Abstract
This pilot, open-protocol study examined whether scalp application of red and near-infrared (NIR) light-emitting diodes (LED) could improve cognition in patients with chronic, mild traumatic brain injury (mTBI). Application of red/NIR light improves mitochondrial function (especially in hypoxic/compromised cells) promoting increased adenosine triphosphate (ATP) important for cellular metabolism. Nitric oxide is released locally, increasing regional cerebral blood flow. LED therapy is noninvasive, painless, and non-thermal (cleared by the United States Food and Drug Administration [FDA], an insignificant risk device). Eleven chronic, mTBI participants (26–62 years of age, 6 males) with nonpenetrating brain injury and persistent cognitive dysfunction were treated for 18 outpatient sessions (Monday, Wednesday, Friday, for 6 weeks), starting at 10 months to 8 years post- mTBI (motor vehicle accident [MVA] or sports-related; and one participant, improvised explosive device [IED] blast injury). Four had a history of multiple concussions. Each LED cluster head (5.35 cm diameter, 500 mW, 22.2 mW/cm2) was applied for 10 min to each of 11 scalp placements (13 J/cm2). LEDs were placed on the midline from front-to-back hairline; and bilaterally on frontal, parietal, and temporal areas. Neuropsychological testing was performed pre-LED, and at 1 week, and 1 and 2 months after the 18th treatment. A significant linear trend was observed for the effect of LED treatment over time for the Stroop test for Executive Function, Trial 3 inhibition (p=0.004); Stroop, Trial 4 inhibition switching (p=0.003); California Verbal Learning Test (CVLT)-II, Total Trials 1–5 (p=0.003); and CVLT-II, Long Delay Free Recall (p=0.006). Participants reported improved sleep, and fewer post-traumatic stress disorder (PTSD) symptoms, if present. Participants and family reported better ability to perform social, interpersonal, and occupational functions. These open-protocol data suggest that placebo-controlled studies are warranted.
Key words: : executive function, mTBI, photobiomodulation, treatment for mTBI
Introduction
Each year in the United States, ∼1,700,000 patients are evaluated for traumatic brain injury (TBI); three TBIs every minute.1 It is estimated that there are 5,300,000 Americans living with TBI-related disabilities.1,2 The annual economic cost is estimated to be between $60 and $76.5 billion.1,3 The majority of cases (70–85%) are mild TBI (mTBI).1 Most civilian mTBI patients recover cognitive abilities within 3 months;4 however, the literature reports that between 5 and 22% of individuals have persistent symptoms.5–9
Cognitive dysfunction associated with sports-related mTBI is of increasing concern, both for males and females (including children).10 Within the past 10 years, the diagnosis of concussion in high school sports has increased annually, by 16.5%.11
It is estimated that 15–40% of soldiers returning from Iraq and Afghanistan as part of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) report at least one mTBI.12–14 Closed-head blast injury is the signature injury,12 and the cognitive sequelae, recovery, and rehabilitation are of increasing concern.15 It is estimated that there are as many as 320,000 veterans who have returned with TBI, most with mTBI.16,17 Post-traumatic stress disorder (PTSD) is also a major concern with OEF/OIF soldiers who have experienced mTBI.18 Therefore, a compelling need exists to address chronic deficits in this population.
Cases of chronic mTBI often present clinically with deficits in attention, working memory, cognitive manipulation of temporal information, and general information processing speed.7,8,19–23 The most common complaints are in attention/concentration and working memory; that is, the ability to hold information in the mind, and to manipulate it in light of incoming material.24 At 6 months post-injury, indices of executive function were found to predict persistence of post-concussive syndrome in mild and moderate TBI patients.25 In addition to cognitive problems, these patients are often unable to re-establish family and work relationships.26 Because of the diffuse nature of damage, however, no single behavioral outcome measure captures the multidimensional nature of mTBI outcome.27
Rationale for the present study
The present study examined whether the application of red and near-infrared (NIR) light, utilizing light-emitting diodes (LED) applied directly to the head could improve cognitive function, particularly executive function and verbal memory, in chronic, mTBI patients. Photons in the red and NIR wavelengths have the potential to improve subnormal, cellular activity of brain tissue that has been damaged by brain trauma.
Scalp application of red and NIR light is a new application for LED technology. More than 30 years ago, however, it was observed in human cadaver studies that red (600 nm) and NIR (800–900nm) wavelengths could penetrate through the scalp and skull (∼ 1 cm).28 Two physiological changes associated with exposure of cells to red and NIR wavelengths of light are: 1) Increased production of adenosine triphosphate (ATP) by the mitochondria,29,30 and 2) Increased vasodilation/regional cerebral blood flow (rCBF),31 explained subsequently.
The last enzyme complex (cytochrome c oxidase) of the electron transport chain within the mitochondrial membrane is a photoacceptor for red and NIR photons.32,33 There is mitochondrial damage and dysfunction after TBI.34,35 Increased ATP production by the mitochondria improves cellular respiration, oxygenation, and function. Also, in hypoxic/compromised cells, cytochrome c oxidase is inhibited by non-covalently bound nitric oxide. When the mitochondria are exposed to red/NIR photons, nitric oxide is released and diffused outside the cell wall, promoting local vasodilation and increased blood flow. The effect of the light is non-thermal.36
Multiple animal studies using mice, show significantly better recovery of motor and cognitive function after NIR transcranial low-level laser therapy when treated in the acute post-injury phase.37–40 Most of this work has suggested improved energy kinetics and decreased inflammation as possible mechanisms for acute neuroprotection. Energetics may also have a role in the chronic phase of injury. We have reported that midline and bilateral scalp application of red/NIR LED therapy improved executive function and verbal memory in two patients with chronic TBI.41 One TBI patient with a history of multiple concussions (retired military) who had been on medical disability for 5 months prior to transcranial LED treatments, returned to full-time employment after 4 months of nightly, home LED treatments.
We undertook a pilot, open-protocol study among patients with chronic mTBI who received the same number of specific, transcranial LED treatments. Pre- and post- neuropsychological (NP) testing and psychological measurements were acquired up to 2 months after the last LED treatment, in order to evaluate its potential to improve chronic symptomatology.
Methods
Design
We utilized a case series design.42
Participants
Eleven, chronic, mTBI cases with nonpenetrating brain injury (6 males) participated. Their ages ranged from 26 to 62 years at time of entry (mean, 44.3 years; SD, 13.7) and the time post- mTBI ranged from 10 months to 8 years (mean, 38.2 months., SD, 29.4). Their demographics, including medical history for mTBI, years of education, and work status, are provided in Table 1.
Table 1.
