Abstract
Purpose: To demonstrate that ultraviolet-A (UV-A) and voriconazole combination therapy is more effective than voriconazole single treatment for fungal keratitis.
Methods: The in vitro UV-A (375 nm) fungicidal effect was evaluated on Fusarium solani solutions. Each fungal solution was irradiated with different UV-A irradiation doses. Also, a fungal solution containing voriconazole was also irradiated with UV-A. The in vivo therapeutic effect of UV-A and voriconazole treatment was studied in a rabbit keratitis model. Fungi were injected intrastromally into the cornea of 16 rabbits. Each treatment was initiated 3 days after fungal injection and continued up to 8 days for the following groups: Group 1, control; Group 2, treated with UV-A once a day; Group 3, treated with voriconazole 3 times a day; Group 4, treated with voriconazole 3 times a day and UV-A once a day. On the last day, the sclera–cornea buttons were extracted and microbiological and histological evaluations were performed.
Results: The colony-forming units (CFUs) of fungal solutions in culture significantly decreased with UV-A irradiation. The CFUs of fungal solutions containing voriconazole also decreased with UV-A irradiation. In vivo, clinical scores of Group 3 (P=0.03) and Group 4 (P=0.02) 5 days after treatment were significantly lower compared to that of Group 1. The clinical score of Group 4 (P=0.03) 5 days after treatment was significantly lower compared to that of Group 3. The histopathological scores 5 days after treatment were significantly lower in Group 4 compared to those of Group 1 (P<0.01) and Group 3 (P=0.02). Based on our CFU analysis, only Group 4 showed significantly lower CFUs compared to Group 1 (P=0.04).
Conclusions: UV-A and voriconazole combination treatment could be a safe and effective alternative to voriconazole single treatment for fungal keratitis.
Introduction
Fungal keratitis accounts for 6%–20% of infectious keratitis in the United States, with even greater prevalence in tropical climates.1,2 It can cause permanent corneal opacity leading to severe vision loss and can cause perforations requiring surgical intervention.3,4 Approximately 50% of infectious keratitis that requires treatment with penetrating keratoplasty is fungal keratitis.4
To date, many antifungal agents have been used in the treatment of fungal keratitis, including 5% natamycin, 0.15% amphotericin B, and several azole derivatives such as voriconazole.5 Where available, 5% natamycin is used for fungal keratitis as a first-line treatment.6 However, its efficacy is decreased by the lag time between diagnosis and initiation of treatment, and low compliance is secondary to cost.7 Furthermore, 0.15% amphotericin B is known to have low intraocular penetration.8 Topical and oral formulations of voriconazole have been reported to yield good results, but voriconazole for ocular administration has not yet been developed.9
Recently, a combination treatment with ultraviolet (UV) light and riboflavin (vitamin B2) was suggested as an alternative treatment for fungal keratitis in an animal model.10 Another study applied this method to bacterial keratitis.11 However, this technique is somewhat complicating in its application, resulting in difficult outpatient treatment, and acquisition of an adequate supply of riboflavin is not always guaranteed. Moreover, there may be toxicity to the corneal structure due to the photosensitizing effects of ultraviolet-A (UV-A) with riboflavin. In addition, only UV itself is known to have antifungal effects.12,13
Based upon these considerations, we compared voriconazole single agent treatment with voriconazole plus UV-A combination therapy as a possible treatment for fungal keratitis.
Methods
Isolation of Fusarium solani
Fusarium solani was isolated from a fungal keratitis patient and cultured at 28°C. Each inoculum solution was prepared by washing the growth surface with 0.1% Tween 80. Sterile physiological saline was used to filter the solution through sterile gauze to remove hyphal fragments. Each spore solution was centrifuged at 10,000 g to pellet the conidia. The supernatant was removed with a sterile pipette, and the conidia were resuspended with sterile physiological saline to produce the final inoculum of 8.0×104 colony-forming units (CFUs)/mL. The number of conidia was confirmed by plating on Sabouraud dextrose agar plates.
