.
As shown in Figure 3b, there was a significant interaction between condition and phase for before and after exposure to IS in the duration of time spent in open arms of the EPM, F(1, 18) = 5.466, p = 0.031. Follow up planned comparison of the main effects of condition showed that there was a significant difference between control and TMT before exposure to IS, F(1, 18) = 5.490, p = 0.031. Control rats spent M = 117.037, S = 9.066 s in the open arms of the maze before exposure to IS and M = 12.452, S = 2.787 s after exposure to IS. Treated rats spent M = 10.330, S = 9.066 s in the open arms before exposure to IS and M = 9.066, S = 2.787 s after exposure to IS. There was no significant difference between control and TMT after exposure to IS, F(1, 18) = 0.072, p > 0.5.
3.2.2 Plantar test
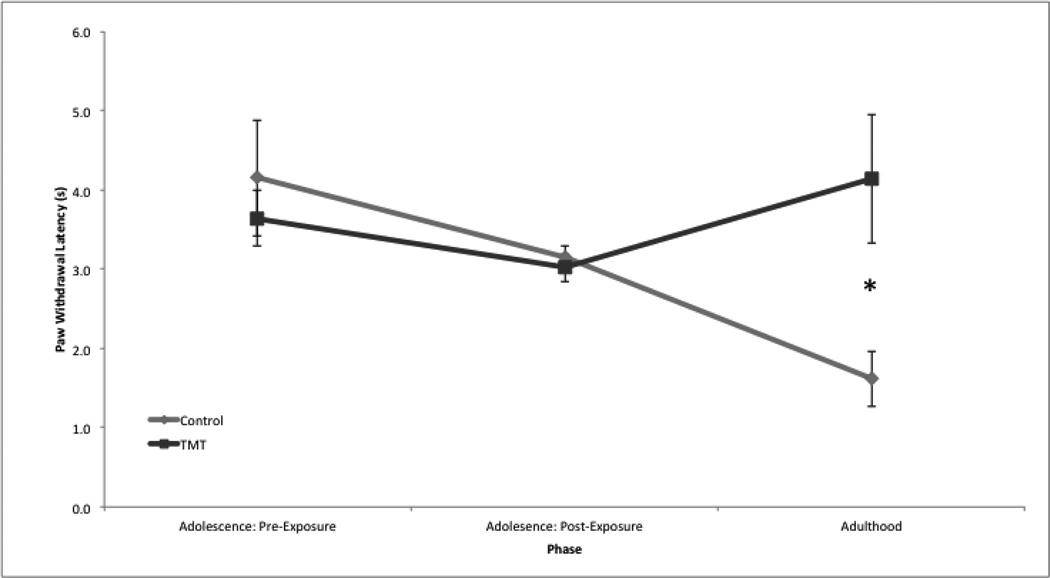
The plantar test data were analyzed with a 2×3 mixed-model ANOVA using the median of the six latency measurements per rat—three per each hind paw—and calculating the mean of those medians by condition and day. A significant effect was found for the interaction (phase×condition), F(1, 18) = 4.077, p = 0.025, as shown in Fig. 4a. No significant main effect for condition irrespective of phase was found, F(1, 18) = 0.636, p > 0.05.
Figure 4.
Interaction of condition and phase. Error bars denote standard deviation. * p < 0.05.
A significant difference in paw withdrawal latency was found between adolescence pre-IS exposure (M = 3.896, S = 1.154) and adolescence post-IS exposure (M = 3.081, S = 0.338), p = 0.017, as displayed in Fig. 4b. A significant difference in paw withdrawal latency was found between adolescent post-IS exposure (M = 3.081, S = 0.338) and adulthood (M = 4.754, S = 1.369), p < 0.001, as shown in Fig. 4b. Additionally, an independent samples t-test showed a significant difference by condition in adulthood, t(18) = 0.983, p = 0.040. As displayed in Fig. 4c, control rats had an average paw withdrawal latency of M = 4.136, S = 0.700 s, and treated rats had an average paw withdrawal latency of M = 5.371, S = 1.618 s.
4. Discussion
In an effort to model early-life trauma, treated juvenile rats were exposed to TMT from PND 23 to PND 27 while control rats were exposed to distilled water. The effectiveness of the stressor was measured by defecation, a traditional measure of fear and anxiety-like behavior (Million, 2000). The present results support similarly published data in which TMT was shown to increase defecation. The higher bolus count of treated rats on all four days of exposure in the metabolic cage indicated the efficacy of TMT in inducing fear (Staples, McGregor, Apfelbach, & Hunt, 2008).
