Abstract
During physiological activities, osteoblasts experience a variety of mechanical forces that stimulate anabolic responses at the cellular level necessary for the formation of new bone. Previous studies have primarily investigated the osteoblastic response to individual forms of mechanical stimuli. However in this study, we evaluated the response of osteoblasts to two simultaneous, but independently controlled stimuli; fluid flow-induced shear stress (FSS) and static or cyclic hydrostatic pressure (SHP or CHP, respectively). MC3T3-E1 osteoblasts-like cells were subjected to 12dyn/cm2 FSS along with SHP or CHP of varying magnitudes to determine if pressure enhances the anabolic response of osteoblasts during FSS. For both SHP and CHP, the magnitude of hydraulic pressure that induced the greatest release of ATP during FSS was 15 mmHg. Increasing the hydraulic pressure to 50 mmHg or 100 mmHg during FSS attenuated the ATP release compared to 15 mmHg during FSS. Decreasing the magnitude of pressure during FSS to atmospheric pressure reduced ATP release to that of basal ATP release from static cells and inhibited actin reorganization into stress fibers that normally occurred during FSS with 15 mmHg of pressure. In contrast, translocation of nuclear factor kappa B (NFκB) to the nucleus was independent of the magnitude of hydraulic pressure and was found to be mediated through the activation of phospholipase-C (PLC), but not src kinase. In conclusion, hydraulic pressure during FSS was found to regulate purinergic signaling and actin cytoskeleton reorganization in the osteoblasts in a biphasic manner, while FSS alone appeared to stimulate NFκB translocation. Understanding the effects of hydraulic pressure on the anabolic responses of osteoblasts during FSS may provide much needed insights into the physiologic effects of coupled mechanical stimuli on osteogenesis.
Keywords: Mechanotransduction, Fluid flow, Hydraulic pressure, Actin cytoskeleton, ATP release, Nuclear factor-kappa B, Osteoblasts
INTRODUCTION
The mechanical environment experienced by osteoblasts consists of multiple types of mechanical stimuli, such as substrate strain, hydraulic pressure, and fluid flow induced shear stress (FSS).1 Each individual form of mechanical stimuli has the ability to induce anabolic responses in osteoblasts that are needed to maintain the mass and structural integrity of bone. In particular, physiologic levels of hydraulic pressure and FSS have been shown to induce anabolic responses in osteoblasts,2–4 while the response to strain requires much higher magnitudes in vitro than physiological strains recorded on bone in vivo.5 Although hydraulic pressure and FSS increase levels of anabolic markers, such as cyclooxygenase-2 (COX-2) production, prostaglandin-E2 (PGE2) release, and osteopontin (OPN) expression,3, 4, 6 the manner in which each form of stimuli initiates mechanotransduction and alter cellular biomechanics.2, 4 As a result, it is difficult to predict how these mechanisms will interact when osteoblasts are subjected to two or more forms of mechanical stimuli.
In response to FSS, the initial influx of extracellular calcium through mechanosensitive cation-selective channels (MSCC) and L-type voltage sensitive calcium channels (L-VSCC) induce vesicular release of ATP and phosphorylation of mitogen activated protein kinases.7–10 Purinergic signaling through ATP release has emerged as an essential component of the mechanotransduction pathway in osteoblasts.8, 9, 11 Extracellular ATP can bind to either P2Y receptors, which are G-protein coupled receptors, or P2X receptors, which are ligand gated ion channels. Activation of P2Y receptors in particular regulates intracellular calcium release from the endoplasmic reticulum (ER) by activating phospholipase-C (PLC) to cleave D-myo-inositol 1,4,5-trisphosphate (IP3) from phosphatidylinositol 4,5 bisphosphonate (PIP2) in the membrane, leaving diacylglyceride (DAG).10 In response to FSS, the PLC/IP3 mediated calcium response contributes to the nuclear translocation of nuclear factor kappa B (NFκB).12, 13 Once in the nucleus, the cis-activating element of NFκB can bind to several gene promoters that can regulate inflammatory and immune responses, cell proliferation, and apoptosis.14 In particular, the production of COX-2 is dependent on PLC/IP3 mediated NFκB translocation to the nucleus.12, 13, 15 Both COX-2 production and NFκB translocation are indicative of the anabolic response among osteoblasts and contribute to the osteogenic process.
Although hydraulic pressure and fluid flow have been shown to invoke similar mechanisms involved in osteogenesis, such as ATP release,4 it is often assumed that the addition of hydraulic pressure during FSS only enhances the anabolic response of osteoblasts. When osteoblasts were subjected to FSS in a gravity-driven flow system, where a gauge hydraulic pressure of 15mmHg was present, the anabolic responses were attributed only to FSS and the contribution of hydraulic pressure was neglected.16 However, there are few reports supporting this assumption. Given the range in magnitudes of hydraulic pressure and FSS osteoblasts are reported to experience,17, 18 the interplay between hydraulic pressure and fluid flow is essential to understanding the anabolic response of osteoblasts during physiological conditions.
The anabolic response of osteoblasts to specific forms of mechanical stimuli can be affected by the organization of the cytoskeleton. In response to either FSS or hydraulic pressure, osteoblasts modify the structural organization of the cytoskeleton by increasing actin stress fiber formation (ASFF) that results in increased cell stiffness.4, 19 The increased polymerization of the actin cytoskeleton in response to mechanical stimuli in various cell types is thought to downregulate the mechanosensitivity as a means to cope with or survive potentially injurious forces.20, 21 In contrast, depolymerization of the actin cytoskeleton enhances the mechanosensitivity of osteoblasts by increasing MSCC and L-VSCC activity and the intracellular calcium response induced at the onset of FSS.22 Overall, the actin cytoskeleton contributes to changes in cell shape and cell stiffness to define the mechanical properties of the cell,19, 23 the function of ion channels,24, 25 and the transduction of external forces through focal adhesions.26 These changes in the cytoskeleton are largely dependent on calcium-induced purinergic signaling that activates intracellular calcium release through PLC/IP3.4, 12, 13 Although both FSS and hydraulic pressure can induce changes in ASFF and cell stiffness when applied separately, it remains unclear how changes in hydraulic pressure during the application of FSS will affect changes in the actin cytoskeleton.