Demographics for 11 Chronic, Mild Traumatic Brain Injury (mTBi) Cases Treated with Transcranial, Red/Near-Infrared Light-Emitting Diode (LED) Therapy
| Time post-TBI | |||||||
|---|---|---|---|---|---|---|---|
| ID number | Age at entry | Gender | Yr. | Mo. | Medical history for mTBI | Years education | Work status |
| P1 | 52 | M | 5 | - | Motor vehicle accident (MVA) | 16 | Disabled |
| P2 | 59 | M | 2 | 6 | MVA | 22 | Partially disabled; working only 22 hours/week at entry |
| P3 | 50 | F | 1 | 8 | Pedestrian hit by a car. | 18 | Disabled Unable to return to work |
| P4 | 26 | F | 1 | 4 | Multiple-car MVA, hit from behind. | 14 | Unemployed, disabled |
| P5 | 58 | M | 1 | - | Sports injury, close-range impact. Hit in the head by a baseball. | 16 | Disabled |
| P6 | 62 | F | 7 | - | MVA | 12 | Partial employment; disabled |
| P7a | 49 | F | 3 | - | Ski accident. History of multiple concussions and a prior stroke. | 16 | Disabled |
| P8a | 32 | M | 3 | - | Blast Injury. Additional improvised explosive device (IED), TBIs. | 13 | Disabled, active duty military. Unable to return to unit, since 3 years previously |
| P9a | 44 | M | - | 10 | MVA. Multiple (>10) concussions (falls, sports injuries, accidents). | 16 | Disabled |
| P10 | 27 | M | 1 | - | Industrial/work accident. Complicated mTBI, maxillo-orbital fractures with fragments into left infratemporal fossa. No craniotomy. |
12 | Disabled |
| P11a | 28 | F | 8 | - | Fell off a chair; also a concussion, 12 years prior to entry. | 18 | Working full time. |
History of multiple concussions.
All participants had previously been diagnosed as having mTBI, with loss of consciousness (LOC) lasting ≤30 min (or no LOC); and with a period of altered mental status that could include post-traumatic amnesia: “memory gaps” or confusion lasting up to 24 h. To be included in the study, persistent cognitive problems consistent with a diagnosis of mTBI had to have been present for at least 6 months prior to screening cognitive testing. Exclusion criteria included moderate or severe TBI, penetrating brain injury, or history of craniotomy or craniectomy. Medical records were obtained, and each participant was examined by a single experienced study clinician prior to referral for screening cognitive testing. Participants were requested not to change their medications or dosages during participation in the study.
Prior to official enrollment into the study, all subjects signed informed consent forms approved by the Spaulding Rehabilitation Hospital, Institutional Review Board (IRB). All study procedures complied with the IRB and with The Health Insurance Portability and Accountability Act (HIPAA) standards.
Screening cognitive testing
In addition to the criteria mentioned, in order to qualify for entry into the study, each participant needed to score at least 2 SD below average on one, or 1 SD below average on at least two of the following NP tests (using age and education-adjusted norms). 1) Trail Making Test, Trails A and Trails B, measuring problem-solving, thinking flexibility, and planning.43 2) Controlled Oral Word Association Test (COWAT)/FAS Test, total words generated for the letters F, A, and S (1 min per letter), measuring verbal fluency and categorical generative capacity.44,45 3) California Verbal Learning Test-II, examining aspects of verbal learning, organization, and memory.46 4) Stroop Test for Executive Function, examining attention, mental speed, mental control, inhibition, and inhibition switching.47
NP tests administered pre-/post- LED treatment series
The participants were tested four times during participation in the study: 1) pre- testing, within 1 week before the first LED treatment; 2) post- testing, within 1 week after the final (18th) LED treatment; 3) at 1 month after the final LED treatment; and 4) at 2 months after the final LED treatment. The tests administered at all four testing times included the following: 1) Stroop Test for Executive Function;47 2) California Verbal Learning Test-II (CVLT-II) with Alternating Versions, with Short Delay Free Recall, Short Delay Cued Recall, Long Delay (20 min later) Free Recall, and Long Delay Cued Recall, for each testing session;46 3) Delis–Kaplan Executive Function (D-KEF) - Trails Test;47 4) Controlled Oral Word Association Test (COWAT)/FAS Test, total words generated, for the letters F, A and S;44,45 and Digit Span, Forwards and Backwards, Wechsler Adult Intelligence Scale (WAIS)-IV.48 Some of the tests administered at screening were carried over to serve as pretreatment/baseline scores at Time 1.
In order to avoid practice effects, alternating versions of the NP tests were used at the post-LED testing times, when possible. This included the CVLT-II, Alternating Versions for the 16 words presented;46 and the alternate form of the FAS test, using the three different letters B, H, and R.47 A consistent practice effect for repeated presentations of the Stroop test has not been demonstrated.49
In addition, psychological measurements included the PTSD Checklist–Civilian (PCL-C);50 the Beck Depression Inventory-II (BDI);51 and the Visual Analog Scale (VAS) for pain (0–10, verbal report).52
LED device and LED treatment method
Two identical, LED Console Units were used (MedX Health, Model 1100, Toronto). Each Console Unit had three LED cluster heads. This device was cleared by the United States Food and Drug Administration (FDA) in 2003 as posing an insignificant risk (FDA-cleared for home treatment, 2005). A sample LED cluster head is shown in Figure 1. Each LED cluster head had a 5.35 cm diameter (9 red diodes, 633 nm, and 52 NIR diodes, 870 nm were embedded into each LED cluster head); 22.48 cm2 in size; 500 mW total power; 22.2 mW/cm2 power density; continuous wave. The power output (500 mW) for each LED cluster head was verified before and after the LED treatments using the MedX MEDRAD200X Radiometer System.
FIG. 1.
