Abstract
Understanding why some terminally ill patients may seek a hastened death (a construct referred to as “desire for hastened death” or DHD) is critical to understanding how to optimize quality of life during an individual’s final weeks, months or even years of life. Although a number of predictor variables have emerged in past DHD research, there is a dearth of longitudinal research on how DHD changes over time and what factors might explain such changes. This study examined DHD over time in a sample of terminally ill cancer patients admitted to a palliative care hospital. A random sample of 128 patients who completed the Schedule of Attitudes toward Hastened Death (SAHD) at two time points approximately 2–4 weeks apart participated. Patients were categorized into one of four trajectories based on their SAHD scores at both time points: low (low DHD at T1 and T2), rising (low DHD at T1 and high DHD at T2), falling (high DHD at T1 and low DHD at T2) and high (high DHD at T1 and T2). Among patients who were low at T1, several variables distinguished between those who developed DHD and those who did not: physical symptom distress, depression symptom severity, hopelessness, spiritual well-being, baseline DHD, and a history of mental health treatment. However, these same medical and clinical variables did not distinguish between the falling and high trajectories. Overall, there appears to be a relatively high frequency of change in DHD, even in the last weeks of life. Interventions designed to target patients who are exhibiting subthreshold DHD and feelings of hopelessness may reduce the occurrence of DHD emerging in this population.
Keywords: US, cancer, desire for hastened death, end of life, palliative care
Introduction
Desire for hastened death (DHD) refers to the desire for a quicker death than would occur naturally, typically in the context of a life-shortening medical illness. However, while the desire for a more rapid death may be evidence of despair or psychological distress, it could also represent a response to chronic suffering for patients diagnosed with diseases such as Amyotrophic Lateral Sclerosis (ALS), HIV/AIDS or advanced cancer (Halpin, 2012; Leeman, 2009; Rosenfeld, 2003). Over the past two decades, roughly 20 publications have examined DHD in patients with advanced or terminal illness, revealing a number of consistent findings. For example, elevated levels of depression and hopelessness, and low levels of spiritual well-being, have emerged as the strongest predictors, regardless of illness or disease status (Albert et al., 2005; Breitbart et al., 2000; Chochinov, Tataryn, Clinch, & Dudgeon, 1999; O’Mahony et al., 2005; Rosenfeld et al., 2006). However, demographic variables such as age, gender, education and religiosity have not been consistently associated with DHD. Pain, social support, and sleep disturbance have also been found to predict the desire for hastened death in occasional studies, albeit with less consistency.
Very few studies, however, have examined the course of DHD over time. O’Mahony and colleagues studied 64 cancer patients at baseline and a 4-week follow up assessment (O’Mahony et al., 2005). They found a modest increase in DHD over time but DHD was quite low at both assessments. They found a significant association between changes in DHD and changes in depression, but a decline in pain intensity and pain-related functional interference over time were not associated with changes in desire for hastened death. Breitbart and colleagues examined the impact of treatment for depression on DHD in 42 depressed patients with advanced AIDS (Breitbart et al., 2010). They administered the Schedule of Attitudes toward Hastened Death (SAHD; Rosenfeld et al., 1999) to patients admitted to specialized nursing facilities shortly after admission and at two follow-up assessments (one and two months later). They grouped patients into several trajectories of change, distinguishing those who showed decreases in DHD over time versus those for whom DHD increased or remained stable. Using hierarchical linear models, the strongest predictor of changes in DHD was change in level of depressive symptoms and this effect was stronger (i.e., DHD decreased more) for patients who were prescribed antidepressant medications.
Chochinov and colleagues examined a related construct, “will to live”, in 168 terminally ill cancer patients using a single-item visual analogue scale to measure will to live twice daily for the duration of the patients’ stay in a palliative care facility (Chochinov et al., 1999). The authors calculated maximum and median fluctuations at four time points: 12 hours, 24 hours, 7 days and 30 days, and used cross-sectional data to predict will to live at 6 time points (using stepwise regression models). They found that will to live was highly unstable, but identified four main predictors of will to live in the cross-sectional models: depression, anxiety, shortness of breath, and sense of well-being. However, they did not analyze predictors of changes in will to live. Albert and colleagues conducted monthly interviews with patients diagnosed with advanced ALS, finding that 10 of the 53 patients expressed the wish to die at some point and three actually took actions that reflected an attempt to hasten death (e.g., requested increased sedation and persisted in this request until it was fulfilled; Albert et al., 2005). Interestingly, those patients who acted on their DHD appeared less distressed over time and reported a greater sense of control over the disease when compared to those who expressed the wish to die but did not act on it.