The goal of this study was to determine the influence of hydraulic pressure on the anabolic responses of MC3T3-E1 osteoblasts-like cells during FSS along with the changes in cytoskeleton organization. We postulated that hydraulic pressure enhances the anabolic response of osteoblasts during FSS by increasing the release of ATP and the resulting translocation of NFκB to the nucleus. We also hypothesized that hydraulic pressure contributes to the increase in ASFF reorganization in MC3T3 osteoblasts during FSS. To test these hypotheses, we examined changes in these endpoints in response to FSS while independently varying static and dynamic hydraulic pressures applied to MC3T3-E1 cells.
METHODS
Cell Culture
Mother cultures of MC3T3-E1 osteoblast-like cells (Clone 14, ATCC, Bethesda, MD) were grown in -MEM containing 10% FBS (Gibco, New York, NY), 100 U/ml penicillin, 100 μg/ml streptomycin and 26 mM NaHCO3, and housedin humidified incubators at 37° C with 5% CO2/95% air. For experimental studies, cells were seeded at 1×103 cells/cm2 on glass slides coated with rat-tail type I collagen (50 μg/ml, BD, Franklin Lakes, NJ). Once cells reached 75% confluence, they were serum starved for 24 hours prior to testing with serum free medium. All the compounds, if not otherwise stated, were purchased from Fisher Scientific (Pittsburgh, PA).
In-Vitro FSS with Hydraulic Pressure
MC3T3 cells seeded on glass slides were subjected to FSS with varied magnitudes of hydraulic pressure inside a parallel plate flow chamber. A constant uni-directional laminar flow through the flow chamber was used to generate 12 dynes/cm2 shear stress along the plasma membrane as described previously16. The fluid flow rate to the chamber was controlled by a gravity driven flow system, maintained at 37°C and filled with 25ml of serum free medium with a pH of 7.3 by aerating the system with 5% CO2/95% air prior to each experiment (Fig 1A). During each experiment, the flow system was sealed so that the gauge pressure (defined relative to atmospheric pressure) inside the vertical column and the parallel plate flow chamber could be adjusted. The pressure inside the vertical column was adjusted by compressing air using a custom-built syringe pump. The syringe was driven either to a set distance for changing the SHP, or to follow a sinusoidal waveform to generate CHP. By changing the pressure in the vertical column, the mean pressure in the flow chamber was altered independently from the gravity-driven FSS (12 dynes/cm2). The gauge pressure inside the vertical column was monitored throughout the course of each experiment with a pressure gauge (Omega, Stamford, CT) mounted to the system (Fig 1B). Inhibitors used during mechanical stimulation were supplemented in the media and cells were pretreated for 5 minutes with the appropriate inhibitor prior to testing.
Figure 1. Schematic design of the gravity driven fluid flow system used to subject MC3T3 cells inside a parallel flow chamber to FSS and hydraulic pressure.
A] The pressure inside the column was adjusted so that cells were subject to hydraulic pressure with a magnitude of 0, 15, 50, or 100 mmHg. The magnitude of pressure is the gauge pressure, with 0 representing atmospheric pressure. B] The measured CHP profile applied during FSS reached peak magnitudes of 15, 50 or 100 mmHg (gauge pressure) and oscillated at a frequency of 0.5 Hz or 1 Hz.
For each experiment, cells plated on a glass slide were first loaded into the flow chamber with the inlet and outlet sealed so that the cells were at atmospheric pressure prior to mechanical stimulation. Next, the pressure in the vertical column was adjusted to the appropriate magnitude of SHP or CHP. To begin the experiment, the inlet and outlet valves on the flow chamber were then opened to expose the cells to an instantaneous increase in FSS and hydraulic pressure.
To verify that the hydraulic pressure in the fluid flow chamber reached the appropriate magnitude, a Mikro-Tip catheter pressure transducer (Millar Instruments, Houston, Texas) sealed in a t-connector provided by the manufacturer was connected at the inlet of the flow chamber (Fig. 1). The signal from the pressure transducer was ampli ed through a controller (PCU 2000, Millar Instruments) and recorded at 250 Hz using a NI-USB-6221 A/D board and Labview data acquisition software (National Instruments, Texas). The pressure transducer was calibrated according to the manufacture’s instruction.
In the first series of experiments, cells were subjected to 12 dynes/cm2 FSS with 0, 15, 50 or 100 mmHg of static hydraulic pressure (SHP) applied during FSS. The magnitudes of pressure chosen for this study were derived from in-vivo and in-vitro measurements and mathematical estimates.17, 18, 27–29 The magnitude of FSS (12 dynes/cm2) was selected based on its ability to generate a significant anabolic response in osteoblasts.30–32 Each magnitude of pressure was tested a minimum of three times from different cells passages.
A second series of experiments determined the effect of cyclic hydraulic pressure (CHP) with steady 12 dynes/cm2 FSS. During experiments with CHP, the gauge pressure inside the flow chamber oscillated between 0 and a peak magnitude of 15, 50, or 100 mmHg, based on a sinusoidal waveform with a frequency of either 0.5 or 1.0 Hz. Each magnitude of pressure was tested a minimum of three times for each frequency from different cell passages.
During each series of experiments, a control group denoted as static was also tested. Each control experiment consisted of placing cells inside the parallel plate chamber for the same duration of time, but was exposed to only atmospheric pressure with no FSS.
