Abstract
With the rapid development of new therapies for patients with hematological malignancies, there is an increasing need for patient report of symptom status during all phases of drug testing. The patient’s perspective on new treatments reflects treatment tolerability as well as symptom benefit, and may assist patients and clinicians in choosing treatments. Inclusion of patient-reported outcomes, more common in solid-tumor than hematological trials, provides early information about symptoms to guide decisions about appropriate dosing and supportive care needs. We provide a historical overview of the use of patient-reported outcomes and symptom assessment in solid-tumor and hematological drug development, and offer recommendations about methodological issues in the monitoring of symptoms in the drug development process in hematological clinical trials.
Keywords: cancer clinical trials, drug development, European Medicines Agency, patient-reported outcomes, recommendations, symptom assessment, symptom burden, symptom benefit, US FDA
The survival of patients with hematological malignancies has been significantly extended by a rapid expansion in the availability of new agents, including tyrosine kinase inhibitors, proteasome inhibitors and monoclonal antibodies targeted to hematopoietic cell surface markers. As a result of increased understanding of the molecular basis of hematological diseases, many of which were rapidly fatal in the past, these diseases are becoming chronic, with indefinite periods of remission so long as treatment is maintained [1]. These gains have broadened our view of the outcomes of therapy to include how patients feel and function during extended periods of survival. Given an increasing number of therapies with similar survival outcomes, maintenance of better functioning with fewer treatment-related symptoms becomes an increasing therapeutic advantage, and information about symptomatic status and function obtained in clinical trials is helpful to both patients and their healthcare team in treatment decision making.
There is increasing recognition that identification of symptoms sensitive to changes in disease and to the effects of treatment is important in oncology practice and clinical research. Symptoms are subjective phenomena reported by patients and indicating change in normal functioning, sensation or appearance due to disease and treatment [2]. When treatment only marginally extended survival, life-threatening toxicities were the major concern in making decisions about the acceptability of therapy; however, these toxicities were measured by clinician ratings and did not capture patients’ experience during therapy. Patient-reported outcome (PRO) measures most directly portray patients’ views of the impact of treatment on how they are feeling and functioning. Methodologically sound patient self-report is a critical component of drug evaluation [3–5].
The evolution of patient report: a brief history
In the 1970s, the concept of individual quality of life (QOL) began to appear in the oncology literature. Factors identified as being part of QOL measurement have consistently included daily functioning and symptoms as well as other factors such as general health, emotional well-being and cost [6,7]. Although QOL was recognized as reflecting a patient’s experience, direct assessment by patient report was not sought. QOL was evaluated by objective events, such as hospitalizations, or was subjectively rated by clinicians [8].
Initially, QOL concerns were primarily related to radical surgeries or intensive therapies, such as those for acute leukemia [8,9]. The idea of measuring QOL in clinical trials was advanced in the early 1980s [10]. The focus was on determining if one treatment offered a QOL advantage over another equally efficacious regimen. During this period, results of quantitative clinician assessments of patient functionality were reported [11], patients were asked to judge the quality of their lives [12], and the ideas of operationally defining QOL for uniform measurement and the need to measure QOL longitudinally for a complete understanding of the impact of treatment were introduced [7].
During the 1990s, efforts were made to overcome the obstacles associated with QOL measurement in oncology populations and especially in clinical trials. By this time, patient report was identified as the optimal source of information about QOL and the concept of QOL was narrowed to focused on health-related QOL (HRQOL) in an effort to remove extraneous factors that had nothing to do with disease or treatment [13]. Critical HRQOL issues for patient care are managing symptoms and maintaining functionality [14]. Several oncology-specific measures of HRQOL were developed and became widely used, enabling comparisons of results across clinical trials [15–18]. The value of establishing the psychometric reliability and validity of assessment instruments became more widely understood and accepted. By 2000, the need for reliable HRQOL clinical trial end points to guide the practicing clinician in patient discussion of treatment options was recognized [14].
Although HRQOL is a narrower concept than QOL, it nonetheless includes broad domains that may be affected by many factors beyond a single disease and its treatments [13]. Early in the 2000s, symptoms and the interference caused by symptoms were recognized as the more relevant patient reports for monitoring drug development and evaluation [19]. In an effort to overcome problems associated with HRQOL measurement (e.g., lack of sensitivity to change in disease or treatment) and to make the measures more useful to clinicians, the concept of ‘symptom burden’ as the most relevant component of HRQOL was suggested [19–21]. Symptom burden can be defined as the combined severity of the symptoms of a disease and treatment, and the impact of the symptoms on daily functioning [21].
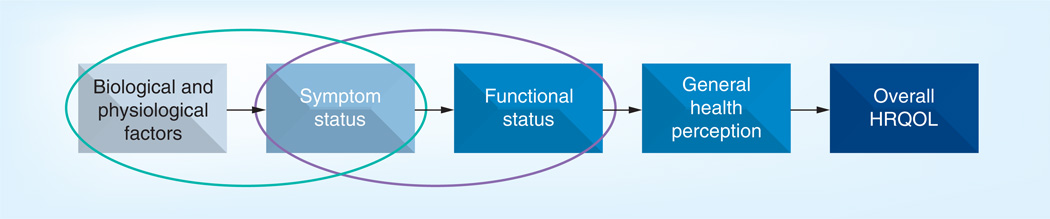
As illustrated in Figure 1, symptom burden (symptom status plus functional status) is a subset of HRQOL. Restricting measurement to patient report of symptom burden allows a focus on the domains most related to a single disease and its treatment, offering the best chance to detect meaningful differences for prognostic significance and treatment effectiveness [19,22]. Patient report of symptom burden allows judgments to be made about the patient’s perception of treatment impact [5,23].
Figure 1. Model of symptom burden as part of health-related quality of life.
Within the HRQOL spectrum, symptoms are the component most closely related to the disease and treatment process (green oval). Symptom burden includes both symptom status and functional status (purple oval).
HRQQL: Health-related quality of life.
This figure can be viewed in full color at: www.future-science.com/doi/full/10.4155/CLI.13.108
With permission from © Cleeland CS (2006) and adapted from Wilson and Cleary (1995).