Although these studies have generated a handful of important findings, the paucity of longitudinal research on DHD in terminally ill populations is striking. Hence, there is a clear need for systematic research examining how DHD changes over time. More importantly, identifying factors that might explain such changes may have important implications for the care of terminally ill patients. The present study sought to fill this void by examining DHD over time in a sample of terminally ill cancer patients admitted to a palliative care hospital.
Method
Participants
Participants were recruited from a palliative care hospital in New York City serving adult patients with advanced cancer and a life expectancy of 6 months or less. All participants were 21 years of age or older, English speaking, and cognitively intact enough to provide informed consent and meaningful responses to study questionnaires (based on a Mini-Mental State Exam [MMSE] score of 20 or greater; Folstein, Folstein, & McHugh, 1975). During the 42 month study period, a total of 1585 individuals were admitted to this facility, 990 of whom obtained an MMSE score of 20 or greater, suggesting cognitive functioning adequate for study participation; 595 individuals could not be assessed, either because they were too ill or too sedated to participate (n=332) or they refused to complete the MMSE or were unable to understand English (n=263). Of the 990 individuals who met inclusion criteria, 439 (44.3%) provided written informed consent and 128 of these (29.2%) provided sufficient follow-up data to be included in the present study; the 311 remaining individuals had either died or deteriorated to the point where study participation was no longer possible (no patients voluntarily withdrew from the study). The study sample was 52.3% female (n=67), had an average age of 66.0 years old (SD=13.87, range: 30 to 90) and 13.3 years of education (SD=3.1, range: 6 to 23 years). The most common cancer diagnoses were lung cancer (n=25; 19.5%), followed by gastro-intestinal (n=13; 10.2%), prostate (n=12; 9.4%), and breast cancers (n=9; 7.0%). The majority (n=87, 68.0%) of participants were white and identified themselves as Catholic (n=64, 50.4%).
Procedures
All patients admitted to the study hospital, who were alert and met inclusion criteria, were invited to participate in this study within a few days after admission. Those individuals who consented to participate were administered an extensive structured interview and completed multiple self-report measures (detailed below). Medical and demographic data were elicited from the patient’s medical record and through interview. All assessments were conducted by clinical psychologists or psychology doctoral students. Because many participants had visual impairments and/or fatigue, self-report measures were typically read aloud and when necessary, assessments were divided into two or more sessions.
The original study design involved three assessment points: baseline, 2-week follow-up and 4-week follow-up, with patients exhibiting clinically significant depressive symptoms at baseline being referred to the staff psychiatrist for antidepressant treatment. However, most patients were either too ill, or deteriorated to rapidly to complete the third assessment and some required multiple, shorter sessions to complete even the second assessment (extending the time between assessments). Thus, there were insufficient data to analyze participants across three time points and, for a handful of participants, the second and third assessment were merged into a single follow-up assessment roughly three to four weeks after the first assessment. In total, 133 participants completed a second assessment within 2 to 4 weeks after the baseline assessment, but 5 of these participants did not complete the SAHD and therefore were not included in these analyses.
The primary dependent variable was the Schedule of Attitudes toward Hastened Death (SAHD), a 20-item self-report measure of current DHD developed for use with patients diagnosed with life threatening illnesses (Rosenfeld et al., 1999; Rosenfeld et al., 2000). The SAHD has been widely used in studies of DHD and has demonstrated high levels of reliability and concurrent validity in samples of patients with HIV/AIDS, cancer and ALS. Although some differences have emerged across study populations, cut-off scores of 7 and 10 have been used to distinguish “low” versus “high” desire for hastened death (Rosenfeld et al., 1999; Rosenfeld et al., 2000). In the present study, we utilized a cut-off of 7 or greater to designate high desire for hastened death since the higher threshold (≥ 10) resulted in too small a sample for the planned analyses.