ATP Assay
The release of ATP by MC3T3 cells was measured in response to 5 minutes of FSS with various magnitudes of SHP and CHP. Following 5 minutes of mechanical stimuli, aliquots of the circulating media were taken and cell lysates were obtained by washing the cells with PBS, then incubating the cells on ice with 80 μl of lysis buffer (5 mM HEPES, 150 mM NaCl, 26% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride). Both lysate and media aliquots were stored at −80°C for further analysis. The protein concentration of each lysate sample was determined using a BCA assay (BCA Protein Assay Kit, Pierce) and a 96-well micro-injector plate reader (POLARstar OPTIMA, BMG LABTECH GmBH). Duplicate readings were taken for each lysate sample and averaged to determine the appropriate protein concentration. The ATP concentration of each media sample was measured using a bioluminescence assay kit (ATP Bioluminescence Assay kit HS II; Roche, Indianapolis, IN). Light emitted as a result of the reaction between D-luciferin and luciferase was detected using the 96 well micro-injector plate reader. The magnitude of ATP release for each sample was first averaged over two measurements, and then normalized by the protein concentration determined from the respective cell lysate. The normalized ATP release for each sample was then normalized to their respective control group to obtain the percent change in ATP release. The average percent change in ATP release for each magnitude of pressure and frequency was measured at least three times and reported along with the standard deviation between each experiment.
Immunohistochemistry
Using immunostaining, both F-actin and NFκB were visually assessed following mechanical stimuli and compared to controls. Previous studies have shown 60 minutes of FSS can increase actin polymerization into ASFF and induce nuclear translocation of NFκB.12, 13, 26 Thus, cells were exposed to 60 minutes of FSS with various magnitudes of SHP or CHP. A positive control for NFκB translocation was conducted by treating cells for 30 minutes with tumor necrosis factor alpha (TNF-α, 100 ng/ml, Sigma). Following treatment or mechanical stimuli, cells were fixed and permeabilized with 3% paraformaldehyde and 0.1% Triton X-100 in PBS for 30 min. Nonspecific binding was then reduced by incubating cells with 3% BSA (Gibco, New York, NY) and 10% goat serum (Gibco, New York, NY) for 45 minutes. NFκB was then labeled with a primary antibody specific to the p-65 subunit of NFκB (AB Cam, Cambridge, MA) for 3 hours at 37°C. Cells were then washed with blocking buffer and the incubated with secondary antibody Cy-3 goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA) and Alexa Fluor 488 phalloidin conjugate (Invitrogen) for 1 hour. Each slide was then mounted with polyvinyl alcohol mounting medium (Fluka-BioChemika) and imaged using confocal microscopy (LSM 510, Zeiss, Germany) with dual excitations (488 nm for F-actin, and 561 nm for NFκB). Cytoskeleton re-organization and NFκB translocation was evaluated for each experimental group described above. Each group was tested a minimum of 3 times from different cell passages, with 4–5 images taken for each test and a total of 12–15 images to evaluate the cytoskeleton organization and the location of NFκB in the cell.
Statistical Analysis
One-way ANOVA was used to detect differences in ATP release between experimental groups of different magnitudes of hydraulic pressure (0, 15, 50, 100 mmHg) or applied frequencies (0.5 or 1.0 Hz) and un-treated controls. Significant differences in cell stiffness between groups were also detected using a one-way ANOVA with a p-value of 0.05. Each experimental group along with their respective controls was conducted a minimum of three times. Statistical significance was defined with a p < 0.05 and a post hoc Tukey-Kramer test was used to detect significant difference among groups.
RESULTS
ATP Release
The gauge pressure measured inside the parallel chamber of a gravity driven flow system was on average 15 mmHg (~2 kPa), while generating a FSS of 12 dynes/cm2. The magnitude of FSS was selected based on previous work demonstrating that at least 10 dynes/cm2 is needed to elicit an anabolic response among osteoblasts.30–32 Under these conditions (FSS/SHP15), MC3T3 cells exhibited a ~7 fold increase in ATP release. Reducing the SHP to atmospheric pressure (FSS/SHP0 with a gauge pressure of zero) significantly attenuated the ATP release to a level equivalent to basal static release (Fig 2A). Increasing the SHP to 50 mmHg (FSS/SHP50) and 100 mmHg (FSS/SHP100) did not enhance ATP release during FSS, but rather caused a slight, but non-significant, decrease compared with the case of 15 mmHg.
Figure 2. Maximal ATP release during FSS was observed at 15 mmHg of SHP or CHP. Reducing the hydraulic pressure to atmospheric pressure (FSS/SHP0) attenuated the release of ATP to basal levels.
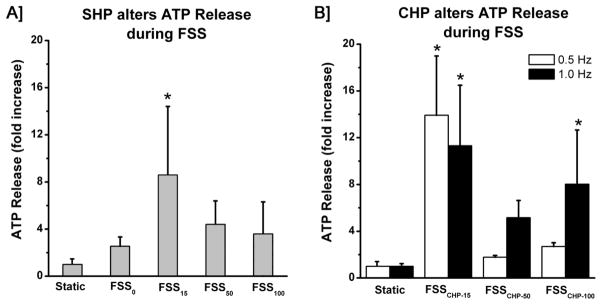
Increased magnitudes of 50 and 100 mmHg of SHP attenuated the release of ATP. Magnitudes of 50 and 100 mmHg of CHP attenuated the overall release of ATP. Increasing the frequency of applied CHP increased the release of ATP for peak magnitudes of 50 and 100 mmHg. Mean +/− std (* indicates p-value < 0.05 compared to static controls)
Since osteoblasts experience cyclic bouts of hydraulic pressure in physiological conditions, we applied CHP during FSS with peak magnitudes of 15, 50, or 100 mmHg at either 0.5 or 1.0 Hz (Fig. 1B). The maximum release of ATP from MC3T3 cells occurred during application of 15 mmHg CHP at both 0.5 and 1.0 Hz, while increasing the magnitude of pressure above this optimal pressure decreased the release of ATP relative to FSS/CHP15 (Fig 2B). Increasing the frequency of applied CHP caused an increase in ATP release for both FSS/CHP50 and FSS/CHP100.