On the fifth day of exposure, the data were not significant in open field measures. This could be largely due to the size of the open field that was used. It has been found that the size of the chamber used for exposure and the concentration of TMT are both important factors to consider when fearful behavior in rats is measured (Wallace & Rosen, 2000). The same amount and concentration of TMT was absorbed onto the cotton ball in the open field as in the metabolic cages, but the open field was much larger than the metabolic cage and thus the scent dispersed more quickly. Therefore, there was less impetus for the rats to demonstrate fear-provoked behaviors associated with the odor, which could account for the lack of statistical significance in this exposure.
In adolescence, all rats were tested before and after IS for anxious behavior using the EPM, which is a reliable, established test that measures anxiety-like behavior, where rats that exhibit freezing behaviors and hide in the closed arms are considered anxious because they demonstrate behaviors of fear and anxiety towards the prospect of exploring the open arms (Pellow, Chopin, File, & Briley, 1985). In the current experiment, it was found that control rats were less anxious than treated rats before IS, but after IS there was no longer a significant difference by condition; the adolescent stressor seemed to elicit a ceiling effect as all rats exhibited higher levels of anxiety-like behavior.
There was no difference by condition in terms of pain sensitivity when the adolescent rats were tested on the plantar test before IS exposure, but there was a lower tolerance for pain exhibited by all rats immediately after IS. Just as all rats experienced greater anxiety-like behavior after IS, all rats also appeared to experience an acute nociceptive effect immediately following the adolescent stressor. Jorum (1988) found that perhaps due to inherent individual differences, some rats after undergoing a tail-flick stressor showed distinct differences in pain threshold. Rats that experienced agitation, termed “hyper emotionality,” were more likely to experience hyperalgesia (Jorum, 1988). With respect to the present experiment, all rats may have demonstrated this type of “hyper emotion” due to the potency of IS; this could have caused the rats to experience hyperalgesia after IS relative to the day before IS, regardless of condition. Similar findings have been replicated in other experiments (Meagher, 2001).
Adult rats were retested for anxiety-like behavior and pain sensitivity on EPM and plantar test respectively, in order to measure the long-term effects of juvenile and adolescent stress. On the EPM, there was no significant difference in anxiety-like behavior by condition, but all adult rats spent less time in the open arms compared to adolescence before IS. This suggests that there was a long-term effect on anxiety-like behavior from the IS 20 days prior. In support of this, van Dijken, Mos, van der Heyden, and Tilders (1992) found that foot-shocked rats exhibited increased defecation, increased freezing, and decreased activity compared to controls 11, 14, and 28 days after a stressor. Tsoory and Richter-Levin (2006) found that juvenile-stressed rats showed decreased exploration and poor avoidance learning, with 28% exhibiting learned helplessness in a novel environment when tested in adulthood. Conversely, adolescent-stressed rats did not exhibit the same learned helplessness but did show decreased exploration and poor avoidance learning during adulthood (Tsoory & Richter-Levin, 2006). While they do not offer a clear mechanism for the effect of stress during development on later anxiety-like behaviors, these results in conjunction with our own suggest a dependent relationship between long-term anxiety and the developmental time period during which a stressor occurs, particularly highlighting a distinction between juvenility and adolescence.
In regards to adulthood pain tests, TMT-treated rats had a higher pain tolerance compared to controls, suggesting a type of long-term analgesia. Similar results have been reported previously. D’Amato, Mazzacane, Capone, and Pavone (1999) found that mice pups that experienced maternal separation demonstrated analgesia in adulthood whereas control mice did not. In a study considering the differences between prenatal and postnatal stress, groups included pregnant mice that were restrained (prenatal stress), postnatal mice that were handled (postnatal stress), or mice that experienced both prenatal and postnatal stress to examine longterm nociceptive effects in adulthood (Sternberg & Ridgway, 2003). Adult mice stressed in either prenatal or postnatal life showed increased pain thresholds, while those mice that experienced both stressors did not demonstrate a nociceptive effect (Sternberg & Ridgway, 2003). The types of stressors employed should be noted: the mice that experienced prenatal restraint coupled with the postnatal handling may have exhibited no change in pain tolerance because the handling may have negated the adverse effects of restraint if it was considered motherly or nurturing to the maternally-separated pups. The rats in the present experiment that experienced both a phylogenetically-relevant juvenile stressor and a nociceptive adolescent stressor were found to have a higher long-term pain tolerance compared to the rats that were stressed only in adolescence.
