Abstract
Background
This study aimed to characterize and differentiate the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy 2011 cut points through the modified Medical Research Council dyspnea scale (mMRC) and chronic obstructive pulmonary disease (COPD) assessment test (CAT).
Methods
Analysis of COPD patient data from the 2012 Adelphi Respiratory Disease Specific Program was conducted in Europe and US. Matched data from physicians and patients included CAT and mMRC scores. Receiver operating characteristic curves and kappa analysis determined a cut point for CAT and mMRC alignment and thus defined patient movement (“movers”) within GOLD groups A–D, depending on the tool used. Logistic regression analysis, with a number of physician- and patient-reported covariates, characterized those movers.
Results
Comparing GOLD-defined high-symptom patients using mMRC and CAT cut points (≥2 and ≥10, respectively), there were 890 (53.65%) movers; 887 of them (99.66%) moved from less symptomatic GOLD groups A and C (using mMRC) to more symptomatic groups B and D (using CAT). For receiver operating characteristic (area under the curve: 0.82, P<0.001) and kappa (maximized: 0.45) recommended CAT cut points of ≥24 and ≥26, movers reduced to 429 and 403 patients, respectively. Logistic regression analysis showed variables significantly associated with movers were related to impact on normal life, age, cough, and sleep (all P<0.05). Within movers, direction of movement was significantly associated with the same variables (all P<0.05).
Conclusion
Use of current mMRC or CAT cut points leads to inconsistencies for COPD assessment classification. It is recommended that cut points are aligned and both tools administered simultaneously for optimal patient care and to allow for closer management of movers. Our research may suggest an opportunity to investigate a combined score approach to patient management based on the worst result of mMRC and CAT. The reduced number of remaining movers may then identify patients who have greater impact of disease and may require a more personalized treatment plan.
Keywords: GOLD classification, mMRC, CAT, cut points, kappa analysis, receiver operating curves
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex global disease, which requires a simple approach to management. In recognition of these complexities, the Global initiative for Chronic Obstructive Lung Disease (GOLD) group has provided recommendations for appropriate diagnosis and treatment of COPD. In 2011, the GOLD strategy1 moved away from a linear, one-dimensional classification of severity groups, defined solely by degree of airflow limitation (forced expiratory volume [FEV1]), to a two-dimensional assessment that takes into account exacerbation risk, measured by exacerbation history and/or FEV1, and current symptoms, measured by either the modified Medical Research Council dyspnea scale (mMRC) or the COPD assessment test (CAT).2 An updated GOLD report was released in January 2014 based on further published scientific research since completion of the 2011 document but maintains the same treatment paradigm.3
This new classification creates four GOLD groups stratified by exacerbation risk (low, high) and symptoms (low, high), with patient treatment recommendations dependent on group allocation: A: low risk, low symptoms; B: low risk, high symptoms; C: high risk, low symptoms; D: high risk, high symptoms.2 Exacerbation risk is defined by the greater level of either history of exacerbations in the previous year (0–1, low risk and 2+, high risk) or by spirometry-determined airflow limitation (GOLD stages 1 and 2, low risk [FEV1≥50% predicted] and stages 3 and 4, high risk [FEV1 <50% predicted]). However, it should be noted that one or more hospitalizations for COPD exacerbations should be considered high risk.3 Current symptoms are measured by either the mMRC (0 or 1, low symptoms and 2+, high symptoms) or the CAT (<10, low symptoms and ≥10, high symptoms). GOLD recognizes that COPD is multi-symptomatic and that mMRC measures breathlessness only. It therefore recommends that additional comprehensive symptom assessment is undertaken using questionnaires such as CAT.3
Real-world evidence has become an established method for examining treatment practices in primary and specialist care across a large number of disease areas, and demand continues for better evidence of the practical and real-world effects of treatments.4 It is complementary to data provided by randomized clinical trials and addresses gaps in current knowledge. In this research, the matched elements of physician-reported (exacerbations/FEV1) and patient-reported (mMRC and CAT) variables provided a unique data source to enable the categorization of patients into the GOLD groups A to D.
This research sought to assess the implications of the revised GOLD strategy on the consulting population by understanding the degree of CAT and mMRC alignment given the cut points recommended by GOLD. Additionally, it aimed to characterize the patients who have a different GOLD classification depending on which instrument is used – the movers – and to determine a CAT cut point that would more closely align the two populations in order to provide a consistent approach to patient classification and treatment.
Materials and methods
Study design and population
Data were from a retrospective analysis of the Adelphi Real World Respiratory Disease Specific Program (DSP) conducted from September to December 2012 in France, Germany, Spain, Italy, UK, and USA. These surveys are large, multinational, and cross-sectional and collect data from physicians and patients in real-world clinical settings thereby providing a holistic picture of a disease and its treatment. They do not aim to demonstrate cause and effect and have no set hypothesis. The DSP is an established method for investigating multicenter treatment practices across a variety of disease areas. The full methodology has been published previously.5
Physicians were a geographically representative sample of primary care physicians and pulmonologists, who had qualified between 1977 and 2006, were actively involved in COPD management, and saw three or more COPD patients per physician in a typical week. Included subjects were the next six consulting patients of 40 years of age and older, with a history of smoking and with a confirmed diagnosis of COPD (COPD-only or with mixed COPD and asthma). For the purposes of this analysis, patients with a mixed asthma diagnosis were excluded. It was also deemed irrelevant for the purposes of this observational survey if physicians had applied, or were even aware of, GOLD criteria to their patients as GOLD categorization was performed retrospectively on the dataset.
