Abstract
“As the clarification and development of neurophysiological biomarkers continues, shifts in our approach to diagnosis and treatment decisions should follow. After all, the success of precision medicine lies within these neuroscientific advances, and will likely be the roadmap to a next-generation brain-based Diagnostic and Statistical Manual.”
Keywords: mismatch negativity, neuroplasticity, psychosis, risk
Following the release of the recently updated fifth edition of the Diagnostic and Statistical Manual (DSM-V), psychiatry has fallen under fire from critics outside and even within the field [101,102]. Among the most frequently mentioned criticisms is that diagnosis and treatment decisions are based largely on patient reports, behavioral observation and our ability to make inferences about patients’ true inner experiences (e.g., clinical judgment), rather than objective laboratory tests. Psychiatric researchers have long recognized that our current symptom-based diagnostic approach is inconsistent with our emerging understanding of the overlapping neural networks that subserve multiple psychiatric illnesses [1]. To address these and other shortcomings, the National Institutes of Mental Health (NIMH) has launched the Research Domain Criteria Project (RDoC) as a framework for the next generation of neuropsychiatric research. In this forthcoming RDoC era, researchers are encouraged to directly assay deficiencies in neural systems in order to guide diagnosis, develop and inform treatments, and predict and track outcomes. The RDoC aims to further expand our knowledge of brain–behavior relationships, and ultimately infuse this understanding of neural dysfunction into clinical practice and accelerate the development of more effective treatments. This paradigmatic shift toward “precision medicine” joins brain-based disruption with clinical observation, serving to align patient and provider treatment goals for more effective outcomes. Here, we provide an example of a translatable EEG biomarker, mismatch negativity (MMN), that offers great promise for improving our understanding, treatment, and perhaps even the prevention of a severely disabling and frequently intractable condition: psychosis.
Many candidate biomarkers have provided critical insights into the pathophysiology of schizophrenia and related psychotic disorders. Some of these biomarkers include: prepulse inhibition of the acoustic startle reflex [2,3], and EEG-based measures such as the P300 event-related potential amplitude [4] and cortical oscillatory dynamics [5]. In this paper, we focus on MMN [6]. In concert with efforts to further infuse neuroscience into psychiatric assessment and care, an expert consensus panel formed as part of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative highlighted MMN as one of the more “mature” biomarkers that is “promising” and ready for immediate incorporation into multisite clinical trials [7]. Recently, this measure has been described as a “break-through biomarker” [8] that is “translatable” [9] and potentially “the one we’ve been waiting for” [10] in neuropsychiatry.
Auditory MMN: critical findings in psychosis
Auditory MMN reflects an automatic change detection process that is elicited in response to unattended and infrequent sound stimuli embedded in a sequence of frequently presented standard stimuli. The MMN is elicited when a stimulus physically differs (e.g., in duration, pitch, intensity) from the context of the standard trials, and also during a sequential pattern violation [11]. Importantly, because MMN does not require sustained task engagement or even consciousness [12,13], it is thought to reflect an initial step from bottom-up sensory information processing leading to the conscious awareness of environmental change. MMN amplitude reduction in schizophrenia was first reported over 20 years ago [14], and subsequent studies have consistently shown a reduction of MMN in chronic (effect size Cohen’s d = 1.00 [14–23]), recent onset [21–30] and even unmedicated schizophrenia patients [16,25,28,31,32]. Over the past two decades, other studies have demonstrated robust relationships among MMN deficits and clinical and functional impairments (e.g., [33–35]). MMN amplitude exhibits utility as a repeated measure with high test–retest stability over short and long (e.g., 12-month) retest intervals in both healthy subjects and schizophrenia patients (retest correlation = 0.90 [36]), comparable to or exceeding reliability levels observed in common neuropsychological tasks [37]. Additionally, MMN testing is well-tolerated by a wide range of patients [32,38]. Based on this collection of attributes, MMN fulfills criteria for use as a biomarker in clinical outcome studies [37]. Moreover, MMN accounts for substantial portions of variance in cognition [6,39,40], psychosocial functioning [29,41–43] and level of independence in community living [35].
The vast majority of MMN studies in psychosis, however, have been cross-sectional characterizations of deficits in patients who have already experienced a psychotic event. What is the time course of the emergence of MMN deficits? Are deficits present prior to the onset of psychosis? The answers to these critical questions are beginning to be addressed in longitudinal biomarker validation studies [28,44,45].