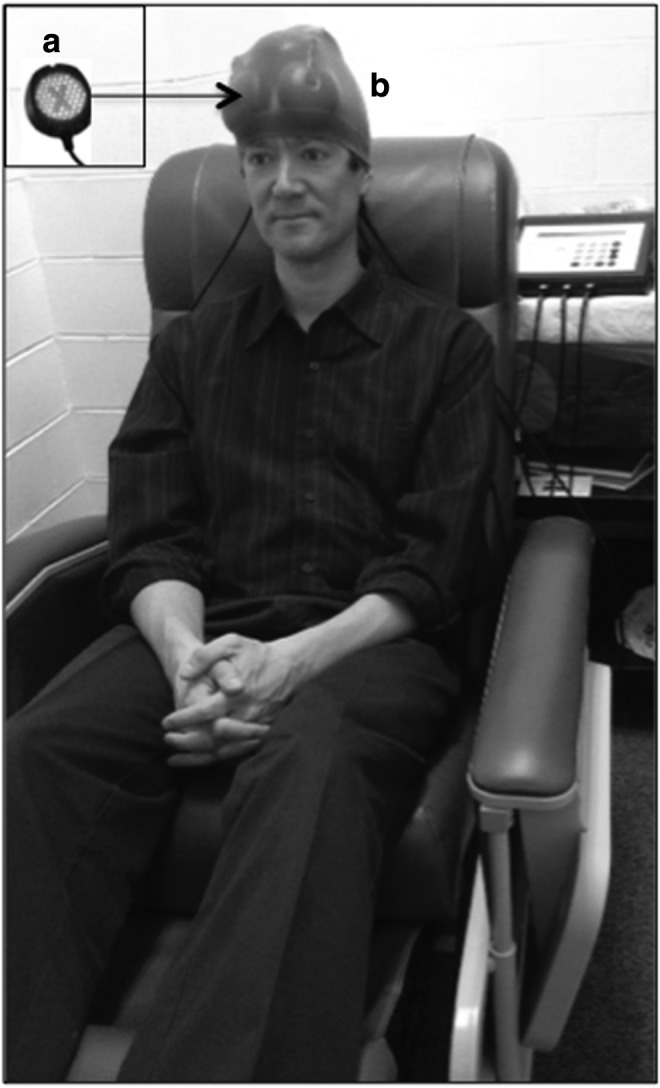
(a) Sample light-emitting diode (LED) cluster head, showing the side that was applied to the skin. The “X” shows location of the nine red diodes embedded within the LED cluster head. The 52 near-infrared (NIR) diodes surrounding the “X” are not visible to the eye. Each red/NIR LED cluster head had a 5.35 cm diameter, and the total power output was 500 mW. (b) View of subject being treated, and example of three LED placement areas on the head from Set A (first, second, and third LED placements described in Table 2). During each treatment, six LED cluster heads were used simultaneously (13 J/cm2, 10 min per LED placement). Immediately after treatment using the Set A LED placements, the LED cluster heads were moved to other placements on the scalp (Set B) for 10 min. The LED cluster heads were held in place with a soft, nylon cap. The total treatment time per visit was 20 min; it was painless, noninvasive, and non-thermal.
Table 2 lists the LED cluster head placements on the head that were used at each visit (Set A for 10 min, followed immediately by Set B for 10 min), and approximate surface brain cortex areas that were impacted with the red/NIR LEDs. The LED placement loci included, in part, a proposed paradigm to target nodes within the default mode network (DMN) and the salience network (SN), areas where functional connectivity MRI studies have reported abnormalities in TBI.53 The LED placement loci also included dorsolateral prefrontal cortex (DLPFC), part of the central executive network (CEN).54 Supplementary Figure 1 (see online supplementary material at http://www.liebertonline.com) shows the location of extracranial bone and suture landmarks on the skull, in relationship to approximate surface brain cortex areas. In the present study, the LED placements were hypothesized to impact the immediate subjacent, surface cortical areas, although this is unknown.
Table 2.
List of LED Cluster Head Placements on the Forehead and Scalp Treated at Each Visit
| Placement order | LED placement loci for each LED cluster head | Approximate, surface brain cortex areas hypothesized to be impacted with the LED cluster heads |
|---|---|---|
| Set A LED placements | ||
| 1st | Midline of face, centered over front hairline (half of LED placement was anterior to hairline on forehead; half of LED placement, posterior to hairline). | L & R dACC, part of SN L & R vmPFC, part of DMN |
| 2nd and 3rd | L & R forehead, between eyebrow and front hairline, centered on pupil line | L & R orbitofrontal cortex; and most anterior, MFG areas |
| 4th | Midline, superior to external occipital protuberance (half-way to vertex); and on alternate treatment days, midline, inferior to external occipital protuberance. | L & R precuneus, with midline placement superior to occipital protuberance. Precuneus areas are part of DMN. Placement inferior to occipital protuberance was used to promote neck muscle relaxation, and treat headache pain, if present. |
| 5th | Midline, vertex of the head | L & R SMA, and PreSMA. PreSMAs are part of SN |
| 6th | Sole of foot (proximal to toes), alternating L & R on different treatment days, as well as alternating with a placement on dorsum of foot (proximal to toes), also alternating L & R on different treatment days. | Red wavelength of low-level laser light applied to a point on the foot has been observed to increase ipsilateral, regional cerebral blood flow to occipital cortex.72 |
| Set B LED placements | ||
|---|---|---|
| 7th and 8th | Immediately posterior to L & R front hairline, centered on pupil line | L & R MFG areas, including DLPFC, part of CEN |
| 9th and 10th | L & R temple areas | L & R IFG, possibly including L & R AI, but unknown because of greater depth of AI, part of SN |
| 11th and 12th | L & R posterior, superior to each ear | Posterolateral IPC, part of DMN |
Each participant received Set A, followed immediately by Set B, at each treatment session. Each LED cluster head had a 5.35 cm diameter. (See also Fig. S1 showing location of external bone and suture landmarks on the skull in relationship to surface brain cortex areas.)
LED, light-emitting diode; dACC, dorsal, anterior cingulate cortex; DMN, default mode network; vmPFC, ventral medial prefrontal cortex; SN, salience network; MFG, middle frontal gyrus; SMA, supplementary motor area; DLPFC, dorsolateral prefrontal cortex; CEN, central executive network; IFG, inferior frontal gyrus; AI, anterior insula; IPC, inferior parietal cortex (PPC, posterior parietal cortex), includes angular gyrus.
At each visit, six, 5.35 cm diameter LED cluster heads were applied simultaneously for 10 min during Set A, and then immediately after Set A, the LED placement loci were changed, and Set B was treated for 10 min (energy density, 13 Joules/cm2 [J/cm2] per each LED cluster head placement). It was estimated that up to 3% (0.4 J/cm2) could reach the surface brain cortex, although this is unknown in humans (M. Hamblin, personal observation).28,55 Patients were treated in a recliner chair, and the total LED treatment time per visit was 20 min. The LED cluster heads were held in place with a soft nylon cap. Because of the elastic tension from the cap, the location of the LED placements did not shift during each 10 min treatment (Fig. 1). LED therapy is noninvasive, painless and non-thermal.36 Each participant received 18 treatments (Monday, Wednesday, and Friday, minimum of 48 h between treatments), for 6 weeks.