Cytoskeleton Organization
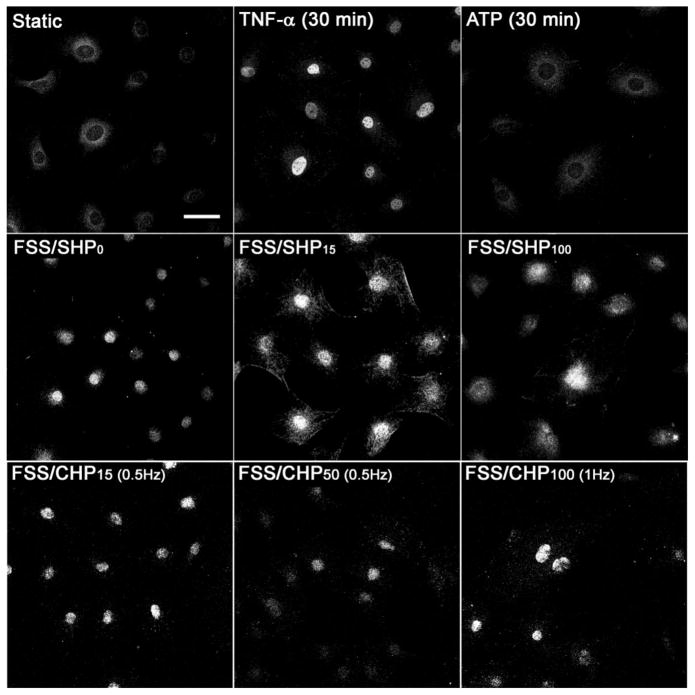
Previously, purinergic signaling has been shown to play a key role in ASFF and cytoskeleton reorganization.4 A similar response in ASFF was observed in response to FSS with various magnitudes of hydraulic pressure. The typical increase in actin polymerization into ASFF in MC3T3 cells in response to FSS/SHP15 was lost when SHP was reduced to atmospheric pressure during FSS/SHP0 (Fig 3). Increasing the magnitude of SHP during FSS to 100 mmHg (FSS/SHP100) caused a slight increase in ASFF parallel alignment compared to atmospheric pressure (FSS/SHP0), but not to the same degree as FSS/SHP15. The addition of CHP during FSS caused a similar response, with FSS/CHP15 at 0.5 Hz causing the largest degree of ASFF (Fig 3). However, increasing the magnitude of pressure to 50 mmHg at 0.5 Hz, the cytoskeleton became distinctly disorganized. This disorganization of the cytoskeleton was prevented by increasing the magnitude of pressure and rate of application to 100 mmHg and 1.0 Hz respectively during FSS. Osteoblasts treated with TNF-α (100 ng/ml), which is a positive control for NFκB translocation to the nucleus, did not exhibit an increase in actin polymerization.
Figure 3. Increased ASFF and reorganization in MC3T3 cells is dependent on the presence of hydraulic pressure during FSS.
The actin cytoskeleton of MC3T3 cells was imaged following 60 minutes of FSS with different magnitudes of SHP (0, 15, 100 mmHg gauge pressure) or CHP (15 mmHg at 0.5 Hz, 50 mmHg at 0.5 Hz, and 100 mmHg at 1 Hz). Cells were also treated with either 100 ng/ml of TNF-α or 200 μM of ATP in static conditions for 30 minutes. Each image is representative of 12–15 images taken for each group. Bar indicates 50 μmm.
NFκB Translocation
The anabolic response of osteoblasts to FSS has been shown to be dependent on NFκB activation and translocation to the nucleus.12, 33 As a positive control, MC3T3 cells were treated with TNF-α (100 ng/ml) that induced NFκB translocation to the nucleus within 30 minutes. Unlike ATP release, 60 minutes of FSS induced translocation of NFκB to the nucleus independent of the magnitude of hydraulic pressure when compared to static controls (Fig 4). In the same way, the magnitude and rate of applied CHP during FSS did not impact translocation of NFκB to the nucleus. These data suggest that NFκB translocation in response to mechanical loading is not exclusively regulated through purinergic signaling. This possibility is supported by our observation that treatment of MC3T3 cells with exogenous ATP (100 μM, Sigma) or Benzoylbenzoyl-ATP (BzATP, 100 μM, Sigma) did not induce NFκB translocation, suggesting that load-induced NFκB translocation is not mediated through direct activation of purinergic signaling (Fig 4 and 5).
Figure 4. Nuclear translocation of NFκB in MC3T3 cells is independent of the hydraulic pressure during FSS.
The presence and location of NFκB in MC3T3 cells was imaged following 60 of FSS with various magnitudes of SHP (0, 15, or 100 mmHg gauge pressure). Cells were also treated with either 100 ng/ml of TNF-α or 200 μM of ATP in static conditions. Each image is representative of 12–15 images taken for each group. Bar indicates 50 μm.
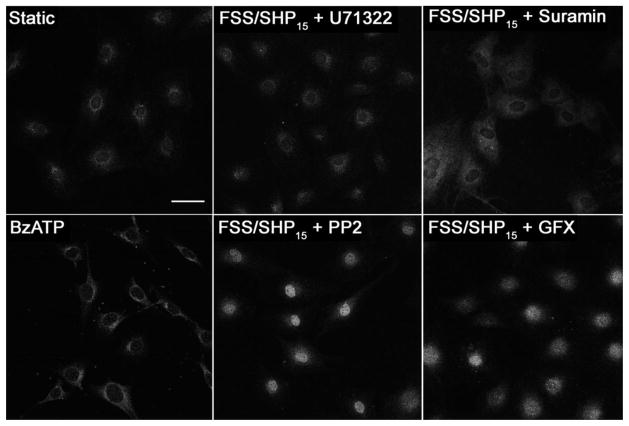
Figure 5. Nuclear translocation of NFκB in MC3T3 cells is mediated by PLC activation, but is independent of Src activation during FSS.