Using biomarkers to develop preemptive interventions for psychosis
There has been a recent surge of interest in improving the prediction of psychosis onset in individuals at high risk for developing schizophrenia (for review see [9], also [45,46]). In the past decade, several research groups have developed clinical criteria to identify individuals at clinical high-risk for psychosis. Under the Criteria of Prodromal Syndromes (COPS) [47] or the comparable At-Risk Mental States criteria (ARMS) [48], 18–36% of the individuals identified as clinical high-risk for psychosis subsequently developed a psychotic disorder within a 2–3-year follow-up period [49,50]. This means that approximately 65–80% of individuals identified as being at high risk do not convert to psychosis. This low hit rate is a major barrier for attempting prophylactic pharmacologic interventions, particularly with antipsychotic medications, which cause metabolic or motor side effects. Ultimately, this lack of predictive power has raised doubts about the utility of the clinical high-risk syndrome [51].
Recently, longitudinal studies have shown that the prediction of the onset of psychosis in individuals at clinical high-risk can be considerably improved by MMN recordings [9,45]. In the first of these studies, Bodatsch et al. compared high-risk participants who converted to psychosis relative to nonconverters during a follow-up period of approximately 3 years [28]. At baseline, converters had significantly smaller MMN amplitude comparable to that in early-illness psychosis patients; MMN in non-converters was comparable to that of healthy age-matched controls. As an illustration of MMN as a biomarker, greater severity of MMN deficits contributed to higher estimates of individualized risk. Furthermore, Perez and colleagues [45] showed that attenuated MMN amplitude can be used to forecast the time lag to psychosis onset in high-risk individuals – those with more severe MMN abnormalities more imminently developed psychosis. These and related studies [30,44,45,52] demonstrate the feasibility of identifying biomarkers that are associated with disease vulnerability, predicting the development of psychosis, estimating the interval to psychosis onset, and enhancing individualized risk-estimation/prevention strategies [10].
Predicting therapeutic response: towards biomarker-informed treatment stratification
While it is now widely recognized that neurocognitive impairments present in most psychosis patients contribute to the severity of psychosocial disability, we can now be optimistic in our ability to ameliorate these impairments. Emerging findings indicate that the impaired neural systems of psychiatric illnesses are not fixed, but may be modified by carefully designed training interventions that harness neuroplasticity-based learning mechanisms [53–55]. One promising intervention, targeted cognitive training (TCT), is designed to sharpen the accuracy and fidelity of auditory information processing in psychosis via daily computer-based cognitive exercises [53,56]. Plastic changes within the neural systems that subserve early perceptual processing are thought to feed forward to enhance higher order cognition [56]. Studies in psychosis patients who completed 50 h (1 h/day, 5 days/week) of TCT demonstrated large effect size gains that generalized to auditory-dependent cognitive domains (verbal learning and memory; effect size Cohen’s d = 0.86–0.89), as well as global cognition (effect size Cohen’s d = 0.86) and quality of life [53]. Although TCT is efficacious at the group level, individual participant responses vary considerably; some patients show little or no benefit after even a full course of training [53]. As such, there is a need to identify predictive biomarkers of response to this daily, resource-intensive intervention. Since MMN is regarded as a robust, reliable and sensitive index of central auditory system plasticity [57] with important relationships to cognition and psychosocial functioning [33,35,36,58], could it also serve as a biomarker that predicts or corresponds to changes following TCT? Studies are underway to investigate this application, with notable precedents showing that MMN predicts response to intensive computerized cognitive training [59] and psychosocial skills training [41] in clinical populations.
