Abstract
The aim of this study was to explore the association between polymorphisms of five cytokine genes and clinical parameters in patients with Philadelphia-positive (Ph+) chronic myeloid leukemia (CML) treated with imatinib. We analyzed five cytokine genes (interleukin [IL]-6, IL-10, gamma interferon [IFN-γ], transforming growth factor beta-1 [TGF-β1], and tumor necrosis factor-alpha [TNF-α]) in 60 cases with Ph+ CML and 74 healthy controls. Cytokine genotyping was performed by the polymerase chain reaction-sequence-specific primer. All data were analyzed using the de Finetti program and SPSS version 14.0 for Windows. No significant differences were detected between the CML group and healthy controls with respect to the distributions and numbers of genotypes and alleles in TNF-α, TGF-β1, IL-10, and IFN-γ. However, the GG genotype associated with high expression in IL-6 was found to be significantly more frequent in CML as compared to controls (p=0.010). The median follow-up time was 49.3 months (range 6.1–168.4) and the median duration of imatinib treatment was 39.5 months (range 5.2–103.4) for these patients. On multivariateanalysis, only IL-10 GCC/GCC highly produced haplotypes were significantly associated with a shorter event-free survival. The relationship between cytokine genotypes/haplotypes and clinical parameters in CML has not been investigated before. Our results suggest that IL-10 may be a useful marker for CML prognosis and theGG genotype of the IL-6 gene may be associated with susceptibility.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder of clonal origin with an annual incidence of about 1 in 50,000 (Chen and Li, 2013). CML patients usually present in the chronic phase of the disease, during which there is a gradual expansion of mature myeloid cells in the bone marrow and peripheral blood. Without treatment, patients inevitably progress through an accelerated phase of disease (4–6 years on average after diagnosis) to a terminal acute phase known as blast crisis, which is characterized by a massive increase in undifferentiated blasts that can be either myeloid or lymphoid in nature (Mayani et al., 2009). In their study, Nowell and Hungefort (1960) described the origin of CML as a common chromosomal abnormality that they found in CML patients and suggested a “causal relationship between the chromosomal abnormality observed and chronic granulocytic leukemia.” This unique chromosomal abnormality, known as the Philadelphia chromosome (Ph), was later shown to be a reciprocal translocation between the long arms of chromosomes 9 and 22 [t(9:22)(q34;q11)] and leads to the fusion of the breakpoint cluster region (BCR) and human ABL1 genes (Rowley, 1973). The resulting BCR-ABL fusion gene codes for BCR-ABL transcripts and fusion proteins with unregulated tyrosine kinase activity (Hochhaus, 2008). Since 1998, patients with Ph-positive (Ph+) CML have been treated successfully with the abl-tyrosine kinase inhibitor, imatinib. More than 95% of the patients have achieved a complete hematologic response, and more than 80% have achieved a complete cytogenetic response (CCR) (Aliano et al., 2013). However, a proportion of patients demonstrate resistance or suboptimal response to imatinib therapy; in many cases, the mechanism is unknown (Aliano et al., 2013; Chen and Li, 2013).
The initiating event in the pathogenesis of CML is the well-known chromosomal translocation (9;22) that drives the aberrant differentiation of hematopoietic stem cell prefentially toward the myeloid lineage. Although necessary, BCR-ABL expression alone is not sufficient for the progression from chronic phase to accelerated phase or blast crisis CML. BCR-ABL expression persists in the bone marrow of patients who have achieved and maintained CCR for up to 10 years (Chomel et al., 2000). It has also been detected in leukocytes of healthy normal persons who never go on to develop CML (Bose et al., 1998). These findings would suggest that additional different gene mutations are needed for progression to leukemia to occur (Rise and Jamieson, 2010). It has been hypothesized that genetic factors other than histocompatibility disparity could play a role in the predisposition or prognosis for CML. In this regard, T helper types 1 and 2 (Th1 and Th2), cytokines, and the polymorphisms in their genes seem to be important (Humlová et al., 2006). Cytokines released from activated lymphocytes, monocytes, and macrophages modify the intensity of the immune inflammatory response. These molecules function as chemical mediators among cells and are recognized by specific receptors on the target cells. In addition to supporting the immune response locally and systemically, cytokines also regulate hematopoiesis. Differences in cytokine production are related to sequence variants in their genes. Previous studies have shown that polymorphisms in regulatory regions of cytokine genes could affect transcription, resulting in variations of cytokine protein production. Recently, the association between cytokine genes and leukemias has been examined intensively (Bidwell et al., 2001; De Oliveira, 2007).