Data collection
Physicians completed a detailed patient record form, which included spirometry and exacerbation history, for the next six consecutive consulting COPD patients. The same patients were invited to fill out a voluntary, confidential, anonymized patient self-completion form. Unique numerical identifiers allowed matching of physician and patient recorded data. Questions related primarily to demographics, symptomology, quality of life, and disease burden, including EuroQol-5D6 and the Work Productivity and Activity Impairment Questionnaire.7 Mandatory questions for inclusion in this analysis included completion of both the CAT8 and the mMRC.9,10
The mMRC is a patient-reported ordinal-rating scale measuring levels of dyspnea. Patients are asked which of five descriptions of breathlessness best describes their impairment, with a choice of 0, “only breathless with serious exercise”, to 4, “I am too breathless to leave the house”; high symptoms according to current GOLD recommendations equal ≥2.2 The CAT questionnaire measures the health status of COPD patients. Eight statements describe best and worst case scenarios; patients decide where they fit on a scale of 0–5 (best to worst) for each statement. The total score range is 0–40.8 High symptoms according to current GOLD recommendation are ≥10.3
Symptom evaluation for a GOLD group (A–D) is defined by the results of one of these two questionnaires. For the purposes of this research, patients who were classified into different symptom groups, depending on the symptom questionnaire and cut point being used, were termed as movers.
The survey was performed according to the European Pharmaceutical Market Research Association guidelines11 and in full accordance with the US Health Insurance Portability and Accountability Act 199612 and European equivalents.11,13 Each patient provided consent for deidentified and aggregated reporting of research findings as required by the guidelines. Data were deidentified prior to receipt by Adelphi.
Statistical analysis
Receiver operating characteristic (ROC) curves analysis14 was conducted to determine the optimal cut point of CAT to match the mMRC cut point of ≥2 recommended by the GOLD strategy. We employed nonparametric estimation of the ROC curve and selected the most appropriate CAT cut point by maximizing the Youden index,15–17 the sum of sensitivity and specificity. The area under the curve (AUC) was calculated using the Mann–Whitney–Wilcoxon test method; this can range between 0 and 1, representing expected performance. An AUC of 0.5 is equivalent to random guessing or the lack of a relationship, whereas an AUC of 1 is equivalent to the reference standard. Preliminary ROC curves analysis was also conducted to determine the optimal cut point of mMRC to match the CAT cut point of ≥10 recommended by the GOLD strategy. However, in all further analysis, mMRC was selected as the reference as it is more evenly split between low and high, and CAT has more granularity and potential cut points.
Furthermore, the kappa statistic18 was used as a measure of interrater agreement for qualitative (categorical) items, scaled to be 0 when the amount of agreement is what would be expected to be observed by chance and 1 when there is perfect agreement. We determined which CAT cut point maximized the agreement with the mMRC cut point of ≥2 in determining presence of current symptoms (low, high) per patient, as recommended by the GOLD group.
In order to investigate what characterized a mover and what characterized those who moved left/right, we ran separate logistic regression analyses, respectively, for the following dependent variables: 1) whether or not a patient has been categorized differently by CAT and mMRC cut points (movers versus non movers), and 2) whether the patient moved left or right (movers-left versus movers-right) in the GOLD classification (for those categorized differently by CAT and mMRC cut points).
Standard errors were computed by adjusting for possible intragroup correlation within reporting physician. A list of covariates was as follows (all physician-reported): country, age, sex, ethnicity, smoking status, body mass index, physician comanagement of patients, rapid decliners, and Deyo–Charlson comorbidity index (CCI).19 Additionally, patient-reported variables, general non validated questions in the survey, were included as covariates: presence in past 4 weeks of phlegm, general chest tightness, cough, tiredness through lack of sleep, lack of energy, compromised daily activities, getting up and ready for the day, ability to lead a normal life due to COPD, and the Jenkins Sleep Questionnaire score.20 Variables with two-sided P<0.05 were retained in final regression models.
Regressions were repeated for the following cut points: CAT cut point as recommended by the GOLD group, optimal CAT cut points recommended by the ROC curves analysis, and CAT cut points recommended by the kappa statistic analysis.
Continuous variables were split into three binary variables according to tertiles where possible (or four variables in the case of body mass index). This allowed for nonlinear effects and to make interpretation easier for clinicians. Therefore, all variables used in regression analysis were discrete variables.
Statistical analyses were performed using STATA version 12.1 (StataCorp, College Station, TX, USA).21
Results
General patient population
Primary care physicians (318) and pulmonologists (319) from Europe and the US included a total of 3,830 patients, with each physician completing a patient record form for each patient. Of these patients, 2,054 completed a voluntary patient self-completion questionnaire and from this group, 1,659 were COPD-only and had completed both mMRC and CAT and were therefore included in the analysis. Patients were recruited in France (254, 15.3%), Germany (454, 27.3%), Italy (209, 12.6%), Spain (302, 18.2%), UK (52, 3.13%), and the US (388, 23.39%). Patient characteristics are summarized in Table 1.
Table 1.
Physician-reported patient characteristics (n=1,659 unless otherwise stated)
| Patient characteristics | Category | n (%) or mean (SD) |
|---|---|---|
| Sex | M | 1,122 (67.63) |
| F | 537 (32.37) | |
| Age in years | 65.14 (10.44) | |
| Ethnicity (1,644 patients) | White/Caucasian | 1,517 (91.4) |
| BMI (1,594 patients) | kg · m−2 | 26.77 (4.93) |
| Smoking status | Current smokers | 533 (32.13) |
| Ex-smokers | 1,126 (67.87) | |
| Lung function (FEV1) (1,073 patients) | FEV1 < 30 | 45 (4.19) |
| FEV1 ≥ 30, FEV1 < 50 | 203 (18.92) | |
| FEV1≥50, FEV1<80 | 713 (66.45) | |
| FEV1 ≥ 80 | 112 (10.44) | |
| COPD comanaged (more than one doctor), (1,645 patients) | 685 (41.29) | |
| How quickly disease is progressing (1,631 patients) | Slowly/average | 1,542 (92.95) |
| Rapid | 89 (5.36) | |
| Deyo–Charlson comorbidity index (CCI)a (1,528 patients) | 1.40 (0.74) |
Notes:
Comorbid conditions as described by Deyo–Charlston index19 are mapped from as many as ten reported ICD-9-CM secondary diagnosis codes. A single summary cumulative value is represented. A score of 0 represents no comorbidities. Since COPD was a prerequisite in this research, minimum score was 1. Out of the 17 conditions, this research could accommodate 12: myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, diabetes mellitus, cerebrovascular disease, COPD, connective tissue disease, mild liver disease, any malignancy including lymphoma and leukemia, AIDS, metastatic solid tumor.