The presence and location of NFkB in MC3T3 cells was imaged following 60 minutes of FSS with a SHP of 15 mmHg (FSS/SHP15). Cells were treated for 30 minutes prior to mechanical stimuli with 5 μM of U71322 to inhibit PLC activation, or 10 μM of Suramin to inhibit G-protein activation, 10 μM of PP2 to inhibit Src activation, or 5 μM GF 109203X (GFX) to inhibit protein kinase C. Cells were also treated with BzATP (100 μM) alone to activate P2X receptors. Each image is representative of 12–15 images taken for each group. Bar indicates 50 μm.
Similar to our previous data, inhibition of PLC activation in osteoblasts with U73122 (5 μM, Sigma) completely inhibited NFκB translocation to the nucleus during FSS/SHP15 (Fig 5).12, 13 While addition of exogenous ATP did not activate NFκB, suramin, a G-protein inhibitor that has been used extensively in manipulation of purinergic signaling, was able to block the shear-induced translocation of NFκB (Fig 5). This led us to consider that intigrins may also be involved with NFκB translocation, given their ability to activate G-proteins needed to initiate a PLC/IP3 mediated intracellular calcium release through focal adhesion kinase (FAK).34 Other studies have gone on to demonstrate that FAK specifically activates PLC through Src-kinase.35, 36 We then hypothesized that inhibiting Src-kinase activation with PP2 (10 μM, Sigma) during FSS would inhibit NFκB translocation. However, inhibition of Src-kinase activation with PP2 did not inhibit NFκB translocation during either FSS/SHP15 or FSS/SHP0 as anticipated (Fig 5). Lastly, we tested if protein kinase C (PKC) played a role in NFκB translocation, given its activation in response to intracellular calcium mobilization associated with G-protein coupled purinergic receptors.10 Inhibition of PKC with GFX (5 μM of GF 109203X, Sigma) during FSS/SHP15 did not inhibit NFκB translocation.
DISCUSSION
Dynamic loading of bone results in various forms of mechanical stimuli that mediate osteoblastic responses that maintain bone mass density and its structural integrity. The mechanical forces experienced by osteoblasts includes substrate strain, hydraulic pressure and interstitial fluid flow induced shear stress (FSS).1 Although extensive insight on the anabolic response of bone cells has been established for individual types of mechanical stimuli,1, 37 little attention has been given to the combined effect that multiple forms of mechanical stimuli may have. Hydraulic pressure alone has been shown to induce an anabolic response similar to FSS, such as ATP release and COX-2 production, while causing distinct effects on cytoskeleton organization compared to FSS.4 In this study we varied the static or dynamic pressure experienced by osteoblasts, while being subjected to a constant FSS of 12 dynes/cm2. The results from this study provide the first insight of the biphasic effect hydraulic pressure has on mechanotransduction signaling in osteoblasts during FSS.
The immediate and latent responses of osteoblasts to a constant FSS of 12 dynes/cm2 was evaluated with respect to different magnitudes of applied static or cyclic hydraulic pressures of 0, 15, 50, or 100 mmHg. In agreement with previous observations that dynamic loading has a greater influence on mechanotransduction than constant loads,38 CHP caused a larger release of ATP compared to similar magnitudes of SHP. The higher loading frequency of 0.5 Hz caused a larger release of ATP compared to the smaller loading frequency of 0.25 Hz for each magnitude of pressure. Independent of FSS, magnitudes on the order of 100–500 mmHg of pressure are needed to elicit an anabolic response among osteoblasts, while static hydraulic pressure has not been found to induce a significant response.2, 4 Although higher magnitudes of pressure are needed to induce an anabolic response in osteoblasts independent of FSS, we have gone on to demonstrate under 12 dynes/cm2 of FSS, osteoblasts are more sensitive to magnitudes less than 100 mmHg of SHP and CHP. During normal ambulation, the intramedullary pressure of the mouse femur was measured at ~10 mmHg, while we have demonstrated under dynamic loading the intramedullary pressure can reach magnitudes between 10 and 20 mmHg.17, 27 Overall, the low magnitudes of pressure generated within bone serve to enhance the osteoblasts response to FSS, even though they may not be adequate to elicit a response independently.
Under a constant FSS of 12 dynes/cm2, the addition of 15 mmHg caused the largest release of ATP; resulting in a biphasic effect where increasing or decreasing the magnitude of pressure significantly attenuated this response. Several possibilities exist for this enhanced biphasic response. First, we show that hydraulic pressure increases ATP release and cytoskeletal organization, but has little or no effect on NFκB translocation. This would suggest that 15 mmHg of pressure may activate different cell signaling pathways from those activated by FSS, and when the two stimuli were combined we found the release of ATP to be enhanced. Secondly, it is well established that pre-stress of a cell impacts the ability of the cell to sense and respond to FSS.39, 40 A disorganized cytoskeletal structure and altered cell adhesion are found to attenuate/abolish cellular response to FSS in osteoblasts in particular.12, 13, 26, 41 Therefore, we speculate that the 15mmHg pressure added an additional stress, or pre-stress, to the cytoskeleton that enhances the cells sensitivity to FSS. A third explanation is that increasing the magnitude of hydraulic pressure from 15 mmHg to 50 and 100 mmHg may alter the focal adhesion complex such that their signaling mechanisms initiated under 12 dynes/cm2 of FSS are enhanced. Previous reports in lung tissue and smooth muscle cells have found that an increase in pressure on the order of 1 mmHg decreases focal adhesion activation of the protein kinase, Akt.42, 43 Focal adhesions that anchor the osteoblasts to the extracellular matrix play a key role in translating these mechanical forces to the intracellular cytoskeleton framework and into a biochemical response. The interaction between focal adhesions and the extracellular matrix can change under different mechanical loads due to conformational changes to the integrin structure.44 Current models of focal adhesions have been shown to be sensitive to forces as small as 1–2 pN, and are considered susceptible to buckling under high loads.45 These modifications to the integrin structure and overall focal adhesion complex can have a significant impact on the associated biochemical response inside the cell.39, 40 Given our current understanding of the influence mechanical forces can have on focal adhesion function and interaction with the cytoskeleton, our results suggest purinergic signaling in osteoblasts during FSS is facilitated under an optimal magnitude of hydraulic pressure of 15 mmHg.