In this study, it was aimed (1) to investigate single-nucleotide polymorphisms in five different cytokine genes in 60 chronic phase Ph+ CML patients and 74 healthy controls followed between 2000 and 2008 and (2) to compare the correlations of these results with clinical data and determine their effects on total and progression-free life durations.
Materials and Methods
The study included 74 controls (ethnically matched healthy unrelated individuals), and 60 patients diagnosed with chronic phase Ph+ CML were diagnosed at the Department of Hematology, Medical School Hospital, Gaziantep and Erciyes Universities, Turkey. The study was approved by the Local Ethics Committee. Written informed consent was received from all participants. Of 60 patients with CML, 23 were male and 37 were female. The age of these patients ranged from 20 to 74 years. Ten patients (16.7%) were designated as the low-risk group, 28 patients (46.7%) as the intermediate-risk group, and 22 patients (36.6%) as the high-risk group, according to the Sokal risk score assigned at diagnosis (Table 1) (Sokal et al., 1984). All 60 patients with Ph+ CML in chronic phase (within 1 year of diagnosis) were treated with 400 mg oral imatinib daily. Ten of those patients were also treated with a gamma interferon (IFN-γ) protocol (O'Brien et al., 2003; Aliano et al., 2013). Genomic DNA was extracted from mononuclear cells obtained from EDTA-treated peripheral venous blood using the salting-out method (Miller et al., 1988). Cytokine genotyping was performed by the polymerase chain reaction-sequence-specific primer method, using the Cytokine Genotyping Tray kit according to the manufacturer's instructions (One Lambda). Single-nucleotide polymorphisms for five cytokines (interleukin [IL]-6, IL-10, IFN-γ, transforming growth factor beta-1 [TGF-β1], and tumor necrosis factor-alpha [TNF-α]) were analyzed (Karaoglan et al., 2009). All data were analyzed using SPSS version 14.0 for Windows (SPSS, Inc.). Categorical data were analyzed using Pearson's chi-square analysis. Odds ratio (OR) and 95% confidence interval (95% CI) were also calculated. OR (95% CI) was adjusted by age and sex. The data were analyzed for appropriateness between the observed and expected genotypes as well as for Hardy–Weinberg Equilibrium (HWE) as described elsewhere. All analyses were two tailed, and differences were interpreted as statistically significant when p<0.05.
Table 1.
Clinical Features of Chronic Myeloid Leukemia in Chronic Phase Patients
| n (%) | |
|---|---|
| Number of patients | 60 |
| Age at diagnosis | 41 (20–74)a |
| Age ≥60 years | 6 (10) |
| Male/female | 23/37 (38.3/61.7) |
| Splenomegaly | 42 (70) |
| Hemoglobin 12< g/dL | 42 (70) |
| Leukocytes >50×109/L | 38 (63.3) |
| Platelets >450×109/L | 28 (40) |
| Sokal risk score at diagnosis | |
| Low | 10 (16.7) |
| Intermediate | 28 (46.7) |
| High | 22 (36.6) |
| Initial treatment | |
| Imatinib 400 mg/day | 50 (83.3) |
| Interferon-α→imatinib 400 mg/day | 10 (16.7) |
| Response ELN criteria (18 months) | |
| Optimal | 40 (66.6) |
| Suboptimal | 13 (21.7) |
| Failure | 7 (11.7) |
| Mortality | 2 (3.3) |
| Eventb | 12 (20) |
| Chromosomal abnormalities in addition to the Philadelphia chromosome | 4 (6.7), trisomy 8 [2](3.4), monosomy 7 [1](1.7), trisomy 21[1](1.7) |
| Time after diagnosis, months | 49.3 (6.1–168.4)a |
| Duration of imatinib, months | 39.5 (5.2–103.4)a |
Median.
Death (2), progression to accelerated phase or blastic phase (2), loss of an MCyR (8).
ELN, European Leukemia Net.