Abbreviations: AIDS, acquired immunodeficiency syndrome; BMI, body mass index; COPD, chronic obstructive pulmonary disease; F, female; FEV1, forced expiratory volume in 1 second; ICD-9-CM, international classification of disease, ninth revision, clinical modification; M, male; SD, standard deviation.
Patient-reported COPD symptoms
Patient-reported general symptoms present in the previous 4 weeks included tightness in the chest, cough and coughing up of mucus, effect of their COPD on daily activities on a scale of 1–10 (no effect to prevented daily activities), whether they were restricted in their normal life because of COPD on a scale of 1–5 (not restricted at all to completely restricted), whether they ever lacked energy, and whether they ever experienced tiredness through lack of sleep. Patients also reported on the impact of COPD on getting up and ready for the day. A complete breakdown of patient-reported COPD characteristics can be seen in Table 2.
Table 2.
Patient-reported patient COPD characteristics (n=1,659 unless otherwise stated)
| Questions | Description | n (%) or mean (SD) |
|---|---|---|
| Breathing symptoms present in last 4 weeks, (1,514 patients) | Coughing up mucus and phlegm | 649 (39.12) |
| General tightness in chest | 340 (20.49) | |
| Cough | 878 (52.92) | |
| Breathing condition’s effect on daily activities in past week: (1,631 patients) | 0 No effect on daily activities | 127 (7.66) |
| 1 | 194 (11.69) | |
| 2 | 232 (13.98) | |
| 3 | 262 (15.79) | |
| 4 | 185 (11.15) | |
| 5 | 130 (7.84) | |
| 6 | 151 (9.10) | |
| 7 | 124 (7.47) | |
| 8 | 134 (8.08) | |
| 9 | 55 (3.32) | |
| 10 Condition prevented daily activities | 37 (2.23) | |
| Extent breathing condition has restricted normal life: (1,625 patients) | 1 Not at all restricted | 226 (13.62) |
| 2 | 460 (27.73) | |
| 3 | 482 (29.05) | |
| 4 | 353 (21.28) | |
| 5 Completely restricted | 104 (6.27) | |
| Do you ever experience the following feelings, (1,623 patients) | Constant lack of energy | 588 (35.44) |
| Tiredness through lack of sleep | 476 (28.69) | |
| Impact breathing condition has on getting up and ready for the day: (1,634 patients) | 1 No impact | 413 (24.89) |
| 2 | 412 (24.83) | |
| 3 | 268 (16.15) | |
| 4 | 201 (12.12) | |
| 5 | 167 (10.07) | |
| 6 | 103 (6.21) | |
| 7 Constant impact | 70 (4.22) | |
| Jenkins sleep scale (1,588 patients) | (scale 0–20, where 0= no problems with sleep and 20= high impact on sleep), | 5.12 (4.65) |
Abbreviations: COPD, chronic obstructive pulmonary disease; SD, standard deviation.
ROC curves and kappa statistic analysis
Table 3 provides the results of the ROC and kappa statistic analyses. For each cut point of the CAT score, the sensitivity, specificity, Youden index, and the kappa statistic are presented. The Youden index is maximized at ≥24 and the kappa statistic is maximized at ≥26.
Table 3.
ROC curves analysis and kappa statistic analysis
| Cut point | ROC Points
|
Kappa statistic (95% CI) | ||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Youden index (bootstrap 95% CI) | ||
| ≥0 | 100.00 | 0.00 | ||
| ≥1 | 100.00 | 0.65 | 0.01 (0.00–0.01) | 0.00 (0.00–0.01) |
| ≥2 | 100.00 | 1.47 | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) |
| ≥3 | 100.00 | 2.40 | 0.02 (0.01–0.03) | 0.02 (0.01–0.02) |
| ≥4 | 100.00 | 3.96 | 0.04 (0.03–0.05) | 0.03 (0.02–0.04) |