In vitro viability test by UV-A (375 nm)
Each F. solani solution was irradiated with different UV-A doses (0.5, 1.0, 1.5, 2.0, 2.5 J/cm2) under sterile conditions. In addition, a fungal solution containing voriconazole (10 μg/mL) was irradiated with UV-A (2.0 J/cm2). Irradiated solutions were cultured on Sabouraud dextrose agar plates and incubated at 28°C for 2 days. The quantity of colonies was determined in all cultures.
Animals and injections
Sixteen New Zealand white rabbits weighing 2.5–3 kg were used. All rabbits were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Before inoculation, the eyes of the rabbits received subconjunctival injections of 0.8 mg dexamethasone sodium phosphate for 5 days under anesthesia. Injection of fungal solutions was performed on the last day of these inoculations. The rabbits were anesthetized with 8 mg/kg of intramuscular Zoletil® and 5 mg/kg of xylazine hydrochloride before all interventions. Corneal anesthesia was achieved through the use of 0.5% topical proparacaine hydrochloride. The F. solani solution (8.0×103 CFUs/0.1 mL) was midstromally injected into the central cornea in 1 eye of each rabbit using a 30-gauge needle.
Treatment with UV-A and voriconazole
The treatment was initiated 3 days after the injection of F. solani. The rabbits were divided randomly into 4 groups: Group 1, control group; Group 2, treated with UV-A once a day; Group 3, treated with voriconazole 3 times a day; Group 4, treated with voriconazole 3 times a day and UV-A once a day. The rabbits were anesthetized with 8 mg/kg of intramuscular Zoletil and 5 mg/kg of xylazine hydrochloride before UV-A irradiation. The corneas of the rabbits were irradiated with UV-A at a distance of 5 cm for 12 min, which approximately corresponded to a dose of 2.0 J/cm2.
Clinical evaluation
The eyes were examined at day 0 (fungal inoculation), day 3 (before treatment), and day 8 (5 days after treatment) using a portable slit-lamp biomicroscope. The extent of keratitis was evaluated by a masked observer. Conjunctival hyperemia, corneal clouding, corneal neovascularization, diameter of corneal infiltration, and hypopyon level were evaluated using the Schreiber scoring system.14 Conjunctival hyperemia was graded as follows: 0, no hyperemia; 1, low hyperemia; 2, middle hyperemia; 3, high hyperemia. Corneal clouding was graded as follows: 0, clear cornea; 1, minor clouding; 2, corneal clouding in 2 quadrants of the cornea; 3, total corneal clouding. Corneal neovascularization was graded as follows: 0, no neovascularization; 1, minimal neovascularization at the limbus; 2, moderate neovascularization not reaching the center; 3, neovascularization reaching the center. The level of hypopyon and the diameter of corneal infiltration were measured in mm using a portable slit-lamp biomicroscope.
Microbiological and pathological analyses
At 5 days after treatment, the sclera–corneal buttons were extracted after euthanasia and microbiological and pathological examinations were done. For microbiological evaluation, corneal rupture was performed in a sterile Petri dish, and the minced solutions were cultured on Sabouraud dextrose agar plates and incubated at 28°C for 2 days. The quantity of colonies was determined in all cultures. For histopathological examination, periodic acid-Schiff staining was performed. The degree of inflammation in microscopic corneal cross sections was graded by an examiner blinded to any knowledge of the experimental procedure. The examiner used a scale from 0 to 4, using a previously described inflammation score15 with minor modifications as follows: 0, no signs of inflammation; 1, minimal inflammatory cell infiltration and minimal structural changes; 2, mild inflammatory cell infiltration and mild structural changes; 3, moderate inflammatory cell infiltration and moderate structural changes; 4, severe inflammatory cell infiltration and severe structural changes.