Symptom assessment in cancer research
Several factors have led to a greater emphasis on symptom understanding and management in cancer in the last decade. Newer and more-intensive therapies have resulted in better disease control and the emergence of cancer as a chronic disease [24–28]. However, these newer therapies are sometimes associated with unexpected side effects that clinicians are not prepared to manage [29–33]. Furthermore, newer therapies are often administered orally and may require long-term use to control cancer. Symptoms that are not controlled can interfere with patient compliance with prescribed therapies and leave patients functionally impaired – and faced with the dilemma of choosing between quality versus quantity of life [34–37]. Consideration of multiple treatment options, different symptomatic side effects associated with various treatments and the ability of treatments to control both disease progression and cancer symptoms [38] have become important elements of the treatment decision process for patients and clinicians. Patients and families are demanding more information about a given treatment’s ability to control cancer symptoms, the symptoms to expect with different therapies and methods of managing symptoms to maintain functionality and quality of life.
Without adequate measurement, however, symptoms cannot be understood or their management evaluated and improved [39]. Selection of the appropriate measure(s) for a cancer clinical trial is critical for detecting differences that occur in symptoms or patient functioning related to the disease and treatment. In cancer clinical trials in the USA, the current standard measure of adverse events is the National Cancer Institute physician-rated Common Toxicity Criteria for Adverse Events. Whereas the Common Toxicity Criteria for Adverse Events is a reliable measure of objective adverse events such as abnormal laboratory values, decreased oxygenation and weight loss, it is an unreliable measure of symptoms and is currently undergoing revision to capture adverse events from the patient’s perspective [40]. Research has shown that clinician reporting of symptoms and changes in QOL in clinical trials is delayed and lacks sensitivity and specificity compared with PRO reporting [41,42]. PROs are more sensitive to significant changes in clinical trials than are clinician estimates and foster earlier detection of symptoms, before they reach serious levels [19,43].
Clinical evaluation of new agents is complex and expensive and gathering symptom and other PRO data have traditionally been seen as adding more difficulty and cost to the performance of clinical trials. As a result, symptom data (if gathered at all) customarily contributed to a secondary or exploratory end point and their collection was given a low priority. In recent years, however, the use of PROs in randomized clinical trials has been given greater credibility by regulatory bodies such as the US FDA and the European Medicines Agency [44,45]. The FDA has issued ‘Guidance for Industry: Patient-Reported Outcome Measures: Use In Medical Product Development To Support Labeling Claims’ [46] and ‘Draft Guidance For Industry: Qualification Process For Drug Development Tools’ [47], each of which provide additional information on using PROs as outcomes measures. The FDA recommends that the content of PRO measures used in medical product development to support labeling claims be defined with qualitative patient interviews and psychometrically validated using generally accepted methods for establishing reliability, sensitivity and validity [46].
Solid tumors
The most consistent measurement of patient-reported HRQOL or symptoms has occurred in clinical trials for treatment of solid tumors [22]. However, many of these trials have failed to show a significant difference in symptom outcomes, often because of design issues related to the selection of the instrument to measure the research outcome, the frequency with which measurement occurred, or the methods of analysis. There are notable examples of successful trial outcomes in solid tumors that have shown symptom benefit from treatments or have provided information to guide patients and clinicians in treatment decision making.
Tannock et al. showed that pain in patients with advanced prostate cancer could be relieved by the palliative administration of mitoxantrone and prednisone [38]. This research provided evidence that led to an FDA label indication for the palliative use of mitoxantrone in advanced prostate cancer. Similarly, the FDA approved gemcitabine in 1996 for treatment of locally advanced or metastatic pancreatic cancer on the basis of results from two clinical trials with composite end points that included pain and analgesic use [48]. Several multicenter, randomized-controlled trials showed that combination therapies significantly improved survival compared with standard single-agent therapies, without negatively affecting HRQOL in patients with solid tumors [49–51]. A more selective and potent VEGF receptor inhibitor was shown to significantly extend progression-free survival and delay symptom worsening compared with a currently approved VEGF receptor inhibitor [52]. Another multicenter, randomized clinical trial showed that, compared with single-agent therapy, combination therapy for metastatic pancreatic cancer significantly delayed deterioration in HRQOL and that baseline HRQOL scores improved survival estimates when combined with clinical and demographic information [53].
Experience in solid-tumor trials shows that comparison of various treatments to control both tumor progression and symptoms and to maintain functionality and HRQOL provides important information to clinicians and patients making treatment decisions.
Hematological malignancies
In the early days of treatment for hematological malignancies, the concept of QOL was recognized as important in treatment decision making because of the intensity of the therapies needed to control the disease and the very small chance of cure. Supportive care measures to increase patient comfort and maintain maximum functioning were studied as an alternative to intensive therapies [8]. However, as cure of hematological malignancies became more common and measures to support patients through intense therapies improved, the concepts of HRQOL, symptoms and patient functioning during treatment were considered less frequently than they were for solid tumors [54]. Research was reported on the HRQOL and functionality of patients after treatment [55,56], especially in pediatric literature [57,58]. But with few exceptions [59–62], patient report of HRQOL and symptom burden was rarely measured during the acute phase of treatment, often because patients were considered too ill to respond to questionnaires. Therefore, little is known about the patient experience of intense therapies for hematological malignancies.
The advent of less-intense but highly effective targeted therapies for hematological malignancies, such as tyrosine kinase inhibitors for chronic myeloid leukemia [24], has created the need for better understanding of treatment-related symptom burden and management, and for increased consideration of HRQOL. Some patients with hematological malignancies can be successfully treated with targeted therapies but are unable to tolerate them; as a result, doses may be reduced, delayed or temporarily stopped. Effective therapy may be changed or discontinued earlier than planned when symptoms such as peripheral neuropathy develop [63–66]. Many of the targeted therapies are administered orally, and there is concern that symptoms, such as fatigue and diarrhea, may lead to nonadherence to treatment regimens [34,67].
In recent years, examination of the impact of treatment on symptoms and patient functioning in studies of hematological malignancies has increased [68–70]. A recent clinical trial of the effectiveness and clinical benefit of Janus kinase inhibition in patients with myelofibrosis showed substantial rapid improvement in symptoms of weight loss, fatigue, night sweats and pruritus, along with improvement in patient functioning [71]. This proof of clinical benefit led to the inclusion of symptom reduction as a component of FDA labeling indications for ruxolitinib for the treatment of myelofibrosis, indicating the powerful effect that symptom assessment can have in hematological malignancy clinical trials [72].