| ≥5 | 100.00 | 5.53 | 0.06 (0.04–0.07) | 0.04 (0.03–0.05) |
| ≥6 | 100.00 | 7.00 | 0.07 (0.06–0.08) | 0.05 (0.04–0.06) |
| ≥7 | 99.83 | 9.12 | 0.09 (0.07–0.11) | 0.06 (0.05–0.07) |
| ≥8 | 99.83 | 10.97 | 0.11 (0.09–0.13) | 0.08 (0.06–0.09) |
| ≥9 | 99.83 | 15.21 | 0.15 (0.13–0.17) | 0.11 (0.09–0.13) |
| ≥10 | 99.48 | 18.25 | 0.18 (0.15–0.20) | 0.13 (0.11–0.15) |
| ≥11 | 98.95 | 22.95 | 0.22 (0.19–0.24) | 0.16 (0.14–0.19) |
| ≥12 | 98.61 | 27.93 | 0.27 (0.24–0.29) | 0.20 (0.18–0.23) |
| ≥13 | 97.91 | 32.26 | 0.30 (0.27–0.33) | 0.23 (0.21–0.26) |
| ≥14 | 96.86 | 35.67 | 0.33 (0.30–0.36) | 0.25 (0.22–0.29) |
| ≥15 | 95.99 | 39.54 | 0.36 (0.32–0.38) | 0.28 (0.25–0.31) |
| ≥16 | 94.60 | 42.49 | 0.37 (0.34–0.41) | 0.30 (0.27–0.33) |
| ≥17 | 93.38 | 47.28 | 0.41 (0.37–0.44) | 0.33 (0.30–0.39) |
| ≥18 | 90.07 | 50.14 | 0.40 (0.36–0.44) | 0.34 (0.30–0.37) |
| ≥19 | 88.15 | 54.84 | 0.43 (0.39–0.47) | 0.37 (0.33–0.40) |
| ≥20 | 86.06 | 58.53 | 0.45 (0.41–0.49) | 0.39 (0.35–0.43) |
| ≥21 | 81.71 | 62.49 | 0.44 (0.40–0.48) | 0.39 (0.35–0.43) |
| ≥22 | 78.40 | 67.56 | 0.46 (0.42–0.50) | 0.42 (0.38–0.46) |
| ≥23 | 73.34 | 71.61 | 0.45 (0.40–0.50) | 0.42 (0.42–0.38) |
| ≥24 | 70.38 | 76.13 | 0.47 (0.42–0.51) | 0.45 (0.40–0.49) |
| ≥25 | 64.46 | 80.00 | 0.44 (0.40–0.49) | 0.44 (0.40–0.49) |
| ≥26 | 59.93 | 84.06 | 0.44 (0.40–0.49) | 0.45 (0.40–0.50) |
| ≥27 | 53.48 | 87.83 | 0.41 (0.36–0.46) | 0.44 (0.40–0.48) |
| ≥28 | 46.86 | 91.34 | 0.38 (0.34–0.42) | 0.42 (0.37–0.46) |
| ≥29 | 39.20 | 94.01 | 0.33 (0.29–0.38) | 0.38 (0.33–0.42) |
| ≥30 | 33.97 | 96.13 | 0.30 (0.26–0.34) | 0.35 (0.30–0.39) |
| ≥31 | 29.27 | 97.70 | 0.27 (0.23–0.30) | 0.32 (0.28–0.36) |
| ≥32 | 25.09 | 98.43 | 0.24 (0.20–0.27) | 0.28 (0.24–0.32) |
| ≥33 | 19.34 | 99.26 | 0.19 (0.15–0.22) | 0.23 (0.19–0.27) |
| ≥34 | 15.51 | 99.63 | 0.15 (0.12–0.18) | 0.19 (0.15–0.22) |
| ≥35 | 11.32 | 99.83 | 0.11 (0.09–0.14) | 0.14 (0.11–0.17) |
| ≥36 | 7.49 | 99.83 | 0.07 (0.05–0.09) | 0.09 (0.07–0.12) |
| ≥37 | 5.57 | 99.83 | 0.05 (0.03–0.07) | 0.07 (0.05–0.09) |
| ≥38 | 3.83 | 99.91 | 0.04 (0.02–0.05) | 0.05 (0.02–0.07) |
| ≥39 | 2.79 | 99.91 | 0.02 (0.01–0.04) | 0.03 (0.02–0.05) |
| ≥40 | 1.39 | 99.91 | 0.01 (0.00–0.02) | 0.02 (0.00–0.03) |
| ≥41 | 0.00 | 100.00 | ||
Notes: ROC: 1,659 patients; Area under the curve (standard error) =0.82 (0.01); 95% CI 0.80, 0.84. Bold font indicates where the Youden index and kappa statistic are maximized.
Abbreviations: CI, confidence interval; ROC, receiver operating characteristic.
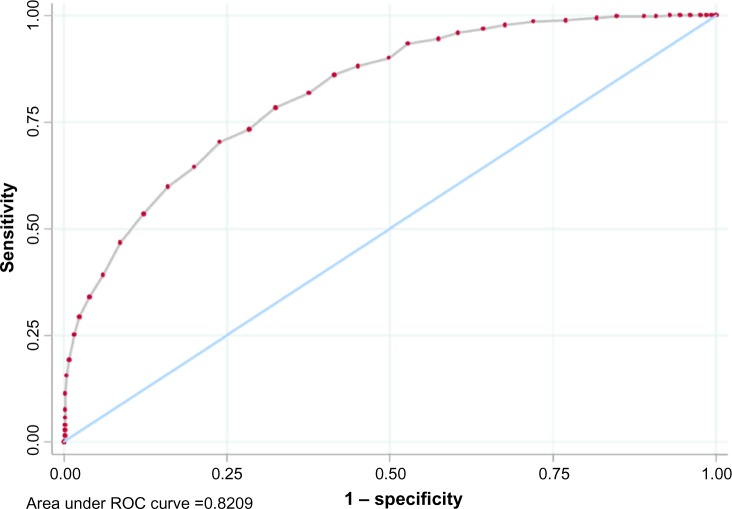
The AUC of 0.82 (with 95% confidence interval [CI] 0.80–0.84) in Figure 1 is significantly greater than 0.5 (P<0.001), which indicates that the CAT score has good discriminating power for the GOLD-recommended mMRC cut point of ≥2.
Figure 1.
Graphical representation of the ROC curves analysis for CAT and mMRC.
Abbreviations: CAT, Chronic Obstructive Pulmonary Disease (COPD) Assessment Test; mMRC, modified Medical Research Council dyspnea scale; ROC, receiver operating characteristic.
Of note, for an mMRC cut point of ≥1, the Youden index is maximized at the CAT cut point of ≥19 (0.52, 95% CI 0.47–0.56), with an AUC of 0.83 (95% CI 0.81–0.85), P<0.001. The kappa measure of agreement is maximized at the CAT cut point of ≥17 (0.47, 95% CI 0.42–0.51).