Statistical analysis
The effect of LED treatments over time was examined for the following NP tests: Stroop Test for Executive Function (D-KEF); CVLT-II, Alternating Versions; D-KEF Trails Test; COWAT/FAS Test; and Digit Span Forwards and Backwards. The psychological measures including BDI, PCL-C, and VAS for pain were also examined. These data were analyzed in a series of univariate one way, repeated measures analyses of variance (ANOVA) with trend analysis to examine changes following treatment and the pattern of change over time: pre-LED, and at 1 week, 1 month, and 2 months after the 18th LED treatment (SPSS, v.20). In the few instances where there was a missing data point, the group mean for that time point was used to estimate that score. To reduce the number of dependent variables, the collinearity of those measures was computed (see Supplementary Table 1 for all bivariate correlations) (see online supplementary material at http://www.liebertonline.com). When r ≥0.8, only one of that pair of variables was analyzed. In order to correct for the number of comparisons, a conservative p value (p<0.025) was adopted. As this was a pilot study, several NP tests were used in order to identify a subset of outcome measures for a future, more statistically robust controlled study.
Results
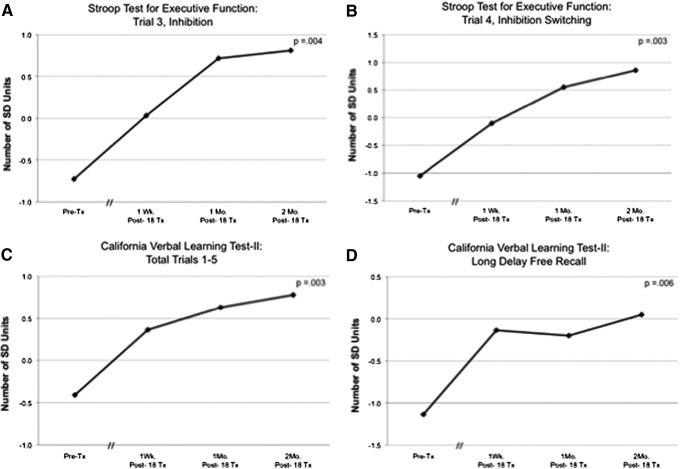
There were no significant correlations between age or years of education, and the pre-LED scores on the NP tests or psychological measures. Results showed a significant linear trend for the effect of LED treatment over time for the following NP tests: inhibition (Stroop test for executive function, Trial 3; F=14.228, df=1, p=0.004); and inhibition switching (Stroop Trial 4; F=16.091, df=1, p=0.003); verbal learning and memory (CVLT-II, Total Trials 1–5; F=14.470, df=1, p=0.003); and CVLT-II, Long Delay Free Recall (F=11.873, df=1, p=0.006) (see Figure 2A–D). Pre- and post-treatment NP data for each participant are provided in Supplementary Tables 2–5, and Supplementary Figures 2 and 3 (see online supplementary material at http://www.liebertonline.com).
FIG. 2.
Graphs showing a significant linear trend over time for the effect of LED treatments on specific neuropsychological tests. (A) Stroop Test for Executive Function, Trial 3 inhibition (p=0.004); (B) Stroop, Trial 4 inhibition switching (p=0.003); (C) California Verbal Learning Test (CVLT)-II, Total Trials 1–5 (p=0.003); and (D) CVLT-II, Long Delay Free Recall (p=0.006).
No other NP measures showed a significant effect of treatment over time (Supplementary Figure 4A,B) (see online supplementary material at http://www.liebertonline.com).
A trend towards significance was observed for the psychological measure of depression (BDI) at 1-week post-treatment (F=5.412, df=1, p=0.045) (Supplementary Fig. 4C and Supplementary Table 6)(see online supplementary material at http://www.liebertonline.com).
Using PTSD criteria established by Monson,56,57 only four participants initially reported symptoms suggestive of PTSD (scores ≥36/85; for specialized medical clinics, such as TBI or pain). All four cases showed a clinically meaningful decrease or a reliable decrease post-LED (Supplementary Table 7)(see online supplementary material at http://www.liebertonline.com).
No significant decrease or increase in VAS Pain Scale scores across time were observed for the five participants who reported pain at pretreatment, when scores ranged from 2 to 4.5 on a 0–10 scale (F=1.398, df=1, p=0.303). Reported changes in psychosocial adjustments post-LED from the participants and families are provided in Table 3. There were no adverse events or negative side effects.
Table 3.
Psychosocial Changes After Light-Emitting Diode (LED), Reported by Participants and Families
| ID number | Psychosocial changes post-LED |
|---|---|
| P1 | Able to sort bills, write checks and read essays, tasks he had been unable to perform for 5 years, since the MVA. |
| P2 | Able to continue work 22 hours/week, and later, full-time. Headache pain was reduced; no longer required medication for headache pain. |
| P3 | Non-talkative at entry, but became quite verbal and talkative after LED Tx. Husband reported that she was “better adjusted” at home. Beck Depression Index (BDI) remained at moderate level. |
| P4 | Clinically meaningful decrease in post-traumatic stress disorder (PTSD). |
| P5 | Clinically meaningful decrease in PTSD. Wife reported that he was more active around the home and was able to perform errands. Went on a job interview. |
| P6 | Remained disabled. |
| P7a | Remained disabled. |
| P8a | Post- LED treatment series, able to return to the military for further evaluation. |
| P9a | Remained disabled. |
| P10 | Clinically meaningful decrease in PTSD. Pre- LED treatment, the patient reported recurrent nightmares of the mTBI event. After a few weeks of LED treatments, he reported that the nightmares had stopped. |
| P11a | Prior to the post-testing at 1 week, she was promoted to a new position, causing distress. PTSD and BDI were minimal at pre-Tx., and at 2 months post-LED. She reported better sleep. |
History of multiple concussions.
Case analyses
For each participant, Supplementary Table 8(see online supplementary material at http://www.liebertonline.com) shows the pre-LED scores and the amount of change at 2 months post-LED, for three NP tests and the psychological measures of depression and PTSD.
NP tests
The level of severity at entry (pre-LED) for each participant was examined in relationship to the amount of change present at 2 months post-LED treatment. For Stroop, Trial 4 inhibition switching, five participants entered with pre-LED scores of −1 to −3 SD below their age- and education-adjusted norms, and all five improved by +1 to +4 SD at 2 months post-LED (Supplementary Table 8). Four of the nine participants entered with pre-LED scores of 0 or +0.5 SD (average scores for their age and education), and two of these participants improved by +1.5 and +2 SD; two participants showed no change. Therefore, five out of five participants who entered the study with more severe deficits on Stroop, Trial 4 inhibition switching (−1 to −3 SD) improved by at least 1 SD. Only half of the participants (two out of four participants) who entered with average scores (0–0.5 SD) improved by at least 1 SD.
For CVLT-II, Total Trials 1–5, 3 of the 10 participants entered with pre-LED scores of −1 to −1.5 SD below their norms, and all three improved by +1 to +2 SD at 2 months post-LED (Supplementary Table 8). Six of the 10 participants entered with pre-LED scores of 0 or −0.5 SD (average scores), and three improved by +1.5 to +3 SD; three participants showed no change. One participant entered at the level of +1 SD, and remained at that level, post-LED (P1). Therefore, three out of three participants who entered the study with more severe deficits on CVLT-II, Total Trials 1–5 (−1 to −1.5 SD) improved by at least 1 SD. Half of the participants (three out of six) who entered with average scores (0 to −0.5 SD), improved by at least 1 SD.
