Abstract
Purpose: To investigate the geometry, penetration force, and cutting profile of the novel and old needle of the drug delivery system (DDS) for Ozurdex injections in a standardized laboratory setting.
Methods: In this experimental study, the normative geometrical data of the DDS needle were systematically analyzed according to nomenclature DIN 13097 (ISO 7864) and ISO 9626. The force to penetrate a standardized 0.4-mm-thick polyurethane foil was measured by a penetrometer, when the needle was piercing, cutting, and sliding through the foil and plotted as a load-displacement diagram. Magnified images of the consecutive cut were taken after the entire penetration through the foil.
Results: In this experimental study, the mean point length was equal to 3.34 mm (3.28–3.36 mm) for the old DDS needle versus 3.33 mm (3.30–3.36 mm) for the new DDS needle. The secondary bevel length was 1.64 mm (1.42–1.73 mm) for the new and 1.66 mm (1.62–1.69 mm) for the old needle. The primary angle was 9.2° (9.0°–9.5°) for the old and 8.9° (8.5°–9.0°) for the new needle, respectively. The secondary bevel angle was 117.2° (116°–118°) for the old and 111.4° (110°–113°) for the new needle. The mean penetration force of the old DDS needles was significantly higher at all phases of the penetration experiment: The mean piercing force was 0.7 Newton (N) with the old and 0.47 N with the new DDS needle. The mean cutting force was remarkable higher with 1.1 N for the old DDS needle versus 0.78 N for the new DDS needle. The dilatation phase was not statistically significant between 0.94 and 0.99 N in both DDS needles. The friction phase was maintained at significantly higher levels with the old DDS needle of 0.47 N, whereas it returned to the lowest measurements of 0.11 N with the new DDS needle. Both DDS systems induced a characteristic chevron-shaped incision.
Conclusion: A comparison of the old and new DDS needles demonstrated a reduced penetration force with the modified new DDS needle, which may help to achieve a smooth penetration through the human sclera.
Introduction
Intravitreal injections of dexamethasone (DEX-implant, OZURDEX; Allergan, Inc., Irvine, CA) are currently used for the treatment of macular edema.1 The customized drug delivery system (DDS) injects a preloaded dose of 700 μg DEX transconjunctivally using a 22-gauge needle through a small scleral puncture into the vitreous cavity, avoiding an additional surgical suturing of the insertion site.2–6 For a safe application, we previously investigated in a comparative experimental study the velocity and drag force of the applied DEX implant in water and vitreous and calculated the proposed speed at the perpendicular retinal side.7 Although we measured a high initial muzzle velocity, we determined a fast decrease over time and distance making this application a safe and reliable procedure. These experimental measurements were confirmed in a clinical observational study,8 while we determined on 387 injections no retinal damage from the application of the retina.9 However, when numerous physicians complained about the low sharpness of the blunt DDS needle, Allergan decided to supply the DDS applicator with a novel and sharper needle.
The purpose of this experimental study was to investigate the geometry, mechanical penetration force, and cutting profile of the novel and old DDS needle.
Methods
Measurements of the sharpness of Ozurdex needle (MONO) study were performed by standardized penetration force experiments: 5 novel and 5 old DDS Ozurdex applicator systems were obtained in their original state from Allergan. The DDS applicators were carefully examined immediately after unpacking the blister package and removing the protecting plastic cap to detect, under a microscope, unexpected artificial damages before any penetration resistance measurement.
The penetration force measurement was performed according to the Standard DIN 13097-4. The penetration characteristic was recorded as the load/penetration length diagram, while the cannula was piercing the polyurethane foil.10
Nomenclature of the DDS needle tip
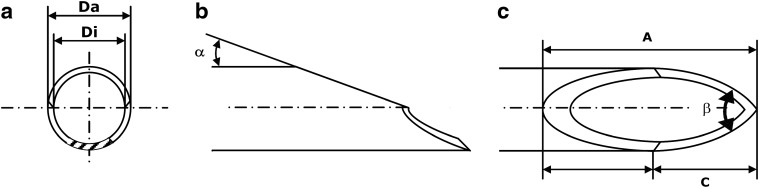
DDS needles are generally composed of a protruded tubular cannula with a facet bevel tip. The cannula size has an outer diameter of 22 gauge. Grinding and debarring of the front end classifies the medical needle. Its bevel has an angled surface on a shaft of the cannula to form the needle point, facilitating an atraumatic penetration through the sclera into the human globe. Each medical needle can be classified according to the design of the needle tip. By definition, the design consists of a characteristic medical bevel: The primary bevel is surfaced at the specific angle α at the front. Two additional bevels, the secondary grind angles β on each side, form the cutting edge. The bevel length is defined as the longest distance of a bevel, measured from the tip of the needle to the most proximate area of grinding behind the heel. The DDS needle contains a typical facet bevel design with 3-beveled tips on the shaft of the cannula to form a slanting edge at the sharpened needle point (Fig. 1a–c).