GOLD groups and definition of mover
GOLD-recommended cut point
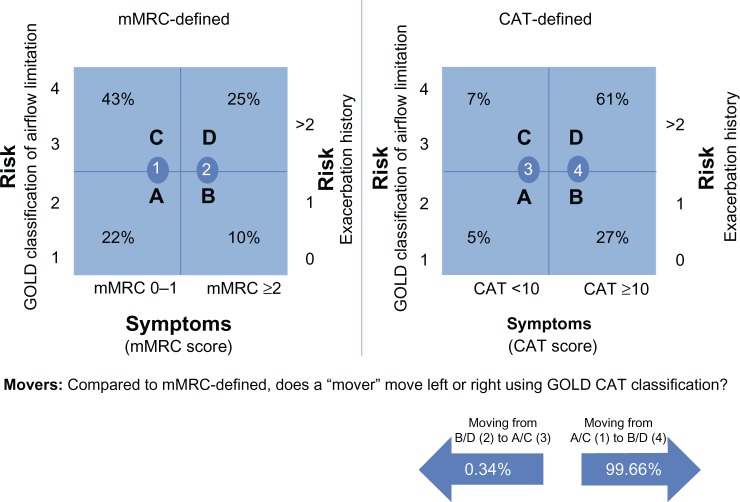
Out of the total 1,659 patients, there were 890 (53.65%) movers under the GOLD-recommended cut points (GOLD-recommended mMRC cut point ≥2 versus the GOLD-recommended CAT cut point of ≥10), three (0.34%) patients moved left, from mMRC higher symptomatic GOLD groups B and D to the lower CAT-defined symptomatic GOLD groups of A and C. Conversely, 887 (99.66%) patients moved right from mMRC lower symptomatic GOLD groups A and C to the higher CAT-defined symptomatic GOLD groups of B and D (see Figure 2).
Figure 2.
mMRC and CAT sized groups as defined by GOLD-recommended cut point (1,653 patients).
Abbreviations: CAT, Chronic Obstructive Pulmonary Disease (COPD) Assessment Test; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council dyspnea scale.
ROC-recommended cut point
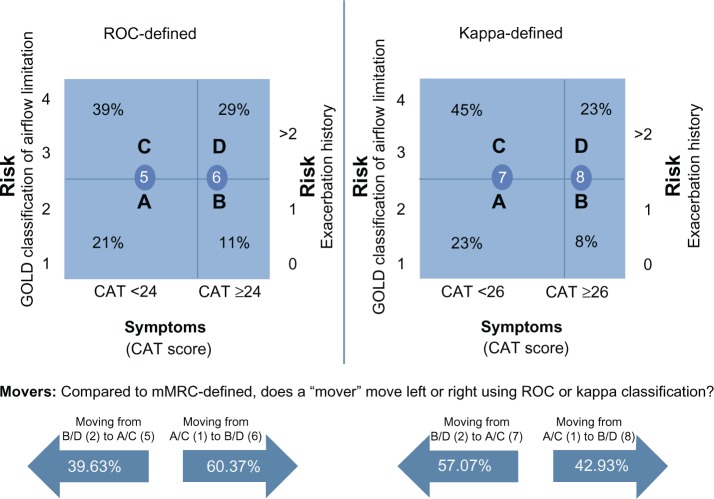
Of the total number of patients who moved under the ROC-recommended cut point of ≥24 (429), 170 (39.63%) moved left from mMRC higher symptomatic groups B and D to the newly defined CAT A and C lower symptomatic groups. The majority of patients, 259 (60.37%), still moved to the right from mMRC lower symptomatic groups A and C to the higher symptomatic ROC CAT-defined groups B and D, albeit to a lesser degree (see Figure 3).
Figure 3.
ROC and Cohen’s kappa-statistic sized segments as defined by ROC and kappa cut point (1,653 patients).
Abbreviations: CAT, Chronic Obstructive Pulmonary Disease (COPD) Assessment Test; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council dyspnea scale; ROC, receiver operating characteristic.
Kappa statistic-recommended cut point
Of the total number of patients who moved under the kappa statistic-recommended cut point of ≥26 (403), 230 (57.07%) patients moved left from mMRC higher symptomatic groups B and D to the newly defined CAT lower symptomatic groups A and C. Fewer patients 173 (42.92%) moved to the right from mMRC lower symptomatic groups A and C to the higher symptomatic groups of B and D defined by kappa statistic (see Figure 3).
Regression analysis on movers
GOLD-recommended cut point
The following were significantly associated with being a mover when defined according to the GOLD-recommended CAT cut point: being less than 70 years of age (P<0.001), being a smoker (P=0.042), having a cough in the previous 4 weeks (P=0.011), COPD having a medium effect (3–5 on a scale of 0–10, where 0 is no effect and 10 prevented from undertaking daily activities) on daily activities in the previous week (P<0.001), COPD having low restriction (1–3 on a scale of 1–5, where 1 is no restriction and 5 is complete restriction) on living a completely normal life (P<0.001), not experiencing constant lack of energy (P=0.001), COPD having no to medium impact (1–4 on a scale of 1–7, where 1 is no impact and 7 is constant impact) on getting up and ready for the day (P<0.001), and an increased Jenkins sleep scale score (≥3 on a scale of 0–20, where 0 is no impact with sleep and 20 is high impact on sleep; P=0.001).
Cut point recommended by ROC analysis
The following were significantly associated with being a mover when defined according to the ROC-recommended CAT cut point: the presence of a cough in the last 4 weeks (P=0.040), COPD having restricted or completely restricted impact (3–5 on a scale of 1–5) on living a normal life (P<0.001), COPD having no to medium impact (1–4 on a scale of 1–7) on getting up and ready for the day (P=0.003), and increased Jenkins sleep scale score (≥3 on a scale of 0–20; P<0.001).