Results
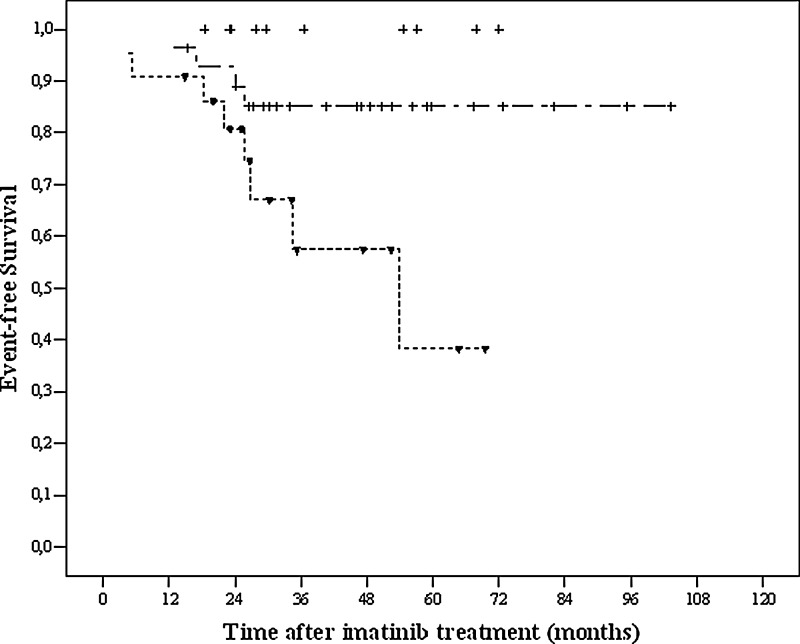
Clinical features of the chronic phase CML patients are given in Table 1. The patients were between 20 and 74 years old and the median age was 41. The median follow-up time was 49.3 months (range 6.1–168.4), and the median duration of imatinib treatment was 39.5 months (range 5.2–103.4) for these patients. Table 2 shows genotype distributions for patients and controls. No significant difference was detected between the CML patients and controls for TNF-α (−308), IL-10 (−592, −819, −1082), INF-γ (+874), and TGF-β1 (codons 10 and 25) polymorphisms (p>0.05). However, the genotype for IL-6 (−174) was significantly different between the patients and the controls. The 6-year probability of overall survival (OS) was 96% and event-free survival (EFS) 73% in all patients (Tables 1 and 3). In the univariate analyses, there was no significant prognostic factor found to influence OS (Table 3). The five factors that were predicted for better EFS from the univariate analysis were younger age (<60) (p=0.023), absence of splenomegaly (p=0.014), lower Sokal risk score at diagnosis (p=0.018), TGF-β1 haplotype of TCGC, TTGC, CCGC, CCCC, CCGG, or TCCC (p=0.019), and IL-10 haplotype of GCC/ACC, GCC/ATA, ACC/ACC, ACC/ATA, and ATA/ATA (p=0.002) (Fig. 1). However, in the multivariate analysis, only the IL-10 (−1082, 819, 592) GCC/GCC haplotype was significantly associated with lower EFS (Fig. 2). The Cox proportional hazard ratio was 0.140 (p=0.019, [95% CI: 0.027–0.726]).
Table 2.