Nonetheless, routine inclusion of symptom or QOL assessment tools in clinical treatment trials for hematological malignancies is still not as common as it is for solid tumors. A recent study adding bortezomib to the standard cytarabine and daunorubicin induction regimen for acute myeloid leukemia in older adults to improve remission rates did not report the impact of the treatment on symptoms, with the exception of peripheral neuropathy [66]. Information about symptom burden in this vulnerable group of patients would be especially useful in making treatment decisions.
One of the barriers to including symptom and HRQOL assessment in clinical trials may be the lack of brief and easy-to-complete PRO measures specific to hematological malignancies and their treatment. However, a recent study of patients undergoing autologous or allogeneic hematopoietic stem cell transplant for hematological malignancies found an 80–100% completion rate for weekly short symptom surveys (median of <5 min to complete) administered primarily electronically from immediately before the start of high-dose therapy to 100 days post-transplant [73]. The authors had a similar experience monitoring patients at baseline (hospital admission), during pretransplant chemotherapy conditioning (2–5 days after admission), on the day of allogeneic transplant (day 0), and twice a week until 30 days post-transplant using a short PRO symptom measure; in this study, the missing-data rate was approximately 1.5% [59].
Recommendations for inclusion of symptom measures in hematological drug development
Along with the FDA guidance on use of PROs as outcome measures to support drug development and labeling claims, there is increasing convergence of expert opinion on how to approach symptom assessment in clinical trials and drug development studies. Consensus-group recommendations include those from the Expert Guidance Document from the Center for Medical Technology Policy [74], the ASCPRO working group [5,19] and a workshop summary on Symptom Measurement in Clinical Trials held by the Friends of Cancer Research [75]. The recommendations are reviewed below as considerations for the inclusion of symptoms as primary, secondary or exploratory outcomes in trial planning and design in hematological diseases.
Assessment of symptoms in early-phase clinical trials
Increasing pressure to include symptom report and other PROs in drug development and evaluation suggests the need to plan for symptom assessment at the earliest stages of the drug-development trajectory [74]. Inclusion of self-report measures in clinical trials for hematological malignancies has been infrequent and often does not occur until pivotal registration trials. This has often meant adding a generic HRQOL instrument, with little or no evidence that the scores obtained from this instrument were sensitive to the symptoms of the type and stage of hematological disease targeted in the trial, the specific symptomatic impact of the treatment, or the potential reduction in symptom burden that might be attributed to that treatment. The importance of having data on the symptom burden or benefit conferred by therapy is often not recognized until after registration trials are completed and questions about symptom burden or symptom benefit arise during the evaluation and approval process of the drug’s clinical effectiveness. It is important that medical advisory boards who develop early trials recognize the need for symptom assessment and include it as an outcome [75]. Persons with expertise in symptom assessment should be included as part of such medical advisory boards.
Early-phase clinical trials present an opportunity to measure symptoms early and often, and to capture signs of toxicity and potential signs of symptom benefit, such as reduction of disease-related symptoms and improved function. This measurement can be done with a simple existing multisymptom assessment instrument and through qualitative interviewing of patients to capture the emergence of additional treatment-related symptoms or symptom benefit. Even though these trials may enroll fewer than 100 patients, systematic use of symptom measurement tools administered at frequent intervals can give incremental information about treatment toxicities or benefits and allow for the identification or development of specific assessment instruments that can be incorporated into planning for registration trials. Early evidence of multiple treatment-related symptoms can serve as a warning sign to drug developers that adherence to treatment may be compromised and the appropriateness of dose selection or plans for supportive care need to be considered [5,75].
Early regulatory approval of trial design & symptom measures
In registration trials, the selection of symptoms to be assessed and measurement tools to be used must be negotiated with the agency that ultimately will be responsible for approval of the drug, for example, the FDA in the USA or the European Medicines Agency Committee for Medicinal Products in Human Use in Europe. This is especially critical if reduction of symptoms or improvement in function is desired as a labeling claim for the agent. The FDA guidance, which emphasizes conceptual models and symptoms [44], advises that including reliable patient report of well-defined components of health status into clinical trials can provide important information about the benefits and risks of treatment [47].
The first line of action in planning a clinical trial seeking a labeling indication is to request a special protocol assessment or similar evaluation by the appropriate regulatory body to obtain a declaration that the trial design, clinical end points and statistical analysis plan are acceptable for the approvals being sought [76]. Regulatory authorities may provide instructive feedback about how the inclusion of PROs could enhance judgments about the agent to be considered for approval and/or labeling [74,75].
Establishment of a conceptual research model
Including symptom assessment in drug development requires a conceptual model of the treatment interacting with the disease that could alter a patient’s symptom status. Several informative symptom assessment scenarios in cancer clinical trials are applicable to drug development in malignant hematology. These include:
-
■
Testing the effectiveness of treatment to reduce disease-related symptoms;
-
■
Measuring delay in the occurrence of symptoms related to disease recurrence or progression;
-
■
Documenting treatment-induced symptoms that need to be considered in the overall evaluation and use of the drug;
-
■
Comparing treatment-related symptoms or the symptom-reduction benefit of two equally effective disease therapies to determine if one produces less symptom burden than the other.
A conceptual model frames the symptom-related hypotheses to be evaluated during development. For example, if an agent might reduce pain or delay the onset of significant pain in multiple myeloma, the conceptual model should identify the pain measure to be used, its frequency of administration and how a ‘significant’ change in pain severity is to be identified in the trial. As a second and more complex example, if a novel agent is expected to be less toxic than one in current use but may not be expected to substantially improve standard clinical trial outcomes, a set of the most common symptoms associated with the current treatment needs to be identified, their measurement specified, and decision rules established to determine if the new treatment is associated with less patient-reported symptom burden. This might be accomplished using a composite symptom score, although the determination of which symptoms to include in a composite score is not without problems and requires collaboration among experienced clinicians and symptom researchers [19].