Cut point recommended by the kappa statistic
The following were significantly associated with being a mover when defined according to the kappa-recommended CAT cut point: being 55 or more years of age (P=0.017), COPD having restricted or complete impact (3–5 on a scale of 1–5) on living a completely normal life (P<0.001), COPD having a medium impact (3–4 on a scale of 1–7) on getting up and ready for the day (P<0.001), and increased Jenkins sleep scale score (≥3 on a scale of 0–20; P=0.003). Results from the three regression analyses are shown in Table 4.
Table 4.
Regression analysis on movers
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| GOLD-recommended cut points | |||
| Age <70 years | 1.62 | 1.25–2.10 | <0.001 |
| Smoker | 1.31 | 1.01–1.70 | 0.042 |
| Cough in last 4 weeks | 1.47 | 1.08–1.98 | 0.013 |
| Condition having medium (3–5 on scale 0–10)a effect on daily activities in past week | 1.79 | 1.34–2.40 | <0.001 |
| Condition having low restriction (1–3 on scale 1–5)b on living a normal life | 2.76 | 1.86–4.09 | <0.001 |
| Not experiencing constant lack of energy | 1.77 | 1.30–2.42 | <0.001 |
| Condition having no/low impact (1–2 on scale 1–7)c on getting up and ready for the day | 2.84 | 1.75–4.60 | <0.001 |
| Condition having medium impact (3–4 on scale 1–7)c on getting up and ready for the day | 1.85 | 1.17–2.93 | 0.009 |
| Increased Jenkins sleep scale score (≥3 on a scale of 0–20)d | 1.78 | 1.28–2.47 | 0.001 |
| ROC-recommended cut points | |||
| Presence of cough in last 4 weeks | 1.34 | 1.01–1.76 | 0.040 |
| Condition having medium restriction (3 on a scale 1–5)b on living a normal life | 4.15 | 2.79–6.17 | <0.001 |
| Condition having high restriction (4–5 on a scale 1–5)b on living a normal life | 2.23 | 1.36–3.67 | 0.001 |
| Condition having no/low/medium impact (1–4 on scale 1–7)c on getting up and ready for the day | 1.96 | 1.27–3.04 | 0.003 |
| Increased Jenkins sleep scale score (≥3 on a scale of 0–20)d | 2.06 | 1.45–2.91 | <0.001 |
| Kappa statistic-recommended cut points | |||
| Age ≥55 years | 1.56 | 1.08–2.24 | 0.017 |
| Condition having medium/high restriction (3–5 on a scale 1–5)b on living a normal life | 3.27 | 2.19–4.89 | <0.001 |
| Condition having medium impact (3–4 on scale 1–7)c on getting up and ready for the day | 1.66 | 1.21–2.28 | 0.002 |
| Condition having high impact (5–7 on scale 1–7)c on getting up and ready for the day | 0.64 | 0.41–0.99 | 0.047 |
| Increased Jenkins sleep scale score (≥3 on a scale of 0–20)d | 1.72 | 1.20–2.47 | 0.003 |
Notes:
Where 0= no effect and 10= prevented from undertaking daily activities;
where 1= no restriction and 5= complete restriction on normal living;
where 1= no impact and 7= constant impact on getting up and ready for the day;
where 0= no impact on sleep and 20= high impact on sleep.
Abbreviations: CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ROC, receiver operating characteristic.
Regression analysis on moving left (from B/D to A/C) or right (from A/C to B/D)
There were insufficient patients moving left (n=3) for the GOLD-recommended cut points to run the regression. However, analysis of the ROC-recommended cut points showed the following were significantly associated with moving to the right: not being from France (P=0.014), being less than 55 years of age (P=0.005), the presence of a cough in the last 4 weeks (P=0.003), COPD having little or no impact (1–2 on a scale of 1–7) on getting up and ready for the day (P=0.032), and an increased Jenkins sleep scale score (≥7 on a scale of 0–20; P=0.001).
Analysis of the kappa statistic-recommended cut points showed the following were significantly associated with moving to the right: not being from France (P=0.047), being less than 55 years of age (P=0.002), being a smoker (P=0.018), the presence of a cough in the last 4 weeks (P=0.001), COPD having little or no impact (1–2 on a scale of 1–7) on getting up and ready for the day (P=0.007), and an increased Jenkins sleep scale score (≥7 on a scale of 0–20) (P=0.001). Detailed results are presented in Table 5.
Table 5.
Regression analysis on moving left or righta
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| ROC-recommended cut points | |||
| Not from France | 2.45 | 1.20–5.02 | 0.014 |
| Age <55 years | 4.07 | 1.51–10.96 | 0.005 |
| Presence of cough in last 4 weeks | 2.11 | 1.29–3.43 | 0.003 |
| Condition having little or no impact (1–2 on scale 1–7)b | 1.82 | 1.05–3.16 | 0.032 |
| On getting up and ready for the day | |||
| Increased Jenkins sleep scale score (≥7 on a scale 0–20)c | 2.53 | 1.47–4.37 | 0.001 |
| Kappa statistic-recommended cut points | |||
| Not from France | 2.12 | 1.01–4.46 | 0.047 |
| Age <55 years | 3.78 | 1.62–8.80 | 0.002 |
| Smoker | 1.82 | 1.11–2.99 | 0.018 |
| Presence of cough in last 4 weeks | 2.38 | 1.41–4.02 | 0.001 |
| Condition having little or no impact (1–2 on scale 1–7)b | 2.18 | 1.24–3.84 | 0.007 |
| On getting up and ready for the day | |||
| Increased Jenkins sleep scale score (≥7 on a scale 0–20)c | 2.38 | 1.41–4.02 | 0.001 |
Notes:
There were insufficient patients moving left to run this regression for GOLD-recommended cut points;
where 1= no impact and 7= constant impact on getting up and ready for the day;
where 0= no impact on sleep and 20= high impact on sleep.