For CVLT-II, Long Delay Free Recall, seven of the participants entered with pre-LED scores of −1 to −3.5 SD below their norms, and five of these improved by +1 to +3.5 SD at 2 months post-LED; two participants showed no change (Supplementary Table 8). Two of the 10 participants entered with pre-LED scores of 0 SD (average scores), and both of these participants improved by +1 to +1.5 SD. One participant entered at the level of +1.5 SD, and remained at that level, post- LED (P1). Therefore, five out of seven participants who entered the study with more severe deficits on CVLT-II, Long Delay Free Recall (-1 to −3.5 SD), improved by at least +1 SD. Also, both participants who entered with average scores (0 SD), improved by +1 or +1.5 SD. On each of the abovementioned measures, not a single participant worsened.
Multiple concussions
There were four participants who had a history of multiple concussions. At 2 months post-LED, two participants (P8, P11) improved on Stroop, Trial 4 inhibition switching, by +1 and +4 SD. Three participants (P7, P9, P11) improved on CVLT, Total Trials 1–5 by +1.5 or +3 SD. All four improved on CVLT, Long Delay Free Recall by+1 to+2.5 SD. None of these participants with multiple concussions reported moderate or severe depression, or presence of PTSD.
Psychological measures
Depression
On the BDI, 5 of the 10 participants entered the study with moderate or severe depression scores (Supplementary Tables 6 and 8). Of these five participants, three had a reduced level of depression at 2 months post-LED (P2, P6, P10), either from a severe level to moderate; or from moderate level to minimal or mild. At the 1 week post-LED testing, four participants had reported a reduced level of depression (P2, P3, P6, P10). Three of them continued to report a reduced level at 1 month and 2 months post-LED (P2, P6, P10). For P3, however, the initial reduction in depression at 1 week and 1 month from severe to moderate, reverted back to severe at 2 months post-LED. In summary, two out of five participants remained with severe or moderate depression at 2 months post-LED.
PTSD
Four participants entered the study with PCL-C scores suggestive of PTSD (scores ≥36/85; for specialized medical clinics, such as TBI or pain).56,57 Using criteria established by Monson,56,57 a clinically meaningful decrease in PTSD severity is defined as a change of 10–20 points, and a reliable decrease is defined as a change of 5–10 points. All four participants who initially reported symptoms suggestive of PTSD showed a clinically meaningful decrease or a reliable decrease post-LED, however, data were available for only three of these participants at 2 months post-LED (Supplementary Tables 7 and 8). These three participants first showed reduction in PCL-C scores at 1 week (P5, P10), or at 1 month post-LED testing (P3) and those improvements were still present at 2 months post-LED. It is of note that P4 only had post-LED testing at 1 week; however, at that time she, too, reported a clinically meaningful decrease in PCL-C scores. In summary, all four participants who entered with scores suggestive of PTSD reported a reduced level of PTSD post-LED.
PTSD plus depression
Three participants entered with PTSD plus moderate or severe depression (P3, P5, P10). All three of these participants had a reduction in PCL-C scores, as first reported at the 1 week or 1 month post-LED testing; which was retained at 2 months post-LED. However, only one of these three participants (P10) also reported a reduced level of depression at 1 week, and 1 and 2 months post-LED. Therefore, when PTSD and depression co-occurred, the improvements post-LED were not parallel. Better results were obtained for reducing PTSD, than for decreasing depression at 2 months post-LED in this small sample (Supplementary Tables 6 and 7).
Discussion
This small pilot, open-protocol study using transcranial red/NIR LED therapy noted significant improvements in executive function (Stroop, Trial 3 inhibition; and Stroop, Trial 4 inhibition switching) and in verbal learning and memory (CVLT Total Trials 1–5; and Long Delay Free Recall), in chronic mTBI patients. These participants had experienced persistent cognitive dysfunction, ranging from 10 months to 8 years. As is common with mTBI, heterogeneity was present among the 11 participants,27 including 4 with a history of multiple concussions. These findings are discussed separately, and possible mechanisms associated with beneficial effects post-LED are presented.
Executive function
In the area of executive function (Stroop, Trial 4 inhibition switching) there was variability in the entry levels across our mTBI participants. For example, in five out of nine participants (56%), the pre-LED levels were at least −1 SD below average; whereas four out of nine entered with average scores (age- and education-adjusted norms). All five participants who entered with below-average scores on the Stroop, Trial 4 inhibition switching, improved by+1 to+4.5 SD at 2 months post-LED. Variability in performance on Stroop inhibition switching was also recently observed among a large number of TBI cases who were studied with resting state functional connectivity MRI (rs-fMRI), task-oriented functional MRI (fMRI) and diffusion tensor imaging (DTI).53 In that study, 20/46, (43%) performed poorly on the stop signal reaction time (SSRT) task, with slower response inhibition (higher SSRT). These cases with slower reaction times in the NoGo condition were observed to have failure deactivating the DMN, particularly the precuneus/posterior cingulate cortex (precu/PCC) portion. Failure to properly modulate the DMN during cognitive tasks that require rapid shifting of attention and inhibition has also been observed in other studies with TBI cases.58–61
For the five mTBI participants in the present study who entered with below-average Stroop inhibition switching scores, but who also improved by at least+1 to+4.5 SD post-LED, it is possible that nodes within the SN and/or the DMN were impacted post- LED, thus improving function and/or connections among these nodes. It is also possible that the red/NIR photons affected the DLPFC as well as the anterior cingulate cortex (ACC), both of which have been shown to be active during functional imaging studies of the Stroop effect.62 Further mechanistic rs-fMRI and task-oriented fMRI studies would be warranted to explore these potential relationships.
Verbal memory
The CVLT is a verbal working memory task in which increased activation on task-related fMRI is associated with DLPFC, and/or frontoparietal areas.63,64 In the present study, all three participants who entered with scores at least −1 SD below average on the CVLT Total Trials 1–5 improved by+1 to+2 SD at 2 months post-LED. Also, a total of five out of seven participants who entered with scores at least −1 SD below average on the CVLT Long Delay Free Recall, improved by+1 to+3.5 SD at 2 months post-LED. Although no rs-fMRI, or task-specific fMRI studies were part of this pilot study, specific LED placements may have had a beneficial focal effect on specific nodes within the CEN.