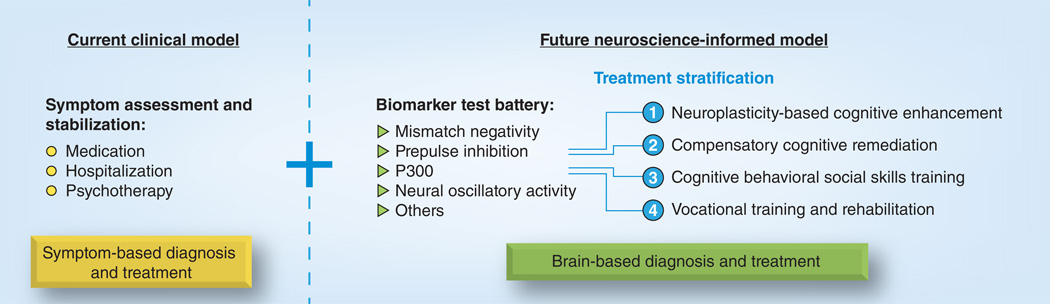
Since MMN improves the prediction of psychosis in clinically high-risk individuals and it reflects the neural systems targeted by TCT, it may prove useful in future treatment stratification algorithms (Figure 1). Current symptom-based models of diagnosis and treatment employ clinical assessment for symptom stabilization using medication, hospitalization and psychotherapy as treatment methods. As illustrated in the figure, the addition of biomarker profiles could indicate neural circuitry patterns subserving/predicting: 1) disruption of auditory processing centers calling for neuroplasticity-based cognitive enhancing treatment such as TCT; 2) neuropsychological impairment to be addressed with compensatory cognitive remediation strategies [60]; 3) maladaptive thoughts and social skills targeted by cognitive behavioral and social skills treatment for psychosis [61]; or 4) impaired role development requiring vocational training and rehabilitation services [62,63]. If successful, biomarker-informed treatment stratification could delineate subgroups of individuals for better responses to even currently available treatments and contribute to future diagnostic classifications.
Figure 1.
Clinical and neuroscience-based models combine to improve diagnosis and develop treatment algorithms for psychiatric illness.
Future directions & unresolved issues
While it is easy to argue that MMN and related neurophysiological biomarkers have tremendous promise, much work is still required for their safe and effective application in clinical settings. For example, with a low base rate of psychosis in the general population and a movement towards implementing more widespread screening procedures in schools and clinics, false positives, the potential for misuse and other problems are a certainty. Aside from the substantial validation that will be necessary to develop protocols for considering false positives, biomarker instrumentation also needs to be greatly simplified for administration by nonspecialists. Studies are underway using low-cost, portable systems reliable for multisite studies, similar to electrocardiography, with even fewer, smaller and easier-to-use electrodes. Biomarker tools could also capitalize on telemetry monitoring, where testing could take place in clinics, with data being uploaded to the cloud for sophisticated offline analyses and expert consultation, if required. Aside from improving the hardware, software and analytic capacity, we still do not know which (if any) parameters are maximally predictive of therapeutic response. If these barriers can be overcome, lengthy government and other regulatory oversight will be required.
Conclusion
The field of neuropsychiatry has made transformative advances in our understanding of the neural dynamics of normal and aberrant brain processes. In addition, many patients benefit from current mental health treatments that reduce seriously impairing symptoms and improve quality of life and daily functioning – facts that are often overlooked by critics of our field. Still, patients and their loved ones have grown impatient with our failure to take some of the promising advances out of the laboratories and into the clinics. Care providers have similarly called for upgrading our therapeutic arsenals to better combat the complex disabling conditions they face at the front lines of their clinics. Given the paradigmatic shift of the NIMH to apply a more dimensional RDoCs template to fuel ongoing research, we can continue to be optimistic about the future utility of biomarkers derived from clinical neuroscience. With many barriers to widespread implementation, a most promising example, MMN, can be used in conjunction with careful clinical assessment to identify individuals at highest imminent risk for developing serious mental illnesses to inform early intervention decisions. To avoid undesirable medication side effects, cognitive training and/or psychosocial interventions may be course-altering early treatment options.
The time is ripe for advancing the use of biomarkers to redefine illness criteria and evaluate treatment efficacy. Qualitative symptom descriptions no longer need to be used as a stopgap for diagnostic clarity. As the clarification and development of neurophysiological biomarkers continues, shifts in our approach to diagnosis and treatment decisions should follow. After all, the success of precision medicine lies within these neuroscientific advances [64], and will likely be the roadmap to a next-generation brain-based Diagnostic and Statistical Manual.
Acknowledgments
This work was supported by the National Alliance for Research on Schizophrenia and Depression/Brain and Behavior Research Foundation, and grants from the UCSD Clinical & Translational Research Institute, Department of Veteran’s Affairs (VISN 22 Mental Illness Research, Education and Clinical Center), and the National Institute of Mental Health (MH079777, MH042228, MH065571, UL RR031980 and UL 1TR000100). NR Swerdlow is a consultant for Neurocrine, Inc.
Biographies

Veronica B Perez

Gregory A Light
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Veronica B Perez, VISN-22 Mental Illness Research, Education & Clinical Center (MIRECC), VA San Diego Healthcare System, CA 92161, USA; Department of Psychiatry, University of California San Diego, La Jolla, CA, USA.