In response to mechanical loading, the cytoskeleton undergoes significant reorganization that is dependent on the type of mechanical loading experienced by the cell.26 The application of 15 mmHg hydraulic pressure caused pronounced actin polymerization and ASFF. Reducing the hydraulic pressure to a gauge pressure of zero, or atmospheric pressure (FSS/SHP0), caused significant loss in cytoskeleton polymerization, while pressure magnitudes of 50 or 100 mmHg produced the same effect on the actin cytoskeleton as that of 15mmHg. Previously, we established that purinergic signaling in response to mechanical stimuli causes an increase in cell stiffness due to changes in the actin-cytoskeleton.4 This dependence on purinergic signaling would explain the loss of ASFF observed when the hydraulic pressure was reduced to atmospheric pressure during FSS/SHP0, given the loss in ATP release. Of the purinergic receptors characterized among osteoblasts, P2Y receptors have the ability to activate G-proteins along with the Rho GTPase pathway to regulate cytoskeleton re-organization.46 Given that depolymerization of the actin cytoskeleton increases the cell mechanosensitivity to successive stimulation;21, 23 purinergic signaling is thought to downregulate the mechanosensitivity of osteoblasts as a negative feedback to the anabolic response. Overall, the increased purinergic signaling during FSS/SHP15 compared to FSS/SHP0 suggests that hydraulic pressure is a key determinate of the sensitivity of osteoblasts to mechanical loading.
Nuclear translocation of NFκB is key component of the anabolic response of osteoblasts to mechanical stimuli,12, 33 and is necessary for both c-fos and COX-2 production.12, 15, 26 In order for NFκB to translocate to the nucleus, one of the two monodimers, IκBα or IκBβ, must be degraded in order to expose the nuclear translocation terminals of NF κB.12, 14 Similar to our previous studies, we found that PLC/IP3 mediated intracellular release of calcium from the ER regulates NFκB translocation to nucleus. We went on to demonstrate that G-protein activation in response to mechanical stimuli is required for NFκB translocation, given the inhibition caused by suramin during FSS (Fig 5). Given that other studies have also found that hydrolyzing extracellular ATP released during FSS with apyrase also inhibits NFκB translocation to nucleus, it would appear that P2Y receptors play a prominent role in this response.47 However, these studies have gone on to demonstrate that not only P2Y, but also P2X receptors regulate IκB degradation needed for NFκB translocation in response to FSS.47 In contrast, we found that the reduction in ATP release during FSS/SHP0 did not inhibit nuclear translocation of NFκB as expected (Fig 5). In addition, BzATP and ATP treatment alone had no effect on NFκB translation (Fig 4 and 5). To our knowledge, direct activation of purinergic receptors with ATP independent of mechanical loading has not investigated prior to our study even though purinergic signaling is considered a key mechanisms through which NFκB translation is regulated. In light of all these findings, NFκB translation to the nucleus in response to FSS appears to be a function of multiple pathways that rely upon a combination of mechanisms as opposed to a linear system of events. The absence of adjacent signaling mechanisms initiated during FSS may render direct purinergic signaling with ATP treatment in sufficient for NFκB translation. Others have also proposed that an anabolic response of osteoblasts to mechanical stimuli is a function of multiple cell-signaling mechanisms as opposed to a single cascade of events.1, 37
Since BzATP and ATP alone were not able to directly induce NFκB translocation in osteoblasts, we then investigated the potential role of focal adhesions. Similar to purinergic receptors, integrin activation of FAK has also been shown to contribute to NFκB translocation to the nucleus in response to FSS.48 The activation of FAK in response to FSS initiates numerous signaling pathways in osteoblasts,6, 26, 41, 48 and is a non-receptor tyrosine kinase with the ability to activate G-proteins and PLC- through c-Src as a result of phosphorylation at Tyr-397, of FAK.34–36, 49, 50 Although FAK activation of Src has been shown to induce PLC/IP3 mediated intracellular calcium release, we found that inhibition of Src activation during FSS/SHP15 and FSS/SHP0 when ATP release was minimal does not inhibit NFκB translocation (Fig 5). Independent of Src, FAK is still able to activate G-proteins,34, 50 which we found necessary for NFκB translocation during FSS.34 More recent studies have gone on to demonstrate that binding between the αv integrin, a common integrin vital to osteoblast function,51 and the P2Y2 receptor, which is a G-protein receptor, enables optimal activation of the receptor along with G-proteins and downstream mobilization of intracellular calcium.52, 53 However, this unique interaction has yet to be specifically established in osteoblasts and requires further investigation to determine if a combination of FAK activation and purinergic signaling through G-proteins are needed for NFκB translocation to occur during FSS. Overall, we have shown that NFκB translocation in response to 12 dynes/cm2 of FSS is regulated through G-proteins and PLC/IP3 mediated intracellular calcium release independent of Src or PKC activation as well as the magnitude of hydraulic pressure.