Summary, NP cognitive tests
Each participant who entered this study (regardless of severity level at entry) improved by at least+1 SD on either the Stroop and/or the CVLT post- LED therapy. For example, on the Stroop (Trial 3 or 4), 9/11 cases improved by at least+1 SD, at the maximum post-LED testing time available (Supplementary Table 8). Also, on the CVLT, 7/11 cases improved by at least+1 SD (Supplementary Table 8). Both of the two participants who did not improve by at least+1 SD on the Stroop post-LED (P7, P9) did improve on the CVLT by at least+1 SD. Therefore, all patients improved by at least+1 SD on either the Stroop and/or the CVLT, post-LED; and 9/11 cases improved by at least+1 SD on both the Stroop (executive function) and the CVLT (verbal memory).
Depression
There was only a trend for significant change in depression at the 1 week post-LED testing (p=0.045), and not an overall linear trend effect at 2 months post-LED. Only five participants had entered the study with moderate or severe depression. The pattern of initial reduction in depression at 1 week post-LED in 4/5 of these participants (but not an overall lasting change at 1 or 2 months post-LED), is similar to results observed in the Schiffer et al. study,65 with 10 severe depression cases, in whom depression was significantly reduced at 2 weeks after a single NIR LED treatment to the left and right forehead areas; however, scores returned toward baseline at 4 weeks post- LED. In both the Schiffer study,65 and our study, however, the post-LED depression scores did not return to the pre-LED levels.
Potential mechanisms
Our study suggests a potential cognitive benefit (and reduction in PTSD symptoms) post-transcranial red/NIR LED therapy in chronic mTBI. Specific underlying physiological changes that occur post-LED therapy in this patient population are largely unknown. Data from animal and cellular studies, however, would suggest increase in ATP,29,33,66,67 diffusion of nitric oxide promoting vasodilation38 and rCBF in cortical areas,31 an increase in antioxidants,68 and decreased inflammation39,69,70 as possible supporting mechanisms for improved function in the chronic stage.
Limitations of the present study
The results of this work should be interpreted with caution. This was a small-sample, open-protocol pilot study with 11 chronic mTBI participants; no controls were studied. Although there was heterogeneity for etiology across the 11 participants, and 4 had a history of multiple concussions, all met the inclusion criteria for persistent cognitive deficits (at least 6 months post-injury), as tested at entry. It is possible that these deficits present at entry could have spontaneously improved without intervention because of the passage of time. A recent study, however, with >140 TBI patients, has reported that the overall problems present at 2 years post-injury (cognitive, communication, behavioral, and emotional problems that were present in 60% of the cases) persisted even at 10 years post-injury.71 Therefore, in a chronic mTBI group such as ours, where 6/11 were >2 years post-TBI, significant improvements in cognition would not be expected. (Each of our 11 participants improved by at least+1 SD, on the Stroop and/or the CVLT, post-LED.) The potential impact of a placebo effect in this chronic mTBI population, however, should not be underestimated. The potential for placebo to impact anxiety and symptoms of well-being is clearly present, and could have impacted the post-LED test results. The unusual cognitive improvement in this chronic mTBI sample, however, suggests that further exploration of the possible efficacy of transcranial red/NIR LED therapy for TBI in a larger, controlled study would be warranted.
Conclusion
A small number of chronic mTBI cases (n=11), with nonpenetrating brain injury from diverse etiologies (motor vehicle accident [MVA], sports-related accident, work or home accident, and blast TBI) all improved by at least+1 SD on the Stroop test for executive function, and/or verbal learning and memory on the CVLT, post-LED therapy. Group statistical analyses with linear trend analysis showed significant improvements over time (out to 2 months post-LED) on the Stroop test for executive function – inhibition (p<0.004); inhibition switching (p<0.003); and verbal learning and memory – CVLT-II, Total Trials 1–5 (p<0.003) and Long Delay Free Recall (p<0.006). In addition, patients who had symptoms compatible with PTSD at entry into the study reported either a clinically meaningful decrease, or a reliable decrease in symptoms post-LED therapy. These results should be interpreted with caution, however, because this was a small, open-protocol study with potential for a placebo effect. Future studies with a larger number of patients, including a control arm, are needed to determine the true effect of LED therapy.
Tests recommended as primary outcome measures in future studies include the Stroop test for executive function, and the CVLT-II (Alternating Versions) for verbal memory and learning; the COWAT/FAS test should also be considered. Future studies should segregate participants with separate mechanisms of traumatic injury, and consider including groups with and without concurrent PTSD.
The optimum transcranial LED placements, as well as optimum LED treatment parameters such as wavelength, power density, and J/cm2 delivered to the scalp should be studied. A series of fMRI studies before and after the LED treatments would help to refine the LED placements, and examine whether changes had been made post-LED therapy in the functional connectivity networks often negatively impacted with TBI, including SN, DMN, and CEN. Additional task-oriented fMRI and DTI studies would also provide invaluable information regarding the possible effects of transcranial red/NIR LED in the treatment of TBI.