Selection of symptoms & selection or development of instruments
Once the conceptual model is developed, the appropriate questionnaire(s) for capturing the impact of treatment on how patients feel and function can be identified. The selection is based on the specific disease to be studied and on regulatory recommendations [46,47,77]. All stakeholders in this process should be engaged early in the design of the trial.
The use of multiple instruments to measure individual symptoms and their effects on function is not practical in clinical trials because of the burden that completing multiple symptom questionnaires places on patients. Therefore, the instruments most useful for clinical trials are those that include multiple symptoms specific to a particular disease and treatment and the effect of the symptoms on patient functioning.
The development of a new instrument may be required by authoritative bodies. However, the development of an entirely new symptom assessment instrument can be extremely time consuming and expensive and may not be an effort that fits well into the clinical trial development process. Acceptable instrument(s) may already exist or may suffice with some level of modification.
Studies of symptoms experienced by patients with cancer have identified a core group of approximately 11–13 symptoms as the most prevalent [78]. Several groups of researchers have developed similar lists of symptoms upon which to focus a program of research [5]. Recent systematic reviews have also produced similar lists of the most prevalent cancer symptoms. One review identified the most prevalent and severe symptoms in studies representing more than 1000 patients receiving active cancer treatment [79]. An earlier systematic review similarly reported the ten most prevalent symptoms across 18 studies of patients with cancer [80]. The Center for Medical Technology Policy has developed recommendations for the inclusion of the assessment of a core group of common symptoms in adult oncology clinical effectiveness studies, especially for patients with advanced disease or those undergoing aggressive therapy, and has provided a list of assessment instruments that include these symptoms [74]. The consistent prevalence of some symptoms in these systematic reviews and recommendations, often using reports from both inpatients and outpatients across several disease sites, supports the general concept of the adoption of a core set of cancer- and treatment-related symptoms for consistent assessment in clinical trials. Although most of these reviews focused on solid tumors, a recent review of symptoms in acute leukemia demonstrates that the most prevalent symptoms are almost the same as those found in advanced solid tumors [81]. For a specific clinical trial, these core symptoms can be augmented with a few additional symptoms that may be unique to a particular disease or treatment.
Well-validated questionnaires that measure multiple symptoms in patients with cancer include the MD Anderson Symptom Inventory (MDASI), the Memorial Symptom Assessment Scale, the Symptom Distress Scale, the Edmonton Symptom Assessment System, the Symptom Reporting Tool, the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events, and the Patient Reported Outcomes Measurement Information System, funded by the NIH. Other more-general HRQOL measures for cancer contain symptom subscales, such as the European Organisation for Research and Treatment of Cancer, Quality of Life Measure (EORTC QLQ-30) and the Functional Assessment of Cancer Therapy. Some measures offer the advantage of having undergone rigorous psychometric validation (see ‘Assessment tool development’, below), including the MDASI, Memorial Symptom Assessment Scale, Symptom Distress Scale, Edmonton Symptom Assessment System and the Adapted Symptom Distress Scale [82].
So-called ‘threats to validity’ must be addressed when selecting, modifying or designing a symptom assessment measure [83]. These threats include:
-
■
The conceptual match between the PRO instrument and the intended claim is unclear. The symptoms included in the PRO instrument may not encompass those that are unique to the agent under development, the disease and stage (e.g., refractory acute myeloid leukemia) being targeted by the agent, or the class of drugs (e.g., tyrosine kinase inhibitors) to which the agent belongs. For this reason, several symptom measurement systems, such as the MDASI and the Functional Assessment of Cancer Therapy, provide modules containing additional disease- or treatment-specific questions that have been developed through the use of both patient input and psychometric assessment (validity, reliability and sensitivity)[84];
-
■
There is no evidence that the most relevant and important item content is contained in the assessment tool. Tool development that adheres to FDA guidance includes both qualitative (stakeholder input) and quantitative (psychometric validation) components that address this concern;
-
■
Direct patient input into the PRO item content from the target population in which the claim is desired was not obtained. Well-documented qualitative studies that elicit patient input on the issue(s) of concern can rectify this problem and determine the adequacy of the assessment tool for the specific use;
-
■
Documentation to support modifications to the PRO instrument is lacking [83]. Any modified instrument should also meet the content and psychometric standards of the parent instrument; specifically, the relevance, importance and sensitivity to expected change because of the treatment under study must be established for all new items. See ‘Assessment tool development’ below for a discussion of these processes.
The ideal assessment tool will be as brief as possible. Intensive therapies such as hematopoietic stem cell transplant often produce rapid development of severe symptoms and then rapid resolution. Without frequent measurement, these changes in symptoms may not be captured. Furthermore, there is strong consensus that PRO assessments should optimize patient and research staff time. It has been recommended that baseline assessments should be accomplished in 20 min or less and that follow-up assessments should require no more than 10–15 min [4]. A brief instrument will minimize patient burden in completing the required study assessments while allowing for frequent measurement when needed to detect rapidly occurring changes in symptoms.
Inclusion of validation in the early drug-development process
Frequently, in the development plan for a new agent, the time and resources required for classic psychometric validation of a new or modified assessment tool are lacking. Much like validation of laboratory measures, instrument validation is an incremental process and can never completely exclude all potential measurement error [85]. Nevertheless, frequent administration of a new or modified measurement tool in early-phase clinical trials can give incremental information about both toxicities and symptom benefit that can be incorporated into planning for registration trials, as well as information about the performance of the tool itself. There is no gold standard for implementing this type of validation, but there is some consensus about the magnitude of change that may be expected to be important to patients, based on the initial distribution of symptoms scores and employing effect sizes based on the baseline standard deviations of the target population [86]. These well-accepted determinations of clinical significance are distribution based and empirically determined [87]. With instruments that have a history of use in clinical trials, these differences are often known for multiple populations. Another approach is to coadminister a measure of patient impression of change (none, or gradations of feeling better or worse) and to correlate the magnitude of symptom change with the patient’s impression of the use of the agent. These are termed anchor-based meaningful change scores.