FIG. 1.
Bevel geometry. The drawing shows the needle from the front (a), side (b), and above view (c). The design is characterized by an angled surface on the shaft of the cannula to form a slanting edge at the sharpened needle point. The needle tip of the bevel is sharply angled. α, primary bevel angle; β, combined secondary bevel angle (facet angle); A, point length; C, secondary bevel length; Da, outside diameter; Di, inside diameter.
Normative geometry
Geometrical data of the 2 DDS needles were masked to the examiners and systematically measured according to the international nomenclature given in ISO 7864 (DIN 13097) and ISO 9626: The inner diameter (Di) and outer diameter (Da) of the DDS needle (Fig. 1a) were measured by a Mitutoyo micrometer caliper (S/N 8225 258; Mitutoyo Corporation, Kanagawa, Japan): Bevel and point length were measured with the Olympus stereomicroscope SZH (S/N 509 130) (Olympus Cooperation, Tokyo, Japan; 30× magnification). The primary bevel angle (α) (Fig. 1b) and combined secondary back bevel angle (β) (Fig. 1c) were analyzed by an optical laser inspection station FACET. The procedure was recently described in greater detail.10 The results of the measurements are listed in Table 1.
Table 1.
Normative Geometrical Data of the Old and New DDS Needles for Ozurdex Injections According to Nomenclature DIN 13097 (ISO 7864) and ISO 9626
| DDS applicator | Da (mm) | Di (mm) | A (mm) | C (mm) | α (°) | β (°) |
|---|---|---|---|---|---|---|
| Specification ISO 9626 22 gauge | 0.698–0.730 | >0.440 | ||||
| Old DDS needle | 0.714 | 0.502 | 3.34(3.28–3.36) | 1.64(1.42–1.73) | 9.2(9.0–9.5) | 117.2(116–118) |
| New DDS needle | 0.719 | 0.505 | 3.33(3.30–3.36) | 1.66(1.62–1.69) | 8.9(8.5–9.0) | 111.4(110–113) |
α, primary bevel angle; β, combined secondary bevel angle (facet angle); A, point length; C, secondary bevel length; Da, outside diameter; Di, inside diameter; DDS, drug delivery system.
Penetration force measurement
All penetration force measurements were performed according to DIN 13097: The tip of each needle was positioned in a perpendicular direction at a distance of 6 mm from a polyurethane foil (ISO 10555-5). The fixed standardized testing velocity of the push pull motor on the needle was 100 mm/min. The penetration resistance was measured and plotted as a load-displacement diagram by the Penetrometer DEKA 9 (DKA 0108) (Melba, Leonberg, Germany) from the piercing (F0), cutting (F1), sliding, and dilatation phase (F2).10 The investigators were blinded to the old and new needle design of the DDS applicator system. All DDS needles were measured 5 times under constant conditions.
Results
Geometry
Geometrical data of all 10 intact DDS needles are listed in Table 1. The mean outer diameter (Da) of the old and new DDS needle was 0.714 and 0.719 mm, respectively. The mean inner diameter (Di) was 0.502 mm for the old and 0.505 mm for the new DDS needle. The mean point length (A) was equal with 3.34 mm (3.28–3.36 mm) for the old DDS needle versus 3.33 mm (3.30–3.36 mm) for the new DDS needle. The secondary bevel length (C) was 1.64 mm (1.42–1.73 mm) for the new and 1.66 mm (1.62–1.69 mm) for the old needle. The primary angle (α) was 9.2° (9.0°–9.5°) for the old and 8.9° (8.5°–9.0°) for the new needle. However, the difference in the secondary grinding bevel angle (β) was 117.2° (116°–118°) for the old and 111.4° (110°–113°) for the new needle, respectively.
Penetration measurements
The mean penetration force of the old DDS needles was significantly higher at all phases of the penetration experiment: The mean piercing force (F0) was 0.7 Newton (N) with the old and 0.47 N with the new DDS needle. The mean cutting force (F1), which reflects the maximal measured force to the foil, was remarkably higher with 1.1 N for the old DDS needle versus 0.78 N for the new DDS needle. The dilatation phase (F2) was not statistically significantly different between 0.94 and 0.99 N in both DDS needles. The friction phase (F3) and sliding of the shaft were maintained at significantly higher levels with the old DDS needle, whereas it returned to the lowest measurements with the new DDS needle (Table 2).