In addition to the SAHD, patients were also administered the depression module from the Structured Clinical Interview for DSM-IV (SCID-D; First, Spitzer, Gibbon, & Williams, 2002) and the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960), two clinician-rated measures of depressive symptoms. Additional self-report measures included the Beck Hopelessness Scale (BHS; Beck, Weissman, Lester, & Trexler, 1974), the Memorial Symptom Assessment Scale (MSAS; Portenoy et al., 1994), the Anxiety subscale from the Hospital Anxiety Scale (HADS-A; Zigmond & Snaith, 1983), the Brief Pain Inventory (BPI; Cleeland & Ryan, 1994), the FACIT Spiritual Well-being Scale (SWB; Peterman, Fitchett, Brady, Hernandez, & Cella, 2002), the Duke-UNC Functional Social Support Questionnaire (FSSQ; Broadhead, Gehlbach, de Gruy, & Kaplan, 1988), and the Karnofsky Performance Status Scale (KPRS; Karnofsky & Burchenal, 1949). The Institutional Review Boards of Calvary Hospital, Memorial Sloan-Kettering Cancer Center, and Fordham University approved this study.
Statistical Analyses
The primary analytic strategy involved comparing patients across trajectories of change in DHD. Because of the modest sample size of individuals for whom follow-up data were available, analyses were conducted separately for those who were high in DHD at baseline (i.e., contrasting those who remained high versus improved over time) and those low in DHD at baseline (i.e., contrasting those who remained low versus worsened – i.e., increased DHD over time). This approach was chosen for both logical, as well as statistical reasons. Foremost, this approach reflects the clinical decision making process facing clinicians who work with terminally ill patients; those who are low in DHD when first interviewed may be at risk of increased DHD later, and identifying the risk factors for developing DHD is critical. Conversely, determining which patients high in DHD are likely to change their minds, and no longer want a hastened death, has equally important implications. Although initial univariate analyses (chi-square test of association and ANOVA) could certainly identify variables that distinguished these four groups (one of which – the Low trajectoryn was much larger than the others), a multivariate prediction model would be far less easily interpreted and even gleaning the important pair-wise effects from 4-group analyses is much harder. Thus, we used univariate tests (chi-square and t-tests) to identify variables that distinguished between two subgroups of patients based on baseline DHD (e.g., those who had low and high DHD at baseline). Variables found to be significant in univariate analyses were subsequently entered into a stepwise logistic regression model to identify variables that provided a unique, statistically significant contribution to distinguishing DHD groups. Because our analysis of the 4 groups as two pairs doubles the number of statistical tests conducted, we utilized the False Discovery Rate (Benjamini & Hochberg, 1995) correction to minimize the possibility of Type I error; findings that were no longer significant using this correction are noted as such in the text. Finally, an exploratory analysis examined differences in the four trajectories based on changes in depression and hopelessness. This analysis used change scores (follow-up score minus baseline) in an ANOVA model, using post-hoc tests to identify pairwise (between trajectories) differences in change scores.
Results
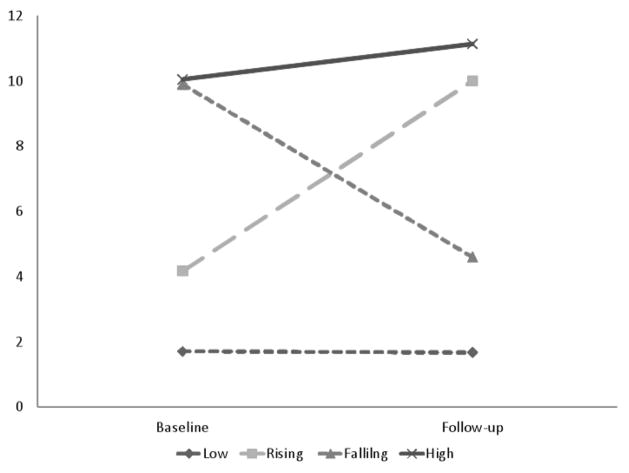
Patients were categorized into four trajectories based on their SAHD scores as baseline and follow-up assessment (see Figure 1 and Table 1). On average, follow-up assessments were conducted 16.3 days (SD=3.1) after the baseline assessment, with a range of 12 to 27; 10 participants (7.8%) completed the follow-up assessment more than 3 weeks after the baseline. The Low trajectory (n=84, 65.6%) included patients with low SAHD score at both time points whereas the Rising trajectory (n=12, 9.4%) included those patients with a low SAHD score at baseline and high SAHD score at follow-up, the Falling trajectory (n=10, 7.8%) included those with high SAHD scores at baseline and lower SAHD scores at follow-up and the High trajectory (n=22, 17.2%) included patients with high DHD at both time points. The mean change between baseline and follow-up for the Low trajectory was 0.0 (SD=1.8), whereas the mean change in the High trajectory was 1.1 (SD=2.9). Not surprisingly, there was significantly greater change in SAHD scores for patients in the Rising (Mean change=5.8, SD=3.9) and Falling (Mean change= −5.3, SD=2.2) trajectories, F(3,124)=45.24, p < .0001.