Safety evaluation
Rabbits were anesthetized before each examination. All 16 eyes were examined using a specular microscope (Topcon SP 3000P™, Tokyo, Japan) before each treatment. Noncontact corneal endothelial photographs of the central 3 mm of the cornea were obtained for analysis. After at least 30 endothelial cells in each image were selected, the cell density, coefficient of variation, and average cell area were calculated using a computer program. Rabbits were divided into 4 groups as previously described. The corneal epithelium was removed on the first day, and each group was treated for 5 days in the previously described manner. Specular microscopic evaluations were performed again at the next day after the last treatment.
Statistical analysis
Clinical scores at 3 and 8 days after inoculation were calculated as the sum of the conjunctival hyperemia, corneal clouding, corneal neovascularization, diameter of corneal infiltration, and hypopyon level scores obtained from each rabbit. The Mann–Whitney U-test was performed to compare the means between the control group and the treatment group on different days. The histological scores of the cornea and the CFUs from each group were compared to the control group using a paired t-test. For the safety evaluation, the Wilcoxon matched sign rank test for 2 related samples was used to compare the mean cell counts. All statistical tests were 2-sided and 95% confidence intervals were used. Tests were performed using the SAS system version 9.13 software (Cary, NC).
Results
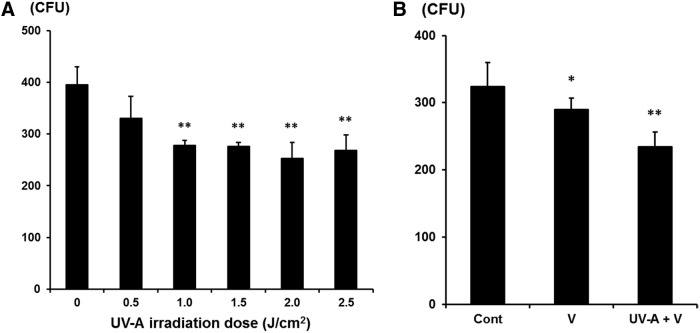
In vitro antifungal efficiency test of UV-A on F. solani showed a significant decrease of CFUs when the dose of UV-A irradiation increased (1.0, 1.5, 2.0, 2.5 J/cm2) and the effect was maximal at 2.0 J/cm2 (Fig. 1A). The fungal solution treated with voriconazole in combination with UV-A irradiation (2.0 J/cm2) was the most effective compared to the control group. However, there was no significant difference between voriconazole single treatment and UV-A and voriconazole combination therapy (Fig. 1B).
FIG. 1.
(A) The CFUs obtained in culture treated with different UV-A (375 nm) doses (0.5, 1.0, 1.5, 2.0, 2.5 J/cm2). Each fungal solution was irradiated with UV-A and cultured on a Sabouraud dextrose agar plate for 2 days. There were statistically significant decreases in CFUs with UV-A (1.0, 1.5, 2.0, 2.5 J/cm2) compared to the control group, as assessed by the t-test. (B) The CFUs obtained in culture with voriconazole single treatment and UV-A plus voriconazole treatment. The CFUs of UV-A plus voriconazole treatment group significantly decreased compared to that of control group, but there was no significant difference between voriconazole and UV-A plus voriconazole group. CFUs, colony-forming units; Cont, control; UV-A, ultraviolet-A; V, voriconazole.