Methods for reducing missing data
Data can be missing for a number of reasons, particularly in longitudinal studies. Priority may not be given to collection of symptom data, especially when it is added at the end of trial planning. The instrument selected may not be appropriate, making it difficult for patients or study staff to see the relevance of the data. Timing of the symptom data collection may not fit well into the data collection schedule for the other trial end points. Thus, symptom assessment needs to be of high priority from the inception of the clinical trial to ensure that the correct instrument is selected, the feasibility of the assessment schedule fits well with other data collection time points, and all study staff recognize the importance of the symptom data.
Historically, PRO data were gathered by paper-and-pencil questionnaires administered at the clinic visit or mailed to patients between visits. These data were often missing because of collection errors, either forgotten due to the pace of the clinic or not followed up if forms were not returned by mail. More critically, data can be missing when patients are too symptomatic to complete forms, and this can be especially true for patients receiving treatment for acute hematological malignancies. These data are not missing at random and will bias the data set with the lack of patient report from those who are the most symptomatic.
Significant progress has been made in the development of brief symptom assessment measures and methods of gathering PROs, including methods that can minimize missing data. Between clinic visits, these data can be obtained via the Internet, computer–telephone-based interactive voice response systems or other devices such as tablet computers, smart phones, and other personal computing devices. Central electronic data monitoring can prompt investigators when data are missing, allowing field staff to follow up with the patient.
Assessment tool development
When it is necessary to develop a new or modified symptom measure, the FDA requires input from a sample of patients from the intended population for the claim, to help in generating the specific items and determining the appropriate recall period, format, and method of administration for the instrument [46]. The FDA suggests patient interviews or focus groups to generate the items and the wording of the items to be used. Cognitive debriefing is also suggested to confirm that the items are understandable to patients and to verify completeness. Steps in the development process must be documented [46]. Although the exact method of item generation is not specified by the FDA, we have adopted a method that we believe is FDA-compliant to ensure patient input into the development of PRO instruments and to document the development [88].
Methods of securing patient input in assessment tool development
To ensure that the instruments are most appropriate for patients with specific hematological malignancies undergoing different treatment modalities, the authors developed a list of characteristics of patients in the intended population that might influence symptom experience and functional interference, such as age, sex, race, disease stage and type of treatment. On the basis of this list, a sampling plan was established for qualitative interviews with 20–40 patients to ensure that the range of symptom experiences in this population was heard. Trained staff conduct and audiotape semistructured interviews lasting 30–45 min to ascertain each patient’s experience of symptoms over the entire course of the disease and its treatment. From the transcripts of the interviews, reports of symptoms are extracted by experienced qualitative analysts and then reviewed with clinicians to eliminate redundant or trivial items. The number of patients spontaneously endorsing each symptom during the interviews and the exact words the patient uses to describe the symptom are recorded. This process has consistently produced a list of 40–50 symptoms from each population sampled. Symptom items are generated by referring to the words the patients used to describe the symptoms.
To further reduce the number of symptom items to only those that are most important, a panel of experts (physicians, nurses, patients or family caregivers) are asked to rate the relevance of each identified symptom to patients with the disease of interest. Symptoms that receive the highest relevance ratings or are endorsed by the highest percentage of patients in the interviews are retained for final psychometric testing.
During the psychometric testing phase, patient input is sought for a third time by cognitive debriefing of a subset of 20–40 patients in the psychometric validation sample. After these patients have completed the instrument, they are asked about the understandability of the questions and the rating scale, the formatting of the instrument, its completeness or redundancy, and their overall satisfaction with it. All patient responses are recorded. These results are compiled and reviewed. Any consistent comment from five or more patients may result in a revision of the instrument (e.g., adding, rewording or deleting a symptom item).
This process also offers an opportunity to modify the instruments through an iterative process, as suggested by the FDA, as treatments evolve and alter disease courses. Our processes of cognitive debriefing or qualitative interviewing can be used to sample patients’ experience of new therapies or changes in disease course.
Psychometric validation
In the classic psychometric development of a PRO, once an instrument is developed or an instrument is modified to fit a specific target patient group or treatment, important additional information is obtained by statistical evaluation of the instrument using a reasonably large sample (100–200 subjects) of the targeted group. This typically includes such methodological steps as evaluation of the internal stability of the component scales of the instrument, test–retest reliability and sensitivity of the scales(s) to variables that should influence symptoms, such as disease stage, clinical status or treatment trajectory [89]. Data from these studies can also provide an ordering of the relative severity of the selected symptoms, and a subset of symptoms can be defined as those that best characterize the disease or treatment selected for clinical study [89,90]. Such analysis can also identify symptom items of low prevalence in the target group that might be eliminated from routine assessment as a way to ease patient burden.
Attribution of symptoms
Many of the symptoms experienced by patients during a clinical trial will be caused by a combination of factors related both to disease and to the toxicities of therapy. Explicit in the FDA guidance is that, if symptomatic benefit is to be claimed for an oncology drug in regulatory review, the symptoms must be produced by the disease and not by the toxicities of therapy. Determining the causes of symptoms is elusive, however. Symptoms can be produced by disease, treatment, both, or neither, and attempts at symptom attribution have been unreliable. For example, a review of clinician attribution of adverse events in a large number of clinical trials found that half of the events attributed to study treatment were recorded for patients who received placebo. Attribution of symptoms to disease is more tenable for trials of first-line therapy, where baseline assessment gives a clear picture of symptomatic status with little or no previous treatment. If the trial has a placebo-controlled group (rarely the case in oncology trials), additional information can be obtained about symptomatic change due to the natural progression of disease. New symptoms arising after the start of therapy are likely treatment related, but disease progression must be considered as well.
Shared symptom outcomes for hematological malignancies
The Eastern Cooperative Oncology Group has developed a database of symptom outcomes and practice patterns in a large group of patients with the four most prevalent solid tumors in the USA. From 2006 to 2008, 3123 outpatients with breast, lung, prostate or colorectal cancer from 38 academic sites and community clinics were enrolled and assessed using the MDASI at two time points. Clinical and demographic information was collected on each patient. This database has proven extremely rich and has already resulted in the publication of six manuscripts on topics ranging from pain and analgesia prescribing to employment among cancer survivors to the core symptoms of cancer and its treatment [91–93], with several other papers in press, under review or in development.