The significant difference in pain sensitivity between the two groups of rats indicates that stress exposure during juvenility has long-term nociceptive effects. The results suggest a long-term stress-induced analgesic mechanism, but because difference in pain sensitivity was significant only in adulthood and not adolescence, adolescent exposure to IS may have acted as a trigger for subsequent analgesia. The early postnatal TMT exposure paired with the additional adolescent shock-induced stress may have precipitated meaningful difference in pain that was not comorbid with a significant difference in anxiety-like behavior compared to controls. These results bolster the argument of Gauriau and Bernard (2002), who found that nociceptive projections from the parabrachial circuits have two main targets in the forebrain, amygdala, and hypothalamus. They suggest that those areas extending to the amygdala may contribute to aversive emotion, fear-evoked avoidance learning, and anti-nociceptive responses. Their findings suggest that there is overlap of pain and fear-related pathways in the brain that may at least partially contribute to the results presented in this study.
Though the results of this study are interesting, interpretation must be tempered by the limitations of the experimental design. The current project was lacking two potentially important control groups: a control that experienced neither TMT exposure nor IS and a control that experienced TMT exposure but not IS. Another potential limitation may include not testing the acute anxiety and nociceptive effects immediately after juvenile TMT exposure on the EPM and plantar test. In further investigations into this effect, the two additional conditions and the anxiety and pain tolerance tests after TMT exposure should be considered in the design.
Rat and human anatomical development are reasonably analogous, allowing animal models to target specific developmental time periods in order to learn how early life stress at various stages may manifest differently in later life (Andersen, 2003). The current project offers avenues of research into future animal models studying the onset of later life trauma- and anxiety-related disorders and their relation to stressful juvenile events. The phylogenetically relevant TMT stressor plus the longitudinal aspect of this experiment should be considered in the design of animal models of psychological disorders in which these circumstances of anxiety and pain sensitivity are found to be disrupted (Fendt & Endres, 2008). Examples of such disorders are PTSD and NSSI. The higher pain tolerance found in adult TMT-treated rats compared to controls, as well as the higher anxiety levels found overall, may have value for research specifically involving NSSI because both are prevalent symptomologies of the disorder, the mechanisms behind the susceptibility to and practice of NSSI in humans are still not fully understood, and a non-pharmacological animal model for this disorder does not exist at this time (Bloom, Holly, & Miller, 2012).
Highlights.
-
·
Animals exposed to stress in juvenility had increased anxiety in adolescence
-
·
A secondary adolescent stressor negated anxiety differences prior to the stressor
-
·
Animals stressed in juvenility demonstrated analgesia in adulthood
Acknowledgements
The authors would like to extend their thanks to Michelle Ouellette, who assisted in data collection. The work published here was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 8 P20 GM103430-12.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportuinity? Neuroscience and Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. International Journal of Neuropsychopharmacology. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Bloom CM, Holly S, Miller AMP. Self-injurious behavior vs. nonsuicidal self-injury. Crisis. 2012;33(2):106–112. doi: 10.1027/0227-5910/a000127. [DOI] [PubMed] [Google Scholar]
- Chan T, Kyere K, Davis BR, Shemyakin A, Kabitzke PA, Shair HN, Wiedenmayer CP. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. The Journal of Neuroscience. 2011;31(13):4991–4999. doi: 10.1523/JNEUROSCI.5216-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, Mazzacane E, Capone F, Pavone F. Effects of postnatal manipulation on nociception and morphine sensitivity in adult mice. Developmental Brain Research. 1999;117:15–20. doi: 10.1016/s0165-3806(99)00090-5. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T. 2,3,5-trimethyl-3-thiazoline (TMT), a component of fox odor – just repugnant or really fear-inducing? Neuroscience and Biobehavioral Reviews. 2008;32:1259–1266. doi: 10.1016/j.neubiorev.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Experimental Physiology. 2002;87(2):251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Jorum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988;32:341–348. doi: 10.1016/0304-3959(88)90046-2. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2012;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Ferguson AR, Crown ED, McLemore S, King TE, Sieve AN, Grau JW. Shock-induced hyperalgesia: IV. Generality. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27(3):219–238. [PubMed] [Google Scholar]
- Million M, Wang L, Martinez V, Taché Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Research. 2000;877:345–353. doi: 10.1016/s0006-8993(00)02719-0. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. doi: http://dx.doi.org/10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biological Psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Ridgway CG. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiology & Behavior. 2003;78:375–383. doi: 10.1016/s0031-9384(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. International Journal of Neuropsychopharmacology. 2006;9:713–728. doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- Van Dijken HH, Mos J, van der Heyden JAM, Tilders FJH. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiology & Behavior. 1992;52:945–951. doi: 10.1016/0031-9384(92)90375-c. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Predator odor as unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behavioral Neuroscience. 2000;114(5):912–922. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]