Neal R Swerdlow, Department of Psychiatry, University of California San Diego, La Jolla, CA, USA.
David L Braff, Department of Psychiatry, University of California San Diego, La Jolla, CA, USA.
Risto Näätänen, Center of Functionally Integrative Neuroscience (CFIN), University of Arhus, Arhus, Denmark; Department of Psychology, University of Tartu, Tartu, Estonia; Institute of Behavioral Sciences, University of Helsinki, Helsinki, Finland.
Gregory A Light, Author for correspondence: VISN-22 Mental Illness Research, Education & Clinical Center (MIRECC), VA San Diego Healthcare System, CA 92161, USA; Department of Psychiatry, University of California San Diego, La Jolla, CA, USA glight@ucsd.edu.
References
- 1.Swerdlow NR. Are we studying and treating schizophrenia correctly? Schizophr. Res. 2011;130:1–10. doi: 10.1016/j.schres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl.) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- 5.Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Näätänen R, Gaillard AW, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. (Amst.) 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 7.Butler PD, Chen Y, Ford JM, et al. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr. Bull. 2012;38:81–91. doi: 10.1093/schbul/sbr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Light GA, Näätänen R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc. Natl Acad. Sci. USA. 2013;110:15175–15176. doi: 10.1073/pnas.1313287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai T, Tada M, Kirihara K, Araki T, Jinde S, Kasai K. Mismatch negativity as a ‘translatable’ brain marker toward early intervention for psychosis: a review. Front. Psychiatry. 2013;4:115. doi: 10.3389/fpsyt.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belger A, Yucel GH, Donkers FC. In search of psychosis biomarkers in high-risk populations: is the mismatch negativity the one we’ve been waiting for? Biol. Psychiatry. 2012;71:94–95. doi: 10.1016/j.biopsych.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Näätänen R, Astikainen P, Ruusuvirta T, Huotilainen M. Automatic auditory intelligence: an expression of the sensory-cognitive core of cognitive processes. Brain Res. Rev. 2010;64:123–136. doi: 10.1016/j.brainresrev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Näätänen R. The mismatch negativity: a powerful tool for cognitive neuroscience. Ear Hear. 1995;16:6–18. [PubMed] [Google Scholar]
- 13.Näätänen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. Neuroreport. 1992;3:493–496. doi: 10.1097/00001756-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol. Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 15.Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 16.Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am. J. Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 17.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual contextdependent processing in schizophrenia: defining the pattern. Arch. Gen. Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 18.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int. J. Psychophysiol. 2001;42:177–194. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- 19.Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol. Psychiatry. 2003;53:1120–1131. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 20.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch. Gen. Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 22.Oknina LB, Wild-Wall N, Oades RD, et al. Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophr. Res. 2005;76:25–41. doi: 10.1016/j.schres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Oades RD, Wild-Wall N, Juran SA, Sachsse J, Oknina LB, Röpcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. doi: 10.1186/1471-244X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch. Gen. Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkötter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr. Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol. Psychiatry. 2006;59:762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol. Psychiatry. 2011;69:959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 29.Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol. Med. 2012;42:85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol. Psychiatry. 2012;71:98–104. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Kirino E, Inoue R. The relationship of mismatch negativity to quantitative EEG and morphological findings in schizophrenia. J. Psychiatr. Res. 1999;33:445–456. doi: 10.1016/s0022-3956(99)00012-6. [DOI] [PubMed] [Google Scholar]
- 32.Rissling AJ, Braff DL, Swerdlow NR, et al. Disentangling early sensory information processing deficits in schizophrenia. Clin. Neurophysiol. 2012;123:1942–1949. doi: 10.1016/j.clinph.2012.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J. Cogn. Neurosci. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J. Int. Neuropsychol. Soc. 2007;13:653–663. doi: 10.1017/S1355617707070816. [DOI] [PubMed] [Google Scholar]
