To the Editor
To increase β-blocker treatment for patients with heart failure and left ventricular systolic dysfunction, recently updated performance measures recommend that oral β-blocker therapy be started by the time of hospital discharge in patients hospitalized for decompensated systolic heart failure.1 These performance measures make clear that patients in whom β-blocker therapy is started “should not be hospitalized in an intensive care unit (ICU), should have no or minimal evidence of fluid overload or volume depletion, and should not have required recent treatment with an intravenous positive inotropic agent.”1 To assess current patterns of β-blocker therapy initiation in these patients at risk for worsening clinical instability from β-blocker use, we examined a large, contemporary cohort of heart failure hospitalizations in the United States.
Methods
We conducted a retrospective cohort study using Perspective, a voluntary, fee-supported database developed by Premier, Inc. for measuring quality and healthcare utilization. As of 2010, Perspective® contained data on more than 130 million cumulative hospital discharges representing approximately 20% of annual acute care hospitalizations in the United States. In addition to the information available in the standard hospital discharge file, Perspective contains a date-stamped log of all billed items at the patient level including diagnostic tests, medications, and therapeutic services. Perspective has been previously used to describe the pharmacologic treatment of hospitalized patients.2,3 Perspective is not publicly available; access to the database was provided under contract with Premier, Inc.
We included hospitalizations from 2009 and 2010 for patients 18 years or older with a principal discharge diagnosis of heart failure by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.xx. We excluded hospitalizations that involved transfers from another acute care facility or that had an unknown admission source because information about treatment at the referring institution was unavailable. We further excluded hospitalizations with a pediatric attending physician to concentrate on care patterns of physicians who treat adults. For sensitivity analyses, we examined 2 additional heart failure cohorts with greater specificity for systolic dysfunction:4,5 (1) heart failure hospitalizations involving patients aged 18 to 49 years and (2) heart failure hospitalizations with acute myocardial infarction as a secondary ICD-9-CM discharge diagnosis.
For each cohort, we identified hospitalizations during which oral β-blocker therapy was started. β-blocker therapy initiation was defined as (1) no evidence of oral β-blocker treatment during hospital days 1 to 2, and (2) oral β-blocker treatment on the day of hospital discharge. Length of stay was therefore 3 days or more for all patients. Oral β-blockers included metoprolol succinate, carvedilol, bisoprolol, and 13 non guideline-approved agents. We calculated the percentage of β-blocker therapy starters receiving these agents in potentially contraindicated situations as defined by the following performance measures: (1) while being cared for in an ICU; (2) while receiving intravenous loop diuretics on the day of discharge, considered to reflect ongoing volume overload; and (3) after having received an intravenous inotrope including dobutamine, milrinone, or dopamine during hospitalization.
We calculated summary statistics using frequencies and percentages with SAS version 9.2 software (SAS Institute Inc.). The Yale University Human Investigation Committee reviewed the protocol and determined that it was not considered human subjects research according to the Office of Human Research Protections.
Results
We identified 217,550, 15,108, and 5,154 heart failure hospitalizations from the primary (P), age-restricted (A-R), and acute myocardial infarction-restricted cohorts (AMI-R), respectively. In the 3 cohorts, the median patient age was 76 (P), 44 (A-R), and 76 (AMI-R) years (eTable 1; jamainternalmedicine.com). Patients in the P cohort were similar to that of a large heart failure registry.6 β-blockers were administered in 71.6% (P), 76.6% (A-R), and 73.4% (AMI-R) of hospitalizations in each cohort during the first 2 hospital days (eTable 2). Following hospital day 2, β-blocker therapy was started in 7.1% (P), 7.3% (A-R), and 10.7% (AMI-R) of hospitalizations in each cohort. The hospitalizations in which β-blocker therapy was started comprised 24.9% (P), 30.9% (A-R), and 40.4% (AMI-R) of all heart failure hospitalizations potentially eligible for β-blocker therapy initiation. Over 40% of β-blocker therapy starters had at least 1 potential contraindication to treatment (Figure). Approximately one-third received concomitant intravenous diuretics on the day of discharge, and up to one-fifth had received intravenous inotropes during hospitalization. Potential contraindications were higher among cohorts with higher specificity for systolic dysfunction.
Figure.