Comparison of Frequencies of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ Gene Polymorphisms Between Patients with Chronic Myeloid Leukemia and Healthy Controls
| CML | Healthy control | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | na | (%) | nb | (%) | OR | 95% CI | p | |
| TNF-α (−308) | GG | 56 | (93.3) | 66 | (89.2) | 0.621c | 0.158–2.445c | 0.496c |
| AG | 3 | (5) | 8 | (10.8) | 0.540c | 0.126–2.319c | 0.407c | |
| AA | 1 | (1.7) | – | (0) | 0.444d | 0.367–0.537d | 0.448d | |
| TGF-β (codons 10) | CC | 12 | (20) | 11 | (14.9) | 1.387c | 0.445–4.228c | 0.565c |
| TC | 32 | (53.3) | 40 | (54.1) | 1.161c | 0.485–2.776c | 0.738c | |
| TT | 16 | (26.7) | 23 | (31) | 1215c | 0.528–2.797c | 0.647c | |
| TGF-β (codons 25) | GG | 52 | (86.6) | 59 | (79.7) | 0.597c | 0.220–1.621c | 0.312c |
| GC | 7 | (11.7) | 15 | (20.3) | 0.507c | 0.180–1.431c | 0.199c | |
| CC | 1 | (1.7) | – | (0) | 0.444d | 0.367–0.537d | 0.448d | |
| IL-10 (−1082) | AA | 31 | (51.7) | 28 | (37.8) | 1.152c | 0.350–2.794c | 0.816c |
| AG | 22 | (36.7) | 36 | (48.6) | 0.882c | 0.264–2.947c | 0.838c | |
| GG | 7 | (11.6) | 10 | (13.6) | 0.895c | 0.247–3.237c | 0.865c | |
| IL-10 (−819) | CC | 25 | (41.7) | 37 | (50) | 1.305c | 0.237–7.187c | 0.759c |
| CT | 32 | (53.3) | 33 | (44.6) | 1.981c | 0.359–10.948c | 0.433c | |
| TT | 3 | (5) | 4 | (5.4) | 2.851c | 0.434–18.743c | 0.276c | |
| IL-10 (−592) | CC | 25 | (41.7) | 37 | (50) | 1.305c | 0.237–7.187c | 0.759c |
| CT | 32 | (53.3) | 33 | (44.6) | 1.981c | 0.359–10.948c | 0.433c | |
| TT | 3 | (5) | 4 | (5.4) | 2.851c | 0.434–18.743c | 0.276c | |
| IL-6 (−174) | GG | 47 | (88.3) | 37 | (50) | 0.347c | 0.155–0.774c | 0.010c |
| GC | 7 | (11.7) | 32 | (43.2) | 0.219c | 0.083–0.579c | 0.002c | |
| CC | 6 | (10) | 5 | (6.8) | 0.687c | 0.177–2.657c | 0.586c | |
| IFN-γ (+874) | TT | 9 | (15) | 9 | (12.2) | 0.700c | 0.236–2.079c | 0.520c |
| TA | 31 | (51.7) | 32 | (43.2) | 0.879c | 0.279–2.767c | 0.826c | |
| AA | 20 | (33.3) | 33 | (44.6) | 0.529c | 0.163–1.721c | 0.290c | |
n=60.
n=74.
OR (95% CI) was adjusted by age and sex.
Fisher's exact test.
95% CI, 95% confidence interval; IFN-γ, gamma interferon; IL, interleukin; OR, odds ratio; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor-alpha; CML, chronic myeloid leukemia.
Table 3.
Univariate Analysis (Logrank Test) of Prognostic Factors in 60 Patients with Chronic Phase CML
| n | 6 years OS % | Log rank p-value | 6 years EFS % (median months) | Log rank p-Value | |
|---|---|---|---|---|---|
| All patients | 60 | 96 | 73 | ||
| Gender | |||||
| Female | 37 | 97 | 71 | ||
| Male | 23 | 96 | 0.705 | 75 | 0.708 |
| Age | |||||
| <60 | 54 | 96 | 77 | ||
| ≥60 | 6 | 100 | 0.643 | 40 (25.8) | 0.023 |
| Sokal risk score at diagnosis | |||||
| Low | 10 | 100 | 100 | ||
| Intermediate | 28 | 100 | 0.148 | 85 | 0.018 |
| High | 22 | 90 | 38 (53.8) | ||
| Low–intermediate–high | 38/22 | 100/90 | 0.050 | 87/38 (53.8) | 0.007 |
| Splenomegaly | |||||
| Yes | 42 | 95 | 61 | ||
| No | 18 | 100 | 0.353 | 100 | 0.014 |
| Hemoglobin (g/dL) | |||||
| <12 | 42 | 95 | 60 | ||
| ≥12 | 18 | 100 | 0.353 | 87 | 0.144 |
| Leukocytes (×109/L) | |||||
| <50 | 22 | 100 | 89 | ||
| ≥50 | 38 | 94 | 0.276 | 61 | 0.087 |
| Platelets (×109/L) | |||||
| <450 | 32 | 97 | 82 | ||
| ≥450 | 28 | 96 | 0.930 | 65 | 0.392 |
| Initial treatment | |||||
| Imatinib | 50 | 96 | 73 | ||
| Interferon-α→imatinib | 10 | 100 | 0.525 | 77 | 0.685 |
| TNF-α (−308) | |||||
| GGa | 56 | 96 | 77 | ||
| GA/AAb | 4 | 100 | 0.717 | 50 (53.8) | 0.943 |
| TGF-β (codons 10, 25) | |||||
| TCGC, TTGC, CCGC, CCCC, CCGG, TCCCa,c | 26 | 100 | 100 | ||
| TTGG, TCGGb | 44 | 95 | 0.372 | 63 | 0.019 |
| IL-10 (−1082, 819, 592) | |||||
| GCC/ACC, GCC/ATA, ACC/ACC, ACC/ATA, ATA/ATAa,c | 52 | 98 | 77 | ||
| GCC/GCCb | 8 | 88 | 0.085 | 42 (25.8) | 0.002 |
| IL-6 (−174) | |||||
| CCa | 6 | 100 | 100 | ||
| GG/GCb | 54 | 96 | 0.643 | 70 | 0.198 |
| IFN-γ (+874) | |||||
| AA/TAa,c | 51 | 96 | 82 | ||
| TTb | 9 | 100 | 0.552 | 42 (53.8) | 0.083 |
Sokal, patient's age, spleen size, percentage of blood blasts and platelets.