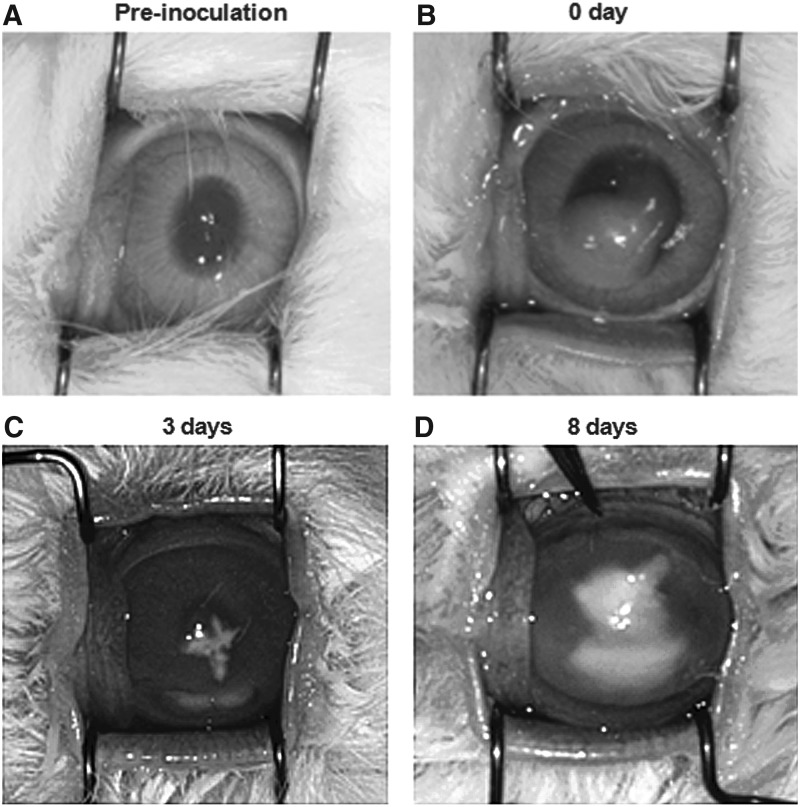
In vivo, F. solani solution was injected into the corneal stroma, and then fungal keratitis developed 3 days after inoculation. At 8 days after inoculation, severe inflammatory changes with corneal infiltration, clouding, and anterior chamber hypopyon were noted (Fig. 2).
FIG. 2.
Induction of Fusarium solani fungal keratitis in rabbit eyes. (A) Preinoculation. (B) Inoculation (0 day). Fungal solution was successfully inoculated in corneal stroma. (C) Three days after inoculation, cornea infiltration, anterior chamber hypopyon, and conjunctival hyperemia were noted. (D) Eight days after inoculation, there were severe corneal infiltrates, clouding, and anterior chamber hypopyon.
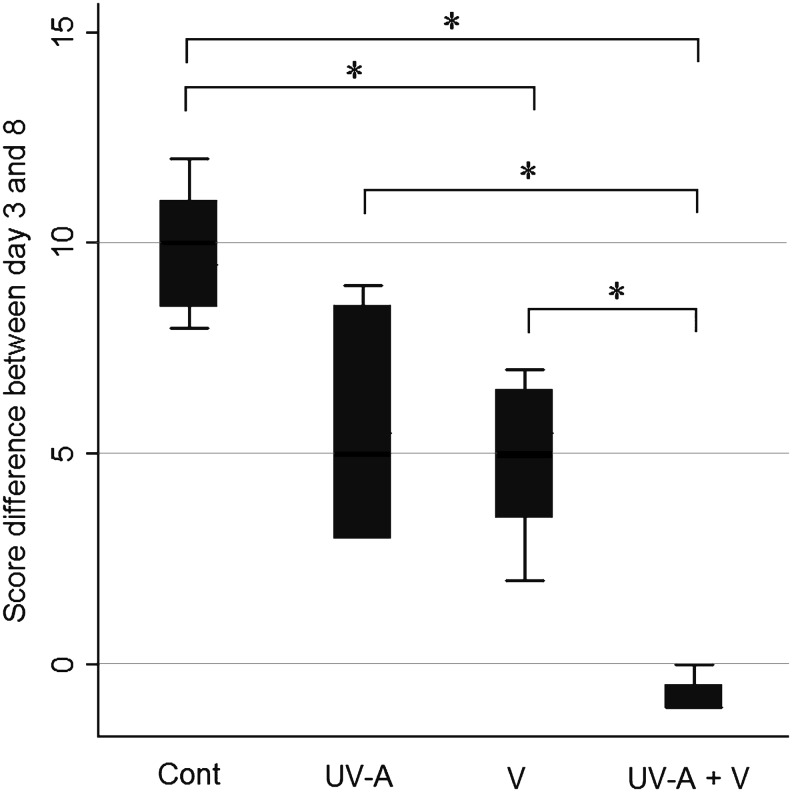
The clinical Schreiber scores obtained at day 3 and 8 after inoculation showed that there were statistically significant differences in Group 3 (P=0.03) and Group 4 (P=0.02) compared to Group 1. In particular, there was a statistically significant difference in Group 4 compared to Group 3 (P=0.03) (Fig. 3). The negative score difference in Group 4 showed improved clinical signs at 8 days after inoculation compared to those at 3 days.