A common aspect of in-vitro systems such as ours is their limitation to mimic the in-vivo environment. Despite our ability to identify the influence varying magnitudes of hydraulic pressure have on the mechanotransduction of osteoblasts, further insight is needed to determine if the biphasic response in ATP release due to 12 dynes/cm2 of FSS holds true for different flow rates. One of the key limitations of our system is that osteoblasts are only subjected to unidirectional fluid flow on a two-dimensional substrate, as opposed to the oscillatory flow osteoblasts experience within bone. Although both uni-directional and oscillatory fluid stimulate an osteogenic response in osteoblasts, the timing and genes associated with this response can differ between the two types of stimuli.54 The advantage to using uni-directional flow in our study was the ability to adjust the hydraulic pressure independent of the FSS. However, further work is needed to evaluate the impact hydraulic pressure has on the mechanotransduction pathways of osteoblasts when combined with magnitudes of FSS other than 12 dynes/cm2. Overall, our system provides a unique means to evaluate mechanotransduction pathways in response to multiple forms of stimuli that osteoblasts often experience in-vivo.
In summary, we have demonstrated that the presence of hydraulic pressure during FSS plays a key role in the mechanical behavior of osteoblasts, specifically though cytoskeleton dynamics and reorganization. While other studies have focused on the anabolic response to vary degrees of FSS under a constant static pressure,32 this is the first study to demonstrate that under a constant FSS of 12 dynes/cm2 the hydraulic pressure induces a biphasic effect on ATP release. In addition, FSS at this magnitude is a crucial component to the anabolic response of osteoblasts by enabling purinergic signaling the ability to regulate translocation of NFκB to the nucleus through G-protein activation independent of hydraulic pressure. Thus, osteoblasts require both hydraulic pressure and FSS to regulate their mechanosensitivity and, at the same time, their anabolic response to mechanical loading of bone.
Acknowledgments
This study was supported by funding from NIH/NIAMS (AR043222, AR051901, AR054385, and P30GM103333).
Footnotes
Conflicts of Interest: The authors Joseph D. Gardinier, Vimal Gangadharan, Liyun Wang, and Randall L. Duncan declare that they have no conflicts of interest.
Ethical Standards: No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
References
- 1.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. Faseb Journal. 1995;9:441–5. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 3.Roelofsen J, Klein-Nulend J, Burger EH. Mechanical stimulation by intermittent hydrostatic compression promotes bone-specific gene expression in vitro. J Biomech. 1995;28:1493–503. doi: 10.1016/0021-9290(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 4.Gardinier J, Majumdar S, Duncan R, Wang L. Cyclic hydraulic pressure and fluid flow differentially modulate cytoskeleton re-organization in mc3t3 osteoblasts. Cellular and Molecular Bioengineering. 2009;2:133–143. doi: 10.1007/s12195-008-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122:387–93. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- 6.Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of cox-2 and pge2 release in mc3t3-e1 osteoblasts. J Appl Physiol. 2004;97:135–42. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- 7.Duncan RL, Akanbi KA, Farach-Carson MC. Calcium signals and calcium channels in osteoblastic cells. Semin Nephrol. 1998;18:178–90. [PubMed] [Google Scholar]
- 8.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced atp secretion mediates prostaglandin release in mc3t3-e1 osteoblasts. J Bone Miner Res. 2005;20:41–9. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (erk1/2) by fluid shear is ca(2+)- and atp-dependent in mc3t3-e1 osteoblasts. Bone. 2008;42:644–52. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz S, Boland R, Santillan G. Modulation of erk 1/2 and p38 mapk signaling pathways by atp in osteoblasts: Involvement of mechanical stress-activated calcium influx, pkc and src activation. Int J Biochem Cell Biol. 2006;38:2082–91. doi: 10.1016/j.biocel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322–30. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced nfkappab translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33:399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;278:C989–97. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin AS., Jr The nf-kappa b and i kappa b proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 15.Forwood MR. Inducible cyclo-oxygenase (cox-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–93. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 16.Frangos JA, Mcintire LV, Eskin SG. Shear-stress induced stimulation of mammalian-cell metabolism. Biotechnology and Bioengineering. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- 17.Gardinier JD, Townend CW, Jen KP, Wu Q, Duncan RL, Wang L. In situ permeability measurement of the mammalian lacunar-canalicular system. Bone. 2010;46:1075–1081. doi: 10.1016/j.bone.2010.01.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowin SC, Gailani G, Benalla M. Hierarchical poroelasticity: Movement of interstitial fluid between porosity levels in bones. Philos Transact A Math Phys Eng Sci. 2009;367:3401–44. doi: 10.1098/rsta.2009.0099. [DOI] [PubMed] [Google Scholar]
- 19.Jaasma MJ, Jackson WM, Tang RY, Keaveny TM. Adaptation of cellular mechanical behavior to mechanical loading for osteoblastic cells. J Biomech. 2007;40:1938–45. doi: 10.1016/j.jbiomech.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Kainulainen T, Pender A, D’Addario M, Feng Y, Lekic P, McCulloch CA. Cell death and mechanoprotection by filamin a in connective tissues after challenge by applied tensile forces. J Biol Chem. 2002;277:21998–2009. doi: 10.1074/jbc.M200715200. [DOI] [PubMed] [Google Scholar]
- 21.Glogauer M, Arora P, Yao G, Sokholov I, Ferrier J, McCulloch CA. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. Journal of Cell Science. 1997;110(Pt 1):11–21. doi: 10.1242/jcs.110.1.11. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Ryder KD, Bethel JA, Ramirez R, Duncan RL. Pth-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21:1729–37. doi: 10.1359/jbmr.060722. [DOI] [PubMed] [Google Scholar]