A database of this nature could be extremely useful in hematological malignancies, which are rarer diseases. Clinicians and individual treatment centers have less opportunity to assess large numbers of patients with hematological malignancies and to establish baselines for symptoms associated with standard treatments. Baseline data on symptoms for standard treatments can be extremely useful in planning and evaluating clinical-trials outcomes for new therapies. Combined data may illuminate areas of need for symptom management and functional improvement, increase treatment tolerability and compliance, and enhance QOL for survivors of hematological malignancies.
Conclusion
The characterization of changes in symptoms and the impact of symptoms on function can provide critical information on the patient’s perspective about the impact of a new agent for the treatment of hematological malignancies, just as it does for solid tumors. Symptom assessment in drug development provides critical information about the patient’s perspective of benefit/cost in early trials and in controlled trials, even if a symptom benefit is not included in the label. Patients and the healthcare team learn what to expect from treatment and what treatment-related supportive care measures may be needed. In addition, symptom assessment provides critical information for advisory boards that approve drugs and evaluate reimbursement and quality-of-care issues [74]. Both symptomatic toxicities and symptom reduction may be demonstrated early in the drug development process. As a larger pallet of agents become available, often with marginal differences in standard clinical outcomes, the agent’s impact on symptoms, either positive or negative, will increase in importance in drug evaluation and adjudication of benefit. Signals of symptom benefit or symptom burden can be included early in the development of a drug to guide the development of dosing recommendations and needed supportive care and to inform potential providers and patient consumers – along with those who must approve the agent and those who will determine whether or not to pay for it.
Future perspective
A number of converging factors in the development of agents to treat hematological malignancies indicate that characterization of the symptomatic status of patients in response to new agents will increase in importance in the next 5–10 years. These factors include the increasing demand for the patient perspective in drug approval and reimbursement processes, the need to determine the relative treatment burden and potential symptom relief among drugs with similar effectiveness and the increasing use of drugs indefinitely to maintain remission. The use of symptom assessment in the drug-development process will be facilitated by ever-improving conceptual and measurement models, reducing patient burden in symptom assessment and making use of existing and new technologies to reduce missing data.
Executive summary.
-
■
There is an increasing need to understand symptoms related to newer, more effective therapies for hematological malignancies, in order to improve adherence to treatment and enhance quality of life in survivorship.
-
■
Integrating symptom assessment into all phases of hematological drug development provides comprehensive understanding of the symptom benefit and burden related to individual therapies.
-
■Recommendations for inclusion of symptom measures in hematological drug development include:
-
■Assessment of symptoms in early-phase clinical trials.
-
■Early regulatory approval of trial design and symptom measures.
-
■Establishment of a conceptual research model.
-
■Selection of symptoms and measurement instruments.
-
■Inclusion of validation in the early drug development process.
-
■Incorporation of methods for reducing missing data.
-
■Assessment tool modification or development when needed.
-
–Patient input in assessment tool development.
-
–Psychometric validation.
-
–
-
■Cautious attribution of symptoms.
-
■Mechanisms to share symptom outcomes for hematological malignancies.
-
■
Acknowledgments
This work is supported in part by grants from the National Cancer Institute of the NIH, including R01 CA026582 and P01 CA124787 to C Cleeland, P01 CA049639 to RE Champlin, and MD Anderson Cancer Center Support Grant P30 CA016672. The sponsors played no role in the design, analysis, interpretation, or preparation of this report. CS Cleeland is a consultant for AstraZeneca, Abbott, Genentech, Amgen, Exelixis, Pfizer, and Millennium.
The authors acknowledge the editorial assistance of JF Woodruff, an employee of the Department of Symptom Research, The University of Texas MD Anderson Cancer Center (Houston, TX, USA).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reference
- 1.Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118(12):3123–3127. doi: 10.1002/cncr.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes VA, Watson PM. Symptom distress – the concept: past and present. Semin. Oncol. Nurs. 1987;3(4):242–247. doi: 10.1016/s0749-2081(87)80014-1. [DOI] [PubMed] [Google Scholar]
- 3.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl. 2):S125–S137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Abernethy AP. Supporting clinical practice decisions with real-time patient-reported outcomes. J. Clin. Oncol. 2011;29(8):954–956. doi: 10.1200/JCO.2010.33.2668. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland CS, Sloan JA, Cella D, et al. Recommendations for including multiple symptoms as endpoints in cancer clinical trials: a report from the ASCPRO (Assessing the Symptoms of Cancer Using Patient-Reported Outcomes) Multisymptom task force. Cancer. 2013;119(2):411–420. doi: 10.1002/cncr.27744. [DOI] [PubMed] [Google Scholar]
- 6.Patterson WB. The quality of survival in response to treatment. JAMA. 1975;233(3):280–281. [PubMed] [Google Scholar]
- 7.van Dam FS, Somers R, van Beek-Couzijn AL. Quality of life: some theoretical issues. J. Clin. Pharmacol. 1981;21(Suppl. 8–9):166S–168S. doi: 10.1002/j.1552-4604.1981.tb02592.x. [DOI] [PubMed] [Google Scholar]
- 8.Burge PS, Prankerd TA, Richards JD, Sare M, Thompson DS, Wright P. Quality and quantity of survival in acute myeloid leukaemia. Lancet. 1975;2(7936):621–624. doi: 10.1016/s0140-6736(75)90111-7. [DOI] [PubMed] [Google Scholar]
- 9.Oliver H, Blum MH, Roskin G. The psychiatrist as advocate for post surgical ‘quality of life’. Psychosomatics. 1976;17(3):157–159. doi: 10.1016/S0033-3182(76)71136-8. [DOI] [PubMed] [Google Scholar]
- 10.Carter SK. Clinical considerations in the design of clinical trials. Cancer Treat. Rep. 1980;64(2–3):367–371. [PubMed] [Google Scholar]
- 11.Nou E, Aberg T. Quality of survival in patients with surgically treated bronchial carcinoma. Thorax. 1980;35(4):255–263. doi: 10.1136/thx.35.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer BV, Walsh GA, McKinna JA, Greening WP. Adjuvant chemotherapy for breast cancer: side effects and quality of life. Br. Med. J. 1980;281(6255):1594–1597. doi: 10.1136/bmj.281.6255.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 14.Passik SD, Kirsh KL. The importance of quality-of-life endpoints in clinical trials to the practicing oncologist. Hematol. Oncol. Clin. North Am. 2000;14(4):877–886. doi: 10.1016/s0889-8588(05)70316-6. [DOI] [PubMed] [Google Scholar]