FIG. 3.
Effectiveness of each treatment based on the Schreiber scoring system. There were statistically significant differences in Group 3 (P=0.03) and Group 4 (P=0.02) compared to Group 1. There was a statistically significant difference in Group 4 compared to Group 3 (P=0.03). *P<0.05 from the Mann–Whitney U-test. Group 1, control group; Group 2, treated with UV-A once a day; Group 3, treated with voriconazole 3 times a day; Group 4, treated with voriconazole 3 times a day and UV-A once a day.
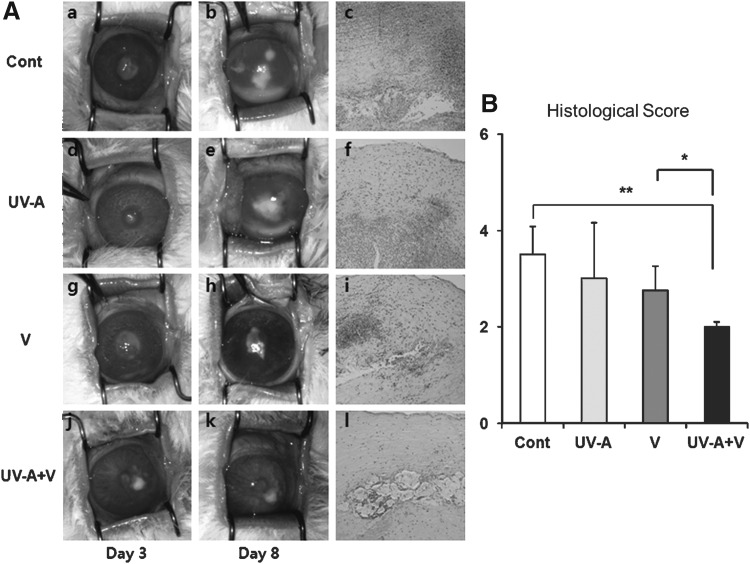
The conditions of the cornea from each group were evaluated at day 3 and 8 after inoculation. In clinical findings, there were severe inflammatory changes in Group 1 and 2 at day 8. In histological findings, Group 4 showed fewer F. solani hyphae, inflammatory cells, and nonspecific stromal changes compared to Group 1 (Fig. 4A). According to histological scoring, there were statistically significant differences in Group 4 compared to Group 3 (P=0.02) and Group 1 (P=0.002) (Fig. 4B).
FIG. 4.
(A) Eyes from each group at day 3 and 8 after inoculation, and histopathology of the corneas at day 8. (a–c), Control group; (d–f), UV-A-only treatment group; (g–i), voriconazole-only treatment group; (j–l), UV-A plus voriconazole combination treatment group. There were severe corneal infiltrates, clouding, and anterior chamber hypopyon in the control group and UV-A-only treatment group. In the UV-A plus voriconazole combination treatment group, less severe inflammatory changes were noted on histopathological examination. (B) The histological scores of the corneas from each group 8 days after inoculation. *P<0.05; **P<0.01.