Table 2.
Penetration Load Tests (DIN 13097-4) of the Old and New DDS Needles for Ozurdex Injections
| DDS applicator | Size (mm) | F0 (min–max) (N) | F1 (min–max) (N) | F2 (min–max) (N) | F3 (min–max) (N) |
|---|---|---|---|---|---|
| Old DDS needle | 22 gauge (0.7×15) | 0.70 (0.40–0.80) | 1.1 (0.87–1.2) | 0.94 (0.87–1.05) | 0.47 (0.29–0.63) |
| New DDS needle | 22 gauge (0.7×15) | 0.47 (0.36–0.58) | 0.78 (0.76–0.88) | 0.99 (0.91–1.11) | 0.11 (0.08–0.14) |
F0, maximum of the piercing phase; F1, maximum of the cutting phase superposed with F0; F2, maximum of the dilatation phase; FR, mean value of the friction phase.
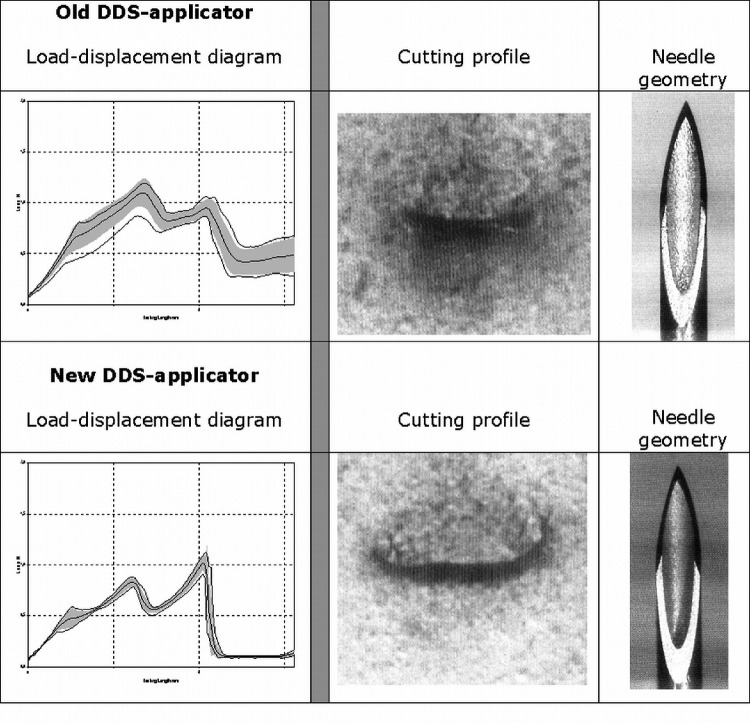
The corresponding load-displacement diagram with the old DDS needle demonstrated a significant peak as the needle passes through the foil (F0) (Fig. 2). Once the cannula of the DDS needle passed through, there was no significant peak in the penetration force (F0) measurement. The penetration force decreased significantly from the old to the new DDS needle as the needle heel penetrated the foil and also decreased immediately afterward.
FIG. 2.
The upper 3 images present results with the old drug delivery system (DDS) applicator, the lower 3 images with the new DDS applicator. The left graphs show the load-displacement diagram [y-axis load (N), x-axis testing length (mm)]. The images in the middle column present the cutting profiles of both needles. The right images show magnified image of the old and new DDS needles.
Geometric cutting profile
Both DDS systems induced a characteristic chevron-shaped incision. The novel cut seemed to keep the shaft tight to the edge of the penetration hole, maintaining the force at lower levels (Fig. 2).