- 15.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J. Clin. Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 16.Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30-item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncol. 1992;31(3):311–321. doi: 10.3109/02841869209108178. [DOI] [PubMed] [Google Scholar]
- 17.Cella DF. Overcoming difficulties in demonstrating health outcome benefits. JPEN J. Parenter. Enteral Nutr. 1992;16(Suppl. 6):106S–111S. doi: 10.1177/014860719201600613. [DOI] [PubMed] [Google Scholar]
- 18.Ferrans CE. Development of a quality of life index for patients with cancer. Oncol. Nurs. Forum. 1990;17(Suppl. 3):15–19. [PubMed] [Google Scholar]
- 19.Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J. Pain Symptom. Manage. 2010;39(6):1077–1085. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS, Reyes-Gibby CC. When is it justified to treat symptoms? Measuring symptom burden. Oncology. 2002;16(9) Suppl. 10:64–70. [PubMed] [Google Scholar]
- 21.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J. Natl Cancer Inst. Monogr. 2007;37(37):16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan DR, O’Mara AM, Kelaghan JW, Minasian LM. Quality-of-life assessment in the symptom management trials of the National Cancer Institute-supported Community Clinical Oncology Program. J. Clin. Oncol. 2005;23(3):591–598. doi: 10.1200/JCO.2005.12.181. [DOI] [PubMed] [Google Scholar]
- 23.Ganz PA, Goodwin PJ. Health-related quality of life measurement in symptom management trials. J. Natl Cancer Inst. Monogr. 2007;37(37):47–52. doi: 10.1093/jncimonographs/lgm010. [DOI] [PubMed] [Google Scholar]
- 24.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J. Clin. Oncol. 2009;27(28):4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103(8):2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 26.Cortes J, Giles F, O’Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-α. Blood. 2003;102(1):83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 27.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 28.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2(5):561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 29.Solomon BM, Jatoi A. Epidermal growth factor receptor (EGFR) inhibitor-induced rash: a consecutive patient series that illustrates the need for rigorous palliative trials. J. Palliat. Med. 2011;14(2):153–156. doi: 10.1089/jpm.2010.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target. Oncol. 2009;4(2):107–119. doi: 10.1007/s11523-009-0114-0. [DOI] [PubMed] [Google Scholar]
- 31.Jatoi A, Rowland K, Sloan JA, et al. Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB) Cancer. 2008;113(4):847–853. doi: 10.1002/cncr.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon BM, Jatoi A. Rash from EGFR inhibitors: opportunities and challenges for palliation. Curr. Oncol. Rep. 2008;10(4):304–308. doi: 10.1007/s11912-008-0048-1. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10(5):345–356. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 34.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J. Clin. Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotay C, Dunn J. Adherence to long-term adjuvant hormonal therapy for breast cancer. Expert. Rev. Pharmacoecon. Outcomes. Res. 2011;11(6):709–715. doi: 10.1586/erp.11.80. [DOI] [PubMed] [Google Scholar]
- 36.Noens L, van Lierde MA, De BR, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 37.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1–2):1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 38.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J. Clin. Oncol. 1996;14(6):1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 39.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Basch E. The missing voice of patients in drug-safety reporting. N. Engl. J. Med. 2010;362(10):865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J. Clin. Oncol. 2004;22(17):3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Huschka MM, Mandrekar SJ, Schaefer PL, Jett JR, Sloan JA. A pooled analysis of quality of life measures and adverse events data in north central cancer treatment group lung cancer clinical trials. Cancer. 2007;109(4):787–795. doi: 10.1002/cncr.22444. [DOI] [PubMed] [Google Scholar]
- 43.Morton RP, Sloan JA, Grothey A, et al. A comparison of simple single-item measures and the common toxicity criteria in detecting the onset of oxaliplatin-induced peripheral neuropathy in patients with colorectal cancer. Presented at: American Society of Clinical Oncology 41st Annual Meeting; 13–17 May 2005; Orlando, FL, USA. [Google Scholar]
- 44.Bottomley A, Jones D, Claassens L. Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. Eur. J. Cancer. 2009;45(3):347–353. doi: 10.1016/j.ejca.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 45.Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. Cancer J. Clin. 2007;57(5):278–300. doi: 10.3322/CA.57.5.278. [DOI] [PubMed] [Google Scholar]
- 46.US FDA. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Rockville, MD, USA: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US FDA. Guidance for industry. Qualification process for drug development tools (draft guidance) Rockville, MD, USA: 2010. [Google Scholar]
- 48.King RS. Gemcitabine. New first-line therapy for pancreatic cancer. Cancer Pract. 1996;4(6):353–354. [PubMed] [Google Scholar]
- 49.Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(12):937–944. doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- 50.van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized Phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J. Clin. Oncol. 2005;23(28):6881–6889. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 51.Krasner CN, Poveda A, Herzog TJ, et al. Patient-reported outcomes in relapsed ovarian cancer: results from a randomized Phase III study of trabected in with pegylated liposomal doxorubicin (PLD) versus PLD alone. Gynecol. Oncol. 2012;127(1):161–167. doi: 10.1016/j.ygyno.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 52.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised Phase III trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 53.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J. Clin. Oncol. 2013;31(1):23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 54.Efficace F, Novik A, Vignetti M, Mandelli F, Cleeland CS. Health-related quality of life and symptom assessment in clinical research of patients with hematologic malignancies: where are we now and where do we go from here? Haematologica. 2007;92(12):1596–1598. doi: 10.3324/haematol.11710. [DOI] [PubMed] [Google Scholar]