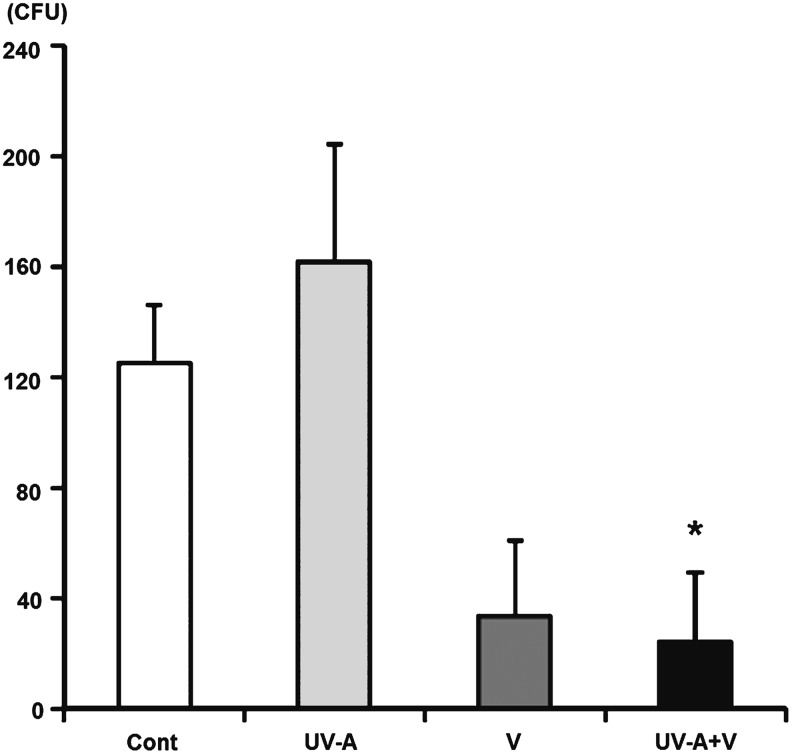
The fungal colonies cultured on agar from the extract of whole corneal buttons were counted. There was a statistically significant difference in Group 4 (P=0.04) compared to Group 1. Group 3 also had lower CFUs compared to Group 1; however, the difference was not statistically significant (Fig. 5).
FIG. 5.
The CFUs obtained in culture from each group. Each corneal button was obtained 8 days after inoculation, minced with PBS, and cultured on a Sabouraud dextrose agar plate for 2 days. *There was a statistically significant decrease in CFUs in Group 4 compared to Group 1. Group 1, control group; Group 4, treated with voriconazole 3 times a day and with UV-A once a day.
To confirm the safety of each treatment, we evaluated endothelial changes using specular microscopy. The cell density, coefficient of variation, and average cell area for each group were determined, and there were no significant changes in any of these parameters before and after treatment (Table 1).
Table 1.
Specular Photomicroscopy Results
| Cell density | Coefficient of variation | Average cell area | ||||
|---|---|---|---|---|---|---|
| Before treatment (0 day) | After treatment (5 days) | Before treatment (0 day) | After treatment (5 days) | Before treatment (0 day) | After treatment (5 days) | |
| Control | 2,545±373 | 2,489±264 | 34±14 | 39±21 | 397±58 | 404±42 |
| UV-A | 2,780±548 | 2,819±255 | 22±11 | 29±6 | 367±72 | 363±33 |
| V | 2,559±277 | 2,563±178 | 36±4 | 54±36 | 393±42 | 391±28 |
| UV-A+V | 2,447±378 | 2,502±66 | 47±23 | 47±28 | 414±64 | 399±10 |
Cell density (cells/mm2), coefficient of variation and average cell area (unit/mm2) were evaluated before and after treatment in each group. There was no statistically significant difference between before and after treatment in all parameters based on the Wilcoxon matched-pairs signed-ranks test.
UV-A, ultraviolet-A; V, voriconazole.