Discussion
Friedenwald described the “ocular rigidity as a measure of the resistance, which extents to distending forces” and he developed a formula for ocular rigidity. The Friedenwald equation was the first attempt to quantify ocular rigidity by manometric measurements.11 The form and length of the induced cut are frequently influenced by the rigidity and thickness of the sclera, size of the globe, as well as the needle tip sharpness.12,13 The necessary manual force to puncture the sclera and the area of any needle point is predominantly influenced by the composition of its tissue.14 The initial puncture, which starts with the insertion, is accomplished when the DDS needle tip applies enough pressure (F0) to cause the collagen lamella of the sclera to displace (F2). The form and length of the induced cut are frequently influenced by the needle design. Pioneer studies inserted the DEX implant into the vitreous cavity using a 1.15 mm pars plana incision, which required additional suturing. Although the approach was well tolerated, the surgical procedure may induce vitreous hemorrhages. Subsequently, the DDS applicator with a 22-gauge needle was designed to simplify the intravitreal placement, to be more convenient creating a self-sealing wound, eliminating the need for suturing the insertion site, and reducing ocular trauma associated with the drug insertion.15
A sharp needle is mandatory for a safe and painless intravitreal injection. A brief comparison of the 27-gauge Becton Dickinson needle and 27-gauge prefilled Macugen syringe needle was previously reported in an observational study on 70 eyes by Kozak et al.16 They determined that the Becton needle was steeper and longer than the Macugen needle, thus resulting clinically in less force to penetrate the sclera and consecutively less pain for the patients. A larger survey on intravitreal anti-VEGF injections determined 2 lens damages [incidence rate 0.006% (2/32318)] and 5 retinal detachments [incidence rate 0.013% (5/35943)] during 35943 injections.13,14
In this study, we investigated the old and novel needle of the DDS applicator and determined significant modifications in the manufacturing techniques and sharpness of the needle tip. While the old DDS needle frequently required a high penetration force, the new DDS needle had remarkable low piercing and cutting forces. The chevron-shaped incision demonstrated proper wound closure even in vitrectomized eyes. The mean penetration force (F0) increased significantly when the needle tip came in contact with the foil and penetrated the foil. The steep angle of the tip and the pin pointed shape of the cut might be responsible for this profile. Subsequently, a low increase in force was observed, when the cannular tip reached and passed through the foil. The current innovations accomplished less resistance of the needle to the sclera, achieving a smooth blade insertion and facilitating a more efficient wound closure with better healing characteristics. This has been observed by 3 physicians, who tested both DDS needles in a masked fashion on the human sclera obtained from an eye bank. They were independently asked to penetrate the human sclera in a rectangular and angled fashion. All 3 examiners were able to determine the overall smoother penetration with the new—presumably sharper—DDS needle. However, due to the extremely short duration of the entire penetration process, they were not able to tell which interval of the injection (piercing, cutting, or dilatation) became predominantly easier. This observation was confirmed in a greater survey among treating physicians: A survey among the German retinal vein study group showed than all surgeons (n=8) had noticed a significantly easier penetration of the novel DDS needle during intravitreal application (n=293) compared with the old DDS needle design. Our measured values of the piercing, cutting, and dilatation forces were at a lower level with the new DDS needle compared with the old DDS needle.
The same setting was previously used to measure the sharpness of 23-gauge trocar systems for pars plana vitrectomy.10 A comparison between the here investigated 22-gauge DDS applicator systems and 23-gauge pars plana trocar systems demonstrated lower penetration forces (F0) (0.7 N for the old and 0.47 N for the new DDS applicator needles), whereas 23-gauge trocar systems like the spatula bevel tip required significantly higher penetration forces 1.6 N (range 1.59–1.73 N) to penetrate the same foil under standardized conditions according to ISO 10555-5.10,11 On the other hand, other models demonstrated similar measurements of conventional medical needle lower forces to penetrate the standardized 0.4 mm foil: the measured values were 0.54 N for F0, 0.58 N for F1, 0.76 N for F2 for a 29-gauge Klinion needle and 0.42 N for F0, 0.43 N for F1, 0.63 N for F2 for a 31-gauge Klinion needle. All these results underline the benefit of a sharp needle, small gauge diameter, and an enhanced needle design. The novel DDS applicator is a well-established applicator system to apply sustained release of DEX-implants into the vitreous cavity. With favorable confidence in intravitreal DEX injections (17,18,19) among treating physicians, the sharper novel DDS needle will increase in use as confirmed by our measurements in this experimental study.
Conclusion
The new DDS needle represents a significant improvement, reducing the amount of required force to penetrate the sclera. The accomplished cutting profile achieved a good wound architecture. These findings, evaluated by standardized ISO measurements, will support ongoing innovations to improve the needle design for intravitreal injections. Recent advances in the novel needle design make this technology reliable and promising to increase the ease of the injection and reduce the burden of repeated injections for our patients.
Author Disclosure Statement
The required DDS applicator systems containing the old and new needles were obtained from Allergan. Dr. Meyer serves as a consultant and speaker for Novartis, Bayer, and Allergan. Dr. Rodrigues received research grants for Allergan.