- 35.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch. Gen. Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 36.Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am. J. Psychiatry. 2005;162:1741–1743. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- 37.Light GA, Swerdlow NR, Rissling AJ, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS ONE. 2012;7:e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi H, Rissling AJ, Pascual-Marqui R, et al. Neural substrates of normal and impaired preattentive sensory discrimination in large cohorts of nonpsychiatric subjects and schizophrenia patients as indexed by MMN and P3a change detection responses. NeuroImage. 2012;66C:594–603. doi: 10.1016/j.neuroimage.2012.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldeweg T, Klugman A, Gruzelier J, Hirsch SR. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr. Res. 2004;69:203–217. doi: 10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Näätänen R, Kujala T, Kreegipuu K, et al. The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134:3435–3453. doi: 10.1093/brain/awr064. [DOI] [PubMed] [Google Scholar]
- 41.Kawakubo Y, Kamio S, Nose T, et al. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–265. doi: 10.1016/j.psychres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol. Psychiatry. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr. Bull. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaikh M, Valmaggia L, Broome MR, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophr. Res. 2012;134:42–48. doi: 10.1016/j.schres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Perez VB, Woods SW, Roach BJ, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.038. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy JR, Rawdon C, Kelleher I, et al. Reduced duration mismatch negativity in adolescents with psychotic symptoms: further evidence for mismatch negativity as a possible biomarker for vulnerability to psychosis. BMC Psychiatry. 2013;13:45. doi: 10.1186/1471-244X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 48.Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust. NZ J. Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- 49.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 51.Yang LH, Wonpat-Borja AJ, Opler MG, Corcoran CM. Potential stigma associated with inclusion of the psychosis risk syndrome in the DSM-V: an empirical question. Schizophr. Res. 2010;120:42–48. doi: 10.1016/j.schres.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS ONE. 2013;8:e54080. doi: 10.1371/journal.pone.0054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merzenich M, Wright B, Jenkins W, et al. Cortical plasticity underlying perceptual, motor, and cognitive skill development: implications for neurorehabilitation. Cold Spring Harb. Symp. Quant. Biol. 1996;61:1–8. [PubMed] [Google Scholar]
- 55.Rogowsky BA, Papamichalis P, Villa L, Heim S, Tallal P. Neuroplasticity-based cognitive and linguistic skills training improves reading and writing skills in college students. Front. Psychol. 2013;4:137. doi: 10.3389/fpsyg.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Näätänen R. Mismatch negativity (MMN) as an index of central auditory system plasticity. Int. J. Audiol. 2008;47(Suppl. 2):S16–S20. doi: 10.1080/14992020802340116. [DOI] [PubMed] [Google Scholar]
- 58.Rissling AJ, Park SH, Young JW, et al. Demand and modality of directed attention modulate ‘pre-attentive’ sensory processes in schizophrenia patients and nonpsychiatric controls. Schizophr. Res. 2013;146:326–335. doi: 10.1016/j.schres.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovio R, Halttunen A, Lyytinen H, Näätänen R, Kujala T. Reading skill and neural processing accuracy improvement after a 3-hour intervention in preschoolers with difficulties in reading-related skills. Brain Res. 2012;1448:42–55. doi: 10.1016/j.brainres.2012.01.071. [DOI] [PubMed] [Google Scholar]
- 60.Twamley EW, Vella L, Burton CZ, Heaton RK, Jeste DV. Compensatory cognitive training for psychosis: effects in a randomized controlled trial. J. Clin. Psychiatry. 2012;73:1212–1219. doi: 10.4088/JCP.12m07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Granholm E, McQuaid JR, McClure FS, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am. J. Psychiatry. 2005;162:520–529. doi: 10.1176/appi.ajp.162.3.520. [DOI] [PubMed] [Google Scholar]
- 62.Twamley EW, Baker DG, Norman SB, Pittman JO, Lohr JB, Resnick SG. Veterans Health Administration vocational services for Operation Iraqi Freedom/Operation Enduring Freedom Veterans with mental health conditions. J. Rehabil. Res. Dev. 2013;50:663–670. doi: 10.1682/jrrd.2012.08.0137. [DOI] [PubMed] [Google Scholar]
- 63.Twamley EW, Vella L, Burton CZ, Becker DR, Bell MD, Jeste DV. The efficacy of supported employment for middle-aged and older people with schizophrenia. Schizophr. Res. 2012;135:100–104. doi: 10.1016/j.schres.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braff L, Braff DL. The neuropsychiatric translational revolution: still very early and still very challenging. JAMA Psychiatry. 2013;70:777–779. doi: 10.1001/jamapsychiatry.2013.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Frances A. NIMH vs DSM-5: no one wins, patients lose. 2013 www.huffingtonpost.com/allen-frances/nimh-vs-dsm-5-no-one-wins_b_3252323.html.
- 102.Hyman SE. Psychiatric drug development: diagnosing a crisis. 2013 www.dana.org/news/cerebrum/detail.aspx?id=41290. [PMC free article] [PubMed]