Low production.
High production.
Intermediate production.
EFS, event-free survival; OS, overall survival.
FIG. 1.
Kaplan–Meier plots on event-free survival (EFS) time according to the type of Sokal risk score at diagnosis. Low risk 100% (n=10). Intermediate risk 85% (n=28). High-risk median 53.8 months, 38% (n=22). Log rank p=0.018.
FIG. 2.
Kaplan–Meier plots on EFS time according to the type of interleukin (IL)-10 (−1082, 819, 592) haplotypes. ab, Low and intermediate production haplotypes (GCC/ACC, GCC/ATA, ACC/ACC, ACC/ATA, ATA/ATA), c, high production haplotypes (GCC/GCC).
Discussion
In this work, we investigated certain cytokine gene polymorphisms in CML patients. Previous studies have indicated the role of immunologic responses in the mechanisms of prognosis, sepsis, and mortality during CML therapy (Bidwell et al., 2001; Humlová et al., 2006; Hochhaus, 2008). It is thought that increased cytokine levels and complement activation may be responsible for CML (Amirzargar et al., 2005). In addition to its important role in immune response and inflammatory processes, the cytokine IL-6 is crucially involved in carcinogenesis (www.ncbi.nlm.nih.gov/omim?term=IL6). IL-6 is associated with poor prognosis in various malignancies (Deans et al., 2007; DeMichele et al., 2009). Nevertheless, it was reported that this cytokine was not associated with multiple myeloma or primary cutaneous melanoma (Martinez-Escribano et al., 2003; Duch et al., 2007). No data are available on a possible association with CML. When the CML and control groups were compared in this study, the IL-6 (−174) polymorphism exhibited a significant difference both in terms of allele frequency and genotype frequency; there was a deviation from HWE in the CML group as well (Table 2). The most frequent genotype in our CML patients was IL-6 GG at position −174 (88.3% CML vs. 50% control, p=0.010). In contrast, the frequencies of the genotype IL-6 GC at position −174 (11.7% vs. 43.2%, p=0.002) were very low in the CML patients. This substitution could result in high levels of IL-6 secretion. The high expression of IL-6 in the results is in accordance with the literature (Martinez-Escribano et al., 2003; Lehrnbecher et al., 2005; Snoussi et al., 2005; Deans et al., 2007; Ambruzova et al., 2009; Bhattacharyya et al., 2009).
TGF-β1, a multifactorial cytokine, is the strongest known growth inhibitor of normal and transformed cells (Deans et al., 2007; Noori-Daloii et al., 2007). Increased TGF-β1 expression and EGFR amplification accompany the emergence of highly aggressive human carcinomas. The literature contains data supporting the fact that the polymorphisms of this gene could be associated with susceptibility to the disease, but contrasting data in the literature suggest that such polymorphisms could be protective (Noori-Daloii et al., 2007; Castillego et al., 2009; Hawinkels et al., 2009; Qian et al., 2009). No associations were detected between TGF-β1 genotype/allele frequencies and CML in our study. However, the low to intermediate production of TGF-β1 was shown to improve EFS in univariate analysis, and no associations were found in multivariate analysis. No similar analyses were performed up to date.