Figure 1.
SAHD Total scores for DHD trajectories
Table 1.
Means (SD) of Desire for Hastened Death by trajectory (theoretic range of scores is 0–20)
| Variable | Trajectory | Falling (n=10) | High (n=22) | |
|---|---|---|---|---|
| Low (n=84) | Rising (n=12) | |||
| Baseline | 1.65 (1.7) | 4.17 (2.0) | 9.90 (2.1) | 10.05 (2.7) |
| Follow-up | 1.65 (1.4) | 10.00 (2.9) | 4.60 (1.3) | 11.13 (2.9) |
Of the demographic variables studied (gender, age, race, and years of education), only race, Chi-square=11.40, df=3, p=.01, differed significantly across the four trajectories, with a greater proportion of ethnic minority participants in the low trajectory (41.7%) compared to the other three groups (15.8%). Age approached significance, F(3,124)=2.62, p=.054; those in the Low trajectory were younger (M=63.7) than patients in the other three trajectories (Rising=68.1; Falling=70.6; High=71.8).
The overall sample was moderately distressed, with a mean HDRS score of 10.37 (SD=6.2; range: 0 to 29) and a mean BHS score of 5.59 (SD=4.8; range: 0 to 19); 23 (18.0%) met criteria for a Major Depressive Episode based on the SCID-D. Depressed participants were significantly more likely to be prescribed antidepressant medications (either an SSRI or psychostimulant) than were non-depressed participants (72.7% versus 20.2%; data were missing for two participants), chi-square=24.16, df=1, p < .001. There was a significant difference in the rate of antidepressant medication prescribed for patients in the four DHD trajectories, Chi-square=8.47, df=3, p=.04.
Correlates of DHD Trajectories
A number of psychological distress variables significantly distinguished the four trajectories (see Table 2) including measures of depression (HDRS), hopelessness (BHS), spiritual well-being (SWB), quality of life (QOL), and physical symptom distress (MSAS). However, several variables that were anticipated to differentiate these groups did not, including social support (FSSQ), physical functioning ability (KPRS), and average pain intensity (BPI).
Table 2.
Means (SD) of Correlates of DHD Trajectories
| Variable | Trajectories of Desire to Hasten Death | F | p | |||
|---|---|---|---|---|---|---|
| Low (n=84) | Rising (n=12) | Falling (n=10) | High (n=22) | |||
| Depression (HDRS) | 8.35 (5.1) | 14.17 (6.8) | 12.00 (5.9) | 15.32 (6.5) | 11.65 | .0001 |
| Hopelessness | 3.88 (3.5) | 7.50 (5.5) | 10.60 (3.5) | 10.81 (4.3) | 24.82 | .0001 |
| Spiritual Well-Being | 3.22 (0.8) | 2.61 (0.8) | 2.53 (1.2) | 1.78 (1.0) | 16.46 | .0001 |
| Anxiety | 4.01 (4.1) | 4.50 (3.9) | 6.30 (5.1) | 6.37 (5.3) | 1.96 | .12 |
| Quality of Life | 6.40 (2.8) | 4.83 (2.4) | 5.10 (1.8) | 3.82 (3.2) | 1.22 | .001 |
| Social Support | 3.55 (0.6) | 3.66 (0.6) | 3.42 (0.8) | 3.29 (0.8) | 1.12 | .34 |
| Phys. Symptom Distress | 1.25 (0.7) | 1.78 (0.9) | 1.51 (0.7) | 1.50 (0.8) | 5.60 | .002 |
| Pain Intensity | 4.90 (2.7) | 4.00 (1.3) | 3.75 (1.3) | 4.70 (1.8) | 1.86 | .55 |
| Phys. Functioning | 40.06 (7.3) | 39.75 (10.6) | 40.00 (13.1) | 41.82 (8.9) | 0.15 | .93 |
Differentiating Low and Rising DHD Trajectories
Analysis of putative correlates of DHD trajectories among the 96 individuals who were low on DHD at baseline revealed that no demographic variables (e.g., gender, age, race, education) significantly differed between those with rising DHD versus those who remained low at both time points. Antidepressant medication use at baseline also failed to differentiate these two subgroups, as 33.3% of the rising patients (4 of 12) were prescribed antidepressant medication versus 25.0% of the low patients (21 of 84). However, several other medical and clinical variables distinguished the low and rising trajectories including the MSAS Global Distress Index, HDRS, BHS, SWB, baseline SAHD scores, and a history of mental health treatment. When the six variables that differed significantly between the two DHD trajectories were entered into a stepwise logistic regression analysis, only two variables emerged as unique contributors: baseline SAHD score (B=0.69, Exp(B)=2.0, p=.001) and MSAS Global Symptom Distress Index (B=1.23, Exp(B)=3.4, p=.02). These two variables correctly classified 91% of cases and accounted for 21 to 40% of the variance in group classifications (based on the Cox & Snell and Nagelkerke R2 estimates respectively).