Abbreviations: CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ROC, receiver operating characteristic.
Discussion
Our data would suggest that relying on one marker of COPD symptoms may potentially result in under treatment for many patients if based on mMRC. Indeed, GOLD recommends further symptom assessment in conjunction with mMRC administration. However, alignment of the two measures by moving the cut point will only be partially successful in better categorizing and stabilizing patients to their respective groups. There will likely still be some movers, though greatly reduced as we have shown, and it is these patients who will need additional and possibly more personalized treatment.
When modifying the cut point from the GOLD-recommended values (mMRC ≥2; CAT ≥10) to those which identified best alignment using the kappa statistic cut point (mMRC ≥2; CAT ≥26), the number of movers declines from 890 to 403. These movers are significantly associated with being older, with their COPD condition restricting or completely restricting their lives, with COPD having a medium or high impact on their ability to get up and ready for the day, and with an increased Jenkins sleep scale score. These remaining movers may represent patients who are more difficult to treat and who may require additional follow-up.
It is worth noting that movers from B/D to A/C are also significantly likely to be from France. However, as this is a borderline significance, it should be viewed with caution, as geographic regions should not affect COPD characteristics.
The results of our research concur broadly with a number of studies published in the last 2 years since GOLD 2011, which have shown that the heterogeneity of COPD does not easily translate to “grouping”; that the presence of comorbidities complicates diagnosis;22 and that group C, with less symptoms, does not necessarily translate into better outcomes.23 Since the GOLD strategy 2011, four large retrospective analyses of COPD cohorts24–27 have examined the relationships among the four GOLD groups. These have been synthesized by Agusti et al, finding that the prevalence of patients in each group may depend on the population studies and whether they are treated through a primary or secondary health setting.24
Further research on misalignment has been produced in analyses by Jones et al,29,30 which indicate as our current research does, that the two cut points for mMRC and CAT are not equivalent. Jones et al29 suggested that an mMRC score of ≥1 is approximately equal to a CAT score of ≥10 when diagnosing low symptom patients. Having examined the current GOLD cut points and noted, in Jones’ more limited analysis, the suggested mMRC/CAT score equivalent of ≥1/≥10, we chose to use as our starting point the baseline of mMRC cut point ≥2 since this is the midpoint between low and high symptoms. CAT offers more granularity and breadth of potential cut points to align against mMRC at ≥2 and thus, harmonizing the tools via this approach makes for a potentially more accurate “fine-tuning” of the cut points.
A study published in 2009 by Lacoma et al31 concluded that the influence of a number of factors, such as disease severity, comorbidities, and treatment, was more important in COPD management, with individual monitoring, than establishing cut points for the COPD population as a whole.31 While we acknowledge this point, we believe that our research offers the potential for more accurate grouping, while also advocating more personalized management for those who move groups and are likely more disease impacted patients than nonmovers. Moreover, our research suggests that with better aligned cut points, those in need of such individualized support will be a smaller number than those who currently move groups with the GOLD recommended cut points.
It may be questioned whether such studies present the clinician with more questions than answers. However, COPD is a global disease, and as an editorial by Franssen and Han32 reminds us, GOLD is a global strategy, and a simple, more uniform approach to COPD disease assessment, management, and treatment is key. Any COPD classification must therefore be easy to use but also provide sufficient information on the disease. Would a more precise alignment of mMRC and CAT therefore provide greater accuracy of grouping?
Ultimately, mMRC is a less sensitive tool than CAT for classifying COPD patients. It provides a baseline measurement of a patient’s COPD status through measuring breathlessness. Indeed, GOLD recommends use of a second measure of symptoms, such as CAT, in addition to mMRC for comprehensive symptom assessment.3 CAT addresses a broader scope of COPD factors, is sensitive to change and consequently management of exacerbations,8 and is thus well placed as an ongoing assessment tool. Our analysis shows that the two tools misclassify a substantial number of patients; a combination of the two, particularly if realigned, could therefore potentially provide more stable and uniform management and treatment of COPD patients.
Data collection through the DSP has some limitations. The collected sample is not truly random as it uses the next six eligible patients who consult the physician. It may, therefore, not represent the overall COPD population as it would capture those patients who may be more symptomatic or consult their doctors more frequently. Patient selection bias is therefore possible. Additionally, only data from those patients who chose to complete the voluntary patient self-completion form were used in this analysis. There could be potential differences between those patients who chose to complete the form and those who do not, possibly introducing additional bias. The quality of data collected depended on accurate reporting of information by both physicians and patients. However, as data for these analyses were collected at the time of the consultation and relate only to that consultation, recall bias is unlikely to be an issue. Finally, while minimal inclusion criteria governed the selection of participating physicians, inclusion will have been influenced by willingness to take part and practical considerations of location.
Despite these limitations, our research provides valuable real-world physician and patient matched data. The misalignment shown and statistical evidence for a possible change of cut points, to measure disease severity, would indicate that further research is necessary to understand, characterize, and differentiate the GOLD classification through mMRC and CAT.
Conclusion
Use of mMRC or CAT leads to inconsistencies for COPD assessment classification. The optimal clinical management of COPD patients, and a closer and more personalized approach to the treatment of movers in particular, may require alignment of assessment tool cut points, coadministration of these tools, and consideration of whether a higher cut point for CAT is appropriate. Our research may suggest an opportunity to investigate a combined score approach to patient management based on the worst result of mMRC and CAT. Subsequently, the reduced number of remaining movers may then identify patients who have greater impact of disease and may require a more personalized treatment plan.