Discussion
Based upon the results of our study, we conclude that combining UV-A treatment with voriconazole eyedrops was more effective than voriconazole alone in inhibiting disease progression and reducing corneal complications. This combined therapeutic tool could be a useful coadjuvant treatment for other medical treatments in fungal keratitis. We showed for the first time that UV-A additive treatment with conventional voriconazole antifungal treatment was effective in reducing fungal pathogens and eventually decreasing corneal inflammations and the subsequent intractable complications.
In this study, there were significant differences in clinical and histopathological scoring between voriconazole single treatment and UV-A additive treatment although there was no significant difference in the final CFU counts. Although the difference was not statistically significant in vitro, additive UV-A treatment lowered fungal burdens more effectively than voriconazole single treatment (Fig. 1B). We speculate that additive UV-A treatment reduced more fungal burdens than voriconazole single treatment in the early treatment period of fungal keratitis, which helped reduce subsequent inflammatory reactions. Although the group that received voriconazole 3 times a day showed a decrease of fungal burden eventually, early fungal pathogenic inflammations would result in more irreversible changes in corneal architectures.
Voriconazole eyedrops are generally used more than 3 times per day. However, due to the toxicity of antifungal eyedrops, we tested whether UV-A treatment had any additive effect on treating fungal keratitis, which would compensate for the decreased use of voriconazole eyedrops. In vivo, voriconazole treatment 3 times per day was not very effective, and clinical scores increased (Fig. 3). Moreover, voriconazole eyedrops penetrated well into the human aqueous humor when instilled at 6-hourly intervals (4 times per day).16 Rabbits have a low blink rate and a large epithelial eye surface, which enhances the penetration of lipophilic drugs such as voriconazole.17 Therefore, we chose 3 times per day voriconazole treatment to evaluate any additional effect of UV-A.
We selected 2.0 J/cm2 as an appropriate UV-A irradiation dose concerning its efficacy and safety. In in vitro test, UV-A was effective in reducing fungal pathogens at this irradiation dose (Fig. 1A). Regarding the safety concerns, a daily dose of 2.02 J/cm2 UV-A irradiation was found to be metabolically safe,18 and there were no metabolic changes with a daily dose of 2.02 J/cm2. Additionally, we confirmed the structural safety using specular photomicroscope. Although the rabbit corneal endothelium was quickly regenerated after traumatic damage, several days were necessary for full recovery.19 We checked the state of the corneal endothelium the next day after a 5-day irradiation because we supposed that 1 day was insufficient for the complete recovery of a damaged endothelium. We confirmed that corneal endothelial parameters, including cell density, coefficient of variation, and average cell area, did not change after UV-A and voriconazole treatment.
This UV-A plus voriconazole treatment has advantages over the previous UV-A plus riboflavin treatment. First, the extent of the photosensitization side effects of riboflavin and UV-A combination therapy is still unknown. Electrons activated in riboflavin by UV-A photosensitization can be harmful to the corneal metabolism and microstructures. Currently, this technique has been recommended only if the corneal thickness is over 400 μm.20 However, this harmful effect does not occur when UV-A is added to voriconazole treatment. Second, UV-A treatment once daily is less complicating as an outpatient procedure than UV-A and riboflavin treatment. Furthermore, riboflavin is relatively expensive and oftentimes unavailable in developing countries.
In conclusion, UV-A and voriconazole combination treatment could be effective in reducing fungi and decreasing complications in fungal keratitis. Regarding the toxicity of antifungal eyedrops, UV-A additive treatment could lower the side effects of voriconazole eyedrops. This combination therapy would be safer and less expensive than other recently developed treatments such as corneal crosslinking, and we anticipate that this UV-A additive treatment could be a coadjuvant treatment for ocular antifungal treatments.
Acknowledgment
This study was supported by a TEPIK grant (A121861).