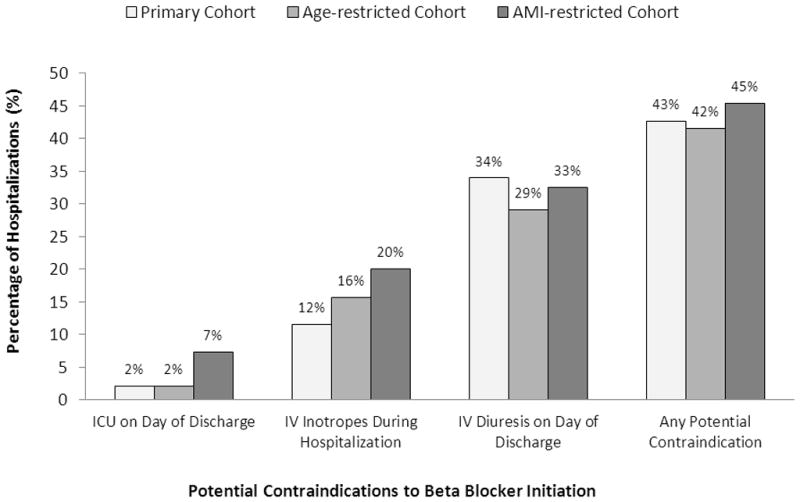
Potential contraindications to therapy. Proportion of patients hospitalized for heart failure receiving a new β-blocker prescription at discharge despite potential contraindications to therapy. AMI indicates acute myocardial infarction; ICU, Intensive care unit; and IV, intravenous.
Discussion
Even before performance measures encourage the further use of β-blockers during hospitalization for heart failure, there is evidence from a large, contemporary database that these agents are frequently started in patients with markers of clinical instability. Further research is needed to confirm these care patterns and examine the outcomes associated with β-blocker therapy initiation in settings with and without potential contraindications to treatment. In the interim, to avoid unintended consequences that may result from the unselective application of this performance measure,7,8 it may be prudent to explore metrics that also assess medication overuse to avoid treating those at higher risk for adverse consequences of therapy.
Supplementary Material
Acknowledgments
Funding/Support: This project was supported by grant DF10-301 from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Connecticut; grant UL1 RR024139-06S1 from the National Center for Advancing Translational Sciences in Bethesda, Maryland; and grant U01 HL105270-02 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute in Bethesda. Dr. Dharmarajan is supported by grant HL007854 from the National Heart, Lung, and Blood Institute and is also supported as a Centers of Excellence Scholar in Geriatric Medicine at Yale by The John A. Hartford Foundation and the American Federation for Aging Research.
Role of the Sponsors: The funding sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Address requests for reprints to 1 Church Street, Suite 200, New Haven, Connecticut 06510.
Conflicts of Interest and Financial Disclosures: Drs. Masoudi and Spertus report serving on the writing committee for the development of the American College of Cardiology Foundation/American Heart Association/American Medical Association-Physician Consortium for Performance Improvement 2011 Performance Measures for Adults with Heart Failure. Dr. Masoudi also reports having contracts with the Oklahoma Foundation for Medical Quality and the American College of Cardiology Foundation. Dr. Krumholz reports that he is the recipient of a research grant from Medtronic, Inc. through Yale University and is chair of a cardiac scientific advisory board for UnitedHealth.
Conflicts of Interest and Financial Disclosures: Drs. Masoudi and Spertus report serving on the writing committee for the development of the American College of Cardiology Foundation/American Heart Association/American Medical Association-Physician Consortium for Performance Improvement 2011 Performance Measures for Adults with Heart Failure. Dr. Masoudi also reports having contracts with the Oklahoma Foundation for Medical Quality and the American College of Cardiology Foundation. Dr. Krumholz reports that he is the recipient of a research grant from Medtronic, Inc. through Yale University and is chair of a cardiac scientific advisory board for UnitedHealth.
Author Contributions:
Drs. Dharmarajan, Li, and Krumholz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dharmarajan, Krumholz
Acquisition of data: Li and Krumholz
Analysis and interpretation of data: Dharmarajan, Masoudi, Spertus, Li, Krumholz
Drafting of the manuscript: Dharmarajan, Li, and Krumholz
Critical revision of the manuscript for important intellectual content: Dharmarajan, Masoudi, Spertus, Krumholz
Statistical analysis: Dharmarajan and Li
Obtained funding: Krumholz
Administrative, technical, or material support: Krumholz
Study supervision: Krumholz
References
- 1.Bonow RO, Ganiats TG, Beam CT, et al. ACCF/AHA/AMA-PCPI 2011 Performance Measures for Adults With Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2012;125(19):2382–2401. doi: 10.1161/CIR.0b013e3182507bec. [DOI] [PubMed] [Google Scholar]
- 2.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 3.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105(11):1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33(7):1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153(6):1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Kanwar M, Brar N, Khatib R, Fakih MG. Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-h antibiotic administration rule. Chest. 2007;131(6):1865–1869. doi: 10.1378/chest.07-0164. [DOI] [PubMed] [Google Scholar]
- 8.Baker DW, Qaseem A. Evidence-based performance measures: preventing unintended consequences of quality measurement. Ann Intern Med. 2011;155(9):638–640. doi: 10.7326/0003-4819-155-9-201111010-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