Supplementary Material
Acknowledgments
M. Naeser was supported by the Clinical Sciences Research and Development, Department of Veterans Affairs. M.R. Hamblin was supported by United States National Institutes of Health (NIH) grant R01AI050875. W.P. Meehan was supported by an American Medical Society for Sports Medicine (AMSSM) Young Investigator Award and an American College of Sports Medicine-American Medical Society for Sports Medicine Foundation Award. The authors thank Laura Burns for assistance with participant enrollment, Iris Monge for the LED treatments, Anita Saltmarche for assistance with acquisition of the LED units and LED methodology, and Michael D. Ho for assistance with manuscript preparation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M. and Coronado V.G. (2013). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Center for Disease Control and Prevention: Atlanta [Google Scholar]
- 2.Zaloshnja E., Miller T., Langlois J.A., and Selassie A.W. (2008). Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 23, 394–400 [DOI] [PubMed] [Google Scholar]
- 3.Maas A.I., and Menon D.K. (2012). Traumatic brain injury: rethinking ideas and approaches. Lancet Neurol. 11, 12–13 [DOI] [PubMed] [Google Scholar]
- 4.Carroll L.J., Cassidy J.D., Peloso P.M., Borg J., von Holst H., Holm L., Paniak C., and Pepin M. (2004). Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 84–105 [DOI] [PubMed] [Google Scholar]
- 5.Rimel R.W., Giordani B., Barth J.T., Boll T.J., and Jane J.A. (1981). Disability caused by minor head injury. Neurosurgery 9, 221–228 [PubMed] [Google Scholar]
- 6.Frenchmen K.A., Fox A.M., and Mayberry M.T. (2005). Neuropsychological studies of mild traumatic brain injury: a meta-analytic review of research since 1995. J. Clin. Exp. Neuropsychol. 27, 334–351 [DOI] [PubMed] [Google Scholar]
- 7.Binder L.M. (1997). A review of mild head trauma. Part II: Clinical implications. J. Clin. Exp. Neuropsychol. 19, 432–457 [DOI] [PubMed] [Google Scholar]
- 8.Binder L.M., Rohling M.L., and Larrabee G.J. (1997). A review of mild head trauma. Part I: Meta-analytic review of neuropsychological studies. J. Clin. Exp. Neuropsychol. 19, 421–431 [DOI] [PubMed] [Google Scholar]
- 9.McMahon P., Hricik A., Yue J.K., Puccio A.M., Inoue T., Lingsma H.F., Beers S.R., Gordon W.A., Valadka A.B., Manley G.T., Okonkwo , The Track-Tbi Investigators Including, D.O. , Casey S.S., Cooper S.R., Dams–O'Connor K., Menon D.K., Sorani M.D., Yuh E.L., Mukherjee P., Schnyer D.M., and Vassar M.J. (2014). Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI Study. J. Neurotrauma 31, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C., Onate J.A., Yang J., and Kelly J.P. (2003). Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290, 2556–2563 [DOI] [PubMed] [Google Scholar]
- 11.Lincoln A.E., Caswell S.V., Almquist J.L., Dunn R.E., Norris J.B., and Hinton R.Y. (2011). Trends in concussion incidence in high school sports: a prospective 11-year study. Am. J. Sports Med. 39, 958–963 [DOI] [PubMed] [Google Scholar]
- 12.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 13.Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K., Scally K., Bretthauer R., and Warden D. (2009). Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23 [DOI] [PubMed] [Google Scholar]
- 14.Morissette S.B., Woodward M., Kimbrel N.A., Meyer E.C., Kruse M.I., Dolan S., and Gulliver S.B. (2011). Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabil. Psychol. 56, 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogdanova Y., and Verfaellie M. (2012). Cognitive sequelae of blast-induced traumatic brain injury: recovery and rehabilitation. Neuropsychol. Rev. 22, 4–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lew H.L., Vanderploeg R.D., Moore D.F., Schwab K., Friedman L., Yesavage J., Keane T.M., Warden D.L., and Sigford B.J. (2008). Overlap of mild TBI and mental health conditions in returning OIF/OEF service members and veterans. J. Rehabil. Res. Dev. 45, xi–xvi [PubMed] [Google Scholar]
- 17.Tanielian T., and Jaycox L.H. (eds) 2008. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation: Santa Monica, CA [Google Scholar]
- 18.Vasterling J.J., Verfaellie M., and Sullivan K.D. (2009). Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin. Psychol. Rev. 29, 674–684 [DOI] [PubMed] [Google Scholar]
- 19.Levin H.S. (1990). Memory deficit after closed head injury. J. Clin. Exp. Neuropsychol. 12, 129–153 [DOI] [PubMed] [Google Scholar]
- 20.Levin H.S., Li X., McCauley S.R., Hanten G., Wilde E.A., and Swank P. (2013). Neuropsychological outcome of mTBI: a principal component analysis approach. J. Neurotrauma 30, 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuss D.T., Ely P., Hugenholtz H., Richard M.T., LaRochelle S., Poirier C.A., and Bell I. (1985). Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery 17, 41–47 [DOI] [PubMed] [Google Scholar]
- 22.Fork M., Bartels C., Ebert A.D., Grubich C., Synowitz H., and Wallesch C.W. (2005). Neuropsychological sequelae of diffuse traumatic brain injury. Brain Inj. 19, 101–108 [DOI] [PubMed] [Google Scholar]
- 23.Himanen L., Portin R., Isoniemi H., Helenius H., Kurki T., and Tenovuo O. (2006). Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology 66, 187–192 [DOI] [PubMed] [Google Scholar]
- 24.McAllister T.W., Flashman L.A., McDonald B.C., and Saykin A.J. (2006). Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J. Neurotrauma 23, 1450–1467 [DOI] [PubMed] [Google Scholar]
- 25.Hartikainen K.M., Waljas M., Isoviita T., Dastidar P., Liimatainen S., Solbakk A.K., Ogawa K.H., Soimakallio S., Ylinen A., and Ohman J. (2010). Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J Clin Exp Neuropsychol 32, 767–774 [DOI] [PubMed] [Google Scholar]
- 26.Chew E., and Zafonte R.D. (2009). Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J. Rehabil. Res. Dev. 46, 851–879 [DOI] [PubMed] [Google Scholar]
- 27.Zafonte R., Friedewald W.T., Lee S.M., Levin B., Diaz–Arrastia R., Ansel B., Eisenberg H., Timmons S.D., Temkin N., Novack T., Ricker J., Merchant R., and Jallo J. (2009). The citicoline brain injury treatment (COBRIT) trial: design and methods. J. Neurotrauma 26, 2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan S., Parrish J.A., Anderson R.R., and Madden M. (1981). Transmittance of nonionizing radiation in human tissues. Photochem. Photobiol. 34, 679–681 [DOI] [PubMed] [Google Scholar]
- 29.Karu T.I., Pyatibrat L.V., and Afanasyeva N.I. (2005). Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 36, 307–314 [DOI] [PubMed] [Google Scholar]
- 30.Lane N. (2006). Cell biology: power games. Nature 443, 901–903 [DOI] [PubMed] [Google Scholar]
- 31.Nawashiro H., Wada K., Nakai K., and Sato S. (2012). Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed. Laser. Surg. 30, 231–233 [DOI] [PubMed] [Google Scholar]
- 32.Eells J.T., Henry M.M., Summerfelt P., Wong–Riley M.T., Buchmann E.V., Kane M., Whelan N.T., and Whelan H.T. (2003). Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. U. S. A. 100, 3439–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong–Riley M.T., Liang H.L., Eells J.T., Chance B., Henry M.M., Buchmann E., Kane M., and Whelan H.T. (2005). Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J. Biol. Chem. 280, 4761–4771 [DOI] [PubMed] [Google Scholar]