- 23.Takai E, Costa KD, Shaheen A, Hung CT, Guo XE. Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Ann Biomed Eng. 2005;33:963–71. doi: 10.1007/s10439-005-3555-3. [DOI] [PubMed] [Google Scholar]
- 24.Shao Y, Czymmek KJ, Jones PA, Fomin VP, Akanbi K, Duncan RL, Farach-Carson MC. Dynamic interactions between l-type voltage-sensitive calcium channel cav1.2 subunits and ahnak in osteoblastic cells. Am J Physiol Cell Physiol. 2009;296:C1067–78. doi: 10.1152/ajpcell.00427.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charras GT, Horton MA. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys J. 2002;82:2970–81. doi: 10.1016/S0006-3495(02)75638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in mc3t3-e1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275:C1591–601. [PubMed] [Google Scholar]
- 27.Stevens HY, Meays DR, Frangos JA. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone. 2006;39:565–72. doi: 10.1016/j.bone.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Qin YX, Lam H. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J Biomech. 2009;42:140–5. doi: 10.1016/j.jbiomech.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.W Li, Gardinier JD, Price C, Wang L. Does blood pressure enhance solute transport in the bone lacunar-canalicular system? Bone. 2010;47:353–9. doi: 10.1016/j.bone.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin e-2 by primary bone cells is shear stress dependent. Journal of Biomechanics. 2001;34:671–677. doi: 10.1016/s0021-9290(00)00231-1. [DOI] [PubMed] [Google Scholar]
- 31.Wadhwa S, Godwin SL, Peterson DR, Epstein MA, Raisz LG, Pilbeam CC. Fluid flow induction of cyclo-oxygenase 2 gene expression in osteoblasts is dependent on an extracellular signal-regulated kinase signaling pathway. Journal of Bone and Mineral Research. 2002;17:266–274. doi: 10.1359/jbmr.2002.17.2.266. [DOI] [PubMed] [Google Scholar]
- 32.Donahue TL, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36:1363–71. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 33.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappab. Nat Med. 2009;15:682–9. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan RS, Jacamo RO, Jiang XH, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor activation rapidly stimulates focal adhesion kinase phosphorylation at ser-843 - mediation by ca2+, calmodulin, and ca2+ calmodulin-dependent kinase ii. Journal of Biological Chemistry. 2005;280:24212–24220. doi: 10.1074/jbc.M500716200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Chattopadhyay A, Ji QS, Owen JD, Ruest PJ, Carpenter G, Hanks SK. Focal adhesion kinase promotes phospholipase c-gamma1 activity. Proc Natl Acad Sci U S A. 1999;96:9021–6. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura I, Lipfert L, Rodan GA, Le TD. Convergence of alpha(v)beta(3) integrin- and macrophage colony stimulating factor-mediated signals on phospholipase cgamma in prefusion osteoclasts. J Cell Biol. 2001;152:361–73. doi: 10.1083/jcb.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–58. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 38.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 39.Ingber DE, Tensegrity i. Cell structure and hierarchical systems biology. Journal of Cell Science. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 40.Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001;98:7765–70. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young SR, Gerard-O’Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24:411–24. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue Z, Zhang W, Desai LP, Gao H, Gunst SJ, Tepper RS. Increased mechanical strain imposed on murine lungs during ventilation in vivo depresses airway responsiveness and activation of protein kinase akt. J Appl Physiol. 2013;114:1506–10. doi: 10.1152/japplphysiol.01460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai LP, Wu Y, Tepper RS, Gunst SJ. Mechanical stimuli and il-13 interact at integrin adhesion complexes to regulate expression of smooth muscle myosin heavy chain in airway smooth muscle tissue. Am J Physiol Lung Cell Mol Physiol. 2011;301:L275–84. doi: 10.1152/ajplung.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science. 2007;316:1148–53. doi: 10.1126/science.1137592. [DOI] [PubMed] [Google Scholar]
- 45.Wu D, Ganatos P, Spray DC, Weinbaum S. On the electrophysiological response of bone cells using a stokesian fluid stimulus probe for delivery of quantifiable localized piconewton level forces. J Biomech. 2011;44:1702–8. doi: 10.1016/j.jbiomech.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of rho in g protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–89. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- 47.Genetos DC, Karin NJ, Geist DJ, Donahue HJ, Duncan RL. Purinergic signaling is required for fluid shear stress-induced nf-kappa b translocation in osteoblasts. Experimental Cell Research. 2011;317:737–744. doi: 10.1016/j.yexcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young SR, Gerard-O’Riley R, Harrington M, Pavalko FM. Activation of nf-kappab by fluid shear stress, but not tnf-alpha, requires focal adhesion kinase in osteoblasts. Bone. 2010;47:74–82. doi: 10.1016/j.bone.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- 50.Brown EJ. Integrin-associated proteins. Current Opinion in Cell Biology. 2002;14:603–607. doi: 10.1016/s0955-0674(02)00360-5. [DOI] [PubMed] [Google Scholar]
- 51.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. Journal of Bone and Mineral Research. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 52.Erb L, Liu J, Ockerhausen J, Kong QM, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Perez LI, Gonzalez FA, Gresham HD, Turner JT, Weisman GA. An rgd sequence in the p2y(2) receptor interacts with alpha(v)beta(3) integrins and is required for g(0)-mediated signal transduction. Journal of Cell Biology. 2001;153:491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao Z, Seye CI, Weisman GA, Erb L. The p2y2 nucleotide receptor requires interaction with alpha v integrins to access and activate g12. Journal of Cell Science. 2007;120:1654–62. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Case N, Sen B, Thomas JA, Styner M, Xie Z, Jacobs CR, Rubin J. Steady and oscillatory fluid flows produce a similar osteogenic phenotype. Calcified Tissue International. 2011;88:189–197. doi: 10.1007/s00223-010-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]