IL-10 is a multifunctional cytokine with both immunosuppressive and antiangiogenic functions and could have both tumor-promoting and tumor-inhibiting properties. A large number of polymorphisms (primarily single-nucleotide polymorphisms) have been identified in the IL-10 gene promoter (Martinez-Escribano et al., 2003; Wilkening et al., 2008). Convincing evidence that all of these polymorphisms are associated with differential expression of IL-10 in vitro, and in some cases in vivo, was obtained, and a number of studies investigated associations between IL-10 polymorphisms and cancer susceptibility and prognosis (Martinez-Escribano et al., 2003; Cacev et al., 2008; Wilkening et al., 2008). However, because of the marginal success of the initial clinical trials using recombinant IL-10, some of the interest in this cytokine as an anti-inflammatory therapeutic agent has diminished (Hawinkels et al., 2009). IL-10 was reported to be a poor prognostic factor in colorectal, gastroesaphageal, and sporadic colon cancers and advanced melanoma, while no associations were reported between IL-10 and nasopharyngeal or breast carcinoma (Martinez-Escribano et al., 2003; Pratesi et al., 2006; Vuoristo MS, 2007; Bogunia-Kubik et al., 2008). In our study, no associations could be detected between IL-10 and CML in terms of allele/genotype frequency. Low to intermediate production of IL-10 was demonstrated to be associated with better EFS in the univariate analysis, and high production of IL-10 was found to be associated with lower EFS in the multivariate analysis (Fig. 2). Previous studies indicate that IL-10 could be a preferred survival marker, and our results support this fact (Hempel et al., 1997; Martinez-Escribano et al., 2003; Amirzargar et al., 2005).
Genetic polymorphisms in the promoter region of the TNF-α gene are involved in the regulation of its expression levels and have been associated with various inflammatory and malignant conditions (Jevtovic-Stoimenov et al., 2008; Castillego et al., 2009). Previous studies demonstrated that TNF-α −308 polymorphism is not associated with chronic lymphocytic leukemia (Au et al., 2006). Also, there is no association between TNF-α and myeloma/myelodisplastic syndrome (Gyulai et al., 2005; Au et al., 2006). Although TNF-α polymorphism association in colorectal and NSCLC has been reported, there are no studies on the association between TNF-α polymorphisms and CML in the literature (Colakogullari et al., 2008; Wu et al., 2008). This is the first study in this field, and it was shown that TNF-α polymorphisms do not play a role in the prognosis of CML.
IFN-γ is a proinflammatory cytokine playing a pivotal role in both innate and adaptive immune responses (Halma et al., 2004). A significant association has been reported between IFN-γ polymorphisms and cervical and pancreatic cancers (Halma et al., 2004; Gangwar et al., 2009), whereas no associations have been detected for breast, lung, and cervical cancers in particular populations (Halma et al., 2004; Gonullu et al., 2007; Colakogullari et al., 2008). Also, no significant associations were detected between allele/genotype frequencies and clinical parameters in our CML patient group.
In conclusion, the relationship between cytokine polymorphisms and clinical parameters in Ph+ CML was investigated in this study for the first time. Our results suggest that IL-10 could be a useful marker for CML prognosis and IL-6 polymorphisms could be associated with susceptibility. These results must be replicated in larger populations, which are needed to elucidate the role of IL-6 and IL-10 in CML.
Author Disclosure Statement
No competing financial interests exist.