- 34.Verweij B.H., Muizelaar J.P., Vinas F.C., Peterson P.L., Xiong Y., and Lee C.P. (2000). Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 93, 815–820 [DOI] [PubMed] [Google Scholar]
- 35.Lifshitz J., Sullivan P.G., Hovda D.A., Wieloch T., and McIntosh T.K. (2004). Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4, 705–713 [DOI] [PubMed] [Google Scholar]
- 36.Uozumi Y., Nawashiro H., Sato S., Kawauchi S., Shima K., and Kikuchi M. (2010). Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 42, 566–576 [DOI] [PubMed] [Google Scholar]
- 37.Oron A., Oron U., Streeter J., de Taboada L., Alexandrovich A., Trembovler V., and Shohami E. (2007). low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma 24, 651–656 [DOI] [PubMed] [Google Scholar]
- 38.Khuman J., Zhang J., Park J., Carroll J.D., Donahue C., and Whalen M.J. (2012). Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J. Neurotrauma 29, 408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Q., Xuan W., Ando T., Xu T., Huang L., Huang Y.Y., Dai T., Dhital S., Sharma S.K., Whalen M.J., and Hamblin M.R. (2012). Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg. Med. 44, 218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xuan W., Vatansever F., Huang L., Wu Q., Xuan Y., Dai T., Ando T., Xu T., Huang Y.Y., and Hamblin M.R. (2013). Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One 8, e53454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naeser M.A., Saltmarche A., Krengel M.H., Hamblin M.R., and Knight J.A. (2011). Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed. Laser Surg. 29, 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitaker H.J., Farrington C.P., Spiessens B., and Musonda P. (2006). Tutorial in biostatistics: the self-controlled case series method. Stat. Med. 25, 1768–1797 [DOI] [PubMed] [Google Scholar]
- 43.Reynolds C.R. (2002). Comprehensive Trail-Making Test (CTMT). Psychological Assessment Resources, Austin, TX [Google Scholar]
- 44.Spreen O., and Benton A. (1977). Neurosensory Center Comprehensive Examination for Aphasia (NCCEA). University of Victoria Neuropsychology Laboratory: Victoria, Australia [Google Scholar]
- 45.Benton A., and Hamsher K. (1989). Multilingual Aphasia Examination. AJA Associates: Iowa City, IA [Google Scholar]
- 46.Delis D.C., Kramer J.H., Kaplan E., and Ober B.A. (2000). California Verbal Learning Test-2nd ed. Adult Version. Manual. The Psychological Corporation: San Antonio [Google Scholar]
- 47.Delis D.C., Kaplan E., and Kramer J.H. (2001). Delis–Kaplan Executive Function System (D-KEFS): Examiner's Manual. The Psychological Corporation: San Antonio [Google Scholar]
- 48.Wechsler D. (2008). WAIS-IV-Wechsler Adult Intelligence Scale-4th ed. Pearson Education, Inc.: San Antonio [Google Scholar]
- 49.MacLeod C.M. (1991). Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 109, 163–203 [DOI] [PubMed] [Google Scholar]
- 50.Blanchard E.B., Jones–Alexander J., Buckley T.C., and Forneris C.A. (1996). Psychometric properties of the PTSD Checklist (PCL). Behav. Res. Ther. 34, 669–673 [DOI] [PubMed] [Google Scholar]
- 51.Beck A.T., and Steer R.A. (1993). Manual for Beck Depression Inventory. The Psychological Corporation: San Antonio [Google Scholar]
- 52.Pain Assessment Visual Analog Scale. (2008). FibroAction. Available at: http://www.fibroaction.org/Articles/Scales-and-Assessments-for-Measuring-and-Recording-Pain.aspx (last accessed April16, 2014)
- 53.Bonnelle V., Ham T.E., Leech R., Kinnunen K.M., Mehta M.A., Greenwood R.J., and Sharp D.J. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. U. S. A. 109, 4690–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 [DOI] [PubMed] [Google Scholar]
- 55.Detaboada L., Ilic S., Leichliter–Martha S., Oron U., Oron A., and Streeter J. (2006). Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg. Med. 38, 70–73 [DOI] [PubMed] [Google Scholar]
- 56.Monson C.M., Gradus J.L., Young–Xu Y., Schnurr P.P., Price J.L., and Schumm J.A. (2008). Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol. Assess. 20, 131–138 [DOI] [PubMed] [Google Scholar]
- 57.Weathers F.W. (1993). Using the PTSD Checklist (PCL). National Center for PTSD, Posttraumatic Stress Disorder, PTSD Checklist and Handout. Available at: http://www.ptsd.va.gov/professional/pages/assessments/ptsd-checklist.asp (last accessed April16, 2014)
- 58.Bonnelle V., Leech R., Kinnunen K.M., Ham T.E., Beckmann C.F., De Boissezon X., Greenwood R.J., and Sharp D.J. (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 31, 13,442–13,451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer A.R., Mannell M.V., Ling J., Gasparovic C., and Yeo R.A. (2011). Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 32, 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayer A.R., Yang Z., Yeo R.A., Pena A., Ling J.M., Mannell M.V., Stippler M., and Mojtahed K. (2012). A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain Imaging Behav. 6, 343–354 [DOI] [PubMed] [Google Scholar]
- 61.Johnson B., Zhang K., Gay M., Horovitz S., Hallett M., Sebastianelli W., and Slobounov S. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 59, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carter C.S., and van Veen V. (2007). Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn. Affect. Behav. Neurosci. 7, 367–379 [DOI] [PubMed] [Google Scholar]
- 63.Smith E.E., and Jonides J. (1998). Neuroimaging analyses of human working memory. Proc. Natl. Acad. Sci. U. S. A. 95, 12,061–12,068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curtis C.E., and D'Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 7, 415–423 [DOI] [PubMed] [Google Scholar]
- 65.Schiffer F., Johnston A.L., Ravichandran C., Polcari A., Teicher M.H., Webb R.H., and Hamblin M.R. (2009). Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav. Brain Funct. 5,46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eells J.T., Wong–Riley M.T., VerHoeve J., Henry M., Buchman E.V., Kane M.P., Gould L.J., Das R., Jett M., Hodgson B.D., Margolis D., and Whelan H.T. (2004). Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 4, 559–567 [DOI] [PubMed] [Google Scholar]
- 67.Pastore D., Greco M., and Passarella S. (2000). Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 76, 863–870 [DOI] [PubMed] [Google Scholar]
- 68.Sompol P., Xu Y., Ittarat W., Daosukho C., and St Clair D. (2006). NF-kappaB-associated MnSOD induction protects against beta-amyloid-induced neuronal apoptosis. J. Mol. Neurosci. 29, 279–288 [PubMed] [Google Scholar]
- 69.Rojas J.C., and Gonzalez–Lima F. (2011). Low-level light therapy of the eye and brain. Eye Brain 3, 49–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aimbire F., Albertini R., Pacheco M.T., Castro–Faria–Neto H.C., Leonardo P.S., Iversen V.V., Lopes–Martins R.A., and Bjordal J.M. (2006). Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed. Laser Surg. 24, 33–37 [DOI] [PubMed] [Google Scholar]
- 71.Ponsford J.L., Downing M.G., Olver J., Ponsford M., Acher R., Carty M., and Spitz G. (2014). Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J. Neurotrauma 31, 64–77 [DOI] [PubMed] [Google Scholar]
- 72.Siedentopf C.M., Golaszewski S.M., Mottaghy F.M., Ruff C.C., Felber S., and Schlager A. (2002). Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci. Lett. 327, 53–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.