Author Disclosure Statement
The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Jurkunas U., Behlau I., and Colby K.Fungal keratitis: changing pathogens and risk factors. Cornea. 28:638–643, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Deorukhkar S., Katiyar R., and Saini S.Epidemiological features and laboratory results of bacterial and fungal keratitis: a five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singapore Med. J. 53:264–267, 2012 [PubMed] [Google Scholar]
- 3.Yildiz E.H., Abdalla Y.F., Elsahn A.F., Rapuano C.J., Hammersmith K.M., Laibson P.R., and Cohen E.J.Update on fungal keratitis from 1999 to 2008. Cornea. 29:1406–1411, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Chang H.Y., and Chodosh J.Diagnostic and therapeutic considerations in fungal keratitis. Int. Ophthalmol. Clin. 51:33–42, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Yavas G.F., Ozturk F., Kusbeci T., Cetinkaya Z., Ermis S.S., Kiraz N., and Inan U.U.Antifungal efficacy of voriconazole, itraconazole and amphotericin b in experimental Fusarium solani keratitis. Graefes Arch Clin Exp Ophthal. 246:275–279, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Loh A.R., Hong K., Lee S., Mannis M., and Acharya N.R.Practice patterns in the management of fungal corneal ulcers. Cornea. 28:856–859, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Pradhan L., Sharma S., Nalamada S., Sahu S.K., Das S., and Garg P.Natamycin in the treatment of keratomycosis: correlation of treatment outcome and in vitro susceptibility of fungal isolates. Indian J. Ophthal. 59:512–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon K.C., Jeong I.Y., Im S.K., Chae H.J., and Yang S.Y.Therapeutic effect of intracameral amphotericin B injection in the treatment of fungal keratitis. Cornea. 26:814–818, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Funakoshi Y., Yakushijin K., Matsuoka H., and Minami H.Fungal endophthalmitis successfully treated with intravitreal voriconazole injection. Intern. Med. 50:941, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Galperin G., Berra M., Tau J., Boscaro G., Zarate J., and Berra A.Treatment of fungal keratitis from Fusarium infection by corneal cross-linking. Cornea. 31:176–180, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Martins S.A., Combs J.C., Noguera G., Camacho W., Wittmann P., Walther R., Cano M., Dick J., and Behrens A.Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest. Ophthalmol Vis. Sci. 49:3402–3408, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Levetin E., Shaughnessy R., Rogers C.A., and Scheir R.Effectiveness of germicidal UV radiation for reducing fungal contamination within air-handling units. Appl. Environ. Microbiol. 67:3712–3715, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes K.A., Lawley B., and Newsham K.K.Solar UV-B radiation inhibits the growth of Antarctic terrestrial fungi. Appl. Environ. Microbiol. 69:1488–1491, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens-Baumann W., and Begall T.Reproducible model of a bacterial conjunctivitis. Ophthalmologica. 206:69–75, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Kitamura K., Farber J.M., and Kelsall B.L.CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J. Immunol. 185:3295–3304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau D., Fedinands M., Leung L., Fullinfaw R., Kong D., Davies G., and Daniell M.Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch. Ophthalmol. 126:343–346, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Thiel M.A., Zinkernagel A.S., Burhenne J., Kaufmann C., and Haefeli W.E.Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob. Agents Chemother. 51:239–244, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cejka C., Platenik J., Buchal R., Guryca V., Sirc J., Vejrazka M., Crkovska J., Ardan T., Michalek J., Brunova B., and Cejkova J.Effect of two different UVA doses on the rabbit cornea and lens. Photochem. Photobiol. 85:794–800, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Hirsch M., Faure J.P., Marquet O., and Payrau P.[Regeneration of corneal endothelium in the rabbit: microscopic study and relation with corneal thickness]. Arch. Ophtalmol. Rev. Gen. Ophtalmol. 35:269–278, 1975 [PubMed] [Google Scholar]
- 20.Spoerl E., Mrochen M., Sliney D., Trokel S., and Seiler T.Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 26:385–389, 2007 [DOI] [PubMed] [Google Scholar]