References
- 1.Haller J.A., Bandello F., Belfort R., Jr., Blumenkranz M.S., Gillies M., Heier J., Loewenstein A., Yoon Y.H., Jacques M.L., Jiao J., Li X.Y., and Whitcup S.M.; OZURDEX GENEVA Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 117:1134–1146, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Lang G.E.Diabetic macular edema. Ophthalmologica. 227Suppl 1:21–29, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Callanan D.G., Gupta S., Boyer D.S., Ciulla T.A., Singer M.A., Kuppermann B.D., Liu C.C., Li X.Y., Hollander D.A., Schiffman R.M., and Whitcup S.M.; Ozurdex PLACID Study Group. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 120:1843–1851, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Querques L., Querques G., Lattanzio R., Gigante S.R., Del Turco C., Corradetti G., Cascavilla M.L., and Bandello F.Repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmologica. 229:21–25, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Guignier B., Subilia-Guignier A., Fournier I., Ballonzoli L., Speeg-Schatz C., and Gaucher D.Prospective pilot study: efficacy of intravitreal dexamethasone and bevacizumab injections in the treatment of macular oedema associated with branch retinal vein occlusion. Ophthalmologica. 230:43–49, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Bezatis A., Spital G., Höhn F., Maier M., Clemens C.R., Wachtlin J., Lehmann F., Hattenbach L.O., and Feltgen N.Functional and anatomical results after a single intravitreal Ozurdex injection in retinal vein occlusion: a 6-month follow-up—the SOLO study. Acta Ophthalmol. 91:340–347, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Meyer C.H., Klein A., Alten F., Liu Z., Stanzel B.V., Helb H.M., and Brinkmann C.K.Release and velocity of micronized dexamethasone implants with an intravitreal drug delivery system: kinematic analysis with a high-speed camera. Retina. 32:2133–2140, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Khurana R.N., Appa S.N., McCannel C.A., Elman M.J., Wittenberg S.E., Parks D.J., Ahmad S., and Yeh S.Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 121:67–71, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Schmitz K., Maier M., Clemens C.R., Höhn F., Wachtlin J., Lehmann F., Bertelmann T., Feltgen N., and Bezatis A.Reliability and safety of intravitreal Ozurdex injections: The ZERO study. Ophthalmologe. 111:44–52, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Meyer C.H., Kaymak H., Liu Z., Saxena S., and Rodrigues E.B.Geometry, penetration force and cutting profile of different 23-gauge trocars systems for pars-plana vitrectomy. Retina. [In press]. [DOI] [PubMed] [Google Scholar]
- 11.Pallikaris I.G., Kymionis G.D., Ginis H.S., HS, Kounis G.A., and Tsilimbaris M.K.Ocular rigidity in living human eyes. Invest. Ophthalmol. Vis. Sci. 46:409–414, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Meyer C.H., Krohne T.U., Chabrel Issa P., and Holz F.G.Routes for drug delivery to the eye and retina: intravitreal injections and vitrectomy, Chapter 12. In: Nguyen Q.D., Rodrigues E.B., Farah M.E., and Mieler W.F., eds. Retinal Pharmacotherapy. Elsevier Publications New York, USA; 2009; pp. 67–73 [Google Scholar]
- 13.Meyer CH, Michels S., Rodrigues E.B., Hager A., Mennel S., Schmidt J.C., Helb H.M., and Farah M.E.Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 89:70–75, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues E.B., Meyer C.H., Grumann A., Jr., Shiroma H., Aguni J.S., and Farah M.E.Tunneled scleral incision to prevent vitreal reflux after intravitreal injection. Am. J. Ophthalmol. 143:1035–1037, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Haller J.A., Dugel P., Weinberg D.V., Chou C., and Whitcup S.M.Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 29:46–51, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Kozak I., Dean A., Clark T.M., Falkenstein I., and Freeman W.R.Prefilled syringe needles versus standard removable needles for intravitreous injection. Retina. 26:679–683, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mayer W.J., Remy M., Wolf A., Kook D., Kampik A., Ulbig M., Reznicek L., and Haritoglou C.Comparison of intravitreal bevacizumab upload followed by a dexamethasone implant versus dexamethasone implant monotherapy for retinal vein occlusion with macular edema. Ophthalmologica. 228:110–116, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Noma H., Funatsu H., Mimura T., Eguchi S., and Shimada K.Inflammatory factors in major and macular branch retinal vein occlusion. Ophthalmologica. 227:146–152, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Zucchiatti I., Lattanzio R., Querques G., Querques L., Del Turco C., Cascavilla M.L., and Bandello F.Intravitreal dexamethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 228:117–122, 2012 [DOI] [PubMed] [Google Scholar]