In order to explore the relevance of baseline SAHD score in identifying rising DHD, we classified some individuals as having “subthreshold DHD” at baseline based on SAHD scores between 4 and 7 (n=20) and differentiated this group from patients with “very low” DHD (SAHD < 4). Within the subthreshold group, 40% (n=8) developed high DHD at the follow-up assessment compared to only 5% (4 of 76) of those with very low DHD at baseline. Thus, having a baseline DHD in the subthreshold range appears to be a strong predictor of developing high DHD over time.
Differentiating Falling and High DHD
Among the 32 individuals with high DHD at baseline, none of the demographic (e.g., gender, age, race, education) variables significantly differed between those who had falling DHD (i.e., were low at follow-up) versus those who remained high at both time points. Similarly, there was no difference in the proportion of patients already prescribed antidepressant medication (at baseline) between the falling (30%; 3 of 10) and high groups (45.5%; 10 of 22). Moreover, unlike the analysis contrasting rising versus low groups, none of the medical or clinical variables analyzed significantly distinguished the falling and high trajectories.
Analysis of change scores
We also analyzed correlates of DHD trajectories using change in HDRS and BHS scores across the four trajectories (see Figure 2). These analyses revealed a significant group difference for BHS scores, F(3,117)=9.59, p < .001, but not HDRS scores, F(3,124)=0.39, p = .76. On average, participants in the Falling trajectory had a decrease in BHS total scores of 4.56 points whereas BHS scores rose for those in the Rising (Mean change=2.58 point increase), Low (Mean change=2.80), and High trajectories (Mean change=0.90). Post-hoc tests confirmed that no significant differences existed between any of the four trajectories in depression change scores, but that the Falling group had significantly greater decreases in hopelessness (p < .001) than the other three groups. No differences in change in hopelessness existed between the other three groups.
Figure 2.
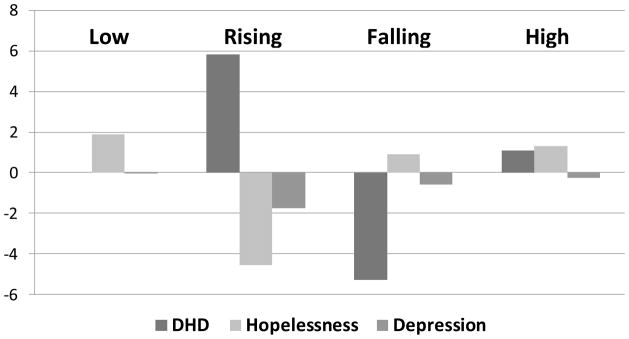
Relationship between changes in distress and DHD
Discussion
This study represents one of the first systematic analyses of change over time in desire for hastened death among terminally ill cancer patients. There are a number of important findings from these data, including the frequency of changes in DHD among patients at this advanced stage of disease. For example, of the 33 individuals with elevated DHD at the baseline assessment, 10 had substantially lower DHD only 2–3 weeks later (a 31% response rate), with a mean reduction of more than 5.3 on the SAHD in this subgroup. This reduction is particularly noteworthy given the severity of illness in our sample, and suggests that important psychological changes can occur even in the last weeks of life. On the other hand, a subset of patients with low DHD at baseline were elevated at the follow-up assessment (n=12, or 12.5%), typically showing large increases in SAHD scores (a mean change of 5.8). This finding is no less important, as it indicates a vulnerability to developing DHD for some terminally ill patients.