- 55.Andrykowski MA, Greiner CB, Altmaier EM, et al. Quality of life following bone marrow transplantation: findings from a multicentre study. Br. J. Cancer. 1995;71(6):1322–1329. doi: 10.1038/bjc.1995.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J. Clin. Oncol. 2005;23(27):6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 57.Felder-Puig R, Peters C, Matthes-Martin S, et al. Psychosocial adjustment of pediatric patients after allogeneic stem cell transplantation. Bone Marrow Transplant. 1999;24(1):75–80. doi: 10.1038/sj.bmt.1701853. [DOI] [PubMed] [Google Scholar]
- 58.Kupst MJ, Penati B, Debban B, et al. Cognitive and psychosocial functioning of pediatric hematopoietic stem cell transplant patients: a prospective longitudinal study. Bone Marrow Transplant. 2002;30(9):609–617. doi: 10.1038/sj.bmt.1703683. [DOI] [PubMed] [Google Scholar]
- 59.Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113(8):2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson KO, Giralt SA, Mendoza TR, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplant. 2007;39(12):759–766. doi: 10.1038/sj.bmt.1705664. [DOI] [PubMed] [Google Scholar]
- 61.Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J. Clin. Oncol. 2002;20(5):1319–1328. doi: 10.1200/JCO.2002.20.5.1319. [DOI] [PubMed] [Google Scholar]
- 62.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90(9):3390–3394. [PubMed] [Google Scholar]
- 63.Zeng Z, Lin J, Chen J. Bortezomib for patients with previously untreated multiple myeloma: a systematic review and metaanalysis of randomized controlled trials. Ann. Hematol. 2013;92(7):935–943. doi: 10.1007/s00277-013-1711-7. [DOI] [PubMed] [Google Scholar]
- 64.Jeter A, Kang Y. Immune modulation therapy in the management of bortezomib-induced peripheral neuropathy. Exp. Hematol. Oncol. 2012;1(1):20. doi: 10.1186/2162-3619-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voorhees PM, Laubach J, Anderson KC, Richardson PG. Peripheral neuropathy in multiple myeloma patients receiving lenalidomide, bortezomib, and dexamethasone (RVD) therapy. Blood. 2013;121(5):858. doi: 10.1182/blood-2012-11-465765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Attar EC, Johnson JL, Amrein PC, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J. Clin. Oncol. 2013;31(7):923–929. doi: 10.1200/JCO.2012.45.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: Definitions and clinical implications. Cancer. 2011;117(4):688–697. doi: 10.1002/cncr.25648. [DOI] [PubMed] [Google Scholar]
- 68.Efficace F, Baccarani M, Breccia M, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554–4560. doi: 10.1182/blood-2011-04-347575. [DOI] [PubMed] [Google Scholar]
- 69.Hahn EA, Glendenning GA, Sorensen MV, et al. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon α plus low-dose cytarabine: results from the IRIS Study. J. Clin. Oncol. 2003;21(11):2138–2146. doi: 10.1200/JCO.2003.12.154. [DOI] [PubMed] [Google Scholar]
- 70.Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim DW. Health-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemia. Leuk. Res. 2012;36(4):438–442. doi: 10.1016/j.leukres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2013;31(10):1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood WA, Deal AM, Abernethy A, et al. Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2013;19(3):450–459. doi: 10.1016/j.bbmt.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J. Clin. Oncol. 2012;30(34):4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 75.Basch E, Minasian L, Burke L, et al. Symptom measurement in clinical trials. Presentated at: The 2011 Conference on Clinical Cancer Research; 10 November 2011; Washington DC, USA. [Google Scholar]
- 76.Williams G, Pazdur R, Temple R. Assessing tumor-related signs and symptoms to support cancer drug approval. J. Biopharm. Stat. 2004;14(1):5–21. doi: 10.1081/BIP-120028503. [DOI] [PubMed] [Google Scholar]
- 77.Acquadro C, Conway K, Hareendran A, Aaronson N. Literature review of methods to translate health-related quality of life questionnaires for use in multinational clinical trials. Value Health. 2008;11(3):509–521. doi: 10.1111/j.1524-4733.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 78.Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr. Oncol. 2007;14(5):173–179. doi: 10.3747/co.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support. Care Cancer. 2013;21(6):1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J. Pain Symptom. Manage. 2009;37(4):715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmermann C, Yuen D, Mischitelle A, et al. Symptom burden and supportive care in patients with acute leukemia. 2013;37(7):731–736. doi: 10.1016/j.leukres.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirkova J, Davis MP, Walsh D, et al. Cancer symptom assessment instruments: a systematic review. J. Clin. Oncol. 2006;24(9):1459–1473. doi: 10.1200/JCO.2005.02.8332. [DOI] [PubMed] [Google Scholar]
- 83.Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR good research practices for evaluating and documenting content validity for the use of existing instruments and their modification pro task force report. Value Health. 2009;12(8):1075–1083. doi: 10.1111/j.1524-4733.2009.00603.x. [DOI] [PubMed] [Google Scholar]
- 84.Cella D, Li JZ, Cappelleri JC, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon-α: results from a Phase III randomized trial. J. Clin. Oncol. 2008;26(22):3763–3769. doi: 10.1200/JCO.2007.13.5145. [DOI] [PubMed] [Google Scholar]
- 85.Cleeland CS, Mendoza TR. Symptom measurement by patient report. In: Cleeland CS, Fisch MJ, Dunn AJ, editors. Cancer Symptom Science. NY, USA: Cambridge University Press; 2011. pp. 268–284. [Google Scholar]
- 86.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ, USA: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 87.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J. Biopharm. Stat. 2004;14(1):73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 88.Williams LA, Agarwal S, Bodurka DC, Saleeba AK, Sun CC, Cleeland CS. Capturing the patient’s experience: using qualitative methods to develop a measure of patient-reported symptom burden: an example from ovarian cancer. J. Pain Symptom Manage. 2013 doi: 10.1016/j.jpainsymman.2013.02.007. Pii: S0885-S3924(13)00183–00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the MD. Anderson Symptom Inventory. Oncologist. 2011;16(2):217–227. doi: 10.1634/theoncologist.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk. Res. 2009;33(9):1199–1203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fisch MJ, Lee JW, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J. Clin. Oncol. 2012;30(16):1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tevaarwerk AJ, Lee JW, Sesto ME, et al. Employment outcomes among survivors of common cancers: the Symptom Outcomes and Practice Patterns (SOAPP) study. J. Cancer Surviv. 2013;7(2):191–202. doi: 10.1007/s11764-012-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013 doi: 10.1002/cncr.28376. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]