References
- Aliano S, Cirmena G, Fugazza G, et al. (2013) Standart and variant Philadelphia translocation in a CML patient with different sensitivity to imatinib therapy. Leuk Res Rep 2:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambruzova Z, Mrazek F, Raida L, et al. (2009) Association of IL6 and CCL2 gene polymorphisms with the outcome of allegeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 44:227–235 [DOI] [PubMed] [Google Scholar]
- Amirzargar AA, Bagheri M, Ghavamzadeh A, et al. (2005) Cytokine gene polymorphism in Iranian patients with chronic myelogenous leukaemia. Int J Immunogenet 32:167–171 [DOI] [PubMed] [Google Scholar]
- Au WY, Fung A, Wong KF, et al. (2006) Tumor necrosis factor alpha promotor polymorphism and the risk of chronic lymphocytic leukemia and myeloma in the Chinese population. Leuk Lymphoma 47:2189–2193 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ishida W, Wu M, et al. (2009) A non-Smad mechanism of fibroblast activation by transforming growth factor-β via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene 28:1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell J, Keen L, Gallagher G, et al. (2001) Cytokine gene polymorphism in human disease: online databases, supplement 1. Genes Immun 2:61–70 [DOI] [PubMed] [Google Scholar]
- Bogunia-Kubik K, Mazur G, Wrobel T, et al. (2008) Interleukin-10 gene polymorphisms influence the clinical course of non-Hodgkin's lymphoma. Tissue Antigens 71:146–150 [DOI] [PubMed] [Google Scholar]
- Bose S, Deininger M, Gora-Tybor J, et al. (1998) The presence of typical and atypical fusion genes in leucocytes of normal individuals: biologic significance and implications fort he assessment of minimal residual disease. Blood 92:3362–3367 [PubMed] [Google Scholar]
- Cacev T, Radosevic S, Krizanac S, Kapitanovic S. (2008) Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis 29:1572–1580 [DOI] [PubMed] [Google Scholar]
- Castillego A, Rothman N, Murta-Nascimento C. (2009) TGFB1 and TGFBR1 polymorphic variants in relationship to bladder cancer risk and prognosis. Int J Cancer 124:608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li S. (2013) Molecular signatures of chronic myeloid leukemia stem cells. Biomark Res 1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel JC, Brizard F, Veinstein A, et al. (2000) Persistence of BCR-ABL genomic rearrangement in chronic myeloid leukemia patients in complete and sustained cytogenetic remission after interferon-alpha therapy orallogeneic bone marrow transplantation. Blood 95:404–409 [PubMed] [Google Scholar]
- Colakogullari M, Ulukaya E, Yilmaztepe-Oral A, et al. (2008) The involment of IL-10, IL-6, IFN-gamma, TNF-alpha, and TGF-beta gene polymorphisms among Turkish lung cancer patients. Cell Biochem Funct 26:283–290 [DOI] [PubMed] [Google Scholar]
- De Oliveira DE, Cavassin GG, Perim Ade L, et al. (2007) Stromal cell-derived factor-1 chemokine gene variant in blood donors and chronic myelogenous leukemia patients. J Clin Lab Anal 21:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans C, Rose-Zerilli M, Wigmore S. (2007) Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro oesaphageal cancer. Ann Surg Oncol 14:329–339 [DOI] [PubMed] [Google Scholar]
- DeMichele A, Gray R, Horn M, et al. (2009) Host genetic variants in the interleukin-6 promotor predict poor outcome in patients with estrogen receptor-positive, node-positive breast cancer. Cancer Res 69:4184–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch CR, Figueiredo MS, Ribas C, et al. (2007) Analysis of polymorphism at site −174 G/C of interleukin-6 promotor region in multiple myeloma. Braz J Med Biol Res 40:265–267 [DOI] [PubMed] [Google Scholar]
- Gangwar R, Pandey S, Mittal RD. (2009) Association of interferon-gamma +874 A polymorphism with the risk of developing cervical cancer in North-Indian population. BJOG 116:1671–1677 [DOI] [PubMed] [Google Scholar]
- Gonullu G, Basturk B, Evrensel T, et al. (2007) Association of breast cancer and cytokine gene polymorphism in Turkish women. Saudi Med J 28:1728–1733 [PubMed] [Google Scholar]
- Gyulai Z, Balog A, Borbenyi Z, Mandi Y. (2005) Genetic polymorphisms in patients with myelodysplastic syndrome. Acta Microbiol Immunol Hung 52:463–475 [DOI] [PubMed] [Google Scholar]