In order to identify correlates of these changes in DHD, we analyzed predictors separately for participants who were low in DHD at the baseline assessment versus those who were high. Despite some loss of power (due to small samples), and the potential for increased type I error, this approach was selected primarily because it mimics the decision process that often occurs in clinical settings. Clinicians who work with terminally ill patients may be confronted with a patient who expresses a desire for hastened death (Meier et al., 1998). The clinician must decide how to respond to such statements, and in particular, whether an intervention might help reduce the patients DHD. Identification of which patients are likely to change their attitude towards hastened death is therefore critical. Conversely, a clinician working with patients who do not express DHD must still remain cognizant of the risk factors for developing DHD. Our analyses were more fruitful with the latter; differentiating consistently low DHD from those who developed high DHD over time.
Analyses demonstrating that baseline DHD, psychological distress variables, and global symptom distress differentiated low from rising DHD suggest that those patients who develop DHD may already be experiencing distress at baseline. In fact, the rising DHD appears to reflect an exacerbation of a subthreshold level of DHD, perhaps fueled by worsening or unresolved symptoms. Although aggressive palliative care is a universal goal for cancer patients approaching the end of life, these findings highlight the importance of effective interventions to reduce or prevent DHD. Importantly, the lack of a significant relationship between changes in depression and DHD trajectories in our sample suggests that treatment may need to focus on reducing hopelessness rather than depression per se, since depressive symptoms decreased (on average) for all four trajectories, yet was not associated with changes in DHD.
Unlike our prior research with advanced AIDS patients (Rosenfeld et al., 2006), there were no significant predictors of falling DHD in this sample of terminally ill cancer patients. This finding may reflect the modest sample size for these analyses (n=34), which precluded examination of multivariate relationships or identifying small effects. Particularly surprising, given our prior research, was the absence of any association with antidepressant medication as had emerged in our prior study with AIDS patients. The failure to observe an effect of antidepressant medication on changes in DHD may be an artifact of our sample, as we had a wide variation in antidepressant treatments provided to those patients with depression. While our initial study design called for standardized prescription practices, the severity of illness in this sample and the high frequency of pre-existing antidepressant medications precluded systematic prescription, dosing, or titration schedules. Many patients were also unable or unwilling to tolerate the addition of another medication to an already complex treatment regimen, or to increase the dosage of a medication even when depressive symptoms were not adequately resolved. These challenges highlight the importance of developing non-pharmacological interventions, not only for patients who articulate a desire for hastened death, but for all advanced cancer patients with psychological and/or physical distress. Our research team has been exploring such interventions (Breitbart et al., 2010; Breitbart et al., 2012), but not with patients in their final weeks of life.
There are, of course, a number of methodological limitations that must be acknowledged, including the variable antidepressant treatment regimen noted above. Another limitation pertains to our inability to generate follow-up data for a large proportion of our sample. Our expectation, based on past research and pilot data, was that we would obtain three data points for a large proportion of study participants, with follow-up data at 2 and 4 weeks. This plan quickly proved untenable, largely due to the rapid physical deterioration in our sample. Patients often needed two or three sessions to complete study measures, and the vast majority of patients deteriorated too quickly to complete even one follow-up assessment, let alone two. This deterioration resulted in a sample size for the longitudinal analyses that was much smaller than anticipated, limiting the nature and sophistication of analyses that could be utilized. Questions of sample representativeness are further compounded by the study site, which provides state of the art inpatient palliative care. It is possible that stronger effects might be observed for many of the health-related variables (e.g., pain and symptom control) in a setting where symptoms are less adequately managed. Given these limitations, the findings from this study must be considered preliminary.
These caveats notwithstanding, this study represents one of only a handful of studies examining changes in DHD. The findings have potentially important implications for guiding clinician responses to patient expressions of DHD, as the potential for changes in DHD appear substantial. Clearly further research is necessary to enhance our understanding of the causes, consequences, and course of desire for hastened death in the context of terminal cancer.
Research Highlights.
This study is the largest systematic examination of desire for hastened death in a terminally population
Desire for hastened death fluctuated substantially during the final weeks of life
Subthreshold DHD was associated with worsening DHD
No variables differentiated patients whose DHD remitted from those that did not Does Desire for Hastened Death Change in Terminally Ill Cancer Patients?