Acknowledgments
The Disease Specific Program, on which the analyses were based, was designed and run by Adelphi Real World. The program was supported by a number of pharmaceutical companies, including Pfizer Inc. This specific analysis, together with this publication, was supported by Pfizer Inc. The decision to publish was made jointly by all authors cited. The authors acknowledge the contributions of Antonio Martin, MD, previously employed by Pfizer, for his contributions to the development of this analysis. Medical writing support and literature searching was provided by Maren White of White Quill Ltd.
Footnotes
Author contributions
DBP provided clinical direction and input to the objective of the analysis, made substantial contributions to the interpretation of data, critical revisions, and approved the final version for submission. CLB made substantial contributions to the design of the study, interpretation of the data, critical revisions, and approved the final version for submission. KHZ made substantial contributions to the statistical analysis and interpretation of the data, critical revisions, and approved the final version for submission. JTB made substantial contributions to the concept and design of the study and interpretation of the data. He was involved in the drafting of the article, critical revisions, and approved the final version for submission. JSP made substantial contributions to the statistical analysis and interpretation of the data, critical revisions, and approved the final version for submission. VSH made substantial contributions to the concept and design of the study and interpretation of the data. She was involved in the drafting of the article, critical revisions, and approved the final version for submission. VSH also takes responsibility for the integrity of the work as a whole, from inception to finished article.
Disclosure
CLB and KHZ are employees of Pfizer Inc, which sponsored this analysis. DBP was a paid consultant to Pfizer in connection with this analysis. VSH, JTB, and JSP are employees of Adelphi Real World. The authors report no other conflicts of interest in this work.
References
- 1.goldcopd.org [homepage on the Internet] Global strategy for the diagnosis, management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Accessed August 22, 2013]. Available from: http://www.goldcopd.org/program.html.
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD 2011) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [Accessed August 22, 2013]. Available from: http://www.goldcopd.org/Guidelines/guidelines-resources.html.
- 3.Global initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Global initiative for Chronic Obstructive Lung Disease; 2014. [Accessed August 22, 2013]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. [Google Scholar]
- 4.Leonard DT. Comparative effectiveness and real-world evidence [webpage on the Internet] American Journal of Managed Care; 2010. [Accessed August 22, 2013]. Available from: http://webcache.googleusercontent.com/search?q=cache:KU4nflw4a9gJ:www.ajmc.com/publications/issue/2010/2010-06-vol16-n06/AJMC_10jun_LeonardCom410to11+&cd=1&hl=en&ct=clnk&gl=ca. [PubMed] [Google Scholar]
- 5.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 6.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 7.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45(9):577–584. [PubMed] [Google Scholar]
- 10.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Society for Opinion and Marketing Research (EsoMAR) ICC/ESOMAR international code of marketing and social research practice. 1995. [Accessed April 25, 2014]. Available from: http://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ICCESOMAR_Code_English_.pdf.
- 12.US Department of Health and Human Services Summary of the HIPAA Privacy Rule May 2003 [webpage on the Internet] [Accessed August 22, 2013]. Available from: http://www.hhs.gov/ocr/privacy/hipaa/understanding/summary/privacysummary.pdf.
- 13.Market Research Society The Data Protection Act 1998 &market research: guidance for MRS members, September 2003. [Accessed August 22, 2013]. Available from: https//www.mrs.org.uk/pdg/DPA_1998_MR.pdf.
- 14.Zou KH, Liu A, Bandos AI, Ohno-Machado L, Rockette HE. Statistical Evaluation of Diagnostic Performance: Topics in ROC Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2011. [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 17.Zou KH, Yu CR, Liu K, Carlsson MO, Cabrera J. Optimal thresholds by maximizing or minimizing various metrics via ROC-type analysis. Acad Radiol. 2013;20(7):807–815. doi: 10.1016/j.acra.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins CD, Stanton B-A, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. Journal of Clinical Epidemiology. 1988;41(4):313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 21.StataCorp . Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 22.Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12:26. doi: 10.1186/1471-2466-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT™) scores. BMC Pulm Med. 2011;11:42. doi: 10.1186/1471-2466-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPD Gene: a prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 26.Soriano JB, Alfageme I, Almagro P, et al. Distribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classification. Chest. 2013;143(3):694–702. doi: 10.1378/chest.12-1053. [DOI] [PubMed] [Google Scholar]
- 27.Agusti A, Edwards LD, Celli B, et al. ECLIPSE Investigators Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42(3):636–646. doi: 10.1183/09031936.00195212. [DOI] [PubMed] [Google Scholar]
- 28.Agusti A, Hurd S, Jones P, et al. FAQs about the GOLD 2011 assessment proposal of COPD: a comparative analysis of four different cohorts. Eur Respir J. 2013;42(5):1391–1401. doi: 10.1183/09031936.00036513. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42(3):647–654. doi: 10.1183/09031936.00125612. [DOI] [PubMed] [Google Scholar]
- 30.Jones PW, Nadeau G, Small M, Adamek L. Characteristics of a COPD population categorised using the GOLD framework by health status and exacerbations. Respir Med. 2014;108(1):129–135. doi: 10.1016/j.rmed.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Lacoma A, Prat C, Andreo F, Dominguez J. Biomarkers in the management of COPD. Eur Respir Res. 2009;18(112):96–104. doi: 10.1183/09059180.00000609. [DOI] [PubMed] [Google Scholar]
- 32.Franssen FM, Han MK. The ABC of GOLD A-B-C-D. Eur Respir J. 2013;42(5):1166–1168. doi: 10.1183/09031936.00107813. [DOI] [PubMed] [Google Scholar]