- Halma MA, Wheelhouse NM, Barber MD, et al. (2004) Interferon-gamma polymorphisms correlate with duration of survival in pancreatic cancer. Hum Immunol 65:1405–1408 [DOI] [PubMed] [Google Scholar]
- Hawinkels LJ, Verspaget HW, vander Reijden JJ. (2009) Active TGF-beta1 correlates with myofibroblasts and malignancy in the colorectal adenoma-carcinoma sequence. Cancer Sci 100:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel L, Körholz D, Nussbaum P, et al. (1997) High interleukin-10 serum levels are associated with fatal outcome in patients after bone marrow transplantation. Bone Marrow Transplant 20:365–368 [DOI] [PubMed] [Google Scholar]
- Hochhaus A. (2008) Prognostic factors in CML. Onkologie 31:576–578 [DOI] [PubMed] [Google Scholar]
- Humlová Z, Klamová H, Janatková I, et al. (2006) Immunological profiles of patients with chronic myeloid leukaemia. I. State before the start of treatment. Folia Biol (Praha) 52:47–58 [DOI] [PubMed] [Google Scholar]
- Jevtovic-Stoimenov T, Kocic G, Pavlovic D, et al. (2008) Polymorphisms of tumor-necrosis factor-alpha −308 and lymphotoxin-alpha +250: possible modulation of susceptibility to apoptosis in chronic lymphocytic leukemia and non-Hodgkin lymphoma mononuclear cells. Leuk Lymphoma 49:2163–2169 [DOI] [PubMed] [Google Scholar]
- Karaoglan I, Pehlivan S, Namiduru M, et al. (2009) TNF-α, TGF-β1, IL-10, IL-6 VE IFN-γ gene plymophisms as risk factor for bruellosis. New Microbiol 32:173–178 [PubMed] [Google Scholar]
- Lehrnbecher T, Bernig T, Hanisch M, et al. (2005) Common genetic variants in the interleukin-6 and chitotriosidase genes are associated with the risk for serious infection in children undergoing therapy for acute myeloid leukemia. Leukemia 19:1745–1750 [DOI] [PubMed] [Google Scholar]
- Martinez-Escribano JA, Moya-Quiles MR, Muro M. (2003) Interleukin-10, interleukin-6 and interferon-gamma gene polymorphisms in melanoma patients. Melanoma Res 63:3066–3068 [DOI] [PubMed] [Google Scholar]
- Mayani H, Flores-Figueroa E, Chávez-González A. (2009) In vitro biology of human myeloid leukemia. Leuk Res 33:624–637 [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nuc Acids Res 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori-Daloii MR, Rashidi-Nezhad A, Izadi P. (2007) Transforming growth factor-beta1 codon 10 polymorphism is associated with acute GVHD after allogenic BMT in Iranian population. Ann Transplant 12:5–10 [PubMed] [Google Scholar]
- Nowell PC, Hungerford DA. (1960) Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst 25:85–109 [PubMed] [Google Scholar]
- O'Brien SG, Guilhot F, Larson RA, et al. (2003) IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Eng J Med 348:994–1004 [DOI] [PubMed] [Google Scholar]
- Pratesi C, Bortolin MT, Bidoli E. (2006) Interleukin-10 and interleukin-18 promotor polymorphisms in an Italian cohort of patients with undifferentiated carcinoma of nasopharynegal type. Cancer Immunol Immunother 55:23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Katsaros D, Lu L. (2009) High miR-21 expression in breast cancer associated with poor disease-free survival in early stage and high TGF-beta1. Breast Cancer Res Treat 117:131–140 [DOI] [PubMed] [Google Scholar]
- Rise KN, Jamieson CHM. (2010) Molecular pathways to CML stem cells. Int J Hematol 91:748–752 [DOI] [PubMed] [Google Scholar]
- Rowley JD. (1973) Letter: a new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature 243:290–293 [DOI] [PubMed] [Google Scholar]
- Snoussi K, Strosberg AD, Bouaouina N, et al. (2005) Genetic variation in pro-inflammatory cytokines (interleukin-1 beta, interleukin-1 alpha and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur Cytokine Netw 16:253–260 [PubMed] [Google Scholar]
- Sokal JE, Cox EB, Baccarani M, et al. (1984) Prognostic discrimination in “good-risk” chronic granulacytic leukemia. Blood 63:789–799 [PubMed] [Google Scholar]
- Vuoristo MS. (2007) The polymorphisms of interleukin-10 gene influence the prognosis of patients with advanced melanoma. Cancer Genet Cytogenet 176:54–57 [DOI] [PubMed] [Google Scholar]
- Wilkening S, Tavelin B, Canzian F, et al. (2008) Interleukin 10 promotor polymorphisms and prognosis in colorectal cancer. Carcinogenesis 29:1202–1206 [DOI] [PubMed] [Google Scholar]
- Wu GY, Wang XM, Kese M, et al. (2008) Association between tumor necrosis factor alpha gene polymorphism and colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 11:567–571 [PubMed] [Google Scholar]