Acknowledgments
We are grateful to the staff and patients of Calvary Hospital, and in particular, to James Cimino and Maryann Santasiero.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert SM, Rabkin JG, Del Bene ML, Tider T, O’Sullivan I, Rowland LP, Mitsumoto H. Wish to die in end-stage ALS. Neurology. 2005;65:68–74. doi: 10.1212/01.wnl.0000167187.14501.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. Journal of Consulting and Clinical Psychology. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Breitbart W, Poppito S, Rosenfeld B, Vickers AJ, Li Y, Abbey J, Cassileth BR. A pilot randomized controlled trial of Individual Meaning-Centered Psychotherapy for patients with advanced cancer. Journal of Clinical Oncology. 2012;30:1304–1309. doi: 10.1200/JCO.2011.36.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Gibson C, Kramer M, Li Y, Tomarken A, Schuster M. Impact of treatment for depression on desire for hastened death in patients with advanced AIDS. Psychosomatics. 2010;51:98–105. doi: 10.1176/appi.psy.51.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Gibson C, Pessin H, Poppito S, Nelson C, Olden M. Meaning-Centered Group Psychotherapy for patients with advanced cancer: A pilot randomized controlled trial. Psychooncology. 2010;19:21–28. doi: 10.1002/pon.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Pessin H, Kaim M, Funesti-Esch J, Galietta M, Brescia R. Depression, hopelessness, and desire for death in terminally ill cancer patients. JAMA. 2000;284:2907–2911. doi: 10.1001/jama.284.22.2907. [DOI] [PubMed] [Google Scholar]
- Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Medical Care. 1988;26:709–723. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Chochinov HM, Tataryn D, Clinch JJ, Dudgeon D. Will to live in the terminally ill. Lancet. 1999;354:816–819. doi: 10.1016/S0140-6736(05)76829-X. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Annals of the Academy of Medicine Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, research version, patient edition. New York, NY: Biometrics Research; 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Halpin M. Accounts of suicidality in the Huntington Disease community. Omega: Journal of Death and Dying. 2012;65:317–334. doi: 10.2190/OM.65.4.e. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. Clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- Leeman CP. Distinguishing among irrational suicide and other forms of hastened death: Implications for clinical practice. Psychosomatics. 2009;50:185–191. doi: 10.1176/appi.psy.50.3.185. [DOI] [PubMed] [Google Scholar]
- Meier DE, Emmons CA, Wallenstein S, Quill T, Morrison RS, Cassel CK. A national survey of physician-assisted suicide and euthanasia in the United States. The New England Journal of Medicine. 1998;338:1193–1201. doi: 10.1056/NEJM199804233381706. [DOI] [PubMed] [Google Scholar]
- O’Mahony S, Goulet J, Kornblith A, Abbatiello G, Clarke B, Kless-Siegel S, Payne R. Desire for hastened death, cancer pain, and depression: Report of a longitudinal observational study. Journal of Pain and Symptom Management. 2005;29:446–457. doi: 10.1016/j.jpainsymman.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy-Spiritual Well Being Scale (FACIT-Sp) Annals of Behavioral Medicine. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Scher H. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld B. Assisted suicide and the right to die. Washington, DC: American Psychological Association Press; 2003. [Google Scholar]
- Rosenfeld B, Breitbart W, Galietta M, Kaim M, Funesti-Esch J, Pessin H, Brescia R. The Schedule of Attitudes toward Hastened Death: Measuring desire for death in terminally ill cancer patients. Cancer. 2000;88:2868–2875. doi: 10.1176/appi.psy.51.2.98. [DOI] [PubMed] [Google Scholar]
- Rosenfeld B, Breitbart W, Gibson C, Kramer M, Tomarken A, Nelson C, Schuster M. Desire for hastened death among patients with advanced AIDS. Psychosomatics. 2006;47:504–512. doi: 10.1176/appi.psy.51.2.98. [DOI] [PubMed] [Google Scholar]
- Rosenfeld B, Breitbart W, Stein K, Funesti-Esch J, Kaim M, Krivo S, Galietta M. Measuring desire for death among patients with HIV/AIDS: The schedule of attitudes toward hastened death. The American Journal of Psychiatry. 1999;156:94–100. doi: 10.1176/ajp